Abstract
Despite the efficacy and widespread use of methylphenidate (MPH) as a treatment for Attention Deficit Hyperactivity Disorder (ADHD), clinical and preclinical findings indicate it has abuse potential. Environmental enrichment reduces susceptibility to cocaine and amphetamine self-administration, and decreases impulsive behavior, but its effects on MPH self-administration are unknown. The present experiments sought to determine the influence of environmental enrichment on MPH self-administration. Male rats were raised in an enriched condition (EC) or isolated condition (IC). They were trained to self-administer MPH (0.3 mg/kg/infusion) and then exposed to varying doses of MPH on either a fixed ratio (FR; Experiment 1) or progressive ratio (PR; Experiment 2) schedule of reinforcement. EC rats earned significantly fewer infusions of MPH at low doses (0.03 and 0.056 mg/kg/infusion) than IC rats under both schedules; however, no differences were observed at high unit doses (0.1–1.0 mg/kg/infusion). During saline substitution at the end of MPH self-administration, EC rats also responded less for saline than IC rats, indicative of more rapid extinction. As with other stimulant drugs with different mechanisms of action, environmental enrichment during development protects against self-administration of MPH at low unit doses, but not at high unit doses.
Keywords: Environmental enrichment, methylphenidate, self-administration, rat, progressive ratio
Introduction
Methylphenidate (MPH) is a widely prescribed psychomotor stimulant for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in children, adolescents and adults. There has been a considerable rise in ADHD diagnoses over the past decade accompanied by an increase in MPH prescription rates (Hoagwood et al. 2000). Currently, MPH comprises approximately 56% of ADHD prescriptions (Setlik et al. 2009). While MPH is effective for managing the symptoms of ADHD, its potential for abuse is high, especially among adolescents and young adults (see Brown et al. 2005; Kollins et al. 2001, for review). For example, misuse of MPH has been reported by students in elementary school, middle school, high school and college and as many as 16% of elementary school children have been asked to sell, trade or give away their prescription MPH (McCabe et al. 2004; Teter et al. 2006; Musser et al. 1998).
There is clear evidence that abuse of MPH stems from its reinforcing properties. Children diagnosed with ADHD will choose capsules containing MPH more often than placebo or no capsules (MacDonald and Kollins 2000). Also, stimulant abusing and non-abusing adults will respond for MPH capsules at rates similar to those responding for amphetamine (Rush et al. 2001; Stoops et al. 2004). MPH also functions as a reinforcer in non-human laboratory animals. For example, rats, dogs and non-human primates will self-administer MPH intravenously in a dose-dependent manner at rates similar to cocaine and amphetamine (Bergman et al. 1989; Botly et al. 2008; Griffiths et al. 1975; Johanson and Schuster 1975; Marusich et al. 2010, 2011a; Risner and Jones 1976). One criterion for drug reinforcement is that subjects respond specifically because of the contingency to earn drug (Meisch, 1987), and previous work from our laboratory has demonstrated that MPH self-administration meets this criterion (Marusich et al. 2010). Also, rats given extended access to MPH self-administration at a low unit dose (0.1 mg/kg/infusion) will show an escalation of intake across sessions (Marusich et al. 2010). This dysfunctional pattern of drug intake (i.e. an escalation in the amount of drug taken over time) is a hallmark sign of substance use disorders (Koob and Kreek 2007). Moreover, drug discrimination studies across species indicate that the discriminative stimulus effects of amphetamine, methamphetamine and cocaine generalize to MPH (Kollins et al. 2001; Rush and Baker 2001; Rush et al. 1998; Sevak et al. 2009).
While genetics and individual characteristics (e.g. age, sex, impulsivity) can influence susceptibility to drug abuse, the physical and social environment can also mediate the reinforcing effects of drugs. The environmental enrichment paradigm has revealed a host of effects on drug-seeking and drug-taking behaviors in rats. With this experimental design, rats are housed for several weeks in either an enriched condition (EC) with novel objects and social cohorts, or an isolated condition (IC) without novel objects or social cohorts (Stairs and Bardo 2009, Simpson and Kelly 2011).
Environmental enrichment appears to have a protective effect against stimulant abuse. EC rats self-administer less cocaine or amphetamine on fixed-ratio (FR) schedules compared to IC rats (Bardo et al. 2001; Gipson et al. 2011; Green et al. 2002; Howes et al. 2000). However, these differences in self-administration are only observed at low unit doses and diminish at higher doses. Similar results have been found using progressive-ratio (PR) schedules. PR schedules measure the reinforcing effect of a drug by systematically increasing the response requirement until the animal no longer responds (Richardson and Roberts 1996; Stafford et al. 1998). IC rats expend more effort than EC rats to receive a single infusion of a low unit dose of cocaine or amphetamine (Bardo et al. 2001; Green et al. 2002; Smith et al. 2009). Additionally, the presence of social cohorts and/or novel objects can affect escalation, extinction and reinstatement of drug-seeking behavior (Chauvet et al. 2009; Gipson et al. 2011; Thiel et al. 2011).
Environmental enrichment may influence drug-taking behavior due to its ability to decrease impulsivity. Clinical and preclinical studies have found that subjects with greater inhibitory control are less prone to self-administer and abuse drugs (see Perry and Carroll 2008, Carroll et al. 2009 for reviews). EC rats show greater response inhibition and less premature anticipatory responding than rats raised in isolation (Ough et al. 1972; Wood et al. 2006). Perry et al. (2008) also showed that acute MPH or amphetamine, both widely prescribed ADHD medications, significantly reduced impulsive choice in IC rats, but not EC rats. However, it remains to be determined if enrichment-induced differences occur with MPH self-administration (Bardo et al. 2001; Green et al. 2002). In contrast to amphetamine, MPH does not reverse the dopamine transporter (DAT) or serotonin transporter (SERT), but rather blocks uptake of both dopamine (DA) and norepinephrine (Han and Gu 2006; Wilens 2008). However, given their common abuse liability, it was hypothesized that environmental enrichment would reduce MPH self-administration using either FR or PR schedules of reinforcement.
Methods
Animals and Rearing Conditions
Subjects were male Sprague-Dawley rats that were 21 days old upon arrival (Harlan Industries, Indianapolis, IN). Rats were assigned randomly to an EC or IC group. Beginning at 21 days of age, rats were housed in their respective conditions. EC rats were housed in groups of 6–12 rats in large stainless steel wire cages (60 × 120 × 45 cm) with solid steel floors and pine bedding. They were exposed to fourteen hard, non-chewable plastic objects placed randomly in the cage. Each day, 7 of the 14 objects were replaced with new plastic objects. The remaining 7 objects were also rearranged to maximize novelty. Plastic objects included tubes, balls, and toys. EC rats were handled daily. IC rats were housed individually in wire mesh hanging cages (17 × 24 × 20 cm) with solid metal side and back walls. These rats were not handled until the experimental protocol began and were not given exposure to plastic objects. Rats were maintained in the same home environment throughout the duration of the experiment. All rats were experimentally and drug naïve prior to the beginning of the experiment. Rats were housed in a colony on a 12:12 hr light/dark cycle (lights on at 7:00 am). They had free access to water and food in the home cage. Experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Kentucky, and followed the principles of laboratory animal care (National Research Council 1996).
Apparatus
Experimental sessions were conducted in standard operant conditioning chambers for rats (28 cm × 24 cm × 25 cm; ENV-001; MED Associates, St. Albans, VT) housed inside sound-attenuating chambers (ENV-018M; MED Associates). Retractable levers (4.5 cm) were located on the front wall 6 cm above the floor on each side of a recessed food receptacle. White stimulus lights (28 V; 3 cm in diameter) were located 3 cm above each lever. Side walls were made of Plexiglas, the front and back walls were aluminum, and floors were made of metal rods. A fan located inside the sound-attenuating chamber produced noise to mask extraneous sounds. Experimental events were arranged and recorded by MED-PC software (Med-Associates) on a computer in the experimental room.
Surgery
Prior to any experimental manipulations, rats were surgically implanted with a chronic indwelling jugular catheter (0.2 mm in diameter) at approximately post-natal day 55. Rats were anesthetized with ketamine and diazepam (100 mg/kg and 5 mg/kg, respectively, i.p.). One end of the catheter was inserted into the jugular vein, and the other end was attached to a metal cannula that exited subcutaneously. The cannula was secured in a head mount adhered to the skull with dental acrylic and metal jeweler’s screws. Rats were given one week to recover before self-administration began. Catheter patency was maintained by 0.2 ml infusions of a mixture containing 20 ml saline, 0.6 ml heparin (1000 USP units/ml), and 0.2 ml gentamicin (10 mg/ml) administered daily after experimental sessions. Prior to daily experimental sessions, cannula were attached to tubing within a flexible, spring covered leash (PHM-120; MED Associates) that was connected to a swivel (PHM-115; MED Associates) outside the operant conditioning chamber. This tubing exited the operant chamber and was connected to an infusion pump (PHM-100; MED Associates) located adjacent to the sound-attenuating chamber.
Experiment 1
Acquisition of MPH self-administration
Following surgery, rats were trained to press a lever for intravenous MPH (0.3 mg/kg/infusion, 0.1 ml/infusion, 5.9 sec/infusion) through the method of autoshaping (Carroll and Lac, 1993). Rats were exposed to 1 hr autoshaping sessions for seven consecutive daily sessions in which the active (drug) lever was extended into the chamber on a random time 60-sec schedule. Following 15 sec of lever extension or a lever press, the lever retracted and an infusion of MPH was delivered. The infusion was paired with a 20-sec timeout signaled by the illumination of both stimulus lights during which lever pressing had no programmed consequence. The inactive lever (no drug) was present continuously, except during timeouts. The side of the operant conditioning chamber corresponding to the active lever was counter balanced across rats. Rats were delivered 10 infusions of MPH during the first 15 min of the session. Rats then remained in the chamber for an additional 45 min with only the inactive lever present (no drug infusions). Autoshaping sessions were paired with a subsequent daily session in which MPH (0.3 mg/kg/infusion) was available on an FR 1 schedule for 60 min, with the active lever being the same one paired with drug during the autoshaping session. The daily autoshaping and FR 1 sessions were separated by approximately 60 min. Following seven consecutive days of paired autoshaping and FR 1 sessions, autoshaping sessions were terminated, but FR sessions continued; rats were given access to MPH (0.3 mg/kg/infusion) on an FR 1 schedule for three consecutive days, followed by three days on FR 2, three days on FR 3, three days on FR 4, and seven days on FR 5. All FR sessions were 60 min in duration.
MPH dose-effect determination using a FR schedule
During the next phase of the experiment, rats were given access to different doses of MPH for self-administration on an FR 5 schedule of reinforcement. Subjects were exposed to 0.03, 0.056, 0.1, 0.56 and 1.0 mg/kg/infusion MPH, with half the rats in each housing group exposed to these doses in ascending order and the other half exposed to these doses in descending order. Rats were given access to each dose for three consecutive sessions. Finally, all rats were tested on saline substitution for seven consecutive sessions.
Experiment 2
Acquisition of MPH self-administration
Autoshaping and initial FR training was similar to that described in Experiment 1.
MPH dose-effect determination using a PR schedule
Following acquisition, rats were given access to different doses of MPH for self-administration on a PR schedule of reinforcement. The response requirement required to earn an infusion increased exponentially (1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268 etc.; Richardson and Roberts 1996) following each infusion. Each 0.1 ml infusion was followed by a 20-sec timeout signaled by the illumination of both stimulus lights. Sessions lasted 5 hr and were ended prematurely if there was no responding on the active lever for at least 1 hr. At the start of this phase, subjects were given access to 0.3 mg/kg/infusion on the PR schedule for a minimum of three consecutive days. Upon reaching stability (i.e. < 20% variability in the number of infusions earned across 3 consecutive sessions) with the training dose, rats were then exposed to 0.03, 0.1, 0.3 and 1.0 mg/kg/infusion MPH in either ascending or descending order. Rats were given access to each dose for three consecutive sessions and the order in which they proceeded through the doses was counterbalanced for each housing condition. Finally, all rats were tested on saline substitution for seven consecutive sessions. Doses were selected based on those from Experiment 1.
Drug
Methylphenidate HCl (Mallinckrodt, St Louis, MO) was prepared in sterile 0.9% NaCl (saline).
Statistical Analyses
The phases were defined as acquisition and dose-effect determination (including saline). Acquisition was further divided into autoshaping and incremental FR phases. To determine acquisition of MPH self-administration, the number of infusions and inactive lever presses were analyzed separately with mixed factor analyses of variance (ANOVAs). Schedule and session served as within-subject factors and environmental condition served as a between-subject factor. Data for statistical and graphical analysis included each of the seven FR 1 sessions during autoshaping and the final three sessions on each FR schedule (FR 1-FR 5) during the FR phase.
For the dose-effect determination phase, the number of infusions earned and inactive lever presses were analyzed with a mixed factor ANOVA, with dose and session as within-subject factors and environmental condition and dose order (ascending or descending) as between-subject factors. Data for statistical and graphical analysis included the final two sessions of exposure to each dose and the final three sessions of exposure to saline. All tests were considered significant at p<0.05. Significant interactions were subject to post-hoc Bonferroni-corrected paired samples t tests that were considered significant at p<0.007 (with 7 comparisons) in Experiment 1, and p<0.01 (with 5 comparisons) in Experiment 2. If a catheter malfunctioned, the rat was removed from the experiment, and all data from that rat were excluded from that phase.
Results
Experiment 1
Acquisition
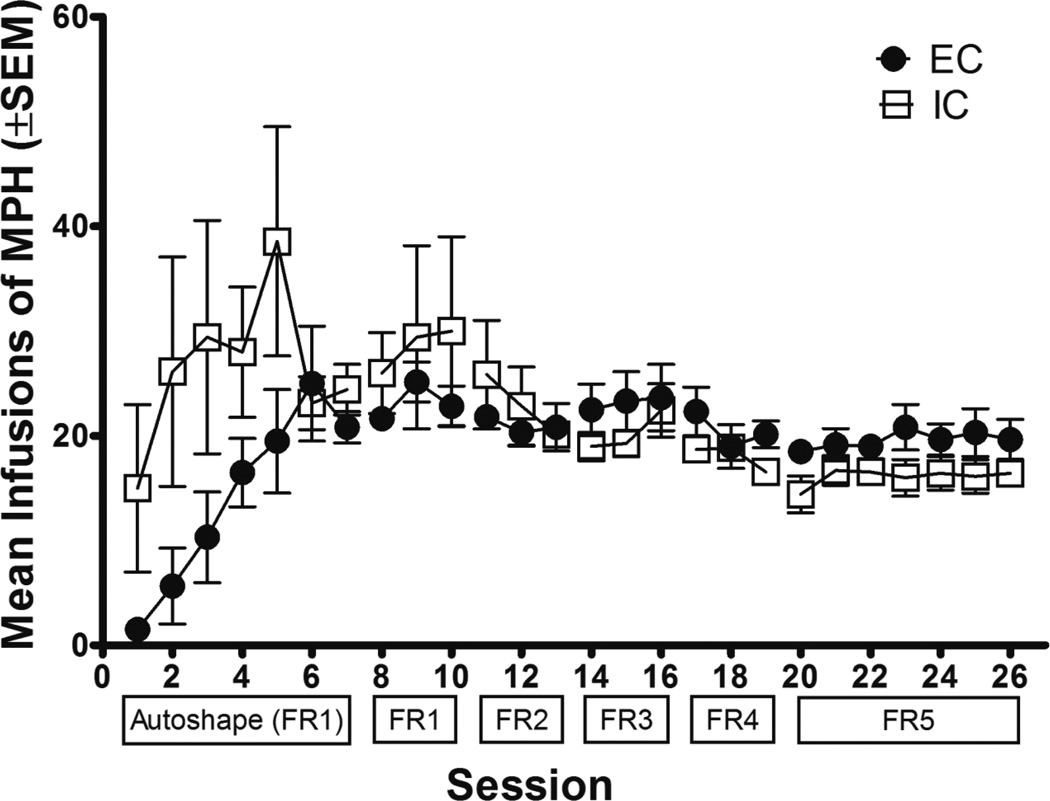
Figure 1 depicts the number of infusions earned during acquisition of MPH self-administration (EC n=9; IC n=9). During the autoshaping phase, a 2-way ANOVA (2 × 7; Environment × Session) revealed a significant main effect of session [F(6, 96)= 4.38, p<0.001], but no significant main effect of environment or interaction. During the incremental FR phase, a 3-way ANOVA (2 × 5 × 3; Environment × Schedule × Session) revealed a significant main effect of schedule [F(4, 64)= 7.68, p<0.001], in which the number of infusions declined with increasing FR values for both EC and IC rats. There were no differences in responding on the inactive lever during any phase of acquisition (results not shown).
Fig. 1.
Number of infusions earned (mean ± SEM) by EC (n=9) and IC (n=9) rats during acquisition of MPH self-administration (0.3 mg/kg/infusion) across incremental FR schedules.
Dose-effect determination
Figure 2 depicts the number of infusions earned for each unit dose of MPH and saline under an FR 5 schedule (EC n=9; IC n=8). A 4-way ANOVA (2 × 7 × 2 × 2; Environment × Dose × Dose Order × Session) revealed a significant main effect of environment [F(1, 13)= 6.32, p<0.05] and dose [F(6, 78)= 24.45, p<0.001], and an environment × dose interaction [F(6, 78)= 4.93, p<0.001]. A Bonferroni corrected pairwise comparison indicated that there was a significant difference between EC and IC rats in the number of infusions earned only at the 0.056 mg/kg/infusion dose and saline. There was significant main effect of dose on responding on the inactive lever [F(6, 78)= 2.81, p<0.05], in which greater responding on the inactive lever occurred at lower doses compared higher doses (results not shown). There was no effect of dose order on either the number of infusions earned or inactive lever presses.
Fig. 2.
Number of infusions earned (mean ± SEM) by EC (n=9) and IC (n=8) rats under a FR5 schedule, plotted as a function of MPH dose. S = saline vehicle; Asterisk (*) = significant difference between EC and IC rats, p< 0.001.
Experiment 2
Acquisition
Figure 3 illustrates the number of infusions earned during acquisition of MPH self-administration (EC n=6; IC n=7). During the autoshaping phase, a 2-way ANOVA (2 × 7; Environment × Session) revealed a significant main effect of environment [F(1, 11)= 4.70, p<0.05] and session [F(6, 66)= 2.47, p<0.05], indicating that EC rats earned fewer infusions than IC rats and that both groups earned more infusions as sessions progressed. During the incremental FR phase, a 3-way ANOVA (2 × 5 × 3; Environment × Schedule × Session) revealed a significant main effect of schedule [F(4, 44)= 2.75, p<0.05], with the number of infusions earned declining as the FR value increased. There were no differences in responding on the inactive lever during either phase of acquisition (results not shown).
Fig. 3.
Number of infusions earned (mean ± SEM) by EC (n=6) and IC (n=7) rats during acquisition of MPH self-administration (0.3 mg/kg/infusion) across incremental FR schedules.
Dose-effect determination
The dose-effect determination for MPH self-administration under a PR schedule is shown in Fig 4 (EC n=6; IC n=7). A 4-way ANOVA (2 × 5 × 2 × 2; Environment × Dose × Dose Order × Session) revealed a significant main effect of dose [F(4, 36)= 75.49, p=0.00] and an environment × dose interaction [F(4, 36)= 4.57, p<0.01]. Post-hoc Bonferroni corrected pairwise comparisons revealed that EC rats earn significantly fewer infusions than IC rats on a PR schedule of reinforcement at the 0.03 mg/kg/infusion unit dose of MPH [t(24)=4.68, p<0.05], and during saline substitution [t(24)=3.84, p<0.05]. There was a significant main effect of dose order [F(1,9)= 16.02, p<0.01] and a dose × dose order interaction [F(4, 36)= 3.22, p<0.05]. Post-hoc Bonferroni corrected pairwise comparisons indicated that rats receiving the doses in descending order earned more infusions at the 0.1 [t(24)=3.58, p<0.05], 0.3 [t(24)=3.62, p<0.05] and 1.0 mg/kg/infusion [t(24)=3.66, p<0.05] doses compared to rats receiving the doses in ascending order. There was also a significant effect of dose on inactive lever presses [F(4, 36)= 5.28, p<0.01], with the most inactive responding occurring at the training dose (0.3 mg/kg/infusion).
Fig. 4.
Number of infusions earned (mean ± SEM) at breakpoint by EC (n=6) and IC (n=7) rats under a PR schedule, plotted as a function of MPH dose. S = saline vehicle; Asterisk (*) significant difference between EC and IC rats, p< 0.001.
Discussion
MPH is a reinforcer in rats (Botly et al. 2008; Marusich et al. 2010, 2011a) and this is the first study, to our knowledge, to examine the influence of environmental enrichment on MPH self-administration. Rats were raised in enriched or isolated conditions and were trained to self-administer MPH (0.3 mg/kg/infusion). Dose-dependent changes in the reinforcing effects of MPH were then determined with FR and PR schedules. Our results indicate that providing enrichment during development protects against self-administration of MPH at low unit doses, but these protective effects do not generalize to higher doses.
The literature supports that environmental enrichment, in the form of social cohorts and/or novel objects, has a number of protective effects across the different stages of drug abuse, including acquisition, maintenance, escalation, extinction/withdrawal and reinstatement (Bardo et al. 2001; Smith et al. 1997; Gipson et al. 2011; Theil et al. 2011; Chauvet et al. 2009; Stairs et al. 2006; Ranaldi et al. 2011). Specifically, others show that rats raised in an enriched environment self-administer less amphetamine and cocaine at low unit doses than rats raised in isolation. For example, EC rats earned fewer infusions of a low dose of amphetamine under both FR and PR schedules than IC rats (Smith et al. 1997; Bardo et al. 2001; Green et al. 2002). Similarly, rats raised with social cohorts respond less than rats raised in isolation for a low dose of cocaine under FR and PR schedules (Boyle et al. 1991; Howes et al. 2000; Smith et al. 1997; Smith et al. 2009). The dose-effect functions under both the FR and PR schedules in this study with MPH mirror the dose-effect functions described in these previous experiments. That is, EC rats earned fewer infusions and had lower breakpoints than IC rats at low doses of MPH (0.03 and 0.056 mg/kg/infusion), suggesting that environmental enrichment reduces sensitivity to the reinforcing effect of MPH. However, because the protective effect exists only at low doses, the translational significance of these findings may be limited.
In contrast to the protective effect seen during dose-effect determination, enrichment did not have a protective effect against the acquisition of MPH self-administration in Experiment 1. However, in Experiment 2, EC rats earned fewer infusions of MPH than IC rats during the initial autoshaping phase; this effect diminished across sessions and was not observed during the FR phase. These findings contrast with previous work showing that EC rats acquire cocaine and amphetamine self-administration more slowly than IC rats (Green et al. 2002; Howes et al. 2000). However, in these latter studies, differential rates of acquisition only occurred with a low unit dose of cocaine or amphetamine. Whether EC or IC rats differ in the acquisition of MPH at doses other than the training dose used here (0.3 mg/kg/infusion) remains to be determined.
Several explanations may account for environment-induced differences in MPH self-administration. First, environmental enrichment may alter neuronal function. EC rats have reduced DAT functioning in mPFC, as indicated by a decrease in maximum velocity of [3DA] uptake, compared to IC rats (Zhu et al. 2004; Wooters et al. 2011). EC rats also have a decrease in DAT cell surface expression in this region (Zhu et al. 2005). As a link within the mesocorticolimbic dopamine neurocircuitry, the mPFC is involved in the rewarding effects of drugs (Tzschentke, 2000). Since the reinforcing effect of MPH is likely due to DAT inhibition (Wilens 2008, Leonard et al. 2004), perhaps the enrichment-induced decrease in DAT function in mPFC may attenuate the ability of MPH to serve as a reinforcer in EC rats.
An alternative explanation for the environment-induced differences in MPH self-administration may involve differences in impulsivity. Impulsivity has largely been considered as a predictor of drug abuse susceptibility (Perry and Carroll 2008; Carroll et al. 2009). EC rats are less impulsive than IC rats on various tasks (Ough et al. 1972; Wood et al. 2006; Perry et al. 2008); therefore, EC rats may self-administer less MPH due to less impulsive behavior. Consistent with this hypothesis, rats with DA depletions in mPFC show enhanced impulsivity compared to non-depleted controls (Sokolowski and Salamone 1994). Because IC rats have increased DAT functioning in the mPFC compared to EC rats (Zhu et al. 2004, 2005), and presumably reduced levels of extracellular DA in this region, this may explain their impulsivity and propensity to self-administer. On the contrary, Marusich et al. (2011b) did not find significant relationships between impulsivity (using the delay-discounting and cued go/no-go tasks) and DA uptake or DAT affinity in mPFC of standard-housed rats.
Regardless of the neural mechanisms, a behavioral explanation for the environment-induced difference in MPH self-administration at low unit doses may involve differences in the rate of extinction or the reinstatement threshold. Stairs and Bardo (2009) suggest that total drug intake must surmount some minimum threshold to engender responding at the beginning of a session. If the unit dose of a drug is too low, responding may extinguish. However, several low dose infusions in rapid succession may surpass the threshold and reinstate responding. Thus, diminished responding by EC rats at low unit doses may be a result of an increased rate of extinction within a session or by an increased reinstatement threshold. For example, IC rats reinstate responding after a period of extinction following a low dose priming injection of amphetamine, whereas EC rats require a high dose priming injection for reinstatement (Stairs et al. 2006). While the current experiment did not examine reinstatement of MPH-seeking specifically, differences in responding at the 0.03 and 0.056 mg/kg/infusion doses support this explanation.
In addition to MPH self-administration, EC rats responded less than IC rats when saline was substituted for MPH at the end of the experiment. This result is in accord with several other reports which found that EC rats emit fewer responses than IC rats under extinction conditions, indicative of enhanced extinction within a session (Green et al. 2002; Smith et al. 2009; Stairs et al. 2006; Gluck and Pearce 1977). Enhanced extinction may be reflective of improved learning by EC rats, as enrichment induces a number of neuroanatomical changes associated with enhanced learning (Diamond 2001; Rosenzweig and Bennett 1996; Kolb et al. 2003). Alternatively, during saline substitution, EC rats may be less sensitive to either the secondary reinforcing effects of the cue light associated with MPH or the incentive motivation provided by the drug-associated lever (Beckmann and Bardo 2012; Robinson and Berridge 2008), or may be less inclined to respond for the novelty provided by the cue lights signaling the time out (Cain et al. 2006). In any case, since EC and IC rats did not differ in responding on the inactive lever, it is likely that environment-induced differences reflect specific behaviors that are goal-directed, rather than nonspecific differences in general activity.
In conclusion, MPH is an effective treatment for reducing the symptoms of ADHD (e.g. impulsivity) in human and non-human subjects (Perry et al. 2008; Brown et al. 2005) and prescription stimulants may protect against the development of substance use disorders (Biederman et al. 1999; Wilens et al. 2003; but see Mannuzza et al. 2003; Herin et al. 2010). Nevertheless, much evidence indicates abuse potential. For example, animals used to model ADHD (e.g., IC rats or spontaneously hypertensive rats) find MPH to be rewarding and will self-administer the drug (Marusich et al. 2011c; Dela Peña et al. 2011). Also, college students with hyperactive symptoms and high scores on measures of sensation-seeking are more likely to report misuse of their prescription stimulant ADHD medication than students without these characteristics (Jardin et al. 2011). They are also more likely to report lifetime use of other illicit substances (i.e. nicotine, marijuana, cocaine, hallucinogens, opiates; Jardin et al. 2011). To the extent that enrichment may reduce signs of ADHD, the current results indicate further that enrichment also may reduce abuse of ADHD medications.
Acknowledgements
The authors thank W. Travis McCuddy, Luke Holderfield, Kate Fischer and Emily Denehy for technical assistance.
Research supported by NIH grants R01 DA12964, P50 DA05312 and T32 DA016176.
Footnotes
Conflicts of Interest
There are no conflicts of interest.
References
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Bardo MT. Environmental enrichment reduces attribution of incentive salience to a food-associated stimulus. Behav Brain Res. 2012;226:331–334. doi: 10.1016/j.bbr.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251:150–155. [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104:e20. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- Botly LCP, Burton CL, Rizos Z, Fletcher PJ. Characterization of methylphenidate self-administration and reinstatement in the rat. Psychopharmacology (Berl) 2008;199:55–66. doi: 10.1007/s00213-008-1093-z. [DOI] [PubMed] [Google Scholar]
- Boyle AE, Gill K, Smith BR, Amit Z. Differential effects of an early housing manipulation on cocaine-induced activity and self-administration in laboratory rats. Pharmacol Biochem Behav. 1991;39:269–274. doi: 10.1016/0091-3057(91)90178-5. [DOI] [PubMed] [Google Scholar]
- Brown RT, Amler RW, Freeman WS, Perrin JM, Stein MT, Feldman HM, Pierce K, Wolraich ML. Treatment of attention-deficit/hyperactivity disorder: overview of the evidence. Pediatrics. 2005;115:749–757. doi: 10.1542/peds.2004-2560. [DOI] [PubMed] [Google Scholar]
- Cain ME, Green TA, Bardo MT. Environmental enrichment decreases responding for visual novelty. Behav Processes. 2006;73:360–366. doi: 10.1016/j.beproc.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, Perry JL. Modeling risk factors for nicotine and other drug abuse in the preclinical laboratory. Drug Alcohol Depend. 2009;104(Suppl 1):S70–S78. doi: 10.1016/j.drugalcdep.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Autoshaping i.v. cocaine self-administration in rats: Effects of nondrug alternative reinforcers on acquisition. Psychopharmacology. 1993;110:5–12. doi: 10.1007/BF02246944. [DOI] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacol. 2009;34:2767–2778. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela Peña IC, Ahn HS, Choi JY, Shin CY, Ryu JH, Cheong JH. Methylphenidate self-administration and conditioned place preference in an animal model of attention-deficit hyperactivity disorder: the spontaneously hypertensive rat. Behav Pharmacol. 2011;22:31–39. doi: 10.1097/FBP.0b013e328342503a. [DOI] [PubMed] [Google Scholar]
- Diamond MC. Response of the brain to enrichment. An Acad Bras Cienc. 2001;73:211–220. doi: 10.1590/s0001-37652001000200006. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology (Berl) 2011;214:557–566. doi: 10.1007/s00213-010-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck JP, Pearce HE. Acquisition and extinction of an operant response in differentially reared rats. Dev Psychobiol. 1977;10:143–149. doi: 10.1002/dev.420100207. [DOI] [PubMed] [Google Scholar]
- Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology (Berl) 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Findley JD, Bray JV, Dolan-Gutcher K, Robinson WW. Comparison of progressive-ratio performance maintained by cocaine, methylphenidate and secobarbital. Psychopharmacologia. 1975;43:81–83. doi: 10.1007/BF00437619. [DOI] [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herin DV, Rush CR, Grabowski J. Agonist-like pharmacotherapy for stimulant dependence: preclinical, human laboratory and clinical studies. Ann NY Acad Sci. 2010;1187:76–100. doi: 10.1111/j.1749-6632.2009.05145.x. [DOI] [PubMed] [Google Scholar]
- Hoagwood K, Kelleher KJ, Feil M, Comer DM. Treatment services for children with ADHD: a national perspective. J Am Acad Chil Adolesc Psychiatry. 2000;39:198–206. doi: 10.1097/00004583-200002000-00020. [DOI] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology (Berl) 2000;151:55–63. doi: 10.1007/s002130000451. [DOI] [PubMed] [Google Scholar]
- Jardin B, Looby A, Earleywine M. Characteristics of college students with attention-deficit hyperactivity disorder symptoms who misuse their medications. J Am Coll Health. 2011;59:373–377. doi: 10.1080/07448481.2010.513073. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Schuster CR. A choice procedure for drug reinforcers: cocaine and methylphenidate in the rhesus monkey. J Pharmacol Exp Ther. 1975;193:676–688. [PubMed] [Google Scholar]
- Kolb B, Gorny G, Soderpalm AH, Robinson TE. Environmental complexity has different effects on the structure of neurons in the prefrontal cortex versus the parietal cortex or nucleus accumbens. Synapse. 2003;48:149–153. doi: 10.1002/syn.10196. [DOI] [PubMed] [Google Scholar]
- Kollins SH, MacDonald EK, Rush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects: A review. Pharmacol, Biochem, Behav. 2001;68:611–627. doi: 10.1016/s0091-3057(01)00464-6. [DOI] [PubMed] [Google Scholar]
- Koob GF, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE, McCartan D, White J, King DJ. Methylphenidate: a review of its neuropharmacological, neuropsychological and adverse clinical effects. Hum Psychopharmacol Clin Exp. 2004;19:151–180. doi: 10.1002/hup.579. [DOI] [PubMed] [Google Scholar]
- MacDonald EK, Kollins SH. Assessing the reinforcing effects of methylphenidate in children diagnosed with ADHD using a choice procedure. Drug Alcohol Depend. 2000;60:S134. [Google Scholar]
- Mannuzza S, Klein RG, Moulton JL., 3rd Does stimulant treatment place children at risk for adult substance abuse? A controlled, prospective follow-up study. J Child Adolesc Psychopharmacol. 2003;13:273–282. doi: 10.1089/104454603322572606. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Beckmann JS, Gipson CD, Bardo MT. Methylphenidate as a reinforcer for rats: contingent delivery and intake escalation. Exp Clin Psychopharmacol. 2010;18:257–266. doi: 10.1037/a0019814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Beckmann JS, Gipson CD, Bardo MT. Cue effects on methylphenidate self-administration in rats. Behav Pharmacol. 2011a;22:714–717. doi: 10.1097/FBP.0b013e32834afed1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Darna M, Charnigo RJ, Dwoskin LP, Bardo MT. A multivariate assessment of individual difference in sensation seeking and impulsivity as predictors of amphetamine self-administration and prefrontal dopamine function in rats. Exp Clin Psychopharmacol. 2011b;19:275–284. doi: 10.1037/a0023897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, McCuddy WT, Beckmann JS, Gipson CD, Bardo MT. Strain differences in self-administration of methylphenidate and sucrose pellets in a rat model of attention-deficit hyperactivity disorder. Behav Pharmacol. 2011c;22:794–804. doi: 10.1097/FBP.0b013e32834d623e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Teter CJ, Boyd CJ, Guthrie SK. Prevalence and correlates of illicit methylphenidate use among 8th, 10th, and 12th grade students in the United States, 2001. J Adolesc Health. 2004;35:501–504. doi: 10.1016/j.jadohealth.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Meisch RA. Factor controlling drug reinforced behavior. Pharmacol Biochem Behav. 1987;27:367–371. doi: 10.1016/0091-3057(87)90584-3. [DOI] [PubMed] [Google Scholar]
- Musser CJ, Ahmann PA, Theve FW, Mundt P, Broste SK, Mueller-Rizner N. Stimulant use and the potential for abuse in Wisconsin as reported by school administrators and longitudinally followed children. J Dev Behav Pediatr. 1998;19:187–192. doi: 10.1097/00004703-199806000-00006. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Ough BR, Beatty WW, Khalili J. Effects of isolated and enriched rearing on response inhibition. Psychonomic Science. 1972;27:293–294. [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: effects of d-amphetamine and methylphenidate. Behav Brain Res. 2008;193:48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranaldi R, Kest K, Zellner M, Hachimine-Semprebrom P. Environmental enrichment, administered after establishment of cocaine self-administration, reduces lever pressing in extinction and during a cocaine context renewal test. Behav Pharmacol. 2011;22:347–353. doi: 10.1097/FBP.0b013e3283487365. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Risner ME, Jones BE. Characteristics of unlimited access to self-administered stimulant infusions in dogs. Biol Psychiatry. 1976;11:625–634. [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kollins SH, Pazzaglia PJ. Discriminative-stimulus and participant-rated effects of methylphenidate, bupropion and triazolam in d-Amphetamine-trained humans. Exp Clin Psychopharmacol. 1998;6:32–44. doi: 10.1037//1064-1297.6.1.32. [DOI] [PubMed] [Google Scholar]
- Rush CR, Baker RW. Behavioral pharmacological similarities between methylphenidate and cocaine in cocaine abusers. Exp Clin Psychopharmacol. 2001;9:59–73. doi: 10.1037/1064-1297.9.1.59. [DOI] [PubMed] [Google Scholar]
- Rush CR, Essman WD, Simpson CA, Baker RW. Reinforcing and subject-rated effects of methylphenidate and d-amphetamine under a modified progressive-ratio schedule. J Clin Psychopharmacol. 2001;21:273–286. doi: 10.1097/00004714-200106000-00005. [DOI] [PubMed] [Google Scholar]
- Setlik J, Bond GR, Ho M. Adolescent prescription ADHD medication abuse is rising along with prescription for these medications. Pediatrics. 2009;124:875–880. doi: 10.1542/peds.2008-0931. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Stoops WW, Hays LR, Rush CR. Discriminative-stimulus and subject-rated effects of methamphetamine, d-Amphetamine, methylphenidate and triazolam in methamphetamine-trained humans. J Pharmacol Exp Ther. 2009;328:1007–1018. doi: 10.1124/jpet.108.147124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J, Kelly JP. The impact of environmental enrichment in laboratory rats – behavioural and neurochemical aspects. Behav Brain Res. 2011;222:246–264. doi: 10.1016/j.bbr.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Smith JK, Neill JC, Costall B. Post-weaning housing conditions influence the behavioural effects of cocaine and d-amphetamine. Psychopharmacology (Berl) 1997;131:23–33. doi: 10.1007/s002130050261. [DOI] [PubMed] [Google Scholar]
- Smith MA, Iordanou JC, Cohen MB, Cole KT, Gergans SR, Lyle MA, Schmidt KT. Effects of environmental enrichment on sensitivity to cocaine in female rats: importance of control rates of behavior. Behav Pharmacol. 2009;20:312–321. doi: 10.1097/FBP.0b013e32832ec568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski JD, Salamone JD. Effects of dopamine depletions in the medial prefrontal cortex on DRL performance and motor activity in the rat. Brain Res. 1994;642:20–28. doi: 10.1016/0006-8993(94)90901-6. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berl) 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Bardo MT. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol, Biochem, Behav. 2009;92:377–382. doi: 10.1016/j.pbb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs DJ, Klein ED, Bardo MT. Effects of environmental enrichment on extinction and reinstatement of amphetamine self-administration and sucrose-maintained responding. Behav Pharmacol. 2006;17:597–604. doi: 10.1097/01.fbp.0000236271.72300.0e. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Glaser PEA, Rush CR. Reinforcing and subject-rated effects of methylphenidate and d-Amphetamine in stimulant abusing humans. J Psychopharmacol. 2004;18:534–543. doi: 10.1177/0269881104047281. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Engelhardt B, Hood LE, Peartree NA, Neisewander JL. The interactive effects of environmental enrichment and extinction interventions in attenuating cue-elicited cocaine-seeking behavior in rats. Pharmacol, Biochem, Behav. 2011;97:595–602. doi: 10.1016/j.pbb.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ. Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration. Pharmacotherapy. 2006;26:1501–1510. doi: 10.1592/phco.26.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. The medial prefrontal cortex as a part of the brain reward system. Amino Acids. 2000;19:211–219. doi: 10.1007/s007260070051. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Wilens TE. Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2008;28:S46–S53. doi: 10.1097/JCP.0b013e318173312f. [DOI] [PubMed] [Google Scholar]
- Wood DE, Siegel AK, Rebec GV. Environmental enrichment reduces impulsivity during appetitive conditioning. Physiol Behav. 2006;88:132–137. doi: 10.1016/j.physbeh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Bardo MT, Dwoskin LP, Midde NM, Gomez AM, Mactutus CF, Booze RM, Zhu J. Effect of environmental enrichment on methylphenidate-induced locomotion and dopamine transporter dynamics. Behav Brain Res. 2011;16:98–107. doi: 10.1016/j.bbr.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem. 2005;93:1434–43. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res. 2004;148:107–117. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]