Abstract
Inflammation of the colon changes motor function of more proximal regions of the gastrointestinal tract. Colitis alters the neurophysiology of enteric neurons within the region of inflammation, which may contribute to altered colonic motor and secretory function. This study seeks to test the hypothesis that colitis alters the neurophysiology of myenteric neurons in the non-inflamed ileum, and that altered neurophysiology coincides with altered small bowel motor function. Trinitrobenzene sulfonic acid (TNBS)-induced colitis was associated with hyperexcitability of AH neurons in the ileum myenteric plexus, demonstrated by depolarized neurons and increased numbers of action potentials, but without changes in the action potential duration or afterhyperpolarization typical of plasticity in these cells. There were no changes in synaptic transmission of either AH neurons or S neurons observed in the current study. The onset of AH neuron hyperexcitability occurred 24h following administration of TNBS, and persisted to eight weeks, a time point following the resolution of colitis. Small bowel transit was reduced as early as 12h after TNBS and resolved by 48h after TNBS. While AH neurons play a central role in coordinating motor function of the ileum, changes in excitability of these neurons did not coincide with changes in small bowel transit.
Keywords: Intracellular Electrophysiology, Enteric Nervous System, Gastrointestinal Motility, Inflammation
Introduction
Inflammation of the colon changes motor function of more proximal regions of the gastrointestinal tract in both man [18,19,26] and in experimental models [1,3,9]. There is some evidence that inflammatory cytokines may be increased in the upper bowel during colitis [2]. Inflammatory cytokines, including IL-1β and IL-6 [10,24,28,31] can directly excite enteric neurons. Additionally, neural reflexes from the distal colon might lead to enhanced excitability of enteric neurons in the proximal gut. Spinal afferents that innervate the inflamed guinea pig intestine are hyperexcitable [20] and may enhance spinal or supraspinal reflexes that could alter motor function in distant non-inflamed regions [12]. Post-ganglionic sympathetic neurons within the prevertebral ganglia that receive synaptic input from viscerofugal neurons in the colon are hyperexcitable during colitis [13] and may also contribute to altered motor function during colitis.
The aim of this study was to test the hypothesis that colonic inflammation alters the excitability of myenteric neurons in the non-inflamed ileum and further that altered excitability contributes to altered motor function of the small bowel. A single enema of trinitrobenzene sulfonic acid (TNBS) was used to induce a limited course of colitis that resolved. Enhanced excitability was observed in AH neurons of the ileum myenteric plexus yet interestingly did not coincide with changes in motor function.
Materials and Methods
Inflammatory models
All methods were reviewed and approved by the Mayo Clinic Animal Use and Care Committee. Colitis was induced by a single enema of 0.3 ml trinitrobenzene sulfonic acid (TNBS; 27 mg ml−1) in 30% ethanol 7 cm proximal to the anus. After the enema, animals were returned to a controlled environment for a period of 12h, 24h, 48h, 6d or 56d before euthanasia by CO2 inhalation. Control animals remained naive to treatment until euthanasia. Inflammatory damage was assessed as previously described [13].
Tissue preparation and electrophysiology
Following euthanasia, a segment of the ileum, within ten centimeters proximal of the cecum was removed and placed in iced Krebs solution (mM: NaCl, 120; KCl, 5.9; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 15.5; NaH2PO4, 1.2; and glucose, 11.4; aerated with a 97%O2/3%CO2). The mucosal, submucosal and circular muscle layers were removed to expose the longitudinal muscle / myenteric plexus preparation that was pinned flat under tension in a recording dish with a glass bottom and thin layer of Sylgard and perfused with 37°C aerated Krebs solution. Myenteric ganglia were visualized at 25× (2.5× eyepiece, 10× objective) with an Olympus IX70 microscope under differential interference contrast optics. Individual neurons were randomly impaled with glass microelectrodes that were filled to the shoulder with 1% (w/v) neurobiotin (Vector Laboratories, Burlingame, CA, USA) in 1.0 M KCl, and the remainder filled with 3.0 M KCl and had resistances in the range of 50–150 MΩ.
The potential difference before impalement and after a stable potential, reached within the first three minutes, was recorded as the resting membrane potential. The average ratio of the potential difference to the injected current (−50 to −100 pA) for three current pulses determined the membrane resistance. The maximum number of action potentials was determined using a 0.5s square-wave current pulse (900pA). A single action potential that peaked after a 0.5–1.5ms current pulse was used to calculate the duration at half of the maximum voltage. The subsequent afterhyperpolarization (AHP) amplitude was measured as the potential difference from before the current pulse to the peak of the AHP. Synaptic inputs were directly stimulated with a single pulse of 1–10V for 0.5 ms applied to interganglionic connectives with monopolar extracellular electrodes made from Teflon-insulated platinum wire. The maximum amplitude of fast excitatory postsynaptic potentials (fEPSP) in response to a supramaximal stimulus was determined as the difference between the membrane potential, held to −90 mV, and the peak of the fEPSP.
Small Bowel Transit
Guinea pigs were fasted for 12h, lightly anaesthetized with isoflurane, and given a gavage of 2 ml 10% charcoal (400 mesh) 5% Arabic gum suspension. After 30min the animals were euthanized using CO2 inhalation. The small intestine was removed from the animal and the mesentery was cut so the intestine could be laid straight. The distance between the pylorus and the cecum was measured as well as the distance from the pylorus to the leading edge of the charcoal meal. These measurements were used to calculate the percent small bowel transit.
Statistical analyses
All statistical analyses were completed using GraphPad Prism software using tests described in the results section. For all tests, P values less than 0.05 was considered significant.
Results
Intracellular recordings were obtained from 57 neurons in 26 preparations. Neurons were classified as AH or S based on the presence or absence of an AHP, two components on the repolarizing phase of the action potential, or fast synaptic input as described previously [4,30]. In all, 35 AH neurons (61%) and 22 S neurons (39%) were randomly impaled from the myenteric plexus of the guinea pig ileum.
Colitis causes hyperexcitability of ileum AH neurons
There was a significant increase in the gross damage scores of the colon at twelve hours post-TNBS that resolved by 56 days (control: 0.70 ± 0.05, N=5; 12h TNBS: 7.6 ± 1.3, N=5; 24h TNBS: 7.9 ± 0.3, N=5; 48h TNBS: 6.3 ± 1.6, N=5; 6d TNBS: 4.5 ± 1.2, N=5; 56d TNBS: 0.79 ± 0.13, N=5; P<0.001 ANOVA Newman-Keuls Multiple Comparison Test). There were no signs of inflammation in the ileum at any time point after TNBS. These data are quite similar to previous studies using the same model and time course [13,14].
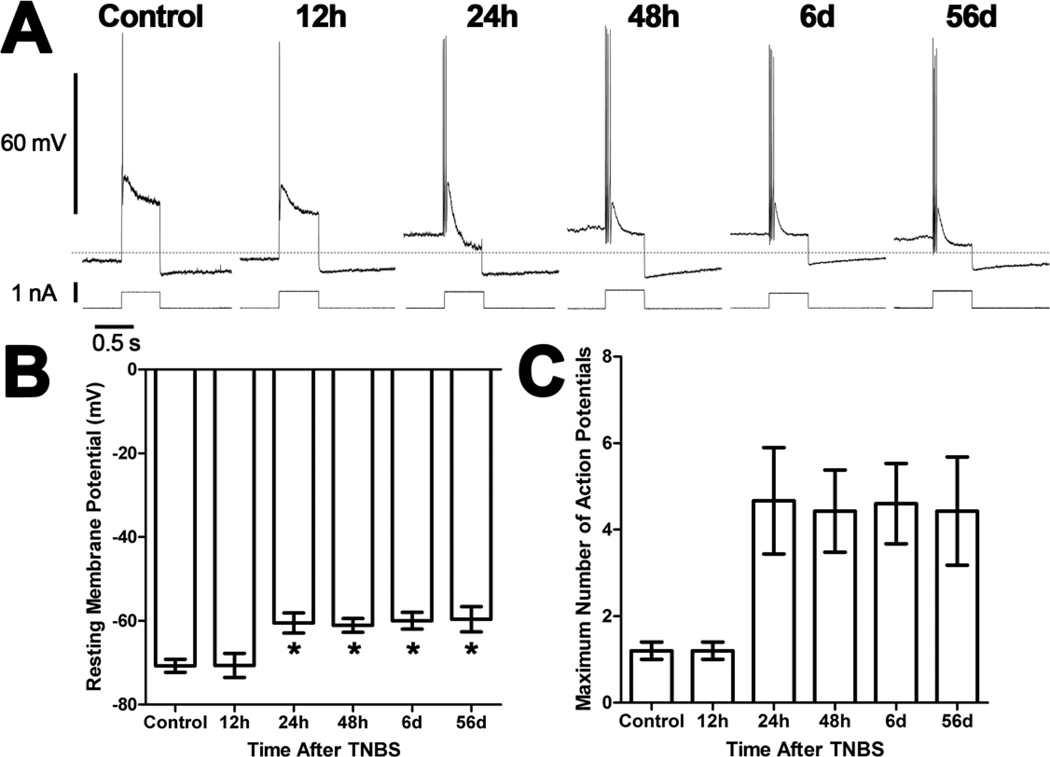
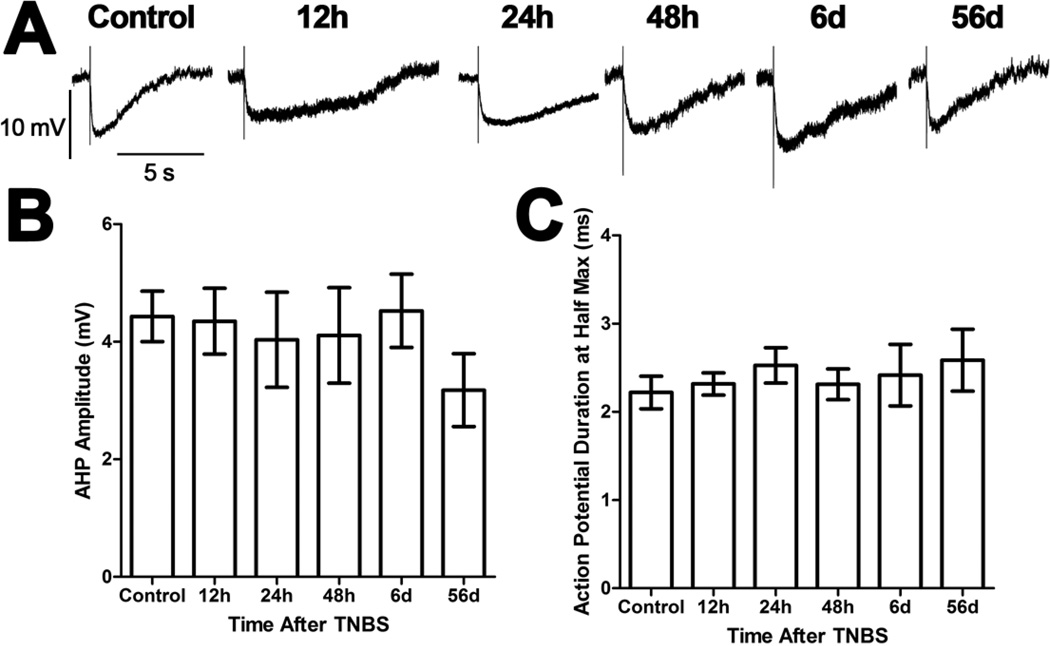
Five AH neurons were recorded from 3 preparations made from control animals. The electrophysiological properties of these neurons were similar to previous reports [7,21,22]. There was a significant time-dependent depolarization of AH neurons from preparations 24h, 48h, six days, and 56 days after TNBS compared to AH neurons from control preparations and preparations 12h after TNBS (P<0.05, ANOVA; Figures 1A and 1B). Interestingly, there was no change in membrane resistance (control: 90 ± 7 MΩ, N=5; 12h TNBS: 87 ± 9 MΩ, N=5; 24h TNBS: 81 ± 7 MΩ, N=5; 48h TNBS: 109 ± 17 MΩ, N=5; 6d TNBS: 92 ± 6 MΩ, N=5; 56d TNBS: 94 ± 12 MΩ, N=5; P>0.05 ANOVA). There were significantly more action potentials in response to a depolarizing current pulse in AH neurons of the ileum from animals with colitis than AH neurons from control preparations and preparations 12h after TNBS (P<0.05, ANOVA; Figures 1A and 1C). Interestingly, however, this increased activity was not accompanied by a change in either the AHP (P>0.05 ANOVA; Figure 2), the duration of the action potential (P>0.05 ANOVA; Figure 2), or the Ih current (data not shown). There were no detectable fEPSPs elicited in AH neurons from any treatment group. In an effort to increase statistical power of contingency table testing, the neurons from control preparations and preparations 12h after TNBS were combined into a single group and the neurons from the remaining groups were combined into a single group. Anodal break action potentials were detected in 1 (control) of 10 AH neurons (10%) from the group with normal excitability. They were more frequent in the group of neurons with increased excitability with 13 (three 24h, four 48h, three 6d and three 56d) of 25 AH neurons (52%) with anodal break action potentials (P<0.05, Fisher’s Exact Test). Likewise, spontaneous action potentials were more frequent in the excitable group with 9 (one 24h, four 48h, two 6d and two 56d) of 25 AH neurons (36%), and none of 10 AH neurons from the group with normal excitability displaying spontaneous activity (P<0.05, Fisher’s Exact Test).
Figure 1. Ileum AH neurons are depolarized and hyperexcitable following TNBS-induced colitis.
A) Representative traces from AH neurons of the guinea pig ileum myenteric plexus illustrating action potentials elicited by 0.5s depolarizing current pulses. All traces are on the same time and voltage or current scales. The dashed line indicates −65 mV. B) A bar graph illustrating the mean (± SEM) resting membrane potential for neurons recorded from each treatment group. Neurons are depolarized from preparations obtained between 24h and 56d after TNBS administration compared to control preparations and preparations 12h after TNBS (*P<0.05 ANOVA Newman-Keuls Multiple Comparison Test). C) A bar graph illustrating the mean (± SEM) maximum number of action potentials for AH neurons recorded from each treatment group. There was a significant difference in the number of action potentials between treatment groups (P<0.05 ANOVA).
Figure 2. The afterhyperpolarization (AHP) and action potential duration of ileum AH neurons were not altered following TNBS-induced colitis.
A) Representative traces from AH neurons of the guinea pig ileum myenteric plexus illustrating the AHP following a single action potential (truncated). While all traces are on the same time and voltage scales, the traces have been aligned at the resting membrane potential. B) A bar graph illustrating the mean (± SEM) amplitude of the AHP recorded from each treatment group. There were no significant differences between the groups (P>0.05 ANOVA). C) A bar graph illustrating the mean (± SEM) duration at half-maximum of action potentials recorded from each treatment group. There were no significant differences between the groups (P>0.05 ANOVA).
Colitis does not alter ileum S neurons
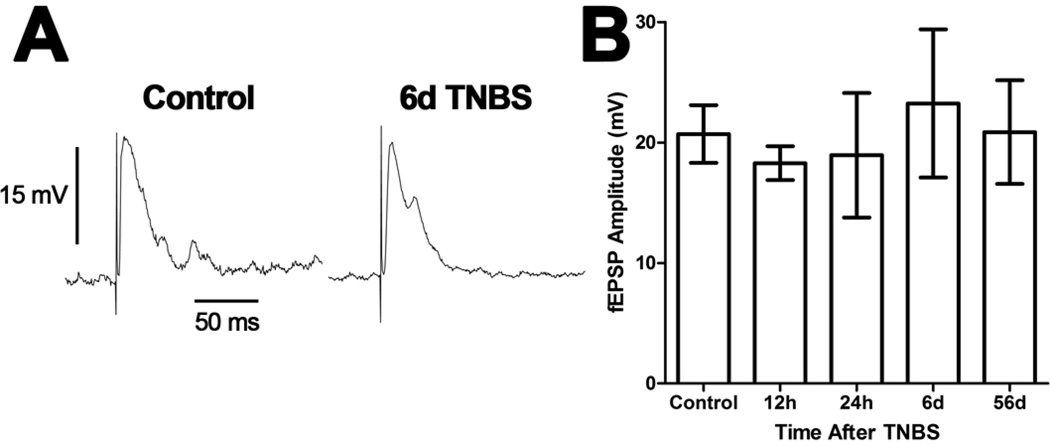
Recordings from S neurons revealed a diverse population of neurons consistent with previous reports [7,21,22]. Nine of 22 S neurons from all groups (one control, two 12h, one 24h, one 48h, two 6d and two 56d) displayed action potentials throughout a 0.5s depolarizing current pulse, while the remaining 13 neurons displayed a single action potential. There was only a single S neuron recorded from the preparations from animals 48h after TNBS and this neuron was not included in statistical analyses. The resting membrane potentials (control: −57 ± 2 mV, N=3; 12h TNBS: −57 ± 3 mV, N=3; 24h TNBS: −57 ± 6 mV, N=4; 6d TNBS: −58 ± 1 mV, N=4; 56d TNBS: −60 ± 4 mV, N=5; P>0.05 ANOVA) and membrane resistances (control: 153 ± 22 MΩ, N=3; 12h TNBS: 131 ± 6 MΩ, N=3; 24h TNBS: 126 ± 14 MΩ, N=4; 6d TNBS: 131 ± 13 MΩ, N=4; 56d TNBS: 182 ± 30 MΩ, N=6; P>0.05 ANOVA) were not different between the treatment groups. Fast synaptic inputs were stimulated in all 22 S neurons tested and the fEPSP amplitude was not different between treatment groups (P>0.05 ANOVA; Figure 3).
Figure 3. Evoked fast synaptic potentials of ileum S neurons were not altered following TNBS-induced colitis.
A) Representative traces from S neurons of the guinea pig ileum myenteric plexus illustrating evoked fast excitatory postsynaptic potentials (fEPSPs). Both traces are on the same time and voltage scales. B) A bar graph illustrating the mean (± SEM) maximum amplitude of fEPSPs in S neurons recorded from each treatment group. There were no significant differences between the groups (P>0.05 ANOVA).
Effect of colitis on small bowel transit
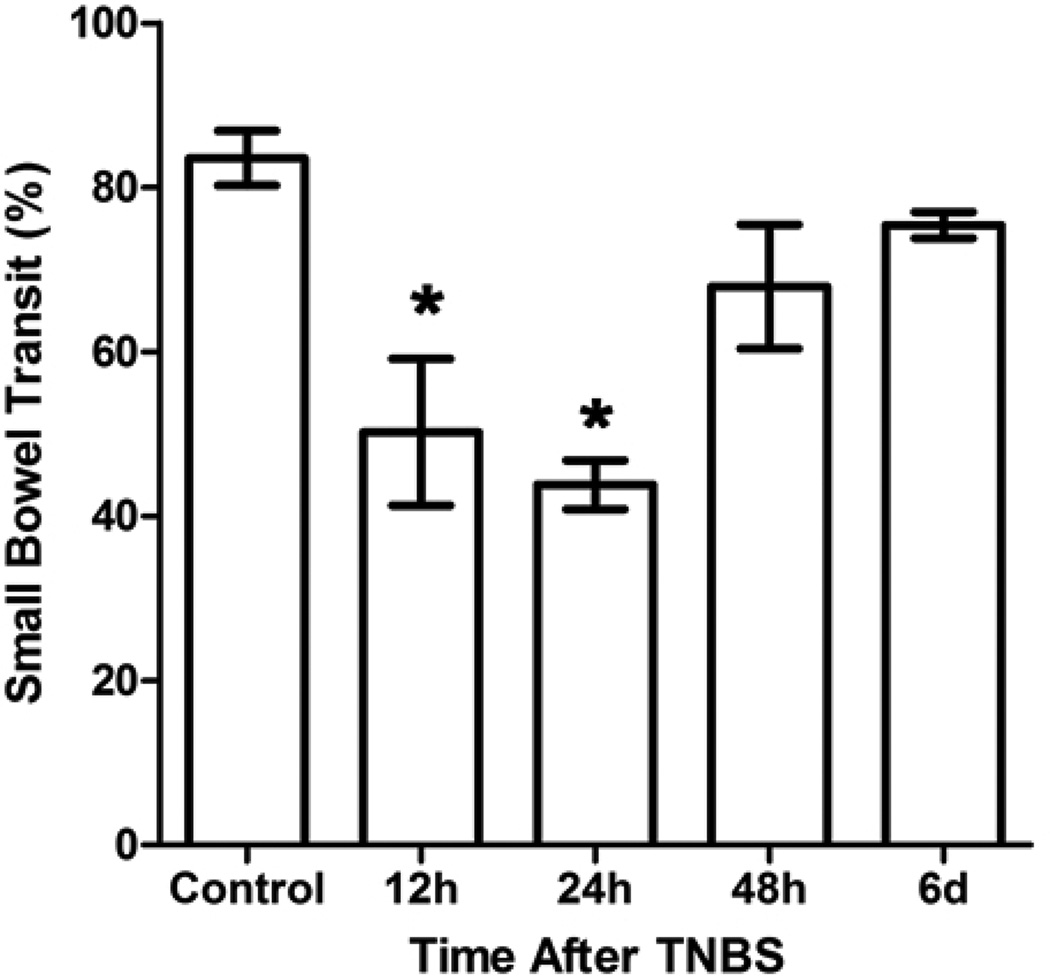
Small bowel transit was reduced in guinea pigs that had been treated with TNBS 12h or 24h before the assay compared to control animals (P<0.05 ANOVA Newman-Keuls Multiple Comparison Test; Figure 4). Interestingly, transit was not different from controls in animals 48h or 6d after TNBS.
Figure 4. Small bowel transit was transiently decreased in guinea pigs with TNBS-induced colitis.
A bar graph illustrating the mean (± SEM) percentage of the small intestine traveled by a charcoal/gum test meal after 30min of each treatment group. There was a significant decrease in transit starting at 12h after TNBS administration that was also present at the 24h time point (*P<0.05 ANOVA Newman-Keuls Multiple Comparison Test). There were no differences in transit between control animals and animals 48h or 6d after TNBS (P>0.05 ANOVA Newman-Keuls Multiple Comparison Test).
Discussion
The results of this study demonstrate that colitis is associated with an increased excitability of intrinsic primary afferent neurons, identified electrophysiologically as AH neurons [6], of the ileum myenteric plexus. This hyperexcitability does not develop immediately as AH neurons from animals 12h after TNBS administration demonstrate normal excitability. Rather, enhanced excitability arises at the 24h time point and persists up to eight weeks, the last time point investigated, when colitis was resolved. These neurons join a growing list of neurons that remain hyperexcitable after the resolution of TNBS colitis, including myenteric [11] and submucosal [17] AH neurons of the colon, spinal afferent neurons that innervate the colon [8], and post-ganglionic sympathetic neurons of the prevertebral ganglia [13].
Several studies have identified enhanced excitability of small intestinal myenteric AH neurons during jejunitis or ileitis caused by various chemical and biological inflammagens [16,22,25]. The most typical measure of excitability altered in these neurons is an increase in the number of action potentials caused by a depolarizing current pulse delivered through the recording electrode, which was also observed in the current study. Unlike previous studies of ileitis, there was no change in the AHP of these neurons during colitis. This is surprising because the AHP is thought to gate the excitability of these neurons and increased action potentials typically follows a reduced AHP [5,6,27]. Another surprising finding of the current study is that AH neurons were depolarized, which is not typically observed during TNBS-induced inflammation [15,16,22,23], without a change in membrane resistance. These findings suggest that the mechanisms contributing to ileum myenteric AH neuron hyperexcitability during colitis is complex, likely involving closing and opening of multiple channels.
AH neurons of the colon during TNBS-induced colitis exhibit cholinergic fast synaptic transmission that is not present in control AH neurons [15,16]. This phenomenon was not observed in the present study or in previous studies of ileum enteric neurons during TNBS-induced ileitis [22] and may reflect differences in neuroplasticity between the ileum and colon. Excitability of myenteric S neurons (interneurons and motor neurons) are typically not altered by an inflammatory insult [15,22,25], which was also observed in the present study. However, S neurons of the colon during TNBS-induced colitis [15,16] exhibit facilitated fast synaptic transmission. This phenomenon was also not observed in the present study or in previous studies of ileum enteric neurons during TNBS-induced ileitis [22] and thus may be related to altered synaptic transmission of AH neurons and reflect differences in inflammation-induced neuroplasticity between the ileum and colon. One previous study observed changes in enteric neurophysiology during inflammation in a distant region [23]. In this study, submucosal AH neurons of the colon displayed similar hyperexcitability and synaptic facilitation during TNBS-induced ileitis [23] as during TNBS-induced colitis [16]. In the present study, hyperexcitability of ileum AH neurons during TNBS colitis was distinct from TNBS ileitis [22].
There was a lack of temporal correlation between altered excitability of ileum myenteric AH neurons and altered small bowel transit. Transit was reduced 12h after TNBS when AH neurons exhibited normal excitability, thus hyperexcitability of AH neurons cannot explain reduced small bowel transit. Likewise, small bowel transit was restored by 48h after TNBS when AH neurons remained hyperexcitable, thus hyperexcitability of AH neurons cannot explain the restoration of normal small bowel transit. These results are surprising given the key role that myenteric AH neurons play in coordinating motor activity in the guinea pig ileum [5,6,27,29]. The onset of reduced small bowel transit observed in the present study is concurrent with the onset of enhanced excitability of prevertebral ganglion neurons [13] that may contribute to altered motor function by altered sympathetic outflow to the small intestine.
Conclusions
The results of this study support the concept that colitis causes hyperexcitability of AH neurons in the ileum myenteric plexus. The onset of hyperexcitability occurred 24h following administration of TNBS, and persisted to eight weeks. There were no changes in synaptic transmission of either AH neurons or S neurons observed in the current study. While AH neurons play a central role in coordinating motor function of the ileum [5,6,27,29], changes in excitability of these neurons did not coincide with changes in small bowel transit. This lack of temporal correlation raises questions regarding the functional significance of altered ileum AH neuron physiology during colitis.
Highlights.
Intrinsic primary afferent neurons of the ileum are hyperexcitable during colitis.
Excitability of ileum myenteric AH neurons persists after colitis has resolved.
There are no changes in fast synaptic transmission in the ileum during colitis.
An early transient reduction in small bowel transit is restored during colitis.
Neither reduced transit nor restoration coincide with changes in AH excitability.
Acknowledgments
I gratefully acknowledge the secretarial support of Ms. Janice Applequist. This work was supported by NIH grant DK76665.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aube AC, Cherbut C, Barbier M, Xing JH, Roze C, Galmiche JP. Altered myoelectrical activity in noninflamed ileum of rats with colitis induced by trinitrobenzene sulphonic acid. Neurogastroenterol. Motil. 1999;11:55–62. doi: 10.1046/j.1365-2982.1999.00137.x. [DOI] [PubMed] [Google Scholar]
- 2.Barada KA, Mourad FH, Sawah SI, Khoury C, Safieh-Garabedian B, Nassar CF, Saade NE. Localized colonic inflammation increases cytokine levels in distant small intestinal segments in the rat. Life Sci. 2006;79:2032–2042. doi: 10.1016/j.lfs.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 3.Blandizzi C, Fornai M, Colucci R, Baschiera F, Barbara G, De GR, De PF, Breschi MC, Del TM. Altered prejunctional modulation of intestinal cholinergic and noradrenergic pathways by alpha2-adrenoceptors in the presence of experimental colitis. Br. J. Pharmacol. 2003;139:309–320. doi: 10.1038/sj.bjp.0705249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bornstein JC, Furness JB, Kunze WA. Electrophysiological characterization of myenteric neurons: how do classification schemes relate? J. Auton. Nerv. Syst. 1994;48:1–15. doi: 10.1016/0165-1838(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 5.Chambers JD, Bornstein JC, Thomas EA. Multiple neural oscillators and muscle feedback are required for the intestinal fed state motor program. PLoS. One. 2011;6:e19597. doi: 10.1371/journal.pone.0019597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furness JB, Jones C, Nurgali K, Clerc N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog. Neurobiol. 2004;72:143–164. doi: 10.1016/j.pneurobio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Hirst GD, Holman ME, Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J. Physiol. 1974;236:303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes PA, Brierley SM, Martin CM, Brookes SJ, Linden DR, Blackshaw LA. Post-inflammatory colonic afferent sensitisation: different subtypes, different pathways and different time courses. Gut. 2009;58:1333–1341. doi: 10.1136/gut.2008.170811. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson K, McHugh K, Collins SM. Experimental colitis alters myenteric nerve function at inflamed and noninflamed sites in the rat. Gastroenterology. 1995;109:718–722. doi: 10.1016/0016-5085(95)90378-x. [DOI] [PubMed] [Google Scholar]
- 10.Kelles A, Janssens J, Tack J. IL-1beta and IL-6 excite neurones and suppress cholinergic neurotransmission in the myenteric plexus of the guinea pig. Neurogastroenterol. Motil. 2000;12:531–538. doi: 10.1046/j.1365-2982.2000.00228.x. [DOI] [PubMed] [Google Scholar]
- 11.Krauter EM, Strong DS, Brooks EM, Linden DR, Sharkey KA, Mawe GM. Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig. Neurogastroenterol. Motil. 2007;19:990–1000. doi: 10.1111/j.1365-2982.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- 12.Krukoff TL. Neuropeptide regulation of autonomic outflow at the sympathetic preganglionic neuron. Anatomical and neurochemical specificity. Ann. N. Y. Acad. Sci. 1990;579:160–167. doi: 10.1111/j.1749-6632.1990.tb48358.x. [DOI] [PubMed] [Google Scholar]
- 13.Linden DR. Enhanced excitability of guinea pig inferior mesenteric ganglion neurons during and following recovery from chemical colitis. Am. J. Physiol Gastrointest. Liver Physiol. 2012;303:G1067–G1075. doi: 10.1152/ajpgi.00226.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linden DR, Couvrette JM, Ciolino A, McQuoid C, Blaszyk H, Sharkey KA, Mawe GM. Indiscriminate loss of myenteric neurones in the TNBS-inflamed guinea-pig distal colon. Neurogastroenterol. Motil. 2005;17:751–760. doi: 10.1111/j.1365-2982.2005.00703.x. [DOI] [PubMed] [Google Scholar]
- 15.Linden DR, Sharkey KA, Mawe GM. Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon. J. Physiol. 2003;547:589–601. doi: 10.1113/jphysiol.2002.035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lomax AE, Mawe GM, Sharkey KA. Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig. J. Physiol. 2005;564:863–875. doi: 10.1113/jphysiol.2005.084285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lomax AE, O'Hara JR, Hyland NP, Mawe GM, Sharkey KA. Persistent alterations to enteric neural signaling in the guinea pig colon following the resolution of colitis. Am. J. Physiol Gastrointest. Liver Physiol. 2007;292:G482–G491. doi: 10.1152/ajpgi.00355.2006. [DOI] [PubMed] [Google Scholar]
- 18.Mackie TT. The medical management of chronic ulcerative colitis. Journal of the American Medical Association. 1938;111:2071–2076. [Google Scholar]
- 19.Manousos ON, Salem SN. Abnormal motility of the small intestine in ulcerative colitis. Gastroenterologia. 1965;104:249–257. doi: 10.1159/000202102. [DOI] [PubMed] [Google Scholar]
- 20.Moore BA, Stewart TM, Hill C, Vanner SJ. TNBS ileitis evokes hyperexcitability and changes in ionic membrane properties of nociceptive DRG neurons. Am. J. Physiol Gastrointest. Liver Physiol. 2002;282:G1045–G1051. doi: 10.1152/ajpgi.00406.2001. [DOI] [PubMed] [Google Scholar]
- 21.Nishi S, North RA. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J. Physiol. 1973;231:471–491. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nurgali K, Nguyen TV, Matsuyama H, Thacker M, Robbins HL, Furness JB. Phenotypic changes of morphologically identified guinea-pig myenteric neurons following intestinal inflammation. J. Physiol. 2007;583:593–609. doi: 10.1113/jphysiol.2007.135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Hara JR, Lomax AE, Mawe GM, Sharkey KA. Ileitis alters neuronal and enteroendocrine signalling in guinea pig distal colon. Gut. 2007;56:186–194. doi: 10.1136/gut.2006.102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Malley D, Liston M, Hyland NP, Dinan TG, Cryan JF. Colonic soluble mediators from the maternal separation model of irritable bowel syndrome activate submucosal neurons via an interleukin-6-dependent mechanism. Am. J. Physiol Gastrointest. Liver Physiol. 2011;300:G241–G252. doi: 10.1152/ajpgi.00385.2010. [DOI] [PubMed] [Google Scholar]
- 25.Palmer JM, Wong-Riley M, Sharkey KA. Functional alterations in jejunal myenteric neurons during inflammation in nematode-infected guinea pigs. Am. J. Physiol. 1998;275:G922–G935. doi: 10.1152/ajpgi.1998.275.5.G922. [DOI] [PubMed] [Google Scholar]
- 26.Rao SS, Read NW. Gastrointestinal motility in patients with ulcerative colitis. Scand. J. Gastroenterol. Suppl. 1990;172:22–28. doi: 10.3109/00365529009091905. [DOI] [PubMed] [Google Scholar]
- 27.Thomas EA, Bornstein JC. Inhibitory cotransmission or after-hyperpolarizing potentials can regulate firing in recurrent networks with excitatory metabotropic transmission. Neuroscience. 2003;120:333–351. doi: 10.1016/s0306-4522(03)00039-3. [DOI] [PubMed] [Google Scholar]
- 28.Tjwa ET, Bradley JM, Keenan CM, Kroese AB, Sharkey KA. Interleukin-1beta activates specific populations of enteric neurons and enteric glia in the guinea pig ileum and colon. Am. J. Physiol Gastrointest. Liver Physiol. 2003;285:G1268–G1276. doi: 10.1152/ajpgi.00073.2003. [DOI] [PubMed] [Google Scholar]
- 29.Wood JD. Intrinsic neural control of intestinal motility. Annu. Rev. Physiol. 1981;43:33–51. doi: 10.1146/annurev.ph.43.030181.000341. [DOI] [PubMed] [Google Scholar]
- 30.Wood JD. Application of classification schemes to the enteric nervous system. J. Auton. Nerv. Syst. 1994;48:17–29. doi: 10.1016/0165-1838(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 31.Xia Y, Hu HZ, Liu S, Ren J, Zafirov DH, Wood JD. IL-1beta and IL-6 excite neurons and suppress nicotinic and noradrenergic neurotransmission in guinea pig enteric nervous system. J. Clin. Invest. 1999;103:1309–1316. doi: 10.1172/JCI5823. [DOI] [PMC free article] [PubMed] [Google Scholar]