Abstract
Although the effects of alcohol on brain-derived neurotrophic factor (BDNF) have been extensively studied in rodents, BDNF levels have rarely been measured in abstinent, alcohol-dependent (AD) individuals. Interpretation of reported group comparisons of serum BDNF levels is difficult due to limited information regarding analytical variance, biological variability, and the relative contribution of platelet and plasma pools to serum BDNF. Analytical variance (intra- and inter-assay coefficients of variation) of the enzyme-linked immunosorbent assay (ELISA) was characterized. Within- and between-subject variability, and group differences in serum and plasma BDNF, was assessed on three separate days in 16, 4-week abstinent AD individuals (7M/9F) and 16 social drinkers (SDs; 8M/8F). Significantly higher mean (±sd) serum BDNF levels were observed for the AD group compared to the SD (p = 0.003). No significant difference in mean baseline plasma BDNF levels was observed between AD and SD groups. The low analytical variance, high day-to-day within-individual stability and the high degree of individuality demonstrates the potential clinical utility of measuring serum BDNF levels. The low correlations that we observed between plasma and serum levels are congruent with their representing separate pools of BDNF. The observation of higher basal serum BDNF in the AD group without a concomitant elevation in plasma BDNF levels indicates that the elevated serum BDNF in AD patients is not due to greater BDNF exposure. Further research is warranted to fully elucidate mechanisms underlying this alteration and determine the utility of serum BDNF as a predictor or surrogate marker of chronic alcohol abuse.
Keywords: Alcoholism, BDNF, ELISA, Plasma, Serum, Abstinence
Introduction
Abstinence from chronic alcohol dependence is associated with alterations in brain stress and reward systems (Sinha, 2008). These adaptations include robust changes in the Hypothalamic-Pituitary-Adrenal (HPA) and autonomic arousal pathways as well as in the mesolimbic dopamine system involved in the rewarding properties of alcohol (Koob, 2003; Sinha et al., 2009). Because basal stress and reward systems adaptations are integral to clinical outcome during early recovery from alcohol abuse, identifying underlying cellular substrates could contribute to the development of more efficacious treatments in alcohol dependence.
The neurotrophin, brain derived neurotrophic factor (BDNF) has been well-characterized within the nervous system for maintaining cellular integrity through its regulation of synaptic plasticity, neurogenesis (Taliaz, Stall, Dar, & Zangen, 2010), neuronal activity and learning and memory processes (Minichiello, 2009). Preclinical models of alcohol dependence have implicated brain-derived neurotrophic factor (BDNF) as a key substrate in modulating alcohol self-administration, alcohol reinstatement and motivational behavior (Jeanblanc et al., 2009; Moonat, Starkman, Sakharkar, & Pandey, 2010). In rodent studies, brain BDNF was observed to be altered in a region-specific manner with alcohol exposure (Tapia-Arancibia et al., 2001; Miller and Mooney, 2004). For example, increased mRNA expression was observed in rat striatum with acute voluntary ethanol consumption (McGough et al., 2004), reduced BDNF mRNA expression was seen in the hippocampus, hypothalamus and cortex with chronic exposure (MacLennan, Lee, & Walker, 1995; Tapia-Arancibia et al., 2001) and elevated expression was observed in the hippocampus and hypothalamus following acute alcohol withdrawal (Tapia-Arancibia et al., 2001).
The relationship between central and peripheral BDNF has been examined in rodent models with recent studies reporting significant positive correlations between serum or whole blood levels (a reflection of platelet BDNF levels) and brain tissue levels (Klein et al., 2010; Sartorius et al., 2009). Moreover, preclinical evidence indicates that peripheral administration of BDNF produces robust cellular and behavioral adaptations in the brain (Schmidt and Duman, 2010). In addition to its role in the nervous system, peripheral BDNF functions as an immunotrophin, ephitheliotrophin and metabotrophin (Chaldakov, Tonchev, and Aloe, 2009). It is expressed in endothelial cells, smooth muscle cells, endocrine cells and several immune cell subtypes (Linker, Gold, & Luhder, 2000). Its presence in multiple sclerosis plaques also suggests a neuroprotective role of peripheral BDNF in autoimmune disorders (Linker et al., 2000).
The role of BDNF in alcohol dependence in humans remains unclear. There have been four reported clinical studies using an enzyme-linked immunosorbent assay (ELISA) to assess peripheral BDNF levels in plasma or serum in AD subjects (Huang et al., 2008; Joe et al., 2007; Lee et al., 2009; Umene-Nakano et al., 2009). Joe et al. (2007) reported lowered plasma BDNF in 30-day abstinent AD individuals. Huang et al. (2008) observed no difference in serum BDNF between AD and healthy subjects when measured on the morning after admission. However, they found that AD patients had a significant increase in serum BDNF levels from admission, after one week of withdrawal. On the other hand, AD patients exhibited higher plasma BDNF levels compared to controls when measured on the morning after admission (Lee et al., 2009), whereas depressed patients with alcohol comorbidity showed lower serum BDNF levels compared to healthy subjects at baseline (Umene-Nakano et al., 2009). Lower peripheral BDNF levels have been reported in bipolar (Monteleone, Serritella, Martiadis, & Maj, 2008; de Oliveira et al., 2009) and depressed (Sen, Duman, & Sanacora, 2008) patients, but the control group mean serum BDNF values have varied substantially with levels ranging from 2 to 4 ng/ml (D’Souza, Pittman, Perry, & Simen, 2009) to 20–40 ng/ml (Sen et al., 2008). None of these studies analyzed both serum and plasma BDNF levels at the same time-points, making interpretation of the findings difficult.
The aims of the present study were to evaluate the analytical variance of the commercially available R&D ELISA BDNF kit, to characterize the intra-individual variation in serum and plasma BDNF, to ascertain group biological variation in BDNF, and to examine simultaneously serum and plasma BDNF levels in early, abstinent AD subjects and in a demographically matched group of social drinkers. Thorough characterization of the analytical variance has been previously neglected and is a necessary precursor to the characterization of the rarely examined within-individual variance. We have previously shown that four-week abstinent alcoholics exhibit a dysregulation in peripheral stress systems and neural responses compared with socially drinking controls (Sinha et al., 2009). Given the influence of stress on brain and peripheral BDNF, and the observation that endogenous BDNF levels are altered following alcohol exposure in animal and clinical studies, we hypothesized that peripheral BDNF levels would be altered in inpatient, treatment-engaged, four-week abstinent, alcoholic individuals compared to a demographically matched group of SDs.
Materials and methods
Participants
Sixteen treatment-seeking, alcohol-dependent individuals (ADs; 7M/9F) and sixteen light-to-moderate social drinkers (SDs; 8M/8F) were recruited from the community through local advertisements. The SD subjects were demographically-matched, healthy individuals with documented multiple negative urine toxicology screens on study admission. The SDs subjects reported drinking 25 drinks or less per month and were classified into light and moderate social drinking categories using the quantity frequency variability index (Cahalan, Cisin, & Crossley, 1969). SDs with current or past diagnoses of any substance dependence or current psychiatric diagnosis, or with any history of being prescribed psychotropic medication, were excluded. Alcoholics met DSM-IV criteria for current alcohol dependence as assessed by the Structured Clinical Interview for DSM-IV (SCID-IV, First, Spitzer, Gibbon, & Williams, 1997) and tested positive for alcohol upon entry into a locked in-patient facility at the Clinical Neuroscience Research Unit (CNRU) of the Connecticut Mental Health Center four weeks prior to their laboratory blood collection sessions. Prior alcohol use history was assessed using the Addiction Severity Index (ASI; McLellan et al., 1992) and the Time-Line Follow-Back (Miller and Del Boca, 1994) for the 90 days prior to inpatient admission. Depressive symptoms were assessed with the 21-item Beck Depression Inventory (BDI) scale administered to all subjects at admission (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961). Alcohol-dependent individuals had no access to alcohol and drugs, limited access to nicotine smoking (1–2 cigarettes) during four (15 min each) smoke breaks and had limited access to visitors. Drug testing was regularly conducted to ensure drug abstinence. Exclusion criteria included dependence on substances other than alcohol or nicotine. The study was approved by the Human Investigation Committee of the Yale University School of Medicine.
Blood collection, serum and plasma separation
The laboratory blood collection sessions for ADs were conducted during three consecutive days and performed 1 month after admission to the inpatient CNRU to minimize acute effects of alcohol withdrawal and the reduced access to tobacco. Blood collections sessions for SDs were performed during an inpatient, three consecutive days stay at the Hospital Research Unit (HRU) of the Yale Center for Clinical Investigation thus providing a similar controlled environment to the ADs. Blood samples were obtained over three consecutive days and collected in the morning between 8.00 and 8.30 am for all participants in order to minimize variance arising from reported circadian variation in BDNF levels (Begliuomini et al., 2008). Blood was collected in anticoagulant-free tubes (serum) and in EDTA-coated tubes (plasma) (BD Biosciences, NJ, USA). To obtain serum, whole blood samples were allowed to coagulate at room temperature (RT) for 30 min and then centrifuged at RT for 10 min at 1000× g. To obtain platelet-poor plasma, blood samples were immediately stored on ice, plasma was separated by centrifugation at 4 °C for 15 min at 1500× g. Aliquots of serum and platelet-poor plasma were stored in polypropylene tubes at −80 °C until assayed.
BDNF assay
BDNF concentrations were quantitatively determined by ELISA using the DuoSet ELISA Development Kit from R&D systems (Minneapolis, MN, USA). ELISA assays were performed according to manufacturer’s directions in 96-well plates (Corning Costar 3590) using a specific human BDNF antibody and no significant cross-reactivity or interference has been observed using this assay (R&D systems, MN, USA). Briefly, the plates were coated overnight with 100 μl of the capture antibody (mouse anti-human BDNF antibody). Wells were washed three times with wash buffer, 100 μl of standards and samples were added in duplicate, incubated for 2 h. Wells were washed extensively with wash buffer, 100 μl of the Detection Antibody (biotinylated mouse anti-human BDNF antibody) was added and incubation performed for 2 h. After washing wells, 100 μl of Streptavidin-Horse Radish Peroxidase was added for 20 min. Wells were washed, 100 μl of Substrate Solution was added and incubated for 20 min. Reaction was stopped by adding 50 μl of Stop Solution and absorbance was determined immediately at 450 nm using a SpectraMax Plus384 plate reader (Molecular Devices, CA, USA), with the correction wavelength set at 540 nm. For serum samples, standard curves were determined using the kit-provided human BDNF diluted in sample diluent at eight concentrations (23.4–1500 pg/ml) and were run in duplicate on each plate. Serum samples were diluted 1:50. Standard curves for plasma BDNF measurements were determined using human BDNF diluted in sample diluent at eight concentrations (11.7–750 pg/ml) run in duplicate and plasma samples were diluted 1:3. Sample BDNF concentrations were then determined by non-linear regression from the standard curves. Measurements were performed in duplicate and averaged to give a value in pg/ml, which was then expressed in ng/ml after correcting for sample dilution. “Low” and “High” concentration quality control (QC) pools were prepared by adding 10 ng or 100 ng to 5 ml portions of human serum (Innovative Research, Novi, MI, USA), giving nominal concentrations of 2 and 20 ng/ml, respectively. Aliquots of 100 ml of each QC were stored in polypropylene microtubes at −80 °C. The ELISA plate templates were planned so that all samples (Day 1, Day 2 and Day 3) from a particular patient were run in the same assay along with the High and Low QCs. Samples from SDs and ADs were balanced within assays, but assayed under blinded conditions. Dilution curves (1:10; 1:20; 1:30; 1:40; 1:50; 1:60; 1:70; 1:80; 1:90; 1:100) for 3 healthy volunteers and 3 AD patients were prepared by combining serum with sample diluent (1% BSA in PBS, pH 7.2–7.4).
Statistical analysis
All the statistical evaluations were performed using SAS version 9 (SAS Institute, Cary, NC). The 3-standard deviation (3-sd) limit of detection of the assay was calculated by first determining the variance (sd) of 6 replicates of the blank. The blank variance (sd in cpm) was multiplied by 3 and then multiplied by the sensitivity calculated for the lowest standard [sensitivity = 23.4 pg/ml divided by the net (i.e., blank subtracted) mean cpm of the 23.4 pg/ml standard]. Variability data was analyzed by calculating the analytical imprecision (CVI), within-subject or individual (CVw), and between-subject (CVG) variation as previously described, with CVw and CVG corrected for the contribution from intra-assay and inter-assay variance, respectively (Fraser and Harris 1989; Lacher, Hughes, & Carroll, 2005). The index of individuality was calculated as (CVw/CVG). Independent t-tests or chi-squares were used to assess group differences in baseline alcohol use and demographic variables. BDNF levels observed over the three days were analyzed by means of a Linear Mixed Effect model (LME; Laird and Ware, 1982) according to a repeated measures design with between-subjects factor of group (SDs vs. ADs) as the fixed effect factor and time (Day 1, Day 2, Day 3) as a within-subjects factor. Secondary analysis was performed to assess whether demographic and individual variables such as age, IQ and smoking status significantly affected BDNF levels and as potential covariates in the LME analysis for group differences. Pearson correlation coefficients were used to assess intra-individual, between-day correlations in BDNF levels and possible associations between BDNF and clinical descriptive variables. Group means are reported with associated standard deviations (±sd).
Results
Demographics and clinical characteristics of alcohol dependent patients (ADs) and social drinkers (SDs)
AD patients were significantly older, but had similar IQ and BDI scores compared to the SDs. As expected, they reported significantly greater years of alcohol use, number of days of alcohol use in the past month, number of drinks consumed in the past month and were more likely to be smokers as compared to the SD group (see Table 1).
Table 1.
Demographics and sample characteristics in the AD and SD groups.
| Subject variable | AD (N = 16) | SD (N = 16) |
|---|---|---|
| Age: mean (sd)a | 39.1 (7.35) | 29.1 (9.82) |
| IQ Shipley: mean (sd) | 112 (9.0) | 108 (12.4) |
| Gender: male | 7 (44%) | 8 (50%) |
| Race: | ||
| African American | 6 (38%) | 4 (25%) |
| Caucasian | 9 (56%) | 10 (63%) |
| Hispanic | 1 (6%) | 2 (13%) |
| Alcohol use: mean (sd) | ||
| Years of alcohol usea | 18.9 (9.0) | 6.3 (6.4) |
| Frequency (days used in prior month)a | 25.9 (6.4) | 3.5 (2.9) |
| Amount (drinks in prior month)a | 383 (212) | 15.9 (15.2) |
| Number of smokersa | 14 (87%) | 2 (13%) |
| Lifetime Mood Disorders | 3 (19%) | 0 |
| Lifetime Anxiety Disorders | 0 | 0 |
p < 0.001.
Analytical characterization of BDNF assay
The analytical characteristics of the commercially available R&D BDNF ELISA assay were assessed.
Linearity: Standard curves were linear from 24 to 1500 pg/ml. We initially performed linearity tests on six different samples in duplicate. Ten dilutions (from 1:10 to 1:100) of the serum samples were tested. The kit instructions recommended a 1:20 dilution, however we found that at this dilution, almost all our serum samples gave values greater than the highest standard. It was determined that for our serum samples, a 1:50 dilution of serum allowed all samples to fall within the assessed and linear range of the standard curve. Dilutions of less than 1:40 gave lower apparent concentrations due to non-linearity in response at concentrations above the 1500 pg/ml standard.
Detection limit: The 3-sd detection limit for BDNF was determined to be 4 pg/ml or 0.2 ng/ml when using the 1:50 dilution of serum.
Analytical variation: Low and High QCs were diluted 1:50 and the intra-assay variation obtained by assaying the QCs in replicates of four across the microtiter plate within the same day. This test was performed six times and gave a mean CV of 5.2% (range 3.1–6.7) for the Low QC, and a mean CV of 4.7% (range 1.8–8.2) for the High QC. Inter-assay (N = 6) variation was determined by running Low and High QC samples on two different microtiter plates, on three different days. The observed CV was 9.4% for the Low QC (mean value 4.37 ng/ml) and 7.2% for the High QC (mean value 27.2 ng/ml).
Biological variation of serum and plasma BDNF
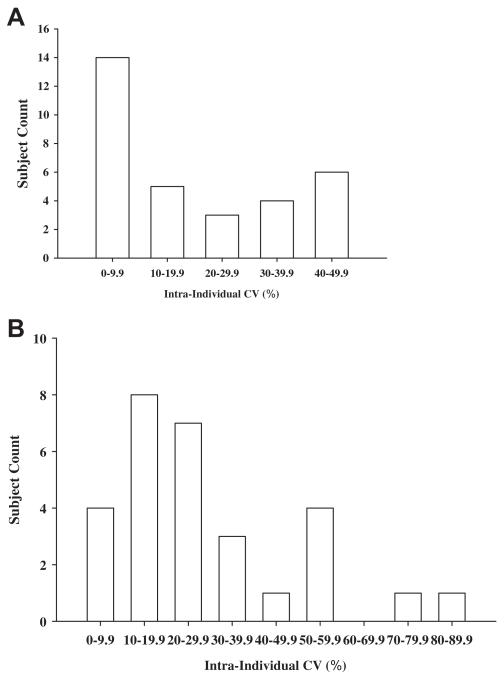
The biological variation consisting of within-person (CVw) and group (CVG) variation was assessed by measuring serum BDNF on three consecutive days in 16 SDs and 16 ADs. A histogram of the uncorrected within-subject (intra-individual) CVs observed in the combined group of subjects (N = 32) is presented in Fig. 1A. Similar distributions were seen in the separate groups, and it was clear that the distributions of the individual variances were non-Gaussian. Mean CVw values (corrected for the contribution of analytical variance) in the SD and AD subjects were 22.6% and 18.8%, respectively. The mean group variations (CVG) observed in the SDs and ADs were 33.4% and 29.1%, respectively. Although analytical variance was corrected for, it actually contributed only about 1% to the uncorrected variances due to its adding geometrically. The indices of individuality (CVw/(CVG) was calculated to be 0.68 and 0.65 in the SDs and AD groups, respectively.
Fig. 1.
A. Distribution histograms of the intra-individual variances (coefficients of variations, CVs) in serum BDNF levels observed across three days in the combined SD and AD group (N = 32 subjects). Bin size of 10% was selected a priori. B. Distribution histograms of the intra-individual variances (coefficients of variations, CVs) in plasma BDNF levels observed across three days in the combined SD and AD group (N = 29 subjects). Bin size of 10% was selected a priori.
For plasma BNDF, the biological variation consisting of within-person (CVw) and group (CVG) variation was assessed by measuring BDNF on three consecutive days in the SD and AD groups. A histogram of the uncorrected within-subject (intra-individual) CVs observed in the combined group of subjects is presented in Fig. 1B. Similar distributions were seen in the separate groups, and it was clear that the distributions of the individual variances were non-Gaussian. Mean CVw values in the SD and AD subjects were 30.4% and 26.5%, respectively. The mean group variations (CVG) observed in the SDs and ADs were 43.8% and 87.7%, respectively. The indices of individuality (CVw/CVG) were calculated to be 0.69 and 0.30 in the SDs and AD groups, respectively.
Day-to-day stability of serum and plasma BNDF in the SD and AD groups
There was no significant day-to-day difference in the mean serum BDNF levels (F2,29 = 1.65; p = 0.21). Moderate, but significant correlations were obtained between days for all subject participants: between Day 1 and Day 2 (n = 32, r = 0.53, p = 0.002); between Day 1 and Day 3 (n = 32, r = 0.60, p = 0.003) and between Day 2 and Day 3 (n = 32, r = 0.48, p = 0.006). correlations were observed in the separate Similar groups. Mean plasma BDNF levels did not significantly differ across days (F2,22 = 0.71; p = 0.5). Significant correlations were obtained between days for all subject participants: between Day 1 and Day 2 (n = 25, r = 0.72, p < 0.001); between Day 1 and Day 3 (n = 25, r = 0.74, p < 0.001) and between Day 2 and Day 3 (n = 25, r = 0.67, p = 0.004). correlations were also seen in the Similar separate groups.
Group comparison of serum BNDF levels in the SD and AD groups
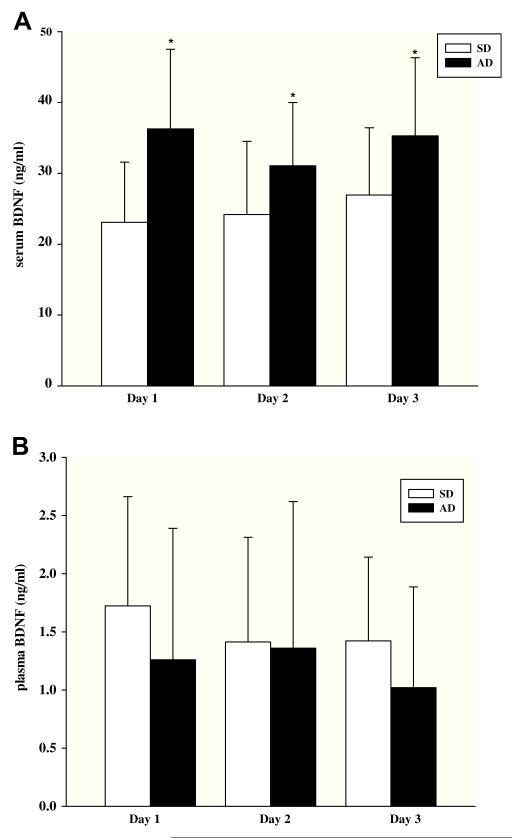
A main effect of group was observed, with abstinent ADs having significantly higher serum BDNF compared with SDs (F1,30 = 10.9, p = 0.003); no significant effects of day or group × day interactions were observed (Fig. 2A). Group differences were substantial, with the AD group mean being 28–56% higher than the SD group. When individual values were collapsed across days, the SD and AD group means were 26.9 ± 9.5 ng/ml versus 35.3 ± 11.1 ng/ml, respectively; an elevation of 30% in the AD group compared to the SD group. Serum BDNF did not correlate significantly with age, IQ, smoking status or prevalence of mood disorders and significant group effects remained significant even when age, IQ or smoking status were included in the LME analyses in secondary analyses. Finally, plasma and serum BDNF values were not significantly correlated in the combined group or in the clinical subgroups (combined group: r = 0.12; p = 0.54: SDs: r = 0.29; p = 0.32; AD: r = 0.23; p = 0.42).
Fig. 2.
A. Baseline serum BDNF concentrations (ng/ml, mean ± sd) measured in social drinkers (SD) and alcohol dependent (AD) patients. Samples were collected between 8:00 and 8:30 am on three consecutive days (N = 16 subjects for both groups). Note; * denotes p < 0.01. B. Baseline plasma BDNF concentrations (ng/ml, mean ± sd) measured in social drinkers (SD) and alcohol dependent (AD) patients. Samples were collected between 8:00 and 8:30 am on three consecutive days; subject N values were 14 and 15 in the SD and AD groups, respectively.
Association of serum BDNF with clinical descriptive variables
We examined correlations between serum BDNF and number of drinks in the previous 30 days, number of drinks in the previous 90 days, days of alcohol use in the previous 30 days, years of alcohol use, and age of onset of alcohol use. The only significant correlation observed was between serum BDNF levels and the number of drinks in the 30 days prior to study admission (r = 0.52, p < 0.003) in the combined group. However, it is important to note that the measures were not correlated in the separate AD (r = 0.35, p = 0.19) and SD (r = −0.11, p = 0.69) groups. We performed also serum BDNF correlations with mood and anxiety scores, as assessed by BDI, obtained at admission in the AD and SD groups, and the combined group. No significant correlations were observed between BDI scores and serum BDNF levels.
Group comparison of plasma BNDF levels in the SD and AD groups
There was no significant effect of group (F1,27 = 0.49, p = 0.49), repeated sampling across days or group × days interaction for plasma BDNF (Fig. 2B). When individual values were collapsed across days, the SD and AD group means were 1.52 ± 0.68 ng/ml versus 1.27 ± 1.12 ng/ml, respectively. Plasma BDNF did not correlate significantly with age, IQ, smoking status or prevalence of mood disorders. Furthermore, secondary analysis including age, IQ or smoking status separately in the LME analysis did not change the non-significant findings.
Association of plasma BDNF with clinical descriptive variables
Plasma BDNF levels were not significantly correlated with any of the clinical or demographic variables in the combined group, or in the SD and AD subgroups.
Discussion
The observed intra- and inter-assay CVs of 5 to 9% indicate that the R&D BDNF ELISA can provide reliable serum and plasma BDNF measurements. A 1:50 dilution of serum samples was found to be necessary to ensure linearity. The reporting of low and high quality assessment samples is recommended and particularly useful when analyzing serum and plasma samples. The analytical variance contributed only slightly to the within-individual and group variation. The mean within-individual variation (CVw) in serum BDNF of approximately 20% indicates that the measure has good short-term stability. However, it did appear that certain subjects display considerably greater day-to-day variation in serum BDNF. The group (CVG) variation for serum BDNF of approximately 30% is typical of many human serum biochemical analytes, while the calculated indices of individuality in the AD and SD groups are indicative of a measure with relatively high degree of individuality. Our observed mean concentration of serum BDNF (27 ng/ml) in the SD group is midrange of reported mean serum BDNF values for healthy controls (Sen et al., 2008).
Higher within-individual and within-group variation was observed for plasma BDNF compared to serum BDNF. The mean within-individual variation of 30% seen for plasma BDNF in the SD group was somewhat higher than the 22% reported by Begliuomini et al. (2008) for healthy male controls sampled at 8 am, three times over 20 days. The same group also reported relatively low group variation in plasma BDNF for their healthy controls (22% versus our CVG of 43.8%). The similarity of CVw and CVG values in the Begliuomini et al. study is surprising; perhaps, the comparatively low CVG value can be partially attributed to greater ethnic homogeneity in their study sample compared to our and others’ samples (see Bocchio-Chiavetto et al., 2010). Our observed mean concentration of plasma BDNF in controls (1.4 ng/ml) and CVg value is close to the median of reported values (Bocchio-Chiavetto et al., 2010; Fujimura et al., 2002; Joe et al., 2007; Lee et al., 2009; Lommatzsch et al., 2005).
We found that serum BDNF levels were significantly higher in 4-week abstinent alcohol dependent subjects compared to healthy, social drinkers. On the other hand, plasma BDNF levels were not significantly different in the abstinent AD group compared to the control group. Before comparing these results to published clinical reports and contemplating possible mechanisms and implications of the observed differences, it is useful to consider the sources of plasma, serum and platelet BDNF. Non-neuronal peripheral cells including vascular smooth muscle cells, endothelial cells lymphocytes and monocytes are the main original sources of circulating (plasma) BDNF (Donovan et al., 1995; Nakahashi et al., 2000). Most of the BDNF in blood is contained in the platelets which actively absorb it from the circulation (Fujimura et al., 2002). Although BDNF cDNA has been detected in platelets (Yamamoto and Gurney, 1990), serum BDNF levels largely reflect the pool of BDNF stored and released from platelets during coagulation. It has been demonstrated that the amount of BDNF in serum is nearly identical to the amount of BDNF found in washed platelet lysates (Fujimura et al., 2002; Lommatzsch et al., 2005) and human serum BDNF levels are typically reported to be 20- to 50-fold higher than plasma levels (Lee and Kim, 2009; Yamamoto and Gurney, 1990). The low correlations that we observed between plasma and serum levels are congruent with their representing separate pools that have little influence on one another.
Our observation of higher serum BDNF levels in early, abstinent AD subjects is consistent with the Huang et al. (2008) report of a small, but significant increase in serum BDNF (from baseline) after one week of alcohol abstinence in AD patients. Umene-Nakano et al. (2009) reported lower serum BDNF in AD subjects; however, their AD subjects had comorbid depression. Although we observed lower mean plasma BDNF levels in AD subjects compared to SDs, the difference was not statistically significant. Our observation differs from the report of Joe et al. (2007) who found substantially lower plasma BDNF levels in 30-day abstinent AD subjects compared to controls. Although the two studies enrolled similar subjects and used the same sampling time-point, one reason for discrepancies across the studies could be that the prior study used heparin-coated blood collection tubes. Heparin has been shown to interfere with the determination of plasma BDNF and to result in a variable lowering of measured values, with levels in heparinized plasma averaging 60% of those in EDTA plasma (Begliuomini et al., 2007).Lee et al. (2009) found higher plasma BDNF in AD patients. However, BDNF was measured in samples obtained the morning after admission and subjects were continually drinking alcohol until admission.
A positive association between serum BDNF and recent number of drinks prior to study admission was observed in the combined group. However, the measures were not correlated in the separate AD and SD groups. The combined group correlation merely reflected the greater drinking and higher group mean level of BDNF in the AD group. Thus, the total group correlation should not be interpreted as suggesting any relationship between serum BDNF and recent number of drinks nor an indicator that serum BDNF could be used as a proxy for number of recent drinks.
Results demonstrating higher basal serum BDNF in the abstinent AD group without an elevation in plasma BDNF levels suggest that the serum elevation is not due to higher BDNF exposure and further studies measuring platelet BDNF levels, platelet uptake and release are required to fully elucidate mechanisms underlying this alteration. Although we did not measure serum levels upon admission, our data are consistent with Huang et al. (2008) findings of increased serum BDNF after one week of abstinence, but not upon admission. BDNF levels may increase during abstinence, but clearly additional studies examining BDNF levels in larger samples of alcohol-dependent subjects during non-abstinence and long-term abstinence are needed to clarify chronic alcohol-related effects during withdrawal and protracted abstinence states.
Decreases in serum and plasma BDNF have been reported in patients with a depressive disorder and BDNF levels increase following antidepressant treatment (Bocchio-Chiavetto et al., 2010; Sen et al., 2008). Even though our sample population did not have any current history of depressive disorders, previous history of depression, lifetime depression or depressive symptoms could influence serum BDNF levels. The lack of correlation between serum BDNF levels and the BDI scores suggests that differences in serum BDNF were unrelated to BDI scores. Nicotine dependence influences BDNF levels and baseline serum (Umene-Nakano et al., 2010) and plasma (Bhang, Choi, & Ahn, 2010) BDNF levels are reported to be lower in smokers compared to non-smokers. Our group differences in serum BDNF remained significant even when smoking status was included in the linear mixed analysis model indicating that smoking status had a minimal influence on serum BDNF levels.
Our findings of elevated BDNF levels after 4 weeks of abstinence during early recovery from alcoholism are consistent with previous reports from our laboratory showing significant alterations in the hypothalamic-pituitary-adrenal axis and autonomic arousal systems responses at baseline and in response to stress and alcohol cue provocation in AD patients (Sinha et al., 2009). These changes are represented as elevations in physiological components such as heart rate, salivary cortisol and plasma ACTH at baseline, in abstinent alcohol dependent patients compared with social drinkers (Sinha et al., 2009). Positive correlations between plasma cortisol levels and circulating BDNF in humans have been well-documented (Begliuomini et al., 2008) and the elevated serum BDNF levels could reflect the homeostatic dysregulation observed in peripheral stress systems during early alcohol abstinence.
Limitations of this study include the relatively small sample size and, as mentioned above, levels in the ADs were assessed only at four weeks after admission. Future studies would benefit from the simultaneous measurement of plasma, serum, and platelet levels, as well as platelet BDNF uptake. Despite the stated limitations, the study has several important strengths compared to prior reports in this area. First, our characterization of within-individual variation of serum and plasma BDNF in ADs and healthy controls indicates that serum BDNF is relatively stable within individuals and that individual variation is low relative to group variation. Serum BDNF can, therefore, be considered a measure with a high index of individuality that characterizes and differentiates individuals. Second, the results clearly show that serum BDNF levels are increased in recently abstinent ADs compared to SDs. Finally, the simultaneous measurement of serum and plasma allowed for increased interpretive power compared to previous studies. Further research is warranted to determine the potential utility of BDNF measures as predictors and surrogates of treatment response in alcoholism. Adaptive changes associated with chronic alcohol abuse contribute to the progressive nature of alcohol dependence, increased craving and vulnerability to relapse (Koob, 2003; Sinha, 2008; Sinha et al., 2009). Clarifying the role of central and peripheral BDNF in alcoholism and alcohol withdrawal could have significant implications for prevention strategies and therapeutic interventions.
Acknowledgments
We thank Adam Hong for excellent assistance with the statistical analysis and Drs. David Russell and Ronald Duman for suggestions and support. This work was supported by NIH grants R01-AA013892, UL1 DE019586, PL1-DA024859 (R. Sinha), PL1 DA024860 (G. Anderson).
Footnotes
Author contributions
CD, RJD, GMA, RS were responsible for study concept and design; CD performed research; RJD contributed to the acquisition of laboratory data, CD and GMA analyzed data; CD, GMA and RS interpreted findings, CD and GMA wrote manuscript, RS and RJD provided revisions.
References
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;29:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Begliuomini S, Casarosa E, Pluchino N, Lenzi E, Centofanti M, Freschi L, et al. Influence of endogenous and exogenous sex hormones on plasma brainderived neurotrophic factor. Human Reproduction. 2007;22:995–1002. doi: 10.1093/humrep/del479. [DOI] [PubMed] [Google Scholar]
- Begliuomini S, Lenzi E, Ninni F, Casarosa E, Merlini S, Pluchino N, et al. Plasma brain-derived neurotrophic factor daily variations in men: correlation with cortisol circadian rhythm. Journal of Endocrinology. 2008;197:429–435. doi: 10.1677/JOE-07-0376. [DOI] [PubMed] [Google Scholar]
- Bhang SY, Choi SW, Ahn JH. Changes in plasma brain-derived neurotrophic factor levels in smokers after smoking cessation. Neuroscience Letters. 2010;468:7–11. doi: 10.1016/j.neulet.2009.10.046. [DOI] [PubMed] [Google Scholar]
- Bocchio-Chiavetto L, Bagnardi V, Zanardini R, Molteni R, Gabriela-Nielsen M, Placentino A, et al. Serum and plasma BDNF in major depression: a replication study and meta-analyses. World Journal of Biological Psychiatry. 2010;11:763–773. doi: 10.3109/15622971003611319. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. American drinking practices. A national study of drinking behaviors and attitudes. Monographs of the Rutgers Center of Alcohol Studies. 1969;6:185–260. [Google Scholar]
- Chaldakov GN, Tonchev AB, Aloe L. NGF and BDNF: from nerves to adipose tissue, from neurokines to metabokines. Rivista di psichiatria. 2009;44:79–87. [PubMed] [Google Scholar]
- D’Souza DC, Pittman B, Perry E, Simen A. Preliminary evidence of cannabinoid effects on brain-derived neurotrophic factor (BDNF) levels in humans. Psychopharmacology. 2009;202:569–578. doi: 10.1007/s00213-008-1333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan MJ, Miranda RC, Kraemer R, McCaffrey TA, Tessarollo L, Mahadeo D, et al. Neurotrophin and neurotrophin receptors in vascular smooth muscle cells. Regulation of expression in response to injury. American Journal of Pathology. 1995;147:309–324. [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for the DSM-IV Axis I disorders—patient edition (SCID-I/P, version 2.0, 4/97 revision) New York State Psychiatric Institute; New York: 1997. [Google Scholar]
- Fraser CG, Harris EK. Generation and application of data on biological variation in clinical chemistry. Critical Reviews in Clinical Laboratory Sciences. 1989;27:409–437. doi: 10.3109/10408368909106595. [DOI] [PubMed] [Google Scholar]
- Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thrombosis and Haemostasis. 2002;87:728–734. [PubMed] [Google Scholar]
- Huang MC, Chen CH, Liu SC, Ho CJ, Shen WW, Leu SJ. Alterations of serum brain-derived neurotrophic factor levels in early alcohol withdrawal. Alcohol and Alcoholism. 2008;43:241–245. doi: 10.1093/alcalc/agm172. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, Carnicella S, Kharazia V, Janak PH, Ron D. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. Journal of Neuroscience. 2009;29:13494–13502. doi: 10.1523/JNEUROSCI.2243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe KH, Kim YK, Kim TS, Roh SW, Choi SW, Kim YB, et al. Decreased plasma brain-derived neurotrophic factor levels in patients with alcohol dependence. Alcoholism: Clinical and Experimental Research. 2007;31:1833–1838. doi: 10.1111/j.1530-0277.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, et al. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. International Journal of Neuropsychopharmacology. 2010;7:1–7. doi: 10.1017/S1461145710000738. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcoholism: Clinical and Experimental Research. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Lacher DA, Hughes JP, Carroll MD. Estimate of biological variation of laboratory analytes based on the third national health and nutrition examination survey. Clinical Chemistry. 2005;51:450–452. doi: 10.1373/clinchem.2004.039354. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lee BC, Choi IG, Kim YK, Ham BJ, Yang BH, Roh S, et al. Relation between plasma brain-derived neurotrophic factor and nerve growth factor in the male patients with alcohol dependence. Alcohol. 2009;43:265–269. doi: 10.1016/j.alcohol.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Lee BH, Kim YK. Reduced platelet BDNF level in patients with major depression. Progress in Neuropsychopharmacology and Biological Psychiatry. 2009;33:849–853. doi: 10.1016/j.pnpbp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Linker RA, Gold R, Luhder F. Function of neurotrophic factors beyond the nervous system. Critical Reviews in Immunology. 2000;29:43–68. doi: 10.1615/critrevimmunol.v29.i1.20. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Schloetcke K, Klotz J, Schuhbaeck K, Zingler D, Zingler C, et al. Brain-derived neurotrophic factor in platelets and airflow limitation in asthma. American Journal of Respiratory and Critical Care Medicine. 2005;171:115–120. doi: 10.1164/rccm.200406-758OC. [DOI] [PubMed] [Google Scholar]
- McGough NN, He DY, Logrip ML, Jeanblanc J, Phamluong K, Luong K, et al. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. Journal of Neuroscience. 2004;24:10542–10552. doi: 10.1523/JNEUROSCI.3714-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters S, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- MacLennan AJ, Lee N, Walker DW. Chronic ethanol administration decreases brain-derived neurotrophic factor gene expression in the rat hippocampus. Neuroscience Letters. 1995;197:105–108. doi: 10.1016/0304-3940(95)11922-j. [DOI] [PubMed] [Google Scholar]
- Miller WR, Del Boca FK. Measurement of drinking behavior using the Form 90 family of instruments. Journal of Studies in Alcohol. 1994;(Suppl. 12):112–118. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- Miller MW, Mooney SM. Chronic exposure to ethanol alters neurotrophin content in the basal forebrain-cortex system in the mature rat: effects on autocrineeparacrine mechanisms. Journal of Neurobiology. 2004;60:490–508. doi: 10.1002/neu.20059. [DOI] [PubMed] [Google Scholar]
- Minichiello L. TrkB signalling pathways in LTP and learning. Nature Reviews Neuroscience. 2009;10:850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Serritella C, Martiadis V, Maj M. Decreased levels of serum brain-derived neurotrophic factor in both depressed and euthymic patients with unipolar depression and in euthymic patients with bipolar I and II disorders. Bipolar Disorder. 2008;10:95–100. doi: 10.1111/j.1399-5618.2008.00459.x. [DOI] [PubMed] [Google Scholar]
- Moonat S, Starkman BG, Sakharkar A, Pandey SC. Neuroscience of alcoholism: molecular and cellular mechanisms. Cellular and Molecular Life Sciences. 2010;67:73–88. doi: 10.1007/s00018-009-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, et al. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Letters. 2000;470:113–117. doi: 10.1016/s0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- de Oliveira GS, Cereser KM, Fernandes BS, Kauer-Sant’Anna M, Fries GR, Stertz L, et al. Decreased brain-derived neurotrophic factor in medicated and drug-free bipolar patients. Journal of Psychiatric Research. 2009;43:1171–1174. doi: 10.1016/j.jpsychires.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Hellweg R, Litzke J, Vogt M, Dormann C, Vollmayr B, et al. Correlations and discrepancies between serum and brain tissue levels of neurotrophins after electroconvulsive treatment in rats. Pharmacopsychiatry. 2009;42:270–276. doi: 10.1055/s-0029-1224162. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35:2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biological Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of New York Academy of Sciences. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Molecular Psychiatry. 2010;15:80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia-Arancibia L, Rage F, Givalois L, Dingeon P, Arancibia S, Beauge F. Effects of alcohol on brain-derived neurotrophic factor mRNA expression in discrete regions of the rat hippocampus and hypothalamus. Journal of Neuroscience Research. 2001;63:200–208. doi: 10.1002/1097-4547(20010115)63:2<200::AID-JNR1012>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Umene-Nakano W, Yoshimura R, Ikenouchi-Sugita A, Hori H, Hayashi K, Ueda N, et al. Serum levels of brain-derived neurotrophic factor in comorbidity of depression and alcohol dependence. Human Psychopharmacology. 2009;24:409–413. doi: 10.1002/hup.1035. [DOI] [PubMed] [Google Scholar]
- Umene-Nakano W, Yoshimura R, Yohii C, Hoshuyama T, Hayashi K, Hori K, et al. Varenicline does not increase serum BDNF levels in patients with nicotine dependence. Human Psychopharmacology. 2010;25:276–279. doi: 10.1002/hup.1113. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Gurney ME. Human platelets contain brain-derived neurotrophic factor. Journal of Neuroscience. 1990;10:3469–3478. doi: 10.1523/JNEUROSCI.10-11-03469.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]