Abstract
Friedreich ataxia is a neurodegenerative disease with an average age of onset of 10 years. We sought to investigate the presence and functional consequences of auditory neuropathy in a group of affected children and to evaluate the ability of personal FM-listening systems to improve perception. Nineteen school-aged individuals with Friedreich ataxia and a cohort of matched control subjects underwent a battery of auditory function tests. Sound detection was relatively normal but auditory temporal processing and speech understanding in noise was severely impaired, with children with Friedreich ataxia typically able to access <40% of the information available to controls. Use of FM-listening devices did, however, improve the speech perception performance of those with Friedreich ataxia to the level of their unaffected peers in conditions designed to replicate the listening environment of the average school classroom.
Keywords: auditory processing, Friedreich ataxia, hearing, speech perception
Introduction
Friedreich ataxia is the most common of the inherited ataxias.1 Clinical features of the disease include progressive limb and trunk ataxia, cardiomyopathy, scoliosis, and an increased rate of diabetes mellitus.2,3 The disease typically presents in childhood, with an average age of onset of 10 years.4 Friedreich ataxia occurs as a result of mutations in the FXN gene.5 In 98% of mutant alleles there is expansion of GAA trinucleotide repeats in intron 1 of the gene, which causes partial silencing of FXN and subsequent deficits in “frataxin,” the encoded protein.3 The other 2% of allele abnormalities are the result of point mutations.1
Hearing, as measured by the ability to detect sound, tends to be unimpaired in individuals with Friedreich ataxia, with most studies showing audiometric thresholds (averaged across the speech frequencies) within the normal range.6–9 Although this finding suggests normal peripheral (cochlear) function, it does not guarantee normal perception and most (adult) Friedreich ataxia listeners described in the literature have, in fact, shown evidence of auditory neuropathy, presenting with either absent or distorted electrophysiologic responses from the VIIIth nerve and auditory brainstem.8,10,11
Auditory neuropathy is known to affect the neural representation of complex acoustic signals.12 In particular, the resulting disruption of neural synchrony can impair temporal resolution—the ability to perceive rapid changes in auditory signals over time.13,14 Psychophysical studies exploring auditory temporal processing in listeners with auditory neuropathy due to Friedreich ataxia have consistently shown impaired detection of brief gaps (silent periods) and rapid envelope variations (sinusoidal amplitude modulation) in continuous stimuli.9
The primary functional consequence of auditory temporal processing deficit is disruption of speech understanding. Discrimination of phonemes (individual speech sounds) in running speech, or even in isolated words, requires that the listener perceive the characteristic spectral shapes of individual sounds, and be able to efficiently update this perception to track changes occurring over the course of only 10s of milliseconds. Difficulties with speech understanding are common in adults with Friedreich ataxia.8,9,15 Perceptual ability varies depending on the degree of temporal processing disorder,9 but most affected adults report impaired communication in everyday listening circumstances15 that worsens with progression of the disease. Furthermore, auditory perceptual deficits pose particular risks for young children, for whom impaired speech understanding can have significant detrimental effects upon general communication, speech/language development, socialization ability, and educational progress.16,17
Amelioration of the functional effects of auditory neuropathy-type hearing loss is challenging. Conventional hearing aids amplify sounds but are not designed to clarify temporally distorted signals and, as such, have been consistently unpopular in trials involving adults with neurodegenerative auditory neuropathy.18 An alternative approach involves the use of FM-listening devices.19 These systems transmit speech signals (detected by a lapel-worn microphone) via radio waves to ear level receivers worn by the listener. Thus, the listener obtains a signal advantage from the proximity of the speaker's mouth to the transmitter microphone.
The aims of this study were to investigate the presence and perceptual consequences of auditory neuropathy in a cohort of school-aged children with Friedreich ataxia and to examine the efficacy of FM-listening systems in this population.
Methods
Subjects
Nineteen children (8 female, age range at assessment, 8 years to 19 years [15.0 ± 3.3 years]) confirmed by genetic testing as being homozygous for GAA expansions of intron 1 of the FXN gene were recruited for the study through the Bruce Lefroy Centre for Genetic Health Research. Patient details are shown in Table 1. Overall disease severity was evaluated using the Friedreich Ataxia Rating Scale.20 The Friedreich Ataxia Rating Scale is scored out of 167, with a higher score representing a greater degree of functional disability. Scores ranged from 31.7 to 108.5, indicating a reasonably broad range of disease severity across the cohort (Table 1).
Table 1.
Friedreich Ataxia Participant Details
| Subject | Age at Assessment (y) | Age at Onset (y) | GAA1 (repeat#) | FARS | 4-Freq Average Hg Level (dB) | ABR |
|---|---|---|---|---|---|---|
| #1 | 15 | 4 | 556 | 56.3 | 20 | NR |
| #2 | 14 | 3 | 659 | 98 | 15 | NR |
| #3 | 15 | 5 | 643 | 108.5 | 25 | NR |
| #4 | 14 | 11 | 593 | 33.3 | 10 | NR |
| #5 | 9 | 3 | 975 | 56 | 10 | Present |
| #6 | 10 | 3 | 1052 | 71 | 15 | Present |
| #7 | 13 | 6 | 1298 | 102 | 15 | NR |
| #8 | 18 | 7 | 1000 | 108.5 | 27.5 | NR |
| #9 | 14 | 10 | 833 | 73 | 8.2 | NR |
| #10 | 8 | 5 | 750 | 62 | 15 | Present |
| #11 | 17 | 12 | 818 | 45 | 16.2 | Present |
| #12 | 19 | 6 | 1015 | 113 | 32.5 | NR |
| #13 | 19 | 14 | 884 | 65.5 | 10 | Present |
| #14 | 16 | 3 | 800 | 105 | 15 | NR |
| #15 | 19 | 9 | 694 | 103 | 10 | Present |
| #16 | 19 | 7 | 998 | 76.5 | 5 | Present |
| #17 | 18 | 9 | 659 | 79.5 | 12.2 | NR |
| #18 | 17 | 16 | 562 | 91.5 | 12.2 | NR |
| #19 | 17 | 16 | 833 | 88 | 17.7 | Present |
Abbreviations: ABR, auditory brainstem response; FARS, Friedreich Ataxia Rating Scale; Freq, frequency; NR, no response.
Each subject underwent bilateral audiometric testing in which sound detection thresholds were established at octave frequencies from 250 Hz to 8 kHz. The ear with better hearing thresholds then underwent a battery of auditory assessments, including auditory brainstem response evaluation, auditory temporal processing and open-set speech perception.
Data from 20 age- and gender-matched children were also obtained. Each control child was matched to within 12 months of their counterpart with Friedreich ataxia at assessment (mean age, 14.8 ± 3.6 years).
Tests of Auditory Function
We completed auditory brainstem response testing using the procedure described in Rance and colleagues.8 If the auditory brainstem response was absent to rarefaction-polarity clicks at maximum presentation levels (90 dB nHL to 100 dB nHL), testing with compression-polarity stimuli was undertaken to confirm the presence of the cochlear microphonic. (The cochlear microphonic can be differentiated from the neural response when it changes phase with alteration in stimulus polarity). Each of the children with Friedreich ataxia showed evidence of abnormal auditory-neural function. In 11 of 19 cases, the auditory brainstem response was absent despite normal preneural potentials and normal or near-normal sound detection (Table 1). In 8 subjects, repeatable but low-amplitude auditory brainstem responses were obtained.
We assessed auditory temporal resolution using a task that sought the minimum detectable depth for sinusoidal amplitude modulation at low (10 Hz) and high (150 Hz) modulation rates. The psychophysical test procedure was as described in Rance and colleagues (2004).13 The modulated (target) stimuli were generated by multiplying a 500 msec noise burst with a dc-shifted sine wave. Depth of modulation (defined as 20 logM) was varied in 3 dB steps, from 0 to −30 dB.
We carried out open-set speech perception testing using recorded consonant-nucleus-consonant words presented at 65 dB SPL(root mean square) or at a comfortable listening level in those ears with impaired sound detection. Subjects were presented with 2 word lists each consisting of 50 stimuli. In one, the words were presented alone (quiet condition). In the other, a competing signal (4-talker babble) was mixed with the speech at a 0 dB signal-to-noise ratio (ie, the speech and noise were at the same level). Subjects were required to imitate the target words and their responses were phonetically transcribed to produce a “percentage phonemes correct” score for each listening condition. Because speech production difficulties (such as dysarthria) have been observed in individuals with Friedreich ataxia,21 a phonetic profile was obtained for each child using the Articulation Survey.8 None of the subjects showed significant production difficulties and, as such, any errors made on the speech perception test were assumed to reflect an inability to hear, rather than reproduce, the stimulus words.
We investigated everyday listening and general communication ability using a hearing disability questionnaire (Abbreviated Profile of Hearing Aid Benefit). This 24-question metric explores 4 aspects of auditory function: (1) communication difficulty, (2) the effect of background noise, (3) the effect of reverberation, and (4) aversion to loud sounds.
Assisted-Listening Device Trial
Nine of the 19 FRDA subjects (and their matched controls) underwent an FM-listening device trial. Each subject was provided with a Phonak Inspiro FM-transmitter paired with bilateral iSense ear-level receivers (Murten, Switzerland). We then conducted speech perception testing (consonant-nucleus-consonant words) in the free-field with the subject seated between 2 loudspeakers. Perceptual ability was assessed in both unaided and aided (FM) conditions as per the technique described in Rance and colleagues.15
Results
Sound Detection
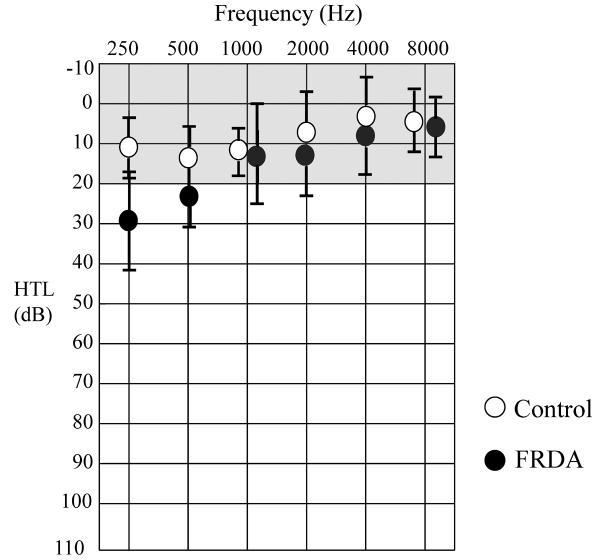
Audiometric thresholds were established in both ears for each subject at octave frequencies between 250 Hz and 8 kHz. Mean thresholds for subjects with Friedreich ataxia and for control subjects are shown in Figure 1. Sound detection at 250 Hz was significantly poorer for those in the Friedreich ataxia cohort (T=4.36, P < .01 [7.12, 21.97]1), but thresholds at each of the higher test frequencies were equivalent (500 Hz: T=0.80, P > .05 [−1.61,3.43]; 1 kHz: T=0.36, P>.05 [−2.34, 3.25]; 2 kHz: T=2.19, P>.05 [−0.04, 4.58]; 4 kHz: T=1.00, P>.05 [−1.12, 2.93]).
Figure 1.
Mean sound detection thresholds at audiometric frequencies for Friedreich ataxia and control subjects. Error bars represent ±1 SD. The shaded area shows the normal hearing range. Abbreviations: FRDA, Friedreich ataxia; HTL, hearing threshold level; SD, standard deviation.
Four-frequency average hearing levels (based upon detection threshold at the 500 Hz, 1 kHz, 2 kHz, and 4 kHz test frequencies) were calculated for each ear to determine overall access to sound in the speech frequency range. Each of the children with Friedreich ataxia showed average hearing at normal or near normal levels in the better hearing ear (see Table 1), although mean 4-frequency hearing levels were poorer for those in the Friedreich ataxia group than for matched controls; Friedreich ataxia, 14.9 ± 7.1; control, 9.5 ± 7.2 (T=3.35, P < .01 [2.02, 8.77]).
Correlation analyses (Pearson r) showed no relation between 4-frequency hearing level and age at assessment, age at disease onset, disease duration, or GAA1 repeat length for those in the Friedreich ataxia cohort (P > .05). In contrast, average hearing level and Friedreich Ataxia Rating Scale score were significantly correlated (r = 0.559, P < .01).
Auditory Temporal Processing
Detection of rapid sinusoidal amplitude modulation was poorer for children with Friedreich ataxia than for matched controls. Whereas mean detection thresholds for the low rate stimulus (10 Hz modulation frequency) were equivalent (Friedreich ataxia, −23.2±2.7 dB [6.9% modulation depth]; control, −23.7±2.6 [6.5% modulation depth]; T=−0.70, P = .562 [−0.77, 0.41]), listeners with Friedreich ataxia required significantly larger depths to identify high-rate (150 Hz) amplitude changes (Figure 2). Mean detection threshold for those in the Friedreich ataxia group in this case was −9.2 ± 6.4 dB (34.7% modulation depth), compared with −18.7 ± 2.4 dB (11.6% modulation depth) for the matched controls (T=6.40, P < .001 [6.42,12.65]).
Figure 2.
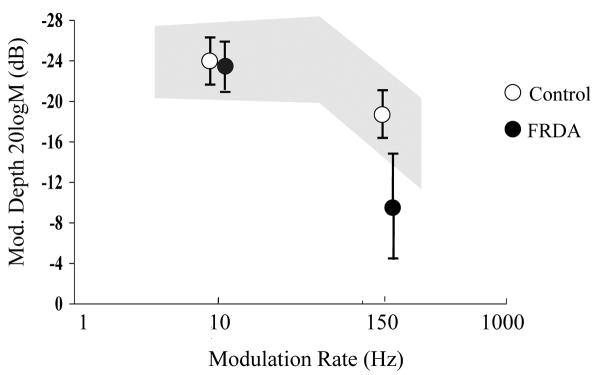
AM detection thresholds for Friedreich ataxia and control subjects (mean ±1 SD). The shaded area represents the normal range for school-aged children from Rance and colleagues.13 Abbreviation: FRDA, Friedreich ataxia; Mod., modulation; SD, standard deviation.
There were no significant correlations between low-rate amplitude modulation detection findings and any of the Friedreich ataxia subject variables. High-rate (150 Hz) amplitude modulation detection threshold was also not correlated with age at assessment, age at disease onset, disease duration, or GAA1 repeat length (P > .05). In contrast, 150 Hz amplitude modulation detection and Friedreich Ataxia Rating Scale score were significantly related (r = 0.628, P < .005).
Speech Perception
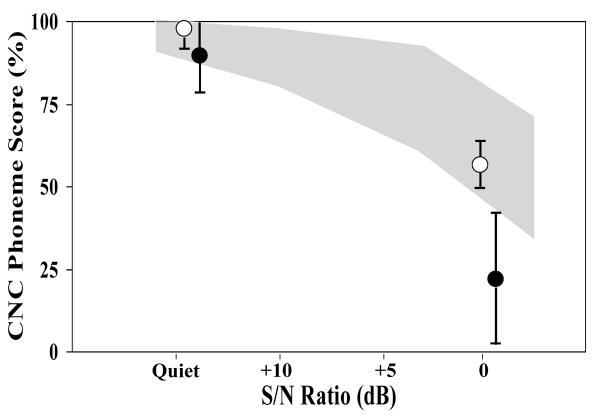
Open-set speech perception was poorer in listeners with Friedreich ataxia than in matched controls. For listening in quiet, mean phoneme score for the Friedreich ataxia group was 88.2 ± 13.6% and for the matched controls was 98.5 ± 1.5% (T=−3.31, P < .01 [−16.79, −3.78] Figure 3). Listening in the presence of background noise (0 dB signal-to-noise ratio) revealed even greater differences. In this condition, mean phoneme score for the Friedreich ataxia cohort was only 22.3 ± 16.0%, whereas the controls scored 55.9 ± 5.6% (T=−8.31, P < .001 [−42.09, −25.15).
Figure 3.
Consonant-nucleus-consonant phoneme scores for Friedreich ataxia and control subjects (mean ±1 SD). The shaded area represents the normal range for school-aged children from Rance and colleagues.22 Abbreviation: CNC, consonant-nucleus-consonant; SD, standard deviation; S/N, signal-to-noise.
Correlation analysis showed no significant relations between phoneme score (in quiet) and any of the Friedreich ataxia subject variables. Similarly, speech perception in background noise was not correlated with age at assessment, age at disease onset, disease duration, or GAA1 repeat length (P > .05). Speech perception (in background noise) and Friedreich Ataxia Rating Scale scores did, however, show a strong inverse relationship (r = −0.585, P < .001).
Hearing Disability
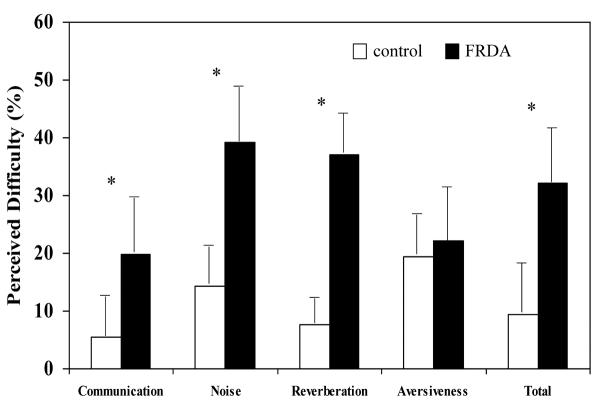
Children with Friedreich ataxia reported a higher degree of everyday listening and communication difficulty than the controls. Abbreviated Profile of Hearing Aid Benefit questionnaire scores (reflecting the proportion of circumstances in which they perceived a problem) were significantly higher for the Ease of Communication (T=4.41, P < .01 [8.18, 23.51), Effect of Background Noise (T=3.96, P < .01, [9.99, 33.36]), and Effect of Reverberation (T=3.30, P < .01 [6.10, 28.29]) metrics and for the Total Score generated from these sub-categories (T=4.48, P < .01 [9.49, 26.74]) (Figure 4). Level of aversion to sudden loud sounds (sirens etc) was equivalent for the Friedreich ataxia and control groups (T=−0.13, P > .05 [−11.49, 10.14]).
Figure 4.
Abbreviated Profile of Hearing Aid Benefit Hearing Disability Questionnaire findings for Friedreich ataxia and control cohorts. Values represent the percentage of everyday listening situations in which the child perceived a difficulty (Mean ±1 SD). Asterisks denote the metrics in which there was a significant difference between subject groups (P < .01). Abbreviation: FRDA, Friedreich ataxia; SD, standard deviation.
There were no significant correlations between the Friedreich ataxia subjects' Abbreviated Profile of Hearing Aid Benefit Total Score and age at assessment, age at disease onset, disease duration, or GAA1 repeat length (P > .05). Abbreviated Profile of Hearing Aid Benefit Total Score and Friedreich Ataxia Rating Scale score were significantly related (r = 0.548, P < .01).
FM Listening Device Evaluation
Speech perception in the free field (ie, with both ears contributing) was also poorer for the children with Friedreich ataxia than for their control counterparts. Mean consonant-nucleus-consonant phoneme score (0 dB signal-to-noise ratio) for participants with Friedreich ataxia was 56.4 ± 25.6% and for the controls was 81.6 ± 3.4% (T=−2.96, P < .05 [−44.84, −5.56]). Provision of the FM-listening device resulted in significant perceptual benefits for children in both groups. Mean FM-aided score for those in the Friedreich ataxia group was 80.7 ± 10.8% and for the control cohort was 92.3 ± 1.5% (T=−3.28, P < .05 [−10.74, −3.44]), which represented an average improvement of 24.5 ± 16.1% for children with Friedreich ataxia and 10.7 ± 2.6% for children in the control group (T=2.51, P<.05 [1.12, 26.42]) (Figure 5).
Figure 5.
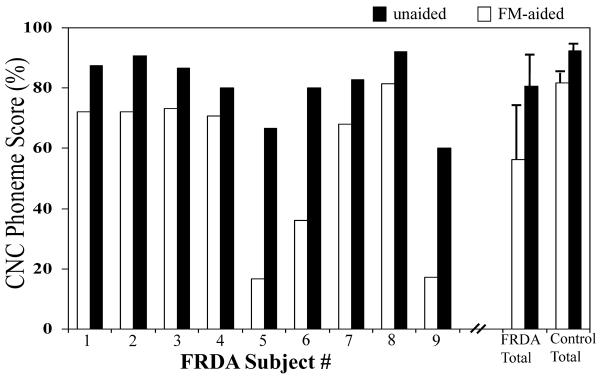
Unaided and FM-aided speech perception scores (CNC words at 0 dB SNR) for each of the Friedreich ataxia subjects. Also shown are the mean ±1 SD scores for the Friedreich ataxia and matched control groups. Abbreviation: CNC, consonant-nucleus-consonant; FRDA, Friedreich ataxia; SD, standard deviation; SNR, signal-to-noise ratio.
Discussion
Each of the children with Friedreich ataxia who participated in this study showed normal or near-normal sound detection. Mean hearing threshold levels were significantly poorer (than matched controls) for low frequency (250 Hz) tones, but were within the normal range for frequencies containing most of the spectral energy required for functional hearing (500 Hz to 8 kHz). As such, their audiometric findings resemble those previously reported for adult subjects with Friedreich ataxia6–9 and suggest that sound detection was unlikely to have affected their auditory processing ability.
All of the participants with Friedreich ataxia did, however, show evidence of auditory neuropathy presenting with normal cochlear responses (cochlear microphonics), but either absent or low-amplitude auditory evoked potentials. This pattern is indicative of auditory nerve axonopathy and is consistent with histological evidence that has shown preserved cochlear structures (organ of corti and hair cells) and selective VIIIth nerve damage in the temporal bones of adults who have Friedreich ataxia.23
Basic auditory processing was disrupted in the children with Friedreich ataxia. While amplitude modulation detection for a low modulation rate (10 Hz) stimulus was normal (suggesting normal sensitivity to amplitude [level] changes), they showed an impaired capacity to detect modulation occurring at a rapid rate (150 Hz). That is, the auditory pathways in these children were less able to encode signal changes occurring over a brief (6 msec to 7 msec) time course. The precise mechanisms underlying this temporal processing deficit are not fully understood, but axonopathy may produce neural desynchrony where inconsistent conduction velocities result from partially damaged (but still functioning) fibers, secondary demyelination, and/or conduction block.24 Desynchrony in the central auditory pathway could, in turn, produce a time-smeared neural representation of acoustic stimuli.14
Speech perception deficits were observed in all listening conditions, but were particularly pronounced when speech was presented in the presence of a competing signal. Mean consonant-nucleus-consonant phoneme score in the 0 dB signal-to-noise ratio condition for the Friedreich ataxia cohort was only 22%, indicating that on average, the children could correctly imitate less than one speech sound per word. In contrast, their normal peers could identify more than half of the phonemes - a level that would allow the listener to use contextual cues and word knowledge to aid understanding in a conversational situation. Reduced ability to cope with background noise is a commonly reported consequence of impaired temporal resolution and is thought to reflect increased masking effects and/or reduced ability to use brief silent periods in a fluctuating noise to access the speech signal.8,14,15,22 Whatever the mechanism(s), the performance levels observed in this study indicate that a high proportion of children with Friedreich ataxia will likely face significant communication and educational challenges, as the listening conditions in a typical school classroom (0 dB to 3 dB signal-to-noise ratio) are similar to that used in the perceptual testing.25
Deficits on formal speech testing were also reflected in the subjects' self-reported hearing disability ratings. Children with Friedreich ataxia considered their listening and general communication to be hampered in >30% of everyday circumstances, whereas their matched controls reported difficulties in <10% of situations. Not surprisingly, participants with Friedreich ataxia found noisy and reverberant (echoic) conditions to be a particular problem (Figure 4).
Speech perception was made significantly easier when wearing the personal FM system. These devices improve listening in noise by increasing the signal-to-noise ratio. That is, the speech is relatively louder than the noise because it is picked-up (by the transmitter microphone) in close proximity to the source. The signal-to-noise ratio advantage afforded by the device is the same for all users (≈6 dB at a typical conversational distance) and hence everyone will enjoy a listening benefit. Significantly, this 6 dB improvement resulted in a bigger perceptual advantage for the children with Friedreich ataxia in this study (24% average FM-aided improvement compared with 10% for the controls), reflecting their specific listening in noise problem. As such, while not allowing perfect perception in noise, the provision of FM-devices did increase the mean speech score for those in the Friedreich ataxia cohort to the unaided level of the control children. This finding suggests intervention strategies that improve the signal-to-noise ratio in the classroom and other challenging communication situations, allow the possibility of relatively normal functional hearing.
Summary and Recommendations
The results of this study suggest that auditory processing abnormality is an early-presenting and pervasive consequence of Friedreich ataxia. All of the school-aged children in our Friedreich ataxia cohort showed at least some functional hearing deficit and those with greater degrees of overall disability were most affected. Performance on each of the auditory measures (particularly temporal processing and speech perception in noise) were correlated with Friedreich Ataxia Rating Scale score, suggesting that auditory function may provide a reliable measure of the natural progression of the disease and may serve as a biomarker for pharmaceutical interventions.
The findings indicate that regular auditory evaluation should form part of the management regimen for all children (and adults) with Friedreich ataxia. This assessment may include audiometric evaluation (as sound detection is a prerequisite for useful hearing) but more importantly, should involve some measure of functional hearing capacity, such as open-set speech perception, which can quantify the degree of processing abnormality and provide a basis for intervention.
Management of auditory neuropathy is not straightforward.18 Conventional hearing aids tend not to be useful for listeners with Friedreich ataxia, as they simply make sounds louder (which is typically not required for this population) and do not overcome the neural distortion problem. FM-listening devices do appear to be effective in providing improved speech understanding (at least in structured listening situations). Further evaluation is required to determine, first, whether affected children will tolerate the systems over an extended period and second, whether they can positively influence long-term speech/language development, socialization and educational outcomes.
Acknowledgments
Data collection for this study was carried out at the University of Melbourne, Department of Audiology and Speech Pathology, in collaboration with the Bruce Lefroy Centre for Genetic Health Research. This paper is based in part on a presentation given at the Neurobiology of Disease in Children Symposium: Childhood Ataxia, in conjunction with the 40th Annual Meeting of the Child Neurology Society, Savannah, Georgia, October 26, 2011. Supported by grants from the National Institutes of Health (2R13NS040925-14 Revised), the National Institutes of Health Office of Rare Diseases Research, the Child Neurology Society, and the National Ataxia Foundation. We thank Melanie Fridl Ross, MSJ, ELS, for editing assistance.
Financial Disclosure/Funding This study was supported by a project grant from the Friedreich Ataxia Research Alliance. G.R. was supported by the Wagstaff Research Fellowship in Otolaryngology.
Ethical approval Approval for the study was obtained from the Royal Victorian Eye & Ear Hospital, Human Research and Ethics Committee (Project #07-747H).
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: Supported by grants from the National Institute of Neurological Disorders and Stroke (5R13NS040925-15), the National Institutes of Health Office of Rare Disease Research, the Child Neurology Society, and the National Ataxia Foundation.
Footnotes
Author Contributions All authors agreed with the conception and design of the study and contributed to the draft and final manuscript. All listed authors met the journal authorship criteria and all contributing authors are indicated. G.R. was responsible for the study design, auditory data collection/analysis and drafting of the manuscript. L.C. and M.D. were involved in subject recruitment, collection of non-auditory data and review of the paper.
Declaration of Conflicting Interests The authors declare no conflicts of interest with respect to the authorship and/or publication of this article.
Numbers in square brackets represent 95% confidence interval for mean difference.
References
- 1.Voncken M, Ioannou P, Delatycki MB. Friedreich ataxia-update on pathogenesis and possible therapies. Neurogenetics. 2004;5:1–8. doi: 10.1007/s10048-003-0170-z. [DOI] [PubMed] [Google Scholar]
- 2.Delatycki MB, Williamson R, Forrest SM. Friedreich ataxia: an overview. J Med Genet. 2000;37:1–8. doi: 10.1136/jmg.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandolfo M. Friedreich ataxia. Arch Neurol. 2008;65:1296–1303. doi: 10.1001/archneur.65.10.1296. [DOI] [PubMed] [Google Scholar]
- 4.Delatycki MB, Paris DB, Gardner RJ, et al. Clinical and genetic study of Friedreich ataxia in an Australian population. Am J Med Genet. 1999;87:168–174. doi: 10.1002/(sici)1096-8628(19991119)87:2<168::aid-ajmg8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Campuzano V, Montermini L, Molto D, et al. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423–1427. doi: 10.1126/science.271.5254.1423. [DOI] [PubMed] [Google Scholar]
- 6.Harding AE. Friedreich's ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain. 1981;104:589–620. doi: 10.1093/brain/104.3.589. [DOI] [PubMed] [Google Scholar]
- 7.Durr A, Cossee M, Agid Y, et al. Clinical and genetic abnormalities in patients with Friedreich's ataxia. New Engl J Med. 1996;335:1169–1175. doi: 10.1056/NEJM199610173351601. [DOI] [PubMed] [Google Scholar]
- 8.Rance G, Fava R, Baldock H, et al. Speech perception ability in individuals with Friedeich ataxia. Brain. 2008;131:2002–2012. doi: 10.1093/brain/awn104. [DOI] [PubMed] [Google Scholar]
- 9.Rance G, Corben L, Barker E, et al. Auditory perception in individuals with Friedreich ataxia. Audiol Neurootol. 2010;15:229–240. doi: 10.1159/000255341. [DOI] [PubMed] [Google Scholar]
- 10.Satya-Murti S, Cacace A, Hanson P. Auditory dysfunction in Friedreich ataxia: result of spiral ganglion degeneration. Neurology. 1980;30:1047–1053. doi: 10.1212/wnl.30.10.1047. [DOI] [PubMed] [Google Scholar]
- 11.Jabbari B, Schwartz DM, MacNeil DM, Coker SB. Early abnormalities of brainstem auditory evoked potentials in Friedreich's ataxia: Evidence of primary brainstem dysfunction. Neurology. 1983;33:1071–1074. doi: 10.1212/wnl.33.8.1071. [DOI] [PubMed] [Google Scholar]
- 12.Starr A, Picton TW, Sininger YS, et al. Auditory neuropathy. Brain. 1996;119(3):741–753. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- 13.Rance G, McKay C, Grayden D. Perceptual characterisation of children with auditory neuropathy. Ear Hear. 2004;25:34–46. doi: 10.1097/01.AUD.0000111259.59690.B8. [DOI] [PubMed] [Google Scholar]
- 14.Zeng F-G, Kong Y-Y, Michaelewski HJ, Starr A. Perceptual consequences of disrupted auditory nerve activity. J Neurophysiol. 2005;93:3050–3063. doi: 10.1152/jn.00985.2004. [DOI] [PubMed] [Google Scholar]
- 15.Rance G, Corben LA, DuBourg E, et al. Successful treatment of auditory perceptual disorder in individuals with Friedreich ataxia. Neuroscience. 2010;171:552–555. doi: 10.1016/j.neuroscience.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Blamey PJ, Sarant JZ, Paatsch LE, et al. Relationships among speech perception, production, language, hearing loss, and age in children with impaired hearing. J Speech Lang Hear Res. 2001;44:264–285. doi: 10.1044/1092-4388(2001/022). [DOI] [PubMed] [Google Scholar]
- 17.Paatsch LE, Blamey PJ, Sarant JZ, et al. Separating contributions of hearing, lexical knowledge and speech production to speech-perception scores in children with hearing impairments. J Speech Lang Hear Res. 2004;47(4):738–750. doi: 10.1044/1092-4388(2004/056). [DOI] [PubMed] [Google Scholar]
- 18.Rance G. Auditory neuropathy/dys-synchrony and its perceptual consequences. Trends Amplif. 2005;9(1):1–43. doi: 10.1177/108471380500900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawkins D. Comparisons of speech recognition in noise by mildly- to moderately-hearing-impaired children using hearing aids and FM systems. J Speech Hear Disord. 1984;49:409–418. doi: 10.1044/jshd.4904.409. [DOI] [PubMed] [Google Scholar]
- 20.Subramony SH, May W, Lynch D, et al. Measuring Friedreich ataxia: interrater reliability of a neurologic rating scale. Neurology. 2005;64:1261–1262. doi: 10.1212/01.WNL.0000156802.15466.79. [DOI] [PubMed] [Google Scholar]
- 21.Blaney B, Hewlett N. Dysarthria and Friedreich's ataxia: what can intelligibility assessment tell us? Int J Lang Commun Disord. 2007;42:19–37. doi: 10.1080/13682820600690993. [DOI] [PubMed] [Google Scholar]
- 22.Rance G, Barker E, Mok M, et al. Speech perception in noise for children with auditory neuropathy/dys-synchrony type hearing loss. Ear Hear. 2007;28(3):351–360. doi: 10.1097/AUD.0b013e3180479404. [DOI] [PubMed] [Google Scholar]
- 23.Spoendlin H. Optic and cochleovestibular degenerations in hereditary ataxias. II. Temporal bone pathology in two cases of Friedreich's ataxia with vestibule-cochlear disorders. Brain. 1974;97:41–48. doi: 10.1093/brain/97.1.41. [DOI] [PubMed] [Google Scholar]
- 24.Brown WF, Watson BV. Pathophysiology of conduction in peripheral neuropathies. In: Brown WF, Bolton CF, Aminoff MJ, editors. Neuromuscular function and disease: basic clinical and electrodiagnostic aspects. WB Saunders Co; Philadelphia, PA: 2002. pp. 56–95. [Google Scholar]
- 25.Crandell CC, Smaldino JJ. Classroom acoustics for children with normal hearing and with hearing impairment. Lang Speech Hear Services in Schools. 2000;31:362–370. doi: 10.1044/0161-1461.3104.362. [DOI] [PubMed] [Google Scholar]