Abstract
This Perspective describes advances from the author’s laboratory on the free radical reactions of organic compounds with molecular oxygen. Polyunsaturated fatty acids (PUFAs) and sterols are particularly prone to undergo radical chain oxidation and evidence suggests that this process, known as lipid peroxidation, occurs in vivo under a variety of conditions that are the result of an oxidative stress. Cyclic peroxides, hydroperoxides, and epoxy-alcohols are major products formed from peroxidation and the basic mechanisms of product formation are now reasonably well understood. These mechanisms include reversible addition of oxygen to carbon radicals, rearrangement and cyclization of allyl and pentadienyl peroxyl radicals and homolytic substitution of carbon radicals on the peroxide bond. A physical organic approach to the problem of free radicals in biology and medicine is highlighted in this Perspective with stereochemical, kinetic and extrathermodynamic probes applied to the study of mechanism. A radical clock permits the determination of free radical propagation rate constants and 7-dehydrocholesterol, the immediate biosynthetic precursor of cholesterol, is found by this clock to be one of the most oxidizable lipids known. The consequences of the extreme reactivity of 7-dehydrocholesterol on human health is the focus of a current research theme in the author’s laboratory.
Gomberg’s report on the existence of triphenylmethyl radical in his 1900 Journal of the American Chemical Society article1 was met with skepticism,2 but over a century has passed and today free radical chemistry and biology is a flourishing field of study. Gomberg also called attention to the fact that his free radical reacted with molecular oxygen. Indeed, a critical piece of evidence supporting the notion that he had prepared triphenylmethyl was the fact that his species reacted with molecular oxygen to form a symmetrical peroxide in a non-chain reaction.3 The color of the radical disappeared when oxygen was admitted to solutions of the dimer, only to appear again once oxygen was excluded and dissociation of the dimer gave more radical. Subsequent experiments by Mike McBride and his coworkers at Yale in the early 1970s on the reactions of triphenylmethyl radical with oxygen shed light on Gomberg’s experiments and showed that a free radical chain reaction of the dimer with oxygen led to products that complicated Gomberg’s analysis. An oil usually accompanied formation of the crystalline symmetrical peroxide and McBride suggested that the oils probably contain the products derived from the induced chain reaction of dimer going to dimer hydroperoxide.4 The Yale group showed that under conditions of oxidation where the triphenylmethyl equilibrium favored the dimer, over 90% of the dimer hydroperoxide was formed from saturated solutions of triphenylmethyl radical as shown in Scheme 1. Thus, the announcement of “trivalent carbon” as an intermediate in Gomberg’s turn of the century paper also gave indications that the radical intermediate was a reactive one, with molecular oxygen being a willing partner for reaction with the trivalent species.
Scheme 1.
Reactions of Triphenylmethyl Radical with Molecular Oxygen
This Perspective, based in part on a James Flack Norris Award in Physical Organic Chemistry address at the 245th National meeting of the ACS in New Orleans, will focus on developments in the free radical chemistry of oxygen with compounds of biological importance, most notably the reactions of oxygen and lipids. There is some irony in discussing Gomberg’s work in a Norris Award symposium since Gomberg and Norris were at odds from the time that Gomberg famously “reserved the field” for himself.1 Norris disputed Gomberg’s claim of trivalent carbon and a number of acrimonious publications authored by the two appeared during the years after Gomberg’s paper. Norris’ National Academies Press Biography concludes “ at almost the beginning of his MIT career, Norris became engaged in, and lost, rather a vitriolic argument with Gomberg about the nature of triphenylmethyl.”5
The reaction of oxygen with organic free radicals has been one of the continuing themes of research that has been of interest to me over the past forty years. There were several ongoing projects in free radical chemistry in the Bartlett research group at Harvard during the mid to late ‘60s when my interests were developing. Mike McBride, the TA in a first-year graduate course I took from Bartlett, and other members of the Bartlett group were unraveling the free radical chemistry of azo compounds.6–8 Radical and bi-radical chemistry was part of Bartlett’s research in step-wise cycloadditions,9–12 and he also had an interest in peroxide, tetroxide and singlet oxygen chemistry during that time.13–16 My project was on bi-radical chemistry and the “spin correlation” effect of singlet and triplet bi-radicals formed from direct and sensitized photolysis of cyclic azo compounds.17 The project hinged on being able to separate diastereomeric product cyclobutanes formed in azo decomposition and a capillary GC column, new to the lab in those days, solved the problem after months of futile work packing 75 foot GC columns in the stairwell of the Converse Labs at Harvard.
The mechanism of azo compound decomposition was a topic of general interest when I started my work at Duke in 1969 and my first independent publications used stereochemistry as a probe, an approach borrowed directly from my thesis research.18,19 The azo compounds we prepared were of interest for a number of reasons and they provided an opportunity to collaborate with Gerhard Closs at an early stage of his work on the theory of 1H and 13C CIDNP.20 We independently carried out some of the first experiments on 15N CIDNP,21,22 all of which showed unequivocally that unsymmetrically substituted azo compounds decompose one bond at a time, the reactions proceeding via a nitrogen containing radical.
My first PhD student at Duke, Larry Marnett, was the principal coworker on the azo project, and he was also instrumental in my decision to step away from the comfort zone that azo chemistry provided and into the broader world of the free radical chemistry of oxygen and lipids. Duke’s Qualifying Exam for the PhD in those days required an independent research proposal and Marnett and I chatted on occasion about a topic for his proposal that would be sufficiently different from his PhD project, but would be of interest to us both. A feature article in Chemical and Engineering News in June of 1971 by Bill Pryor captured our interest since it reviewed the state of affairs of “Free Radical Pathology” and made clear to us the importance of the chemistry of lipids and molecular oxygen.23 The article coincided with the first Gordon Conference on Free Radicals in New Hampshire that Pryor organized to bring together scientists interested in the effects of radicals on living organisms. This conference attracted an international group of chemists interested in the organic chemistry of free radicals for over thirty years. Pryor also helped to establish a California series of Gordon Conferences that endures today, having more of a biology and medical focus than the New Hampshire version. Pryor’s article focused on free radicals, peroxides, antioxidants, and the radical theory of aging and the cover of the C&E News issue was a memorable one that showed a youth morphing into and old man, presumably because of the detrimental effects of free radical oxidation. One measure for lipid peroxidation mentioned in the article was the thiobarbituric acid assay, which measured the formation of malondialdehyde, a by-product of peroxidation of polyunsaturated fatty acids and esters. Marnett proposed a study to determine the mechanism of malondialdehyde formation in his Qualification Exam. The Exam exposed us both to the relevant biochemical literature of the day and in retrospect played an important part in our career decisions for years to come.24
Polyunsaturated Fatty Acid Autoxidation (Peroxidation): Peroxyl Radical Cyclization
Much of the early research on the oxidation of polyunsaturated lipids and isoprenoids was led by Bolland and Bateman at the British Rubber Producers Research Association during the ‘40s and ’50s.25–27 Bolland and Gee25 carried out autoxidation kinetic and product studies on the esters of oleic and linoleic acids. They reported the nearly quantitatively formation of hydroperoxide products and showed that the non-conjugated diene lipid, ethyl linoleate, gave conjugated diene hydroperoxide products as shown in Scheme 2. The reaction was presumed to proceed via an intermediate carbon radical that was formed by abstraction of hydrogen on the carbon flanked by two double bonds. Oxygen addition to this pentadienyl radical at either terminus gave conjugated diene peroxyl radicals that then propagated the chain by another H-atom transfer. In biology, transformations of this sort have come to be known as a peroxidation reaction while chemists still refer to the process as an autoxidation.
Scheme 2.

Autoxidation of Ethyl Linoleate Studied by the British Rubber Producers Research Association
Free radical chemistry in North America was developing rapidly during this period, and the landmark monograph on “Free Radicals in Solution” published in 1957 by Cheves Walling was a reference book that I turned to for an introduction on almost any topic in free radical chemistry.28 Walling attended nearly every national or international conference on free radicals for decades and without fail he would come up with the critical precedent or reference during discussions at these conferences. I have the impression that as Editor of the Journal of the American Chemical Society he doubled as a reviewer for most articles submitted on the topic of free radical chemistry. On more than one occasion I had good anonymous reviews and then Cheves would submit his three-page “review of the reviews” with his commentary and suggestions for revisions.
Another focal point of free radical chemistry in North America was the National Research Council in Ottawa Canada, where Keith Ingold was leading a group that provided absolute rate constants for many important free radical transformations.29–40 Ingold’s publications in the primary literature, his reviews,41,42 one from his collaborator Tony Howard,43 and Walling’s monograph were authoritative texts for anyone starting a program in the free radical chemistry of autoxidation and its inhibition.
While autoxidation of simple olefins like oleic acid or cholesterol gives allylic hydroperoxides and linoleic acid gives conjugated diene hydroperoxides, the autoxidation of lipids containing three or more sites of unsaturation yields product mixtures that are complex. Suggestions had been made in the literature that cyclic peroxides are formed in the autoxidation of isoprenoids like squalene25 but no thorough study of peroxyl radical cyclizations had been reported when we started our work. The discovery that opened the door to systematic investigations of peroxyl radical cyclization for us was that hydroperoxides could serve as precursors to specific intermediate peroxyl radicals, see Scheme 3.44,45 Peroxyl radicals undergo a rapid exchange of hydrogen atoms with hydroperoxides,43 and we recognized that simply initiating a free radical chain with a suitable hydroperoxide precursor provided an effective entry to study cyclization of specific peroxyl radicals. Products of cyclization generally have a hydroperoxide substituted on an exocyclic carbon of a cyclic peroxide ring, as shown in Scheme 3. The peroxyl radical precursor to the product propagates the chain by hydrogen atom abstraction from the acyclic hydroperoxide precursor as shown in the Scheme. Peroxyl radical cyclizations are kinetically controlled, 5 and 6-exo mode products are favored, following the general Beckwith-Houk guidelines for radical cyclization.46,47
Scheme 3.

Peroxyl Radical Cyclization
Peroxyl radical cyclization can occur from fatty acids and esters having three or more double bonds since the carbon framework offers intermediate peroxyl radicals an appropriate double bond target for intramolecular addition. The product radical that derives from the first 5-exo cyclization can follow diverse paths, including the bicyclization and serial cyclization pathways shown in Scheme 4 for a peroxyl radical derived from arachidonic acid. The serial cyclic and bicyclic products shown in the Scheme have five new asymmetric carbons and there are four peroxyl radicals like the one shown in Scheme 4 that can undergo similar serial and bicyclization pathways. It is no wonder that thin layer chromatography of arachidonic acid usually shows a peroxide streak from the origin to the spot of the fatty acid on the plate because literally hundreds of regio- and steroisomeric peroxide compounds are usually present as impurities in arachidonic acid, if it has not been freshly purified. Study of the cyclization in these complex systems can be simplified greatly by our original approach of using a specific hydroperoxide as a precursor to a particular peroxyl radical.48–51
Scheme 4.

Cyclization Mechanisms for Lipid Peroxidation
The stimulus for studying arachidonic acid free radical oxidation was the fact that it is the lipid precursor of the prostaglandins, important mediators of inflammation and a host of other physiological responses. The COX enzymes catalyze the combination of arachidonic acid with two molecules of oxygen by a mechanism that appears to be a stereo-controlled peroxyl radical bicyclization.52–55 The first-formed product of biosynthesis is the bicyclic endoperoxide PGG2 that has a structure identical to one of the stereoisomers formed in the non-enzymatic free radical oxidation of arachidonic acid. The COX enzyme apparently selects only one of the regioisomeric arachidonate peroxyl radicals and controls the configuration of the stereogenic centers formed in the bicyclization.

Our first studies on peroxyl radical bicyclization involved the analysis of products formed in a sequence similar to the pathway shown in Scheme 4.56 The only useful method of product analysis available to us at the time was GC/MS and this severely limited the conclusions that could be drawn from these studies. 1975 was in the early days of HPLC and the NMR available to us was 90 MHz, so we were several years ahead of the time when we could apply more powerful separation and structure identification tools to the problem.57,58 In fact, we did return to this analysis 20 years later when there was renewed interest in these products of arachidonic acid autoxidation.59,60 We nevertheless did conclude from GC/MS fragmentation patterns obtained in our 1975 experiments that compounds having prostaglandin-like structures were formed from peroxyl radical bicyclization, although they were only a part of an extremely complex mixture.56 Bill Pryor published a companion paper to ours in the Journal of Organic Chemistry indicating that malonaldehyde formation was a consequence of the bicyclization pathway.61
Our interest in the peroxidation of arachidonic acid was piqued again in 1992 when Morrow and Roberts reported that prostaglandin-like compounds were found in cells independent of COX enzyme activity. These prostanoid products were formed from arachidonic acid esters present in membrane phospholipids59,60 and were named “isoprostanes” because of the fact that they were a mixture of compounds isomeric with the COX enzyme product, PGF2α. Circulating levels of these compounds increased significantly in animals exposed to an oxidative stress or free radical injury. Isoprostane levels skyrocketed, for example, when rodents were exposed to carbon tetrachloride, a treatment known to generate tricholormethyl radicals in liver. Subsequent studies by Morrow and Roberts at Vanderbilt University concluded that isoprostane levels generally correlate with conditions associated with oxidative stress such as smoking or obesity and a comparative study showed that measurement of isoprostane levels provided the best method of assessing free radical injury in vivo. The standard isoprostane plasma analysis developed by Morrow and Roberts involved a sequence of ester hydrolysis, thin layer chromatography, methylation and TMS formation, followed by GC/MS. Dozens of isomeric products were observed in the assay, confirming the fact that the isoprostanes are not products produced by the COX enzymes.
The developing biomedical interest in the isoprostanes and the powerful analytical tools that became available in the 1990s prompted us to reopen a mechanistic investigation of peroxyl radical bicyclization of arachidonate peroxyl radicals. Triple quad MS systems coupled to HPLC effectively permitted finding a needle in the haystack of lipid peroxidation products by the use of protocols such as selective reaction monitoring (SRM). In SRM, products of a particular m/z are isolated in the first analyzer, these compounds undergo fragmentation in the second cell, and fragment ions that are characteristic of targeted compounds of interest are detected in the last analyzer.
An example of an SRM analysis of isoprostanes is shown in Figure 2. Only those regioisomers that undergo fragmentation similar to PGF2α were selected in this SRM and separation of all but two of the stereoisomers was achieved by HPLC. The analysis showed that PGF2α was formed in less than 10% of the regioisomeric mixture. There are three other sets of regioisomers that were identified by different SRM fragmentation protocols. Taken together, there are 32 isomeric isoprostanes that can form from arachidonic acid autoxidation, with each of these isomers being formed in solution as a racemic mixture of enantiomers. The fact that PGF2α is a minor constituent of the arachidonic acid peroxidation product mixture was anticipated by the earlier studies of O’Connor, Mihelich and Coleman who showed that steroisomers having cis alkyl substitution on the cyclopentane ring are favored in radical bicyclization of linolenic acid, a triene fatty acid. 57,58 In our studies of arachidonic acid oxidation carried out in collaboration with Jason Morrow, all of the stereoisomers of PGF2α were separated and identified by comparison with authentic standards by the use of a combination of reverse- and normal-phase HPLC.62 As seen in Figure 2, 12-epi-PGF2α is the prostaglandin-like stereoisomer formed in the greatest amount in arachidonic acid autoxidation.
Figure 2.

HPLC/MS/MS chromatogram of diastereomeric isoprostanes formed in the oxidation of arachidonic acid. The chromatogram is in SRM mode, selecting only those isoprostanes having the same regioisomeric structure as PGF2α, which is a minor component of the mixture.
With HPLC/MS conditions established for the separation of isoprostane stereoisomers, a stereochemical analysis of these compounds in biological tissues and fluids was now possible. Extensive chromatographic purification of urinary PGF2α followed by HPLC/MS of the compound on a chiral column as shown in Figure 3 gave the surprising result that the enantiomer of PGF2α was two-fold more abundant than the natural COX-produced product. Thus, quantification of urinary PGF2α actually reflects oxidative stress status as opposed to COX activity. Indeed, levels of this compound were shown to be elevated in urine from cigarette smokers and in humans with hypercholesterolemia, two conditions associated with oxidant stress.51,63 While the fact that ent-PGF2α was present in excess of the natural enantiomer in urine was not anticipated, the observation can be understood by the fact that catabolism of this enantiomer is not as efficient as is the case for the natural product. PGF2α is consumed metabolically, the enantiomer is not. As such, the enantiomer is probably a more reliable biomarker of oxidative stress than PGF2α.
Figure 3.

HPLC/MS/MS chromatogram of urinary isoprostanes on a chiral column showed that enantiomeric-PGF2α was two fold more abundant in urine than the COX-product, PGF2α
While the biological relevance of peroxyl radical cyclization has been established for the autoxidation of polyunsaturated fatty acids and esters, it seems likely that this process is also important for other naturally occurring compounds. The degradation of rubber, polybutadiene, and isoprenoid natural products such as squalene, ubiquinones, other prenyl derivatives, etc. involves peroxyl radical intermediates and the 1946 publication of Bolland and Gee suggested that squalene incorporated more than one oxygen molecule into the peroxide products formed during its autoxidation.25 With this history in mind, studies by my coworker Nick Roe found that the autoxidation of isoprenoids did indeed give serial cyclic peroxide products.64,65 In the case of isoprenoids, the favored cyclization is 6-exo, and although Roe’s model allowed for a serial run of only two volumes, squalene could give products containing serial-cyclic peroxide runs of up to five rings. New stereogenic centers are formed in the cyclizations and the stereoisomeric product mixture would provide a formidable analytical problem.
Oxygen Addition to Pentadienyl Carbon Radicals is Reversible: Peroxyl Radical Clocks
Linoleic acid is an essential fatty acid, it has a simple homo-conjugated diene and its free radical oxidation product profile is relatively simple since cyclic peroxide products cannot be formed. Linoleate esters are also present in the highest concentration of any of the fatty acid esters in human circulating low-density lipoprotein (LDL), making its oxidation reactions of some biomedical interest. These facts led us to study the free radical oxidation of linoleic acid and its esters that started in 1978 and continues today.
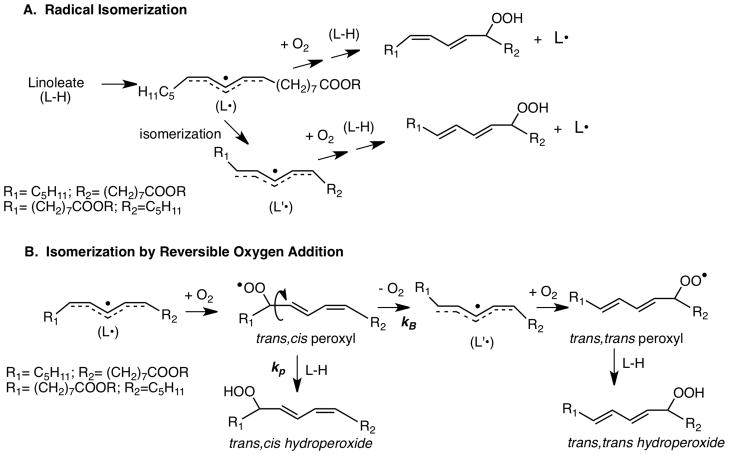
Early work in the field had demonstrated that the oxidation of linoleic acid or its esters gave a mixture of trans,cis and trans,trans conjugated diene hydroperoxide stereoisomers.66,67 Factors that controlled the product ratio of these stereoisomers had not been established but it was assumed68 that an isomerization of the intermediate pentadienyl radical led to the trans,trans product, see Scheme 6A. We were skeptical of the mechanism since it seemed to us that radical isomerization could not compete kinetically with the addition of oxygen to the radical (L•) since the reaction of carbon radicals with oxygen was known to occur at or near the diffusion-limited rate.69 Experiments by Hugo Weenen, a coworker in my lab from the University of Nijmegen, showed that the ratio of trans,cis/trans,trans products was dependent on the concentration of linoleate, higher concentrations of linoleate giving more of the trans,cis products.70 We reasoned that the trans,cis products were kinetic products of the first-formed trans,cis peroxyl radical, while the trans,trans products were thermodynamic, produced after loss of oxygen from the trans,cis peroxyl by β-fragmentation. My groups at Duke and Vanderbilt found several useful applications of this kinetic vs. thermodynamic competition over the course of three decades.71–80
Scheme 6.
Two mechanisms for Linoleate Autoxidation
The rate constant, kp, for the reaction of the trans,cis peroxyl radical with linoleate (L-H in Scheme 6B) was known to be 62 M−1s−1, determined by classical methods.30 Beta-fragmentation of the trans,cis peroxyl radical is a unimolecular process, kB, competing with the kp propagation step. The rate constant kB could therefore be calculated by a competition kinetics analysis and by experiments that determined the dependence of the trans,cis/ trans,trans product ratio on linoleate, L-H in the Scheme. Once kB was known, it was possible to use it to “clock” the rates of other compounds that served as hydrogen atom donors to the linoleate peroxyl radical.81 Measurement of the linoleate trans,cis/ trans,trans product ratio as a function of any H-atom donor present during an oxidation provides a means for determining kp for that donor. The competition kinetics analysis is straightforward.
Free radical clocks have proved to be enormously useful for determining rate constants for the bimolecular reactions of carbon radicals.82–84 Ingold and Newcomb have made particularly good use of these mechanistic probes, with clocks providing important data in several studies of enzymatic transformations. In the case of the linoleate peroxyl radical clock, rate constants for H-atom transfer to peroxyl radicals shown in Figure 4 were determined for a number of compounds, including several polyunsaturated acids and some important sterols.85,86 The rate constant we determined for 7-dehydrocholesterol (7-DHC), 2260 M−1s−1, was surprisingly large. It is the largest propagation rate constant we’ve determined for any lipid, over 200 times that of cholesterol. 7-DHC is the immediate biosynthetic precursor of cholesterol and it is also a key intermediate in the biosynthesis of Vitamin D3. We subsequently studied the peroxidation of this interesting lipid in greater detail, a topic I will return to in this Perspective.
Figure 4.
Rate Constants, kp, Determined by the Linoleate Free Radical Clock for H-atom Transfer to Peroxyl Radicals from the Compounds Shown
The linoleate radical clock idea was also used to assess the efficacy of H-atom donors in model membranes. Oxidation of liposomes containing a “clocking” linoleate phospholipid was used to provide a report of the H-donor activity of any liposomal constituents, based upon the mole fraction of all the H-atom donors present in the liposome.85 Compounds known to be good H-atom donors that were incorporated into the liposome increased the yield of the trans,cis kinetic products, while liposomal constituents that did not serve as good H donors simply acted to dilute the liposome and increased the relative level of the thermodynamic trans,trans products. Not surprisingly, the addition of vitamin E, Nature’s phenolic antioxidant and an excellent H-atom donor, to liposomes containing linoleate phospholipids led to autoxidation product mixtures that contained only the linoleate kinetic products of oxidation, the trans,cis hydroperoxides. In short, the kinetic vs. thermodynamic linoleate product mixture gives a reading of the local reducing environment at the site of oxidation. If vitamin E is present at the site of oxidation, as is the case for the linoleate esters present in human blood plasma, then only the kinetic products are observed.74,78
Rearrangements of Allyl Peroxyl Radicals: Association or Dissociation?
The critical step in translating the linoleate mechanism to a radical clock is the peroxyl radical beta-fragmentation step. There is an overwhelming body of evidence, including the use of oxygen isotopes, indicating that the peroxyl radical releases molecular oxygen87–89 and that oxygen exchanges with the atmosphere during the rearrangement. The rearrangement is clearly dissociative. The mechanistic picture for the [3,2] rearrangement of simple allylic peroxyl radicals was much more difficult to unravel, however, and this provided a stimulus for one thread of our research for a decade.
Allylic [3,2] rearrangements are mechanistically important in the free radical oxidation of oleic acid.90 The oxidation of oleates proceeds by abstraction of an allylic hydrogen at C-8 or C-11 of the acid, as shown in Scheme 7A for abstraction at C-8. The first formed allylic radical has trans,cis substitution of alkyl groups and oxygen addition gives the kinetic products shown in Scheme 7A, i.e. (E)-10 peroxyl-8-ene and (Z)-8-peroxyl-9-ene compounds. Allylic [3,2] peroxyl radical rearrangements shown in Scheme 7B give the two (E) peroxyl radicals that are trapped to give the thermodynamic (E) 8 and 10 hydroperoxides. As shown in Scheme 7B, of the three hydroperoxide products shown, one is a kinetic product, one is a thermodynamic product and one ((E)-10-hydroperoxy-octadeca-8-enoic acid) is formed both kinetically and thermodynamically. Radicals analogous to those shown in Scheme 7 are formed after abstraction of a C-11 hydrogen, giving 9 and 11 hydroperoxides. This results in a total of six hydroperoxide products in the oxidation of oleate.
Scheme 7.
[3,2] Rearrangements of Allylic Peroxyl Radicals in Oleate Oxidation and with Labeled Oxygen. A. Kinetic Oleate Peroxyl Radicals. B. [3,2] Rearrangement of Oleate Peroxyls. C. Labeled Oxygen Studies.
The [3,2] rearrangement of allylic peroxyl radicals was first suggested by Schenck in a study of the mechanism of formation of allylic cholesterol hydroperoxides.91 Brill subsequently reported the rearrangement of acyclic allylic hydroperoxides and confirmed that the mechanism proceeded by intermediate free radicals.92 Very careful experiment work from the Beckwith and Davies groups on the cholesterol system made it clear that the [3,2] rearrangement had interesting mechanistic twists.93,94
Our approach was to focus on acyclic systems and to use stereochemistry and oxygen labels to probe the rearrangement.95–102 The rearrangement proceeds with significant configurational transfer of stereochemistry (>98%) across the three carbon framework, although product selectivity decreases as the temperature of the rearrangement is raised above 40°C. Peroxyl radicals labeled with 18O undergo rearrangement with significant retention of label, although this is also dependent on temperature and on solvent viscosity. Clearly this rearrangement is different from the rearrangement of the conjugated diene peroxyl radicals formed in the oxidation of linoleate, where products of optically pure hydroperoxide precursors are racemic and 18O labels scramble with the environment during rearrangement.
The study described in Scheme 7C provided our best evidence for the mechanism of peroxyl radical [3,2] rearrangements. It involved developing synthetic methods for the preparation of unsymmetrically 18O labeled hydroperoxides103 and strategies for monitoring the regiochemical location of the label in the reactant and product hydroperoxides.101,102 Our studies of the [3.2] rearrangement of unsymmetrically 18O labeled hydroperoxides showed that the terminal oxygen of the peroxyl radical is transferred to the internal site during rearrangement, but the regioselectivity of this transfer decreases at temperatures above 40°C. More atmospheric oxygen is also incorporated into the rearrangement products at elevated temperatures than is the case for reaction at room temperature.
Taken together with the studies on stereochemistry and solvent viscosity, the mechanistic picture that emerges is of a rearrangement that proceeds through the oxygen-allyl radical complex pictured in Scheme 7C. At room temperature, little dissociation of the complex is observed and the regioselectivity of the 18O label transfer is very high. Some dissociation of the complex does occur however, particularly at mildly elevated temperatures, and the result of this dissociative process is decreased stereoselectivity in the rearrangement and partial scrambling of the 18O peroxyl radical oxygen with the environment. The effect of solvent viscosity on the rearrangement suggests that there is a “cage effect” of the allyl radical and molecule oxygen after dissociation, more viscous solvents leading to more recombination of the vicinal radical-oxygen pair. Radicals react with oxygen at or near the diffusion limit so a cage effect is not entirely surprising.69 Some computational work lending credence to such structures has been reported that shows that oxygen-allyl radical complexes are weakly bound species.104,105
The suggestion that an oxygen-allyl radical complex is formed in the dissociation of the allyl peroxyl radical, implies that the reverse reaction, association of allyl radicals with molecular oxygen, may proceed through the same allyl radical-oxygen complex, as shown in Scheme 7C. The notion that an allyl-oxygen complex precedes C-O bond formation in the reaction of allyl radical with oxygen suggests that additional mechanistic tests should be carried out to provide evidence for or against such species. It is fair to say that radical-oxygen complex formation has not historically been a major part of mechanistic conversations about the addition of oxygen to carbon radicals.
What Controls the Site of Reaction of Oxygen with Delocalized Carbon Radicals?
One aspect of linoleate autoxidation that puzzled us for nearly twenty years was the fact that none of the observed products of the reaction were the result of oxygen addition to the center carbon of the intermediate pentadienyl radical, C-11 in Scheme 8. The center carbon of pentadienyl radicals bears more spin density than either of the terminal carbons but only the conjugated diene 9 and 13 hydroperoxides were found in the autoxidation of linoleate or linoleate esters going back to the early studies of Bolland and Bateman.25–27 My colleague Alan Brash at Vanderbilt was the first to identify the C-11 hydroperoxide, which becomes a major product of oxidation when linoleates are co-oxidized in the presence of α-tocopherol, the most reactive constituent of vitamin E.106 Phenolic antioxidants inhibit autoxidation by H-atom transfer to peroxyl radicals, a reaction that occurs with a rate constant of kinh ~ 3 • 106 M−1 s−1 for vitamin E.
Scheme 8.

Kinetic Products of Oxygen Addition to Linoleate Carbon Radicals
After extensive analysis of the products of linoleate oxidation as well as studies of several structural analogs by Keri Tallman, we concluded that the kinetic products of linoleate oxidation are the non-conjugated C-11 hydroperoxide and the conjugated C-9 and C-13 trans,cis diene hydroperoxides. Rearrangement of the C-11 peroxyl (by β-fragmentation and re-addition of oxygen or by the [3,2] associative mechanism) occurs with a rate constant of over 106 s−1, so the C-11 peroxyl radical is trapped by H-atom transfer only if sufficient tocopherol is present. The fragmentation-rearrangement of the trans-cis C-9 and C-13 peroxyl radicals leading to the trans-trans conjugated diene products shown in Scheme 6B is much slower than the rearrangement of the C-11 peroxyl, so in the presence of only amounts of vitamin E no trans,trans conjugated diene products are formed.75,107
Derek Pratt, who came to Vanderbilt as a graduate student after spending time as an undergraduate working with Ingold in Ottawa, provided a theoretical framework for these rearrangement reactions, calculating bond dissociation enthalpies for C-H and C-OO• bonds that proved helpful in understanding the important processes involved.76,108 Our initial thinking about oxygen addition to pentadienyl radicals was that reaction occurs at the sites of highest spin density on the delocalized radical.109,110 Linoleate pentadienyl radicals with a cis configuration at C-9 and C-12 have the highest spin density at C-11 (spin density; C-9, 0.32; C-11, 0.36; C-13, 0.32) and the major kinetic product for oxygen addition to linoleate is at that carbon (oxygen addition: C-9, 0.28; C-11, 0.44; C-13, 0.28). But the linoleate-derived pentadienyl radical with a Δ9–10 trans configuration shown in Scheme 9A gives kinetic products that are inconsistent with the spin density-reactivity suggestion. The ESR spin density of this radical shows the highest spin at C-11, the center carbon of the pentadienyl and the C-11 hydroperoxide is the major product formed, 48% but addition at the cis end of the radical (C-13) amounts to only 18% of the product mixture while 34% of addition occurs at the C-9, the trans end of the radical. This in spite of the fact that the spin density is higher at C-13 than at C-9 and addition at C-13 gives the thermo-dynamically favored trans,trans product while addition at C-9 gives the disfavored trans,cis hydroperoxide. Factors other than spin density must control the regioselectivity of the addition to pentadienyl radicals.
Scheme 9.
Regioselectivity in the Oxygen Addition to Pentadienyl Radicals.
An autoxidation study on a series of seven homoconjugated dienes provided additional mechanistic insight. In this study, a cis n-pentyl substituent was held steady on one end of the diene and the substituent on the remote end was varied. Three pentadienyl radical intermediates from this study are shown in Scheme 9B along with the kinetic hydroperoxide distribution from oxygen addition to each of the radicals. The results show what appears to be a steric effect, oxygen addition occurring at the sterically least crowded end of the radical. Analysis of the product distribution from the seven dienes studied showed a good correlation of product with the Taft steric Es parameter. Large groups at both ends of the pentadienyl radical led to increases of product at the central carbon, large substituents at only one end of the radical skewed the product profile to the central and opposite end of the radical. Spin density plays only a minor role, if any, in determining regioselectivity.
A possible mechanistic picture for oxygen addition to the linoleate trans,cis pentadienyl radical shown in Scheme 9A includes the two oxygen-radical complexes shown in Scheme 9C. Steric effects would play a role in determining which of the two complexes is preferred in the addition, large R groups driving the addition to the remote end of the radical. Similarly, addition would occur at the end of the pentadienyl radical remote from the cis alkyl substituent, which would occupy a pseudo-axial position in the complex and in the transition state for collapse of complex to product. A slightly altered picture from Scheme 9C consistent with the experimental data has been written for oxygen addition transition states without intervening complex formation.79,80
7-Dehydrocholesterol Autoxidation and Smith-Lemli-Opitz Syndrome (SLOS)
The last step in the biosynthesis of cholesterol from squalene is promoted by 7-dehydrocholesterol reductase (DHCR7), an enzyme that reduces the Δ7,8 double bond of 7-dehydrocholesterol (7-DHC), see Scheme 10. 7-DHC is also known as pro vitamin D since it is the substrate for the photochemical ring opening reaction that makes D the sunshine vitamin. We first encountered 7-dehydrocholesterol in 1982 when studying the autoxidation of linoleoyl phosphatidyl-choline liposomes. While cholesterol acted as a simple diluent for linoleate liposomes, 7-DHC proved to be an outstanding H-atom donor in the free radical oxidation reactions of these aggregates.72 Thus, autoxidation of dilinoleoylglycerylphosphatidyl choline (DLPC) liposomes gave linoleate trans,cis and trans,trans oxidation products in a ratio of about 1.2 to 1, but dilution of the liposomes with 7-DHC increased the linoleate product ratio to nearly 3 to 1. Except for phenolic antioxidants like α-tocopherol, 7-DHC had the most profound effect on the linoleate product distribution of any liposome additive we investigated. It was also clear from these studies that 7-DHC was itself very susceptible to free radical chain oxidation. We found it extremely difficult to purify the compound from peroxide impurities.
Scheme 10.

Last Step in Cholesterol Biosynthesis
Twenty-five years after our first encounter with 7-DHC, Libin Xu and Todd Davis were clocking autoxidation rates of biologically important lipids and Libin suggested that we look again at this reactive sterol. His studies confirmed the reactivity of the compound. It is the most reactive lipid as an H-atom donor to peroxyl radicals that we have found, see Figure 4. Arachidonic acid, the isoprostane precursor and a compound considered by the oxidative stress community to be extremely oxidizable, is some 12 times less reactive than 7-DHC as an H-atom donor.85 We initially considered the reactivity of 7-DHC as a curiosity, but our views changed when we learned that this sterol was gaining interest as a key player in a devastating human condition known as Smith-Lemli-Opitz syndrome (SLOS).111 -
The first SLOS patients were identified in the ‘60s and the syndrome has subsequently been characterized as an autosomal recessive disorder with an incidence of approximately 1 in 10,000–30,000 live births.112 The range of severity in the syndrome is wide, with individuals having severe cases not surviving to birth.113–116 The consequence of the genetic defect is a broad spectrum of phenotypes that include neurological deficits and congenital malformations.112 Over 50% of individuals diagnosed with SLOS also have a positive diagnosis for Autism Spectrum Disorder.117 Evidence was presented in the mid ‘90s that elevated levels of 7-DHC and lower than normal levels of cholesterol are observed in tissues and the plasma of patients with SLOS. Subsequent studies confirmed that defects in the gene encoding DHCR7 lead to the altered sterol levels observed in SLOS. The severity of the syndrome is related to the nature of the DHCR7 mutation inherited, over 100 of which have been reported. Two copies of a defective DHCR7 enzyme lead to the syndrome, the carrier incidence of someone having one copy of a deficient enzyme is 1 in 100–300, based upon known mutations.
The current treatment for SLOS is cholesterol dietary supplementation, but since SLOS is a neuro-developmental disorder the neurological deficits and congenital malformations occur before or shortly after birth, at a time when dietary cholesterol has no effect. Indeed, the therapeutic focus in SLOS has been on the deficiency of cholesterol for SLOS individuals instead of on the excess of 7-DHC present. The extreme reactivity of 7-DHC was unknown prior to our 2007 publication reporting the rate constant for its radical chain propagation and this unusual reactivity stimulated us to focus on radical chain autoxidation of 7-DHC as a central feature in SLOS pathogenesis. Libin Xu recognized this as an opportunity to broaden the conversation about SLOS chemistry and biology and he took on the study of 7-DHC autoxidation and the oxysterols that are formed in the process. Over a dozen products were isolated and characterized after azo-initiated autoxidation of 7-DHC in organic solvent. Scheme 11 presents a mechanistic accounting of the oxysterols formed, three of the major products are underlined in the Scheme.118 The formation of all of the products isolated can be understood based upon known transformations of carbon and peroxyl radicals.119 Hydrogen atom abstraction at C-9 of the sterol appears to be a major reaction pathway and subsequent addition of oxygen at either end of the delocalized pentadienyl radical gives a peroxyl radical that can undergo 5-exo cyclization to give the radical designated as “allyl” in the Scheme. Oxygen addition to “allyl” ultimately leads to the two steroisomeric endoperoxide-hydroperoxide species, major products of the oxidation. The “allyl” intermediate can also undergo homolytic substitution (sHi) on the peroxide bond leading to the 5,6-epoxy-9-ol. Such homolytic substitutions are well-known radical transformations of peroxides.120,121
Scheme 11.
Mechanisms for the Autoxidation of 7-Dehydrocholesterol. Major Products are underlined.
Another major product formed in the autoxidation of 7-DHC is the 5,6-epoxide, a product that likely results from a sequence of peroxyl radical addition to the conjugated diene122 and homolytic substitution on the peroxide (sHi) with expulsion of an alkoxy radical. Products were also isolated that appear to come from abstraction of the hydrogen on C-14 of the sterol, although this pathway appears to be less important than abstraction at C-9. It should be noted that the products shown in Scheme 11 are formed under conditions in which 7-DHC is the only H-atom donor present in solution. Co-oxidation of 7-DHC in the presence of α-tocopherol would undoubtedly shift the products from a thermodynamic to a kinetic profile, similar to the effect of antioxidants on the oxidation of linoleate.119 Antioxidants would also prevent the intermolecular addition of a peroxyl radical that is required for the formation of the 5,6-epoxide.
There have been suggestions that the propensity of 7-DHC to undergo oxidation is an important part of the pathology of SLOS,123 as well as reports of elevated peroxide levels in the retina of SLOS rodent models.124 A triene sterol that may be the product of 7-DHC oxidation has been identified in the plasma of SLOS patients125 but the structural link between the products of 7-DHC autoxidation and the SLOS oxysterol profile was not secure at the time we began our studies of 7-DHC autoxidation. To address the link between 7-DHC autoxidation and SLOS, we were fortunate to develop a fruitful collaboration with two neuroscientists, Zeljka Korade and Karoly Mirnics from the Kennedy School at Vanderbilt. Zeljka had an interest in SLOS because of the neurological deficits associated with the syndrome and over the years, she had established cell culture and mouse models for the syndrome. She had acquired cultured human skin cell fibroblasts from SLOS and control individuals as well as a neuroblastoma cell line (Neuro2a) that is Dhcr7-deficient. In addition, Zeljka had established a colony of Dhcr7- knock out (KO) mice that were bred to study, among other things, neurodevelopment in the SLOS embryonic brain. The KO mice have a mutation that is lethal in humans and rodents and they survive for only hours to at most a day after birth.
Analysis of the embryonic brains of KO mice by HPLC/MS/MS showed readily measurable levels of oxysterols in SLOS tissue compared to the assay of wild type (WT) brains, where no detectable 7-DHC oxysterols were found. Similar differences were observed when SLOS and control fibroblasts or control and Dhcr7-deficient Neuro2a cells were analyzed. One compound in particular was present in nearly all of the SLOS samples analyzed but was undetectable in control cells and tissues. This compound, 3β, 5α-dihydroxycholest-7-en-6-one, DHCEO, was found as a minor product of 7-DHC autoxidation but its concentration in embryonic SLOS brain was determined to be on the order of 2–3 μM, based upon the wet tissue weight.126 Levels of DHCEO were also found to correlate well (r2=0.95) with the 7-DHC/cholesterol ratio measured in the samples.
The fact that DHCEO was present in all of the SLOS samples analyzed and that its level correlates with levels of 7-DHC measured in these samples suggests a common mechanism for its formation. To address this point, studies were carried out to link, if possible, the primary oxysterols that were identified in the chemical autoxidation of 7-DHC with the profile of biomarkers found in SLOS cells and tissues.126 In these studies, primary oxysterol autoxidation products were incubated in control or SLOS Neuro2a cells or in control and SLOS human skin fibroblasts. The experiments tested whether metabolism of the oxysterols formed by chemical autoxidation would convert these primary products to the ultimate biomarkers detected in cells and tissues. The results of these studies are shown in Scheme 12. Two of the transformations shown in the Scheme involve epoxide ring opening reactions. The two allylic epoxides (Schemes A and C) are prone to ring open in aqueous media without any assistance from an enzyme, although sterol epoxide hydrolases are well known. Peroxide reduction, allylic oxidation and dehydration reactions account for the other transformations suggested. We have not established which, if any, of these conversions in cells are enzyme-catalyzed. In any case, the metabolism of the 5,6-epoxide, shown in Scheme 12A, accounts for the formation of DHCEO, one of the major SLOS biomarkers in SLOS cells and tissues. The metabolic products underlined in Schemes 12B and C have been observed in cell culture experiments as well, and reasonable mechanisms for their formation from major 7-DHC autoxidation products can be written.
Scheme 12.
Metabolism of Oxysterols formed in 7-DHC Autoxidation in Neuro2a and Human Fibroblasts. Underlined products are found in SLOS cell culture and tissues.
The collaboration with Korade and Mirnics has taken us far afield from free radical chemistry. A description of these efforts is beyond the scope of this Perspective, but the collaborative studies of 7-DHC derived oxysterols has proved to be of some interest in lipid biochemistry and neurobiology.127,128 Most of the major oxysterols formed in 7-DHC autoxidation are toxic to cortical neurons at nM concentrations and the compounds induce profound effects on several important lipid genes. We have also found the field to be ripe for analytical development and the powerful HPLC/MS/MS techniques used in our studies of isoprostanes also proved to be of utility in the assay of human SLOS samples.129
In retrospect, it is striking how the timing of our studies of 7-DHC, the discovery of SLOS and the measurement of one rate constant by our peroxyl radical clock method has opened a productive new line of research for us. SLOS was first reported in the mid-‘60s but the biochemical defect and link to 7-DHC was not established until some thirty years later in the early ‘90s. We first noticed the extreme reactivity of 7-DHC in 1982 but we didn’t measure its autoxidation propagation rate constant until 2007. The reactivity of 7-DHC was nothing more than a curiosity when we first encountered it in the ‘80s, but returning to the problem and the relevant literature 20 years later made it clear that more detailed studies of the compound were warranted.
SLOS results in levels of 7-DHC in human plasma that are up to 5,000 fold greater than the levels found in control populations and since 7-DHC is 200 times more reactive than cholesterol, it seems clear that tissues and fluids of SLOS individuals are more vulnerable to oxidative stresses. A fetus having inherited SLOS mutations begins to make this highly oxidizable compound late during gestation when neuronal development, which relies on cholesterol for myelination, is at a critical stage. The hypothesis that SLOS is an oxidative stress syndrome seems worthy of continued investigation.
A Radical Perspective
I have chosen to focus this Perspective on the free radical chemistry of lipids and molecular oxygen because that theme has been a continuous part of my research program for nearly forty years and it continues to interest me. Research in oxidative stress, reactive oxygen species (ROS), lipid peroxidation and radical enzyme mechanisms has grown steadily during that period. For the biomedical community, the reactivity of molecular oxygen with the molecules of nature, particularly lipids, provides a fertile ground for discovery.130 For the chemist, the beauty of lipid radical chemistry lies in the fact that it is understandable based upon experimentally measurable physical-organic parameters like bond dissociation enthalpies and absolute rate constants. The reactions encountered are reasonably confined to a handful of types, although recognizing those types in new surroundings can sometimes be challenging. After the fact, the products formed in the autoxidation of 7-DHC can be reasonably catalogued based upon mechanistic precedents found in the autoxidation literature. As the structures of the 7-DHC oxysterols were revealed to my group one-by-one, however, the answer to the mechanistic puzzle was not all that obvious.
Interest in free radicals has hardly been limited to biological systems over the past half century, synthetic chemistry being one area that has been advanced at various times by radical-based transformations. My interest in the use of radicals in organic synthesis was stimulated by what seemed to be the generally held opinion in the 1970s that radicals were the “poor cousins” of reactive intermediates. This view was shaken by the studies of Barton and Stork, who expanded the scope and versatility of radical transformations. But the dogma remained in some circles that radicals, being indiscriminant in their reactions, could not find use as intermediates in stereoselective acyclic transformations. Examined from a mechanistic perspective, however, the critical parameter in stereoselectivity is the difference in free energy between transition states leading to stereoisomeric products, and there seemed to be no reason that such energy differences couldn’t be engineered in radical transformations based on the approaches that had been successfully applied in concerted reactions or in enolate alkylations. Bernd Giese in Basel and Dennis Curran at Pitt came to essentially the same conclusion at about the same time and the three of us struck up a very satisfying collaboration that led ultimately to a monograph on stereoselective radical transformations.131,132 Bernd had used competition reactions to provide a foundation for understanding reactivity and selectivity in radical additions and had demonstrated stereoselectivity in the reactions of cyclic substrates. Dennis cut his teeth in radical chemistry developing beautifully sequenced atom transfer additions and cyclizations in natural products synthesis. Each of us believed that advances had to be framed in a basic mechanistic understanding, and each of us took approaches that included mutually beneficial collaborations. In the end, it became clear that free radicals responded to stereocontrol elements in a predictable way and that acyclic diastereoselection could be achieved using many of the same auxiliary groups that were developed for use in other reaction types. My group continued this theme and provided examples of enantioselective radical additions based upon chiral Lewis acid catalysts. Again we found a partner in this work,133 Mukund Sibi at the University of North Dakota, who took the basic idea of chiral catalysis in radical reactions much beyond our initial contributions.
In an earlier review on the stereochemical aspects of radical pair reactions, I made the nearsighted comment that free radicals were the “reactive intermediates of the decade”.134 The decade was the 1980s during a time when there was an increased interest in radicals in organic synthesis. As that decade ended, interest did not subside and new applications and discoveries continued in that area. But radical chemistry has played an increasingly important role in each decade since Gomberg’s discovery over 100 years ago in areas as diverse as polymer and atmospheric chemistry, enzyme mechanisms, biomedicine and materials science. What seems clear now is that the study of reactions that proceed one electron at a time will continue to provide surprises and opportunities for decades to come.
Figure 1.

Keith Ingold, Cheves Walling and Bill Pryor at the Free Radicals in Biology Gordon Conference, Ventura CA, 1998.
Figure 5.

Collaborators in Stereoselective Radical Reactions, Dennis Curran and Bernd Giese circa 2011.
Scheme 5.

Roe’s Isoprenoid Serial Cyclization
Acknowledgments
My wife and personal librarian, Kitty Porter, has been understanding throughout the ups and downs of our careers in the chemical sciences. I am thankful for her love and support. I have mentioned in the text only a few of my many collaborators who have made important contributions to my research program over the years. For all who have worked with me over the years I am grateful for your collaboration. I acknowledge here Duke and Vanderbilt Universities, two great institutions that have provided stimulating environments for academic pursuits. The National Science Foundation and the National Institutes of Health have provided continuous support for my efforts for over 40 years. NIH Career Development and MERIT Awards made it easier to ask new research questions that were outside of my comfort zone.
Biography
 Ned Porter studied with Paul D. Bartlett at Harvard in the field of radical and biradical chemistry. He is currently the Stevenson Professor of Chemistry, Professor of Biochemistry and a member of the Institute of Chemical Biology and the Kennedy Center for Research on Human Development at Vanderbilt University. His research interests include mechanistic studies of free radical transformations.
Ned Porter studied with Paul D. Bartlett at Harvard in the field of radical and biradical chemistry. He is currently the Stevenson Professor of Chemistry, Professor of Biochemistry and a member of the Institute of Chemical Biology and the Kennedy Center for Research on Human Development at Vanderbilt University. His research interests include mechanistic studies of free radical transformations.
References and Notes
- 1.Gomberg M. J Am Chem Soc. 1900;22:757. [Google Scholar]
- 2.McBride JM. Tetrahedron. 1974;30:2009. [Google Scholar]
- 3.Schmidlin J. Ber Dtsch Chem Ges. 1908;41:2471. [Google Scholar]
- 4.Skinner KJ, Hochster HS, McBride JM. J Am Chem Soc. 1974;96:4301. [Google Scholar]
- 5.Roberts JD. Biographical Memoirs. Vol. 45. The National Academies Press; Washington DC: 1974. p. 432. [Google Scholar]
- 6.Bartlett PD, McBride JM. Pure App Chem. 1967;15:89. [Google Scholar]
- 7.Nelsen SF, Bartlett PD. J Am Chem Soc. 1966;88:143. [Google Scholar]
- 8.Bartlett PD, Engel PS. J Am Chem Soc. 1968;90:2960. [Google Scholar]
- 9.Bartlett PD, Schueller KE. J Am Chem Soc. 1968;90:6071. [Google Scholar]
- 10.Bartlett PD, Wallbillich G, Wingrove AS, Swenton JS, Montgomery LK, Kramer BD. J Am Chem Soc. 1968;90:2049. [Google Scholar]
- 11.Hull LA, Bartlett PD. J Org Chem. 1975;40:824. [Google Scholar]
- 12.Bartlett PD, Jacobson BM, Walker LE. J Am Chem Soc. 1973;95:146. [Google Scholar]
- 13.Bartlett PD, Günther P. J Am Chem Soc. 1966;88:3288. doi: 10.1021/ja00966a021. [DOI] [PubMed] [Google Scholar]
- 14.Bartlett PD, Guaraldi G. J Am Chem Soc. 1967;89:4799. [Google Scholar]
- 15.Bartlett PD, McBride JM. J Am Chem Soc. 1965;87:1727. [Google Scholar]
- 16.Bartlett PD, Schaap AP. J Am Chem Soc. 1970;92:3223. [Google Scholar]
- 17.Bartlett PD, Porter NA. J Am Chem Soc. 1968;90:5317. [Google Scholar]
- 18.Porter NA, Landis ME, Marnett LJ. J Am Chem Soc. 1971;93:795. [Google Scholar]
- 19.Porter NA, Marnett LJ. J Am Chem Soc. 1973;95:4361. [Google Scholar]
- 20.Porter NA, Marnett LJ, Lochmueller CH, Closs GL, Shobataki M. J Am Chem Soc. 1972;94:3664. [Google Scholar]
- 21.Green JG, Dubay GR, Porter NA. J Am Chem Soc. 1977;99:1264. [Google Scholar]
- 22.Porter NA, Dubay GR, Green JG. J Am Chem Soc. 1978;100:920. [Google Scholar]
- 23.Pryor W. Chem & Eng News. Vol. 49. The American Chemical Society; Washington DC: 1971. p. 34. [Google Scholar]
- 24.Marnett LJ. J Org Chem. 2012;77:5224. doi: 10.1021/jo300214d. Larry took a postdoc with Bengt Samulsson at the Karolinska Institute followed by faculty positions at Wayne State University and Vanderbilt where I joined him in 1998. A recurring theme in his research has been on the chemistry and biochemistry of electrophiles like malondialdehyde that are formed as byproducts of lipid peroxidation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolland JL, Gee G. Trans Faraday Soc. 1946;42:236. [Google Scholar]
- 26.Bolland JL. Quart Rev. 1949;3:1. [Google Scholar]
- 27.Bateman L. Quart Rev. 1954;8:147. [Google Scholar]
- 28.Walling C. Free Radicals in Solution. John Wiley and Sons; New York: 1957. [Google Scholar]
- 29.Howard JA, Ingold KU. Can J Chem. 1965;43:2729. [Google Scholar]
- 30.Howard JA, Ingold KU. Can J Chem. 1967;45:793. [Google Scholar]
- 31.Howard JA, Ingold KU. Can J Chem. 1967;45:785. [Google Scholar]
- 32.Howard JA, Ingold KU. J Am Chem Soc. 1968;90:1056. [Google Scholar]
- 33.Howard JA, Ingold KU. Can J Chem. 1968;46:2655. [Google Scholar]
- 34.Howard JA, Ingold KU. Can J Chem. 1968;46:2661. [Google Scholar]
- 35.Howard JA, Ingold KU. J Am Chem Soc. 1968;90:1058. [Google Scholar]
- 36.Howard JA, Ingold KU, Symonds M. Can J Chem. 1968;46:1017. [Google Scholar]
- 37.Howard JA, Schwalm WJ, Ingold KU. Adv Chem Ser. 1968:6. [Google Scholar]
- 38.Howard JA, Adamic K, Ingold KU. Can J Chem. 1969;47:3793. [Google Scholar]
- 39.Howard JA. Can J Chem. 1972;50:2298. [Google Scholar]
- 40.Korcek S, Chenier JHB, Howard JA, Ingold KU. Can J Chem. 1972;50:2285. [Google Scholar]
- 41.Ingold KU. Acc Chem Res. 1969;2:1. [Google Scholar]
- 42.Beckwith ALJ, Ingold KU. Rearrangements in Ground and Excited States. Academic Press; New York: 1980. [Google Scholar]
- 43.Howard JA. In: Adv Free Rad Chem. Williams GH, editor. IV. Logos Press; 1972. p. 49. [Google Scholar]
- 44.Funk MO, Isaac R, Porter NA. J Am Chem Soc. 1975;97:1281. doi: 10.1021/ja00838a074. [DOI] [PubMed] [Google Scholar]
- 45.Porter NA, Funk MO, Gilmore DW, Isaac R, Nixon J. J Am Chem Soc. 1976;98:6000. doi: 10.1021/ja00435a037. [DOI] [PubMed] [Google Scholar]
- 46.Beckwith ALJ, Schiesser CH. Tetrahedron. 1985;41:3925. [Google Scholar]
- 47.Spellmeyer DC, Houk KN. J Org Chem. 1987;52:959. doi: 10.1021/jo950884i. [DOI] [PubMed] [Google Scholar]
- 48.Khan JA, Porter NA. Angew Chem Int Edit. 1982;21:217. [Google Scholar]
- 49.Havrilla CM, Hachey DL, Porter NA. J Am Chem Soc. 2000;122:8042. [Google Scholar]
- 50.Yin HY, Havrilla CM, Morrow JD, Porter NA. J Am Chem Soc. 2002;124:7745. doi: 10.1021/ja0201092. [DOI] [PubMed] [Google Scholar]
- 51.Yin HY, Gao L, Tai HH, Murphey LJ, Porter NA, Morrow JD. J Biol Chem. 2007;282:329. doi: 10.1074/jbc.M608975200. [DOI] [PubMed] [Google Scholar]
- 52.Hamberg M, Samuelsson B. Proc Natl Acad Sci, USA. 1973;70:899. doi: 10.1073/pnas.70.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nugteren DH, Hazelfhof E. Biochem Biophys Acta. 1973;71:345. [Google Scholar]
- 54.Samuelsson B, Goldyne M, Granstrom E, Hamberg M, Hammarstrom S, Malmsten C. Annu Rev Biochem. 1978;47:997. doi: 10.1146/annurev.bi.47.070178.005025. [DOI] [PubMed] [Google Scholar]
- 55.Rouzer CA, Marnett LJ. Chem Rev. 2003;103:2239. doi: 10.1021/cr000068x. [DOI] [PubMed] [Google Scholar]
- 56.Porter NA, Funk MO. J Org Chem. 1975;40:3614. doi: 10.1021/jo00912a037. [DOI] [PubMed] [Google Scholar]
- 57.O’Connor DE, Mihelich ED, Coleman MC. J Am Chem Soc. 1981;103:223. [Google Scholar]
- 58.O’Connor DE, Mihelich ED, Coleman MC. J Am Chem Soc. 1984;106:3577. [Google Scholar]
- 59.Morrow J, Awad JA, Boss HJ, Blair I, Roberts LJ. Proc Natl Acad Sci, USA. 1992;89:10721. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morrow JD, Minton TA, Roberts LJ., II Prostaglandins. 1992;44:155. doi: 10.1016/0090-6980(92)90077-7. [DOI] [PubMed] [Google Scholar]
- 61.Pryor WA, Stanley JP. J Org Chem. 1975;40:3615. doi: 10.1021/jo00912a038. [DOI] [PubMed] [Google Scholar]
- 62.Yin HY, Porter NA, Morrow JD. J Chrom B. 2005;827:157. doi: 10.1016/j.jchromb.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 63.Yin HY, Gao L, Porter N, Morrow J. Drug Metabolism Reviews. 2006;38:197. [Google Scholar]
- 64.Porter NA, Roe A, McPhail A. J Am Chem Soc. 1980;102:7574. [Google Scholar]
- 65.Roe AN, McPhail AT, Porter NA. J Am Chem Soc. 1983;105:1199. [Google Scholar]
- 66.Chan HWS, Levett G. Lipids. 1977;12:99. doi: 10.1007/BF02532979. [DOI] [PubMed] [Google Scholar]
- 67.Funk MO, Isaac R, Porter NA. Lipids. 1976 [Google Scholar]
- 68.Frankel EN, Pryde EH, editors. AOCS; Champaign. Ill: 1979. p. 353. [Google Scholar]
- 69.Maillard B, Ingold KU, Scaiano JC. J Am Chem Soc. 1983;105:5095. [Google Scholar]
- 70.Porter NA, Weber BA, Weenen H, Khan JA. J Am Chem Soc. 1980;102:5597. [Google Scholar]
- 71.Porter NA, Wolf RA, Weenen H. Lipids. 1980;14:163. [Google Scholar]
- 72.Weenen H, Porter NA. J Am Chem Soc. 1982;104:5216. [Google Scholar]
- 73.Porter NA, Wujek DG. J Am Chem Soc. 1984;106:2626. [Google Scholar]
- 74.Kenar JA, Havrilla CM, Porter NA, Guyton JR, Brown SA, Klemp KF, Selinger E. Chem Res Toxicol. 1996;9:737. doi: 10.1021/tx9600098. [DOI] [PubMed] [Google Scholar]
- 75.Tallman KA, Pratt DA, Porter N. J Am Chem Soc. 2001;123:11827. doi: 10.1021/ja0169724. [DOI] [PubMed] [Google Scholar]
- 76.Pratt DA, Mills JH, Porter N. J Am Chem Soc. 2003;125:5801. doi: 10.1021/ja034182j. [DOI] [PubMed] [Google Scholar]
- 77.Roschek B, Tallman KA, Rector CL, Gillmore JG, Pratt DA, Punta C, Porter NA. J Org Chem. 2006;71:3527. doi: 10.1021/jo0601462. [DOI] [PubMed] [Google Scholar]
- 78.Liu W, Yin H, Akazawa YO, Yoshida Y, Niki E, Porter NA. Chem Res Toxicol. 2010;23:986. doi: 10.1021/tx1000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tallman KA, Roschek B, Porter NA. J Am Chem Soc. 2004;126:9240. doi: 10.1021/ja049104q. [DOI] [PubMed] [Google Scholar]
- 80.Tallman KA, Rector CL, Porter NA. J Am Chem Soc. 2009;131:5635. doi: 10.1021/ja900040d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Howard JA. Ref 43 The rate of H-atom transfer from donors is relatively independent of peroxyl radical structure. [Google Scholar]
- 82.Griller D, Ingold KU. Acc Chem Res. 1980;13:317. [Google Scholar]
- 83.Newcomb M. Tetrahedron. 1993;49:1151. [Google Scholar]
- 84.Newcomb M, Toy PH. Acc Chem Res. 2000;33:449. doi: 10.1021/ar960058b. [DOI] [PubMed] [Google Scholar]
- 85.Xu L, Davis TA, Porter NA. J Am Chem Soc. 2009;131:13037. doi: 10.1021/ja9029076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cosgrove JP, Church DF, Pryor WA. Lipids. 1987;22:299. doi: 10.1007/BF02533996. [DOI] [PubMed] [Google Scholar]
- 87.Chan HWS, Levett G, Matthew JA. J Chem Soc Chem Commun. 1978:756. [Google Scholar]
- 88.Chan HSW, Levett G, Matthew JA. Chem Phys Lipids. 1979;24:245. [Google Scholar]
- 89.Bascetta E, Gunstone FD, Walton JC. J Chem Soc Perkin Trans. 1983;2:803. doi: 10.1016/0009-3084(83)90022-1. [DOI] [PubMed] [Google Scholar]
- 90.Porter NA, Mills KA, Carter RL. J Am Chem Soc. 1994;116:6690. [Google Scholar]
- 91.Schenck G, Neumuller OA, Eisfeld W. Justus Liebigs Ann Chem. 1958;618:202. [Google Scholar]
- 92.Brill WF. J Am Chem Soc. 1965;87:3286. [Google Scholar]
- 93.Beckwith ALJ, Davies AG, Davison IGE, Maccoll A, Mruzek MH. J Chem Soc, Chem Commun. 1988:475. [Google Scholar]
- 94.Beckwith ALJ, Davies AG, Davison IGE, Maccoll A, Mruzek MH. J Chem Soc, Perkin Tran 2. 1989:815. [Google Scholar]
- 95.Porter NA, Zuraw PJ. J Chem Soc, Chem Commun. 1985:1472. [Google Scholar]
- 96.Dussault P, Porter NA. J Am Chem Soc. 1988;110:6276. doi: 10.1021/ja00226a070. [DOI] [PubMed] [Google Scholar]
- 97.Porter N, Kaplan JK, Dussault PH. J Am Chem Soc. 1990;112:1266. [Google Scholar]
- 98.Mills KA, Caldwell SE, Dubay GR, Porter N. J Am Chem Soc. 1992;114:9689. [Google Scholar]
- 99.Porter NA, Caldwell SE, Mills KA. Lipids. 1995;30:277. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- 100.Porter NA, Mills KA, Caldwell SE, Dubay GR. J Am Chem Soc. 1994;116:6697. [Google Scholar]
- 101.Lowe J, Porter N. J Am Chem Soc. 1997;119:11534. [Google Scholar]
- 102.Allen JL, Paquette KP, Porter N. J Am Chem Soc. 1998;120:9362. [Google Scholar]
- 103.Caldwell SE, Porter NA. J Am Chem Soc. 1995;117:8676. [Google Scholar]
- 104.Olivella S, Solé A. J Am Chem Soc. 2003;125:10641. doi: 10.1021/ja030171e. [DOI] [PubMed] [Google Scholar]
- 105.Hu D, Pratt D. Chem Commun. 2010;46:3711. doi: 10.1039/c0cc00019a. [DOI] [PubMed] [Google Scholar]
- 106.Brash AR. Lipids. 2000;35:947. doi: 10.1007/s11745-000-0604-0. [DOI] [PubMed] [Google Scholar]
- 107.Pratt D, Tallman K, Porter NA. Acc Chem Res. 2011:44. doi: 10.1021/ar200024c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pratt DA, Porter N. Org Lett. 2003;5:387. doi: 10.1021/ol027094x. [DOI] [PubMed] [Google Scholar]
- 109.Bascetta E, Gunstone FD, Walton JC. J Chem Soc, Perkin Tran 2. 1983:603. doi: 10.1016/0009-3084(83)90022-1. [DOI] [PubMed] [Google Scholar]
- 110.Bascetta E, Gunstone FD, Walton JC. J Chem Soc, Perkin Tran 2. 1984:401. [Google Scholar]
- 111.Smith DW, LemIi L, Opitz JM. J Pediatr. 1964;64:210. doi: 10.1016/s0022-3476(64)80264-x. [DOI] [PubMed] [Google Scholar]
- 112.Porter F, Herman GE. J Lipid Res. 2011;52:6. doi: 10.1194/jlr.R009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Witsch-Baumgartner M, Fitzky BU, Ogorelkova M, Kraft HG, Moebius FF, Glossmann H, Seedorf U, Gillessen-Kaesbach G, Hoffmann GF, Clayton P, Kelley RI, Utermann G. Am J Hum Gen. 2000;66:402. doi: 10.1086/302760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Javitt NB. Steroids. 2008;73:149. doi: 10.1016/j.steroids.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 115.Herman GE. Hum Mol Genet. 2003;12(Spec 1):R75. doi: 10.1093/hmg/ddg072. [DOI] [PubMed] [Google Scholar]
- 116.Steiner RD, Linck LM, Flavell DP, Lin DS, Connor WE. J Lipid Res. 2000;41:1437. [PubMed] [Google Scholar]
- 117.Bukelis I, Porter F, Zimmerman AW, Tierney E. American Journal Of Psychiatry. 2007;164:1655. doi: 10.1176/appi.ajp.2007.07020315. [DOI] [PubMed] [Google Scholar]
- 118.Xu L, Korade Z, Porter NA. J Am Chem Soc. 2010;132:2222. doi: 10.1021/ja9080265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yin H, Xu L, Porter NA. Chem Rev. 2011;111:5944. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 120.Porter NA, Nixon JR. J Am Chem Soc. 1978;100:7116. [Google Scholar]
- 121.Porter NA, Cudd MA, Miller RW, McPhail AT. J Am Chem Soc. 1980;102:414. [Google Scholar]
- 122.Matsumoto A, Higashi H. Macromolecules. 2000;33:1651. [Google Scholar]
- 123.Gaoua W, Chevy F, Roux C, Wolf C. J Lipid Res. 1999;40:456. [PubMed] [Google Scholar]
- 124.Richards MJ, Nagel BA, Fliesler SJ. Exp Eye Res. 2006;82:538. doi: 10.1016/j.exer.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chignell CF, Kukielczak BM, Sik RH, Bilski PJ, He YY. Free Rad Biol Med. 2006;41:339. doi: 10.1016/j.freeradbiomed.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 126.Xu L, Korade Z, Dale A, Rosado J, Liu W, Lamberson CR, Porter NA. J Lipid Res. 2011;52:1222. doi: 10.1194/jlr.M014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Korade Z, Xu L, Porter NA. J Lipid Res. 2010;51:3259. doi: 10.1194/jlr.M009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Korade Z, Xu L, Mirnics K, Porter N. J Inherit Metab Dis. 2013;36:113. doi: 10.1007/s10545-012-9504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu W, Xu L, Lamberson C, Merkens L, Steiner R, Elias E, Haas D, Porter N. J Lipid Res. 2013;54:244. doi: 10.1194/jlr.M031732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Davies KJA, Pryor WA. Free Rad Bio Med. 2005;39:1263. doi: 10.1016/j.freeradbiomed.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 131.Porter NA, Giese B, Curran DP. Acc Chem Res. 1991;24:296. [Google Scholar]
- 132.Curran DP, Porter NA, Giese B. Stereochemistry of Radical Reactions. Concepts, Guidelines and Synthetic Applications. VCH; Weinheim: 1995. [Google Scholar]
- 133.Sibi MP, Porter NA. Acc Chem Res. 1998;32:163. [Google Scholar]
- 134.Porter NA, Krebs PJ. In: Topics in Stereochemistry. Elel E, Wilen S, editors. Vol. 18. John Wiley & Sons; New York: 1988. p. 98. [Google Scholar]