Abstract
Previous studies from our laboratory have shown that the activation of G2-M checkpoint after exposure of MCF-7 breast cancer cells to γ-irradiation (IR) is dependent on the activation of extracellular signal-regulated kinase 1/2 (ERK1/2) signaling. Studies presented in this report indicate that IR exposure of MCF-7 cells is associated with a marked increase in expression of breast cancer 1 (BRCA1) tumor suppressor, an effect that requires ERK1/2 activation and involves posttranscriptional control mechanisms. Furthermore, reciprocal coimmunoprecipitation, as well as colocalization studies, indicate an interaction between BRCA1 and ERK1/2 in both nonirradiated and irradiated cells. Studies using short hairpin RNA targeting BRCA1 show that BRCA1 expression is necessary for IR-induced G2-M cell cycle arrest, as well as ERK1/2 activation in MCF-7 cells. Although BRCA1 expression is not required for IR-induced phosphorylation of ataxia telangiectasia mutated (ATM)–Ser1981, it is required for ATM-mediated downstream signaling events, including IR-induced phosphorylation of Chk2-Thr68 and p53-Ser20. Moreover, BRCA1 expression is also required for IR-induced ATM and rad3 related activation and Chk1 phosphorylation in MCF-7 cells. These results implicate an important interaction between BRCA1 and ERK1/2 in the regulation of cellular response after IR-induced DNA damage in MCF-7 cells.
Introduction
DNA damage induced by ionizing irradiation triggers rapid activation of DNA damage checkpoints, resulting in cell cycle arrest and DNA repair. Previous studies have identified several cellular signaling cascades, including signalings mediated by ataxia telangiectasia mutated (ATM) and ATM and rad3 related (ATR), in the activation of DNA damage checkpoint response (1).
BRCA1 tumor suppressor has been associated with increased susceptibility to the development of breast and ovarian cancers (2). Accumulating evidence has suggested a role for BRCA1 in mediating DNA damage checkpoint response (2). After DNA damage, BRCA1 is rapidly phosphorylated by several nuclear kinases, including ATM, ATR, and Chk2 (3–6). Furthermore, cells containing mutated BRCA1 display defective intra-S and G2-M checkpoint responses and increased sensitivity to a range of DNA-damaging agents, including γ-irradiation (IR) and UV (7–10). Other studies have shown that overexpression of BRCA1 in various human cancer cell lines can trigger a G2-M checkpoint response, similar to that induced by DNA damage (11–16). Finally, BRCA1 have been found in complex with a wide range of proteins involved in cell cycle checkpoint control and DNA repair, which include ATM/ATR, BRCA2, CtIP, Chk1/Chk2, p53, and Rad50-Mre11-Nbs1 (17, 18), and BRCA1 is required for ATM- and ATR-mediated phosphorylation/activation of Chk2, Nbs1, and p53 after exposure of cells to IR or UV (19). Whereas a role for BRCA1 in DNA damage checkpoint response has been strongly implicated, the mechanisms involved in this function of BRCA1 are not well understood.
Previous studies in a wide variety of cell types have shown that IR exposure results in the rapid activation of mitogen-activated protein kinase (MAPK) family members, including extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun-NH2-kinase, and p38 (20, 21). Whereas p38γ activation may be essential in IR-induced G2-M arrest in HeLa and U2OS cells (22), studies from our laboratory and others have shown that IR-induced ERK1/2 activation is necessary for the induction of G2-M checkpoint in MCF-7 breast cancer cells and that inhibition of ERK1/2 is associated with increased sensitivity to DNA-damaging agents (23–25).
Because previous studies have indicated that BRCA1 expression and ERK1/2 activation are both necessary for IR-induced G2-M arrest, in this report, we examined the interplay between BRCA1 and ERK1/2 and their involvement in the regulation of checkpoint response after IR-induced DNA damage. The results presented in this report indicate an in vivo association between BRCA1 and ERK1/2 and suggest cooperation between BRCA1 and ERK1/2 in the regulation of IR-induced DNA damage response.
Materials and Methods
Cell culture and drug treatment
MCF-7 human breast cancer cells were obtained from American Type Culture Collection and maintained in DMEM containing 10% fetal bovine serum.
For the studies involving treatment with mitogen-activated protein kinase kinase 1/2 (MEK1/2) inhibitor U0126, log-phase cells were incubated in medium containing U0126 (LC Laboratories), which was dissolved in DMSO. For proteasome inhibitor studies, MCF-7 cells were incubated in medium containing either MG132 (EMD Biosciences) or lactacystin (Santa Cruz Biotechnology) dissolved in DMSO, as described previously (26, 27). Control cells were incubated in medium containing the same amounts of vehicle alone. For experiments involving IR exposure, exponentially growing cells were treated with IR and then incubated at 37°C for the indicated times before analysis. For experiments involving treatment with both inhibitor and IR, cells were incubated with inhibitor for 1 h before IR exposure.
Antibodies and recombinant proteins
All antibodies were obtained from Santa Cruz Biotechnology, unless indicated elsewhere. These include mouse IgG for phosphorylated ATM (Ser1981; Rockland Immunochemical for Research), BRCA1 (D-9), BRCA1 (Ab-1; EMD Biosciences), phosphorylated ERK1/2 (pERK1/2; E-4), Chk1 (G-4), Chk2 (B-4), and p53 (DO-1); rabbit IgG for ATM (Ab-3; EMD Biosciences), BRCA1 (I-20), phosphorylated Chk1 (Ser317; Cell Signaling Technology), phosphorylated Chk2 (Thr68; Cell Signaling Technology), ERK1/2 (C-14-R), pERK1/2 (20G11; Cell Signaling Technology), phosphorylated p53 (Ser20; Cell Signaling Technology), and ubiquitin (FL-76); and goat IgG for ERK1/2 (C-14-G), ATR (N-19), and actin (I-19).
Recombinant p53 protein for ATR kinase assay was a glutathione S-transferase (GST) fusion protein containing full-length human p53 (Santa Cruz Biotechnology); GST was used as a control substrate in all kinase assays and was prepared according to the standard procedure (GE Healthcare Bio-Sciences).
Immunoblotting, immunoprecipitation, and kinase assay
Immunoblotting, immunoprecipitation, and kinase assay were performed as described previously (25, 28–30).
Specific protein signals on Western blots were visualized by chemiluminescence exposed to X-ray film, scanned using EPSON Perfection 4490PHOTO scanner, and analyzed using ImageQuant 5.2 analytical program (GE Healthcare Bio-Sciences).
Short interfering RNA transfection
Short interfering RNA (siRNA) duplexes were obtained from Dharmacon Research. Control nontargeting siRNA, contains at least four mismatches to any human, mouse, or rat gene, as previously determined by the manufacturer. The sequence for Control-siRNA is 5′-UAAG GCUA UGAA GAGA UAC-3′. SMARTpool siRNAs targeting ERK1/2 consists of eight siRNAs targeting multiple sites on ERK1/2 (ERK1/2-siRNAs). The sequences for ERK1-siRNAs are 5′-PUAA AGGU UAAC AUCC GGUC UU-3′, 5′-PAAC UUGU ACAG GUCA GUCU UU-3′, 5′-PAGA GACU GUAG GUAG UUUC UU-3′, and 5′-PUAC UGCA ACUG CGUG UAGC UU-3′, and the sequences for ERK2-siRNAs are 5′-PAAU AAGU CCAG AGCU UUGG UU-3′, 5′-PAGC UUGU AAAG AUCU GUUU UU-3′, 5′-PUUC UACU UCAA UCCU CUUG UU-3′, and 5′-PAAU UUCU GGAG CCCU GUAC UU-3′.
Cells were transfected with siRNAs at 100 nmol/L using Dharma FECT 1 siRNA transfection reagent (Dharmacon Research) according to the manufacturer’s instruction. For experiments involving both siRNA transfection and IR exposure, transfected cells were first incubated for the indicated times and then exposed to IR.
Short hairpin RNA and retrovirus
Retroviral vectors expressing short hairpin RNAs (shRNA) were obtained from Open Biosystems. The sequences for shRNAs targeting BRCA1 include B1, 5′-TTCA TTGC CCCT GAAC TGAG ACGA CAGA CCCA AGTC TATC ATCT CAGT TCAG AGGC AACG AA-3′ and B2, 5′-TTCC TCTC TCGG AAGA CTAG GATT AACA ACCA ATTG TCAA TCCT AGCC TTCC AAGA GAAG AA-3′. The sequence for control shRNA targeting firefly luciferase is 5′-CCCG CCTG AAGT CTCT GATT AA-3′.
Phoenix A retroviral packaging cells (31) were transfected with shRNA retroviral expressing vectors using MBS mammalian transfection kit (Stratagene), according to manufacturer’s instruction. At 48 h posttransfection, medium containing amphotropic retrovirus was collected and filtered through 0.4 µm filter, and MCF-7 cells infected with retroviral vectors in the presence of 4 µg/mL polybrene (Sigma-Aldrich). Clones stably expressing shRNAs were selected in medium containing 2 µg/mL puromycin (Sigma-Aldrich). BRCA1 levels in these clones were determined by Western blot analysis.
Reverse transcription–PCR analysis
MCF-7 cells were incubated with or without U0126 for 1 h and then exposed to 10-Gy IR or, as control, left nonirradiated. Total RNA was isolated from the resulting cells using the RNeasy Mini kit (Qiagen) and analyzed for BRCA1 mRNA by real-time reverse transcription–PCR (RT-PCR) using the RT2 Real-Time Syber Green RT-PCR system (SuperArray Bioscience). The relative mRNA expression of BRCA1 was adjusted with β-actin mRNA levels. The primers used for real-time RT-PCR were BRCA1, 5′-CATA ACAG ATGG GCTG GAAG TAAG G-3′ and 5′-AGCT CTGG GAAA GTAT CGCT GTC-3′; actin, 5′-CACG AAAC TACC TTCA ACTC C-3′ and 5′-CAAA TAAA GCCA TGCC AATC TC-3′.
Cell cycle analysis
Fluorescence-activated cell sorting (FACS) analysis was performed on 20,000 cells using a FACSCalibur instrument (Beckon Dickinson), as described previously (25).
Immunofluorescence staining and microscopy
Immunofluorescence staining was performed as described previously (32) with the following differences: (a) 10% horse serum was used for blocking, (b) 5% horse serum used for antibody dilution, and (c) primary antibody incubation performed at 4°C for 16 h. The antibodies used were I-20 for BRCA1 and C-14-G for ERK1/2 (33, 34). Immunostained cells were analyzed using Zeiss LSM510 confocal laser-scanning microscope.
Results
ERK1/2 activation is required for IR-induced increase in BRCA1 protein stabilization in MCF-7 cells
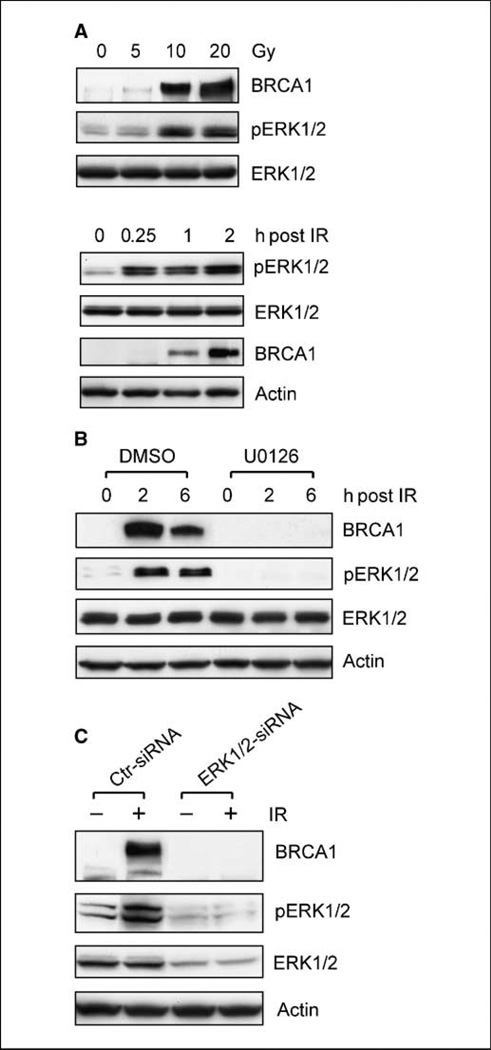
To study the possible relationship between ERK1/2 and BRCA1 in the regulation of IR-induced DNA damage checkpoint response, MCF-7 cells were exposed to increasing doses of IR. As shown in Fig. 1A (top), there was a dose-dependent increase in both BRCA1 protein levels and ERK1/2 phosphorylation in MCF-7 cells after IR exposure. Exposure to 20-Gy IR resulted in a 23-fold increase in BRCA1 protein and a 16-fold increase in ERK1/2 phosphorylation compared with nonirradiated control cells.
Figure 1.
ERK1/2 activation is required for the induction of BRCA1 protein after IR exposure of MCF-7 cells. A, top, log-phase growing MCF-7 cells were exposed to IR at the indicated doses, incubated for 2 h at 37°C, and analyzed for BRCA1 protein and ERK1/2 phosphorylation by immunoblotting. Bottom, cells were exposed to 10-Gy IR, incubated for the indicated time, and analyzed for levels of pERK1/2 and BRCA1. B, after incubation in the presence or absence of 50 µmol/L U0126 for 1 h, MCF-7 cells were exposed to 10-Gy IR, incubated for additionalindicated times, and analyzed for BRCA1 protein and ERK1/2 phosphorylation. Equal loading of all protein samples was confirmed by assessing actin levels. C, MCF-7 cells were transfected with either nontargeting control siRNAs (Ctr-siRNA) or siRNAs targeting ERK1/2 (ERK1/2-siRNA). After incubation for 3 d, transfected cells were treated with or without 10-Gy IR and incubated for additional2 h. Levels of BRCA1, pERK1/2, total ERK1/2, and actin were assessed by immunoblotting.
To correlate the induction of BRCA1 expression with ERK1/2 phosphorylation after IR, we examined the time-dependent changes in both proteins in MCF-7 cells after IR. As shown in Fig. 1A (bottom), while there was a marked increase in ERK1/2 phosphorylation within 15 minutes after IR exposure, an increase in BRCA1 protein levels was detected at 1-hour post-IR. Thus, the increase in ERK1/2 phosphorylation after IR exposure apparently precedes the marked induction in BRCA1 protein expression.
The effect of ERK1/2 inhibition on IR-induced increase in BRCA1 expression was assessed by incubation of MCF-7 cells with the MEK1/2 specific inhibitor U0126. In these studies, cells were preincubated for 1 hour in the presence or absence of 50 µmol/L U0126, a concentration that had been shown in our previous studies to completely inhibit IR-induced ERK1/2 activation in MCF-7 cells (25). As shown in Fig. 1B, inhibition of IR-induced ERK1/2 phosphorylation by U0126 completely abrogated the increase of BRCA1 protein in MCF-7 cells exposed to IR.
To confirm the role of ERK1/2 in the regulation of BRCA1 expression in IR-treated cells, MCF-7 cells were transfected with ERK1/2-siRNA. As shown in Fig. 1C, there was a 65% reduction in ERK1/2 protein levels after transfection of MCF-7 cells with ERK1/2-siRNA for 3 days (ERK1/2). This, in turn, was associated with an abrogation of ERK1/2 phosphorylation in MCF-7 cells after exposure to 10-Gy IR (pERK1/2). Furthermore, ERK1/2-siRNA transfected MCF-7 cells exhibited no detectable increase in BRCA1 protein after exposure to 10-Gy IR (ERK1/2-siRNA: BRCA1). In contrast, transfection of MCF-7 cells with Control-siRNA had no effect on either ERK1/2 protein levels or IR-induced ERK1/2 phosphorylation (Ctr-siRNA: pERK1/2 and ERK1/2), as well as IR-induced BRCA1 expression (Ctr-siRNA: BRCA1) in MCF-7 cells. These studies indicate that ERK1/2 activation is necessary for the increase in BRCA1 protein levels observed in MCF-7 cells after IR exposure.
To investigate the mechanisms involved in the regulation of BRCA1 expression by ERK1/2 signaling after IR exposure, BRCA1 mRNA levels were examined in both control and irradiated MCF-7 cells incubated in the presence or absence of U0126. The results indicate that treatment with either irradiation alone or in combination with U0126 had no detectable effect on BRCA1 mRNA levels in MCF-7 cells for up to 4 hours after treatment (data not shown). Thus, IR-induced increase in BRCA1 expression in MCF-7 cells, which requires ERK1/2 activation, apparently involves posttranscriptional regulatory mechanisms.
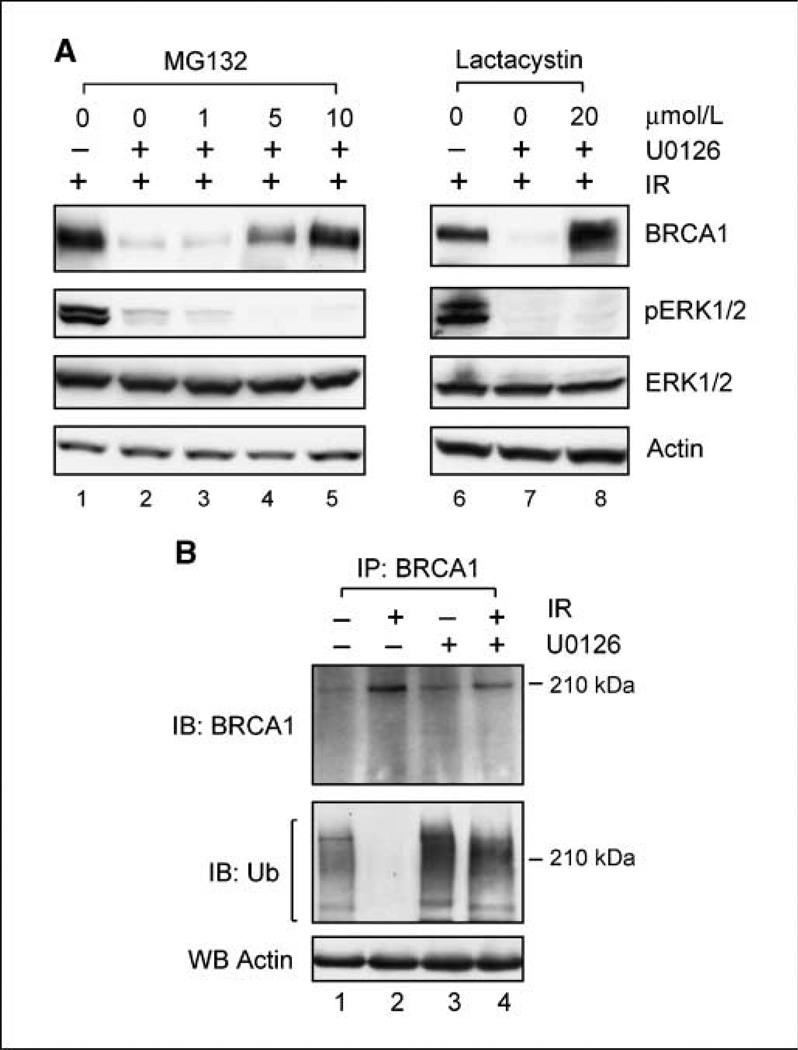
We next examined the influence of proteasome inhibitors on BRCA1 expression in irradiated cells incubated in the presence or absence of U0126. For these studies, MCF7 cells were preincubated for 1 hour with 50 µmol/L U0126 along with increasing doses of the proteasome inhibitor MG132 (26) before exposure to 10-Gy IR. After incubation for an additional 2 hours post-IR, the cells were lysed and analyzed for BRCA1 protein levels (BRCA1) and ERK1/2 phosphorylation (pERK1/2). For comparison, parallel sets of MCF-7 cells were either left untreated (data not shown) or treated with IR alone (Fig. 2A, lane 1), U0126 alone (data not shown), or MG132 alone (data not shown). Whereas incubation of cells with U0126 abolished the increase of BRCA1 protein in MCF-7 cells after IR treatment (Fig. 2A, lane 2 versus lane 1), treatment with the proteasome inhibitor MG132 restored the IR-induced increase in BRCA1 protein levels in cells incubated with U0126 (Fig. 2A, lanes 4 and 5 versus lane 2). The effect of MG132 is dose-dependent, and 10 µmol/L of MG132 were needed to completely restore the increase in BRCA1 protein levels in irradiated MCF-7 cells incubated with U0126 (Fig. 2A, lane 5). Western blot analysis showed that treatment by either MG132 alone of U0126 alone had no detectable effect on endogenous BRCA1 levels (data not shown).
Figure 2.
Proteasome inhibition restores BRCA1 protein expression in MCF-7 cells treated with both IR and U0126. A, left, MCF-7 cells were incubated for 1 hour with increasing doses of the proteasome inhibitor MG132, in the presence or absence of U0126, and then exposed to 10-Gy IR. After incubation for 2 h at 37°C, levels of BRCA1 and ERK1/2 phosphorylation were determined by immunoblotting. Right, cells were incubated for 1 h with or without 20 µmol/L lactacystin in the presence or absence of U0126 and then exposed to 10-Gy IR. After 2-h incubation at 37°C, levels of BRCA1 and ERK1/2 phosphorylation were determined. B, cells were incubated for 1 h with 10 µmol/L MG132 in the presence or absence of U0126 and exposed to 10-Gy IR or, as a control, left nonirradiated. After incubation at 37°C for 2 h, BRCA1 was immunoprecipitated from 1 mg cell lysate using anti-BRCA1 antibody I-20 (recognizing COOH terminus of BRCA1) and analyzed by immunoblotting for BRCA1 with Ab-1 antibody (recognizing NH2 terminus of BRCA1; IB: BRCA1) and ubiquitin-conjugated BRCA1 species with FL-76 anti-ubiquitin specific antibody (IB: Ub). Actin levels in supernatants obtained from immunoprecipitation assays were determined by Western blotting to confirm equal loading of all protein samples (WB Actin).
To confirm the effect of MG132 on IR-induced induction of BRCA1 expression, another proteasome-specific inhibitor lactacystin (27) was used. For these studies, MCF-7 cells were incubated for 1 hour in the presence or absence of 20 µmol/L lactacystin in combination with 50 µmol/L U0126 and then exposed to 10-Gy IR. This dose of lactacystin (20 µmol/L) was selected based on previous studies from our laboratory, which showed that incubation of MCF-7 cells with 20 µmol/L lactacystin completely abrogated the effect of BRCA1 overexpression on the subsequent degradation of Cdc25C protein (16). As shown in Fig. 2A (right), incubation with U0126 abrogated the IR-induced increase of BRCA1 protein (lane 7 versus lane 6), an effect that is completely reversed by simultaneous incubation of cells with lactacystin (lane 8 versus lane 7). In fact, lactacystin treatment of irradiated cells incubated with U0126 resulted in slightly higher BRCA1 levels compared with that in cells received IR treatment alone (Fig. 2A, lane 8 versus lane 6). In contrast, treatment with lactacystin and/or U0126 by itself in the absence of IR exposure did not produce any noticeable change in BRCA1 protein levels in MCF-7 cells compared with nonirradiated control cells (data not shown).
The studies described above suggest that ERK1/2 signaling plays a role in the regulation of proteasome-mediated changes in BRCA1 degradation. Previous studies have indicated that BRCA1 protein levels change during the cell cycle and that this regulation involves changes in ubiquitin-dependent proteasomal degradation of BRCA1 (26). We therefore examined BRCA1 ubiquitination in cells exposed to IR with or without inhibition of ERK1/2 signaling. For these studies, MCF-7 cells were incubated for 1 hour with 10 µmol/L MG132 in the presence or absence of U0126 before exposure to 10-Gy IR. After IR, BRCA1 was immunoprecipitated using an antibody recognizing the COOH terminus of BRCA1 protein and analyzed for the presence of ubiquitin conjugation. As shown in Fig. 2B (IB: Ub), immunoblotting using an ubiquitin-specific antibody reveals the presence of ubiquitin-conjugated BRCA1 species in untreated cells (lane 1). After IR exposure, ubiquitin-conjugated BRCA1 species were markedly diminished in irradiated cells (lane 2). As shown in Fig. 2B (IB: Ub), incubation of irradiated cells with U0126 resulted in a marked increase in ubiquitin-conjugated BRCA1 species compared with irradiated cells incubated in the absence of U0126 (lane 4 versus lane 2). Furthermore, nonirradiated cells incubated in the presence of U0126 also had a noticeable increase in ubiquitin-conjugated BRCA1 compared with control nonirradiated cells incubated in the absence of U0126 (Fig. 2B, IB: Ub, lane 3 versus lane 1). Western blot analysis using an antibody which recognizes the NH2 terminus of BRCA1 protein confirmed the presence of the BRCA1 protein in all immunoprecipitates (Fig. 2B, IB: BRCA1). These results indicate that IR exposure results in a suppression of BRCA1 ubiquitination in MCF-7 cells and that this suppression of BRCA1 ubiquitination requires IR-induced ERK1/2 activation. Conversely, inhibition of ERK1/2 signaling blocks the loss of BRCA1 ubiquitination, which normally occurs after irradiation and this, in turn, abolishes the induction of BRCA1 protein expression in cells after IR treatment.
Collectively, the results described above suggest that the increase of BRCA1 protein levels in MCF-7 cells after IR exposure is due to an increase in BRCA1 protein stability. This effect requires ERK1/2 activation and involves the suppression of BRCA1 ubiquitination and suppression of proteasome-mediated degradation of BRCA1 protein.
BRCA1 interacts with ERK1/2 in MCF-7 cells
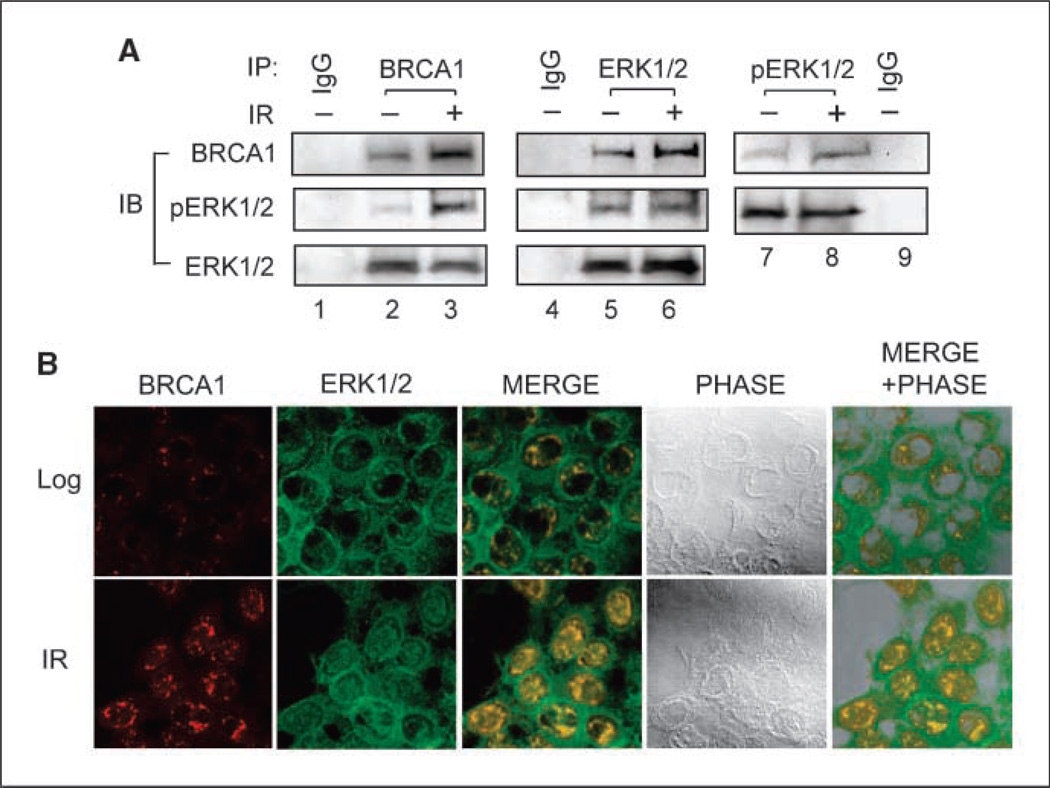
Intracellular interactions between ERK1/2 and BRCA1 were examined using reciprocal coimmunoprecipitation and immunofluorescence assays. As shown in Fig. 3A (left), Western blot analysis indicated that ERK1/2 protein was present in immunoprecipitates derived from MCF-7 cells using anti–BRCA1-specific antibody (ERK1/2). Furthermore, these studies reveal the presence of pERK1/2 in the immunoprecipitates (Fig. 3A, left, pERK1/2). Conversely, BRCA1 protein was detected in immunoprecipitates derived using antibody against either total ERK1/2 (middle, BRCA1) or pERK (right, BRCA1). Whereas coimmunoprecipitation of BRCA1 and ERK1/2 was detected in cell extracts obtained from both log-phase and irradiated cells, relative higher amounts of BRCA1 were detected in immunoprecipitates derived from irradiated cells compared with that obtained from nonirradiated cells (Fig. 3A, BRCA1, lanes 3, 6 , and 8 versus lanes 2, 5 , and 7). In contrast, neither BRCA1 nor ERK1/2 protein was detected in immunoprecipitates derived using nonspecific antibody (IgG). These results suggest a direct physical interaction between BRCA1 and ERK1/2 in MCF-7 cells.
Figure 3.
BRCA1 interacts with ERK1/2 in vivo. A, MCF-7 cells were treated with or without 10-Gy IR and incubated for 2 h at 37°C. Left, BRCA1 was immunoprecipitated from 500 µg lysates using I-20 anti-BRCA1 antibody and analyzed by immunoblotting for BRCA1 with Ab-1 anti-BRCA1 antibody, pERK1/2 with E4 anti-pERK1/2 antibody, and totalERK1/2 with C-14-G anti-ERK1/2 antibody. Middle, ERK1/2 was immunoprecipitated using C-14-R anti-ERK1/2 antibody and analyzed by immunoblotting for BRCA1, pERK1/2, and total ERK1/2 as described above. Right, pERK1/2 was immunoprecipitated using 20G11 anti-pERK1/2 antibody and probed for BRCA1 and pERK1/2, as described above. As a control, immunoprecipitates obtained by incubating nonirradiated cell lysate with preimmune IgG were included in the analysis (IgG). B, cells treated with or without 10-Gy IR were incubated for 4 h at 37°C and then stained with antibodies against BRCA1 (I-20) and ERK1/2 (C-14-G), as described in Materials and Methods. Representative images show the localization of BRCA1 (BRCA1) and ERK1/2 (ERK1/2) using a confocal microscope. Overlapping intracellular distribution of BRCA1 and ERK1/2 in both untreated and irradiated cells was examined and shown as merged images (MERGE). Phase contract images of the examined cell samples were overlapped with relative merged images to visualize the intracellular localization of the examined proteins (MERGE + PHASE).
The intracellular distribution of BRCA1 and ERK1/2 protein was examined by confocal microscopy. As shown in Fig. 3B, in nonirradiated cells, BRCA1 was primarily detected within the nuclei adjacent to the nuclei envelope (Log: BRCA1). After IR exposure, there is an increased expression of BRCA1, which is detected in punctate-appearing loci distributed within the nuclei (IR: BRCA1). As shown in Fig. 3B, ERK1/2 was found mainly in the cytoplasm in nonirradiated cells, although a fraction of ERK1/2 was also found in the nuclei (Log: ERK1/2). This observation is consistent with previous reports showing that ERK1/2 localizes in both cytoplasm and nuclear of the log-phase growing cells (35, 36). After IR exposure, there was an increase in the amount of ERK1/2 found in the nuclei (IR: ERK1/2). Overlap studies showed colocalization of BRCA1 and ERK1/2 within the nuclei of both nonirradiated and irradiated cells with increased expression of both proteins in the nuclear of irradiated cells (Fig. 4B, MERGE and MERGE + PHASE).
Figure 4.
Effect of BRCA1 expression on IR-induced G2-M arrest and ERK1/2 activation. MCF-7 cells were infected with a retroviral vector expressing BRCA1-shRNA and selected by incubation with puromycin. As a control, MCF-7 cells were infected with retroviral vector expressing Luc-shRNA and selected in parallel by incubation in the presence of puromycin. A, top left, clones expressing either Luc-shRNA (Control) or BRCA1-shRNA (B1-4, B1-6, B2-4, and B2-6) were isolated and assessed for BRCA1 protein expression by immunoblotting using an anti-BRCA1 antibody D-9. Actin levels were determined on the same immunoblot to confirm equal loading of all protein samples (Actin). Bottom left, control and BRCA1-shRNA expressing clones were exposed to increasing doses of IR, incubated for 24 h, and analyzed for DNA-content using FACS. Results depict the percentage of cells with 4N-DNA-content and represent the mean ± SD of triplicate samples. Right, control and clone B2-6 cells were exposed to IR at the indicated doses and incubated for 24 h. The cells were fixed, stained with propidium iodide, and analyzed for DNA content by FACS. The location and amount of cells with 4N-DNA content, indicative of G2-M phase of the cell cycle, are indicated. B, top, control (Control) and BRCA1-shRNA–expressing cells (B2-4 and B2-6) were exposed to 10-Gy IR, incubated for 2 h, and assessed for levels of BRCA1 and actin. For ERK phosphorylation determination, control and BRCA1-shRNA expressing cells were exposed to 10-Gy IR, incubated for 15 min, and assessed for pERK1/2 and totalERK1/2 by immunoblotting. Bottom, control and B2-6 cells were exposed to increasing doses of IR, incubated for 15 min, and assessed for pERK1/2 and total ERK1/2 by immunoblotting.
Basal expression of BRCA1 is needed for IR-induced ERK1/2 activation
Previous studies from our laboratory have shown that ectopic BRCA1 expression induces ERK1/2 activation in MCF-7 cells (13, 16) and that ERK1/2 activation is required for BRCA1-induced G2-M arrest in these cells. To investigate the role of BRCA1 in IR-induced ERK1/2 activation, MCF-7 cells were infected with a retroviral vector expressing shRNA-targeting BRCA1 (BRCA1-shRNA) and clones expressing BRCA1-shRNA were isolated. As shown in Fig. 4A (top left), BRCA1 protein levels were reduced 73% in clone B1-4, 74% in clone B1-6, 77% in clone B2-4, and 86% in clone B2-6 cells relative to control MCF-7 cells infected with retrovirus expressing shRNA targeting firefly luciferase (LucshRNA). No difference in BRCA1 protein levels was observed between Luc-shRNA–transduced MCF-7 cells and nontransduced MCF-7 cells (data not shown).
As shown in Fig. 4A (bottom left and right), FACS analysis showed that BRCA1-shRNA–transduced MCF-7 cells displayed a marked attenuation in G2-M arrest after IR exposure compared with control Luc-shRNA–transduced cells, as determined 24 hours after IR. Relative to control cells, exposure to 20-Gy IR resulted in a 63% reduction in G2-M arrest in clone B1-4 cells, a 42% reduction in B1-6 cells, a 50% reduction in B2-4 cells, and a 95% reduction in B2-6 cells (bottom left). No difference in IR-induced G2-M arrest was observed between Luc-shRNA–transduced cells and non-transduced cells (data not shown).
Western blot analysis showed that, after IR treatment, BRCA1-shRNA expressing clones exhibited a marked reduced levels of BRCA1 compared with that in control irradiated MCF-7 cells (Fig. 4B, BRCA1). In addition to an attenuation of IR-induced G2-M arrest, decreased BRCA1 expression in BRCA1-shRNA-transduced cells was also associated with loss of IR-induced ERK1/2 phosphorylation (Fig. 4B, pERK1/2). Relative to control cells, MCF-7 clones expressing BRCA1-shRNA had a marked decrease in ERK1/2 activation after exposure to 10-Gy IR (Fig. 4B, pERK1/2). Furthermore, the abrogation of IR-induced ERK1/2 activation in BRCA1-shRNA–transduced cells was observed at doses up to 20-Gy IR (Fig. 4B, bottom).
The effect of BRCA1 on ATM-mediated signaling after IR exposure
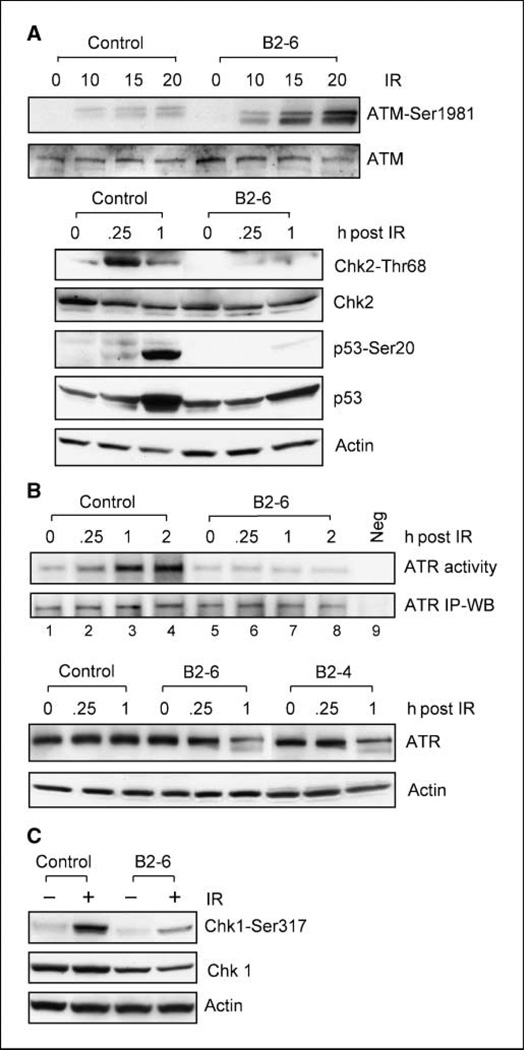
Because previous studies have shown that BRCA1 binds to several components of ATM-mediated signaling including ATM, Chk2, and p53, IR-induced ATM signaling was examined in MCF-7 cells expressing decreased levels of BRCA1. As shown in Fig. 5A (top), IR exposure resulted in a dose-dependent increase in ATM-Ser1981 phosphorylation, an indicator for ATM activation, in both control MCF-7 cells and clone B2-6 cells expressing lower levels of BRCA1. In fact, there was a greater induction of ATM-Ser1981 phosphorylation after IR treatment of B2-6 cells compared with control cells (Fig. 5A, ATM-Ser1981). Thus, after exposure to 20-Gy IR, there was a 19-fold increase in ATM-Ser1981 phosphorylation in B2-6 cells compared with a 4-fold increase in ATM-Ser1981 phosphorylation in control cells (Fig. 5A, ATM-Ser1981). Similar to B2-6 cells, IR treatment of other clones expressing decreased levels of BRCA1 (B1-4, B1-6, and B2-4) also resulted in stronger induction in ATM-Ser1981 phosphorylation compared with that in control cells (data not shown). No difference in IR-induced ATM-Ser1981 phosphorylation was observed between Luc-shRNA–transduced and nontransduced MCF-7 cells (data not shown).
Figure 5.
The effect of BRCA1 expression on IR-induced ATM and ATR signalings. A, top, MCF-7 cells expressing Luc-shRNA (Control) or BRCA1-shRNA (B2-6) were treated with 10-Gy IR, incubated for 15 min, and assessed for ATM-Ser1981 phosphorylation and total ATM by immunoblotting. Bottom, cells were treated as described above, incubated for the indicated times, and then analyzed for phosphorylation of Chk2-Thr68 and p53-Ser20 by immunoblotting. ATM, Chk2, p53, and actin levels in the lysate samples were determined by immunoblotting. B, top, Cells were exposed to 10-Gy IR and incubated for the indicated times. ATR kinase was immunoprecipitated from 500-µg cell lysate and analyzed for ATR activity using recombinant p53 protein as substrate (ATR activity, lanes 1–8), as described in Materials and Methods. As a negative control, ATR activity in irradiated cells was assayed using GST recombinant protein as substrate (ATR activity, lane 9). ATR levels in all immunoprecipitates were assessed by immunoblotting using anti-ATR antibody N-19 (ATR IP-WB). Bottom control (Control) and BRCA1-shRNA–expressing cells (B2-6 and B2-4) were exposed to 10-Gy IR, incubated for the indicated times, and analyzed for ATR protein levels by immunoblotting (ATR). C, cells treated with or without 10-Gy IR were incubated for 1 h and then analyzed for levels of Chk1-Ser317 phosphorylation and total Chk1.
The effect of BRCA1 expression on ATM-mediated downstream signal response was also examined. As shown in Fig. 5A (bottom), exposure to 10-Gy IR resulted in 11-fold increase in Chk2-Thr68 phosphorylation within 15 min in control MCF-7 cells (Control: Chk2-Thr68). In contrast, there was no increase in Chk2-Thr68 phosphorylation after IR exposure of B2-6 cells expressing BRCA1-shRNA (B2-6: Chk2-Thr68). Furthermore, whereas exposure of control cells to 10-Gy IR resulted in a marked increase in p53-Ser20 phosphorylation, there was only a subtle increase, if any, in p53-Ser20 phosphorylation in B2-6 cells after irradiation (Fig. 5A, p53-Ser20).
The effect of BRCA1 on ATR signaling after IR exposure
Previous studies from our laboratory have shown that ATR activity is required for IR-induced G2-M arrest in MCF-7 cells and that inhibition of ERK1/2 kinases abolishes IR-induced ATR activation (25). Because the results described above indicated that BRCA1 expression is required for IR-induced G2-M arrest and ERK1/2 activation in MCF-7 cells (Fig. 4), the effect of BRCA1 expression on ATR signaling after IR treatment was examined. As shown in Fig. 5B (top), there was approximately a 10-fold increase in ATR activity in control cells within 1 hour after exposure to 10-Gy IR (ATR activity, lanes 1–4). In contrast, there was no detectable increase in ATR activity in B2-6 cells expressing BRCA1 shRNA after IR exposure (ATR activity, lanes 5–8). The assay for ATR activity was specific, as no kinase activity was detected in ATR immunoprecipitates obtained from irradiated MCF-7 cells using GST recombinant protein as substrate (ATR activity, lane 9). Western blot analysis confirmed that equal amounts of ATR protein were present in the kinase assays (ATR IP-WB). Taken together, these results suggest an important role for BRCA1 expression in the regulation of ATR activation after IR exposure of MCF-7 cells.
We also examined the effect of BRCA1 expression on ATR protein levels in MCF-7 cells. As shown in Fig. 5B (bottom), whereas IR exposure had no effect on the level of ATR expression in control MCF-7 cells (Control: ATR), IR treatment resulted in a decrease in ATR protein levels in both B2-6 and B2-4 cells that express BRCA1-shRNA (B2-6 and B2-4: ATR). Within 1 hour after 10-Gy IR exposure, the levels of ATR protein decreased 60% in B2-6 and 45% in B2-4 cells relative to that present in control cells.
ATR has been previously implicated in phosphorylation of Chk1 (37, 38). To evaluate the effect of BRCA1 on ATR signaling after IR exposure, Chk1-Ser317 phosphorylation was assessed in control and BRCA1-shRNA-transduced MCF-7 cells. As shown in Fig. 5C, Chk1-Ser317 phosphorylation was markedly induced within 1 hour in control cells after exposure to 10-Gy IR. In contrast, after exposure to IR, Chk1-Ser317 phosphorylation was diminished by 76% in B2-6 cells relative to control cells (Chk1-Ser317). Furthermore, whereas Chk1 levels did not change after IR exposure in both control and B2-6 cells, expression of BRCA1-shRNA in B2-6 cells resulted in a slight decrease (37%) in Chk1 levels compared with that in control cells (Fig. 5C, Chk1). Thus, the diminution in Chk1-Ser317 phosphorylation observed in B2-6 cells after irradiation is, as least in part, related to the decrease in Chk1 protein in B2-6 cells. Taking together, these results suggest that BRCA1 expression is necessary for ATR activation and subsequent activation of Chk1 kinase in MCF-7 cells after IR exposure.
Discussion
Accumulating evidence has suggested that BRCA1 plays an important role in mediating IR-induced intra-S and G2-M DNA damage checkpoint responses (7, 8, 10). Although the precise mechanism for this role of BRCA1 remains unknown, previous studies have shown that BRCA1 can bind to phosphopeptides containing ATM/ATR phosphorylation consensus sites (39) and that BRCA1 is part of a large complex BRCA1-associated genome surveillance complex that serves as a sensor for the recognition of damaged DNA or abnormal DNA structures (17).
Previous studies from our laboratory and others have implicated ERK1/2 signaling in IR-induced G2-M cell cycle checkpoint response (23–25). Studies from our laboratory have shown that BRCA1 overexpression in MCF-7 cells results in ERK1/2 activation and that ERK1/2 signaling is necessary for BRCA1-induced activation of G2-M checkpoint signaling in these cells (16). Results presented in this report show that IR exposure induces a marked increase in BRCA1 expression in MCF-7 cells. Additional studies show that the up-regulation of BRCA1 expression after exposure to IR is mediated by posttranscriptional mechanisms and involves changes in BRCA1 ubiquitination and subsequent inhibition of proteasome-dependent degradation of BRCA1 protein. Furthermore, this IR-induced increase in BRCA1 protein stability requires ERK1/2 activation.
Whereas previous studies have shown an essential role for BRCA1 in DNA damage–induced G2-M checkpoint response (8, 9, 40), studies presented in this report indicate that BRCA1 expression is also necessary for IR-induced ERK1/2 activation and G2-M arrest in MCF-7 cells (see Fig. 4). Furthermore, colocalization and coimmunoprecipitation studies presented in this report indicate a direct interaction between BRCA1 and ERK1/2 in MCF-7 cells (see Fig. 3).
Previous studies have shown that both ATM and ATR play important roles in mediating the DNA damage checkpoint response (1, 41) and that both proteins form complexes with BRCA1 (3, 6, 17, 42, 43). Studies in HCC1937 breast cancer cells, which contain mutant BRCA1 (44), showed that BRCA1 expression is necessary for at least a subset of ATM-dependent and ATR-dependent phosphorylation events (19). Studies presented in this report used BRCA1-shRNA–transduced MCF-7 cells to examine the role of BRCA1 in regulating G2-M response after IR treatment. These studies support the role of BRCA1 in the regulation of IR-induced phosphorylation of Chk1-Ser317, Chk2-Thr68, and p53-Ser20 (see Fig. 5). Although IR-induced phosphorylation of ATM-Ser1981 is not dependent on BRCA1 expression, downstream signaling mediated by ATM after IR exposure is abrogated in MCF-7 cells containing reduced expression of BRCA1 (see Fig. 5A).
Studies presented in this report also indicate that decreased BRCA1 expression is associated with decreased ATR activation, as well as a decrease in ATR protein levels in irradiated cells. Furthermore, the loss of BRCA1 expression is also associated with a loss of subsequent downstream signaling of ATR after IR exposure of MCF-7 cells (see Fig. 5B and C). Although reduced expression of BRCA1 abolishes IR-induced ERK1/2 activation in MCF-7 cells (see Fig. 4B), previous studies from our laboratory have shown that ERK1/2 inhibition by itself has no effect on ATR protein levels in MCF-7 cells (25). Thus, the decrease in ATR protein expression after IR treatment in BRCA1-shRNA expressing cells is apparently not mediated by changes in IR-induced ERK1/2 signaling. Consistent with the influence of BRCA1 expression on the regulation of ATR expression and activation after IR exposure, the phosphorylation of Chk1-Ser317 was not induced after IR exposure of B2-6 cell expressing BRCA1 shRNA (see Fig. 5B and C). These studies support the requirement for BRCA1 expression in the regulation of ATR expression and activation, as well as downstream signaling after IR exposure.
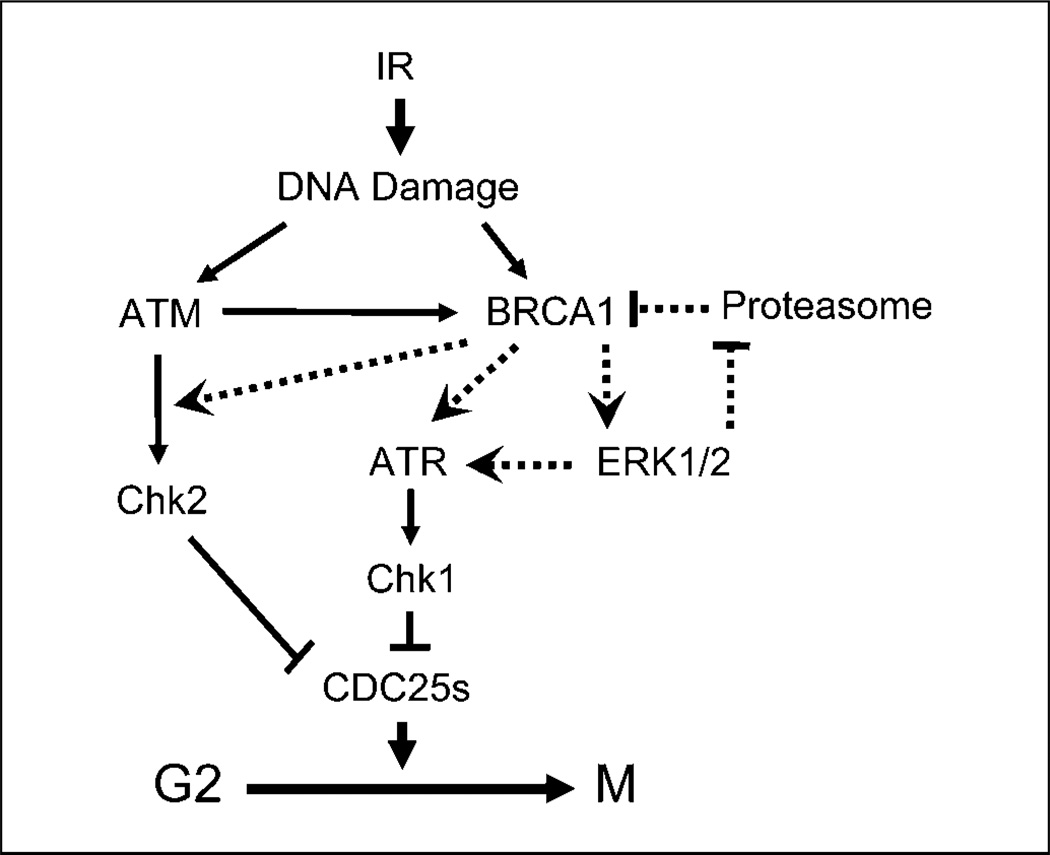
In summary, the results presented in this report suggest a model that incorporates both ERK1/2 and BRCA1 in the control of G2-M DNA damage checkpoint activation after IR exposure of MCF-7 cells (Fig. 6). Whereas basal BRCA1 expression is necessary for IR-induced activation of ERK1/2, ATM/ATR signaling, and subsequent G2-M arrest in MCF-7 cells, ERK1/2 activation after IR is required for IR-induced BRCA1 protein stabilization through suppression of BRCA1 ubiquitination and subsequent proteasome-dependent degradation of BRCA1 protein. Furthermore, studies in this report show a direct interaction between ERK1/2 and BRCA1 in MCF-7 cells, which provides strong evidence supporting the interrelationship between ERK1/2 and BRCA1 in the regulation of IR-induced DNA damage response.
Figure 6.
A model of DNA damage checkpoint that involves both BRCA1 and ERK1/2. The current understanding of protein signaling pathways involved in DNA damage induced G2-M checkpoint control are indicated by solid lines. Dashed lines indicate a feedback regulatory loop between ERK1/2 and BRCA1 in the cellular response to IR exposure, as identified by the studies presented in this report. Results in this report also suggest that both BRCA1 and ERK1/2 play important roles in the activation of ATR after IR exposure. In addition, studies in this report indicate an essential role for BRCA1 in IR-induced Chk2 activation.
Acknowledgments
Grant support: Nebraska DHHS-LB506 grant 2007-45 (Y. Yan).
We thank Dr. Charles Kuzynski, Linda Wilkie, and Victoria Smith for assistance on the flow cytometry analysis; Janice Taylor for assistance with Zeiss LSM510 confocal laser scanning microscope; and Dr. Janina Baranowska-Kortylewicz for assistance on the operation of Mark I 68A Cesium-137 Irradiator.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Iliakis G, Wang Y, Guan J, Wang H. DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene. 2003;22:5834–5847. doi: 10.1038/sj.onc.1206682. [DOI] [PubMed] [Google Scholar]
- 2.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 3.Chen J. Ataxia telangiectasia-related protein is involved in the phosphorylation of BRCA1 following deoxyribonucleic acid damage. Cancer Res. 2000;60:5037–5039. [PubMed] [Google Scholar]
- 4.Lee JS, Collins KM, Brown AL, Lee CH, Chung JH. hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature. 2000;404:201–204. doi: 10.1038/35004614. [DOI] [PubMed] [Google Scholar]
- 5.Cortez D, Wang Y, Qin J, Elledge SJ. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science. 1999;286:1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- 6.Tibbetts RS, Cortez D, Brumbaugh KM, et al. Functional interactions between BRCA1 and the checkpoint kinase ATR during genotoxic stress. Genes Dev. 2000;14:2989–3002. doi: 10.1101/gad.851000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu B, O’Donnell AH, Kim ST, Kastan MB. Phosphorylation of serine 1387 in Brca1 is specifically required for the Atm-mediated S-phase checkpoint after ionizing irradiation. Cancer Res. 2002;62:4588–4591. [PubMed] [Google Scholar]
- 8.Xu B, Kim S, Kastan MB. Involvement of Brca1 in S-phase and G(2)-phase checkpoints after ionizing irradiation. Mol Cell Biol. 2001;21:3445–3450. doi: 10.1128/MCB.21.10.3445-3450.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, Weaver Z, Linke SP, et al. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol Cell. 1999;3:389–395. doi: 10.1016/s1097-2765(00)80466-9. [DOI] [PubMed] [Google Scholar]
- 10.Yarden RI, Pardo-Reoyo S, Sgagias M, Cowan KH, Brody LC. BRCA1 regulates the G2/M checkpoint by activating Chk1 kinase upon DNA damage. Nat Genet. 2002;30:285–289. doi: 10.1038/ng837. [DOI] [PubMed] [Google Scholar]
- 11.MacLachlan TK, Somasundaram K, Sgagias M, et al. BRCA1 effects on the cell cycle and the DNA damage response are linked to altered gene expression. J Biol Chem. 2000;275:2777–2785. doi: 10.1074/jbc.275.4.2777. [DOI] [PubMed] [Google Scholar]
- 12.MacLachlan TK, Takimoto R, El-Deiry WS. BRCA1 directs a selective p53-dependent transcriptional response towards growth arrest and DNA repair targets. Mol Cell Biol. 2002;22:4280–4292. doi: 10.1128/MCB.22.12.4280-4292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan Y, Haas JP, Kim M, Sgagias MK, Cowan KH. BRCA1-induced apoptosis involves inactivation of ERK1/2 activities. J Biol Chem. 2002;277:33422–33430. doi: 10.1074/jbc.M201147200. [DOI] [PubMed] [Google Scholar]
- 14.Thangaraju M, Kaufmann SH, Couch FJ. BRCA1 facilitates stress-induced apoptosis in breast and ovarian cancer cell lines. J Biol Chem. 2000;275:33487–33496. doi: 10.1074/jbc.M005824200. [DOI] [PubMed] [Google Scholar]
- 15.Harkin DP, Bean JM, Miklos D, et al. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell. 1999;97:575–586. doi: 10.1016/s0092-8674(00)80769-2. [DOI] [PubMed] [Google Scholar]
- 16.Yan Y, Spieker RS, Kim M, Stoeger SM, Cowan KH. BRCA1-mediated G2/M cell cycle arrest requires ERK1/2 kinase activation. Oncogene. 2005;24:3285–3296. doi: 10.1038/sj.onc.1208492. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Cortez D, Yazdi P, Neff N, Elledge SJ, Qin J. BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev. 2000;14:927–939. [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida K, Miki Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004;95:866–871. doi: 10.1111/j.1349-7006.2004.tb02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foray N, Marot D, Gabriel A, et al. A subset of ATM-and ATR-dependent phosphorylation events requires the BRCA1 protein. EMBO J. 2003;22:2860–2871. doi: 10.1093/emboj/cdg274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22:5885–5896. doi: 10.1038/sj.onc.1206701. [DOI] [PubMed] [Google Scholar]
- 21.Cui W, Yazlovitskaya EM, Mayo MS, Pelling JC, Persons DL. Cisplatin-induced response of c-jun N-terminal kinase 1 and extracellular signal-regulated protein kinases 1 and 2 in a series of cisplatin-resistant ovarian carcinoma cell lines. Mol Carcinog. 2000;29:219–228. [PubMed] [Google Scholar]
- 22.Wang X, McGowan CH, Zhao M, et al. Involvement of the MKK6-38γ cascade in γ-radiation-induced cell cycle arrest. Mol Cell Biol. 2000;20:4543–4552. doi: 10.1128/mcb.20.13.4543-4552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbott DW, Holt JT. Mitogen-activated protein kinase kinase 2 activation is essential for progression through the G2/M checkpoint arrest in cells exposed to ionizing radiation. J Biol Chem. 1999;274:2732–2742. doi: 10.1074/jbc.274.5.2732. [DOI] [PubMed] [Google Scholar]
- 24.Tang D, Wu D, Hirao A, et al. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J Biol Chem. 2002;277:12710–12717. doi: 10.1074/jbc.M111598200. [DOI] [PubMed] [Google Scholar]
- 25.Yan Y, Black CP, Cowan KH. Irradiation-induced G2/M checkpoint response requires ERK1/2 activation. Oncogene. 2007;26:4689–4698. doi: 10.1038/sj.onc.1210268. [DOI] [PubMed] [Google Scholar]
- 26.Choudhury AD, Xu H, Baer R. Ubiquitination and proteasomal degradation of the BRCA1 tumor suppressor is regulated during cell cycle progression. J Biol Chem. 2004;279:33909–33918. doi: 10.1074/jbc.M403646200. [DOI] [PubMed] [Google Scholar]
- 27.Chen F, Zhang Z, Bower J, et al. Arsenite-induced Cdc25C degradation is through the KEN-box and ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 2002;99:1990–1995. doi: 10.1073/pnas.032428899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarkaria JN, Busby EC, Tibbetts RS, et al. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- 29.Hall-Jackson CA, Cross DA, Morrice N, Smythe C. ATR is a caffeine-sensitive, DNA-activated protein kinase with a substrate specificity distinct from DNAPK. Oncogene. 1999;18:6707–6713. doi: 10.1038/sj.onc.1203077. [DOI] [PubMed] [Google Scholar]
- 30.Yan Y, Mumby MC. Distinct roles for PP1 and PP2A in phosphorylation of the retinoblastoma protein. PP2a regulates the activities of G(1) cyclin-dependent kinases. J Biol Chem. 1999;274:31917–31924. doi: 10.1074/jbc.274.45.31917. [DOI] [PubMed] [Google Scholar]
- 31.Grignani F, Kinsella T, Mencarelli A, et al. High-efficiency gene transfer and selection of human hematopoietic progenitor cells with a hybrid EBV/ retroviral vector expressing the green fluorescence protein. Cancer Res. 1998;58:14–19. [PubMed] [Google Scholar]
- 32.Stewart GS, Maser RS, Stankovic T, et al. The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell. 1999;99:577–587. doi: 10.1016/s0092-8674(00)81547-0. [DOI] [PubMed] [Google Scholar]
- 33.Wellbrock C, Weisser C, Geissinger E, Troppmair J, Schartl M. Activation of p59(Fyn) leads to melanocyte dedifferentiation by influencing MKP-1-regulated mitogen-activated protein kinase signaling. J Biol Chem. 2002;277:6443–6454. doi: 10.1074/jbc.M110684200. [DOI] [PubMed] [Google Scholar]
- 34.Dart DA, Adams KE, Akerman I, Lakin ND. Recruitment of the cell cycle checkpoint kinase ATR to chromatin during S-phase. J Biol Chem. 2004;279:16433–16440. doi: 10.1074/jbc.M314212200. [DOI] [PubMed] [Google Scholar]
- 35.Brunet A, Roux D, Lenormand P, Dowd S, Keyse S, Pouyssegur J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 1999;18:664–674. doi: 10.1093/emboj/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenormand P, Brondello JM, Brunet A, Pouyssegur J. Growth factor-induced p42/p44 MAPK nuclear translocation and retention requires both MAPK activation and neosynthesis of nuclear anchoring proteins. J Cell Biol. 1998;142:625–633. doi: 10.1083/jcb.142.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Q, Guntuku S, Cui XS, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21:4129–4139. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manke IA, Lowery DM, Nguyen A, Yaffe MB. BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science. 2003;302:636–639. doi: 10.1126/science.1088877. [DOI] [PubMed] [Google Scholar]
- 40.Yamane K, Chen J, Kinsella TJ. Both DNA topoisomerase II-binding protein 1 and BRCA1 regulate the G2-M cell cycle checkpoint. Cancer Res. 2003;63:3049–3053. [PubMed] [Google Scholar]
- 41.O’Connell MJ, Cimprich KA. G2 damage checkpoints: what is the turn-on? J Cell Sci. 2005;118:1–6. doi: 10.1242/jcs.01626. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Higuera I, Taniguchi T, Ganesan S, et al. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol Cell. 2001;7:249–262. doi: 10.1016/s1097-2765(01)00173-3. [DOI] [PubMed] [Google Scholar]
- 43.Gatei M, Zhou BB, Hobson K, Scott S, Young D, Khanna KK. Ataxia telangiectasia mutated (ATM) kinase and ATM and Rad3 related kinase mediate phosphorylation of Brca1 at distinct and overlapping sites. In vivo assessment using phospho-specific antibodies. J Biol Chem. 2001;276:17276–17280. doi: 10.1074/jbc.M011681200. [DOI] [PubMed] [Google Scholar]
- 44.Tomlinson GE, Chen TTL, Stastny VA, et al. Characterization of a breast cancer cell line derived from a germ-line BRCA1 mutation carrier. Cancer Res. 1998;58:3237–3242. [PubMed] [Google Scholar]