Abstract
Duchenne muscular dystrophy (DMD) is characterized by severe degeneration and necrosis of both skeletal and cardiac muscle. While many experimental therapies have shown great promise in treating skeletal muscle disease, an effective therapy for Duchenne cardiomyopathy remains a challenge in large animal models and human patients. The current views on cardiac consequences of skeletal muscle-centered therapy are controversial. Studies performed in young adult mdx mice (a mild DMD mouse model) have yielded opposing results. Since mdx mice do not develop dystrophic cardiomyopathy until ≥21 months of age, we reasoned that old mdx mice may represent a better model to assess the impact of skeletal muscle rescue on dystrophic heart disease. Here, we aged skeletal muscle-specific micro-dystrophin transgenic mdx mice to 23 months and examined the cardiac phenotype. As expected, transgenic mdx mice had minimal skeletal muscle disease and they also outperformed original mdx mice on treadmill running. On cardiac examination, the dystrophin-null heart of transgenic mdx mice displayed severe cardiomyopathy matching that of non-transgenic mdx mice. Specifically, both the strains showed similar heart fibrosis and cardiac function deterioration in systole and diastole. Cardiac output and ejection fraction were also equally compromised. Our results suggest that skeletal muscle rescue neither aggravates nor alleviates cardiomyopathy in aged mdx mice. These findings underscore the importance of treating both skeletal and cardiac muscles in DMD therapy.
INTRODUCTION
Duchenne muscular dystrophy (DMD) is the most common X-linked muscle disease caused by genetic mutations in the dystrophin gene. In DMD, dystrophin expression is abolished in all striated muscles. While dystrophin elimination in skeletal muscle results in salient clinical presentations (such as the loss of mobility and death from respiratory failure), the consequences of dystrophin deficiency in the heart are usually subtle at the early stage of disease. Nonetheless, signs of cardiac dysfunction are detected in all DMD patients by their teenage years and up to 40% of patients may die from heart failure or sudden cardiac death (reviewed in 1–3). The molecular pathogenesis of dystrophin-deficient cardiomyopathy is not completely understood. Prevailing hypotheses include weakened sarcolemma integrity, disrupted cellular signaling, abnormal ion channel activity and mitochondrial dysfunction (reviewed in 4–6). Besides these cardiomyocyte-originated mechanisms, it has also been suggested that skeletal muscle disease may play a pivotal role in the development and progression of Duchenne cardiomyopathy (reviewed in 7–10).
The interplay between skeletal muscle disease and cardiomyopathy has been evaluated in dystrophin-null mdx mice by many investigators (11–14). However, there is still no conclusive answer. The most striking difference comes from two studies that examined the cardiac phenotype in skeletal muscle rescued transgenic mdx mice (12,13). Townsend et al. studied skeletal muscle-specific micro-dystrophin transgenic mdx mice generated in the Chamberlain laboratory (13,15). Crisp et al. examined Fiona mice developed by Davies et al. (12,16). Fiona mice are also skeletal specific transgenic mdx mice but they selectively express the full-length utrophin, rather than a micro-dystrophin gene in skeletal muscle (16). As expected, skeletal muscle dystrophy was effectively prevented in these transgenic mdx mice irrespective of the therapeutic gene used (15,16). To investigate the relationship between skeletal muscle disease and cardiomyopathy, Townsend et al. and Crisp et al independently examined cardiac manifestations in skeletal muscle corrected transgenic mdx mice (12,13). Surprisingly, these two groups obtained completely opposite results. Townsend et al. found that selective skeletal muscle correction precipitated heart damage in 4- to 5-month-old micro-dystrophin transgenic mdx mice (13). In sharp contrast, Crisp et al. showed that cardiac function was normalized in 6- to 9-month-old Fiona mice (12). An important caveat of these studies is the use of young adult mdx mice. Mdx mice do not show characteristic dystrophic cardiomyopathy until they reach 21 months of age or older (17–21). We reasoned that studies performed in aged mdx mice might offer more clinically relevant insight. Based on this premise, we designed a study on 23-month-old skeletal muscle corrected transgenic mdx mice. Similar to the mice used by Townsend et al. and Crisp et al. (12,13), skeletal muscle disease was rectified in our experimental mice by targeted expression of micro-dystrophin in skeletal muscle (15,22,23). However, contrary to prior reports, we found that skeletal muscle-rescued mdx mice displayed myocardial fibrosis and dysfunction similar to age-matched non-transgenic mdx mice. Our results suggest that skeletal muscle disease may play a less important role in the development of dystrophic cardiomyopathy in aged mdx mice. We conclude that irrespective of skeletal muscle rescue, cardiomyopathy remains a major health threat in DMD and should be treated.
RESULTS
Skeletal muscle-rescued transgenic mdx mice show robust micro-dystrophin expression in skeletal muscle but not heart
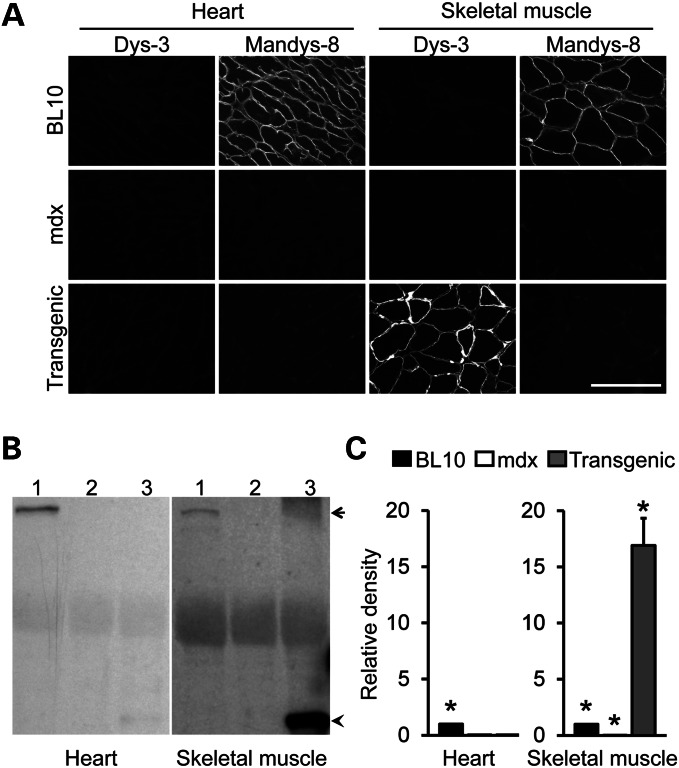
To evaluate the therapeutic effect of the micro-dystrophin gene on skeletal muscle, we have previously generated transgenic mdx mice that expressed human micro-dystrophin under the human skeletal α-actin (HSA) promoter (15,22). Skeletal muscle pathology was ameliorated and force was improved in these mice (15,22,23). To study micro-dystrophin expression in the heart, we performed immunofluorescence staining for dystrophin using two epitope-specific antibodies. One antibody (Dys-3) was used to reveal micro-dystrophin expression because it only recognizes human dystrophin. The other antibody (Mandys-8) was used to detect endogenous full-length mouse dystrophin because it reacts with a region deleted in micro-dystrophin. As expected, micro-dystrophin was only found in skeletal muscle of transgenic mdx mice (Fig. 1A, Supplementary Material, Fig. S1A). Western blot further confirmed this observation (Fig. 1B and C, Supplementary Material, Fig. S1B).
Figure 1.
Transgenic mdx mice show exclusive micro-dystrophin expression in skeletal muscle but not in the heart. (A) Representative photomicrographs of Dys-3 and Mandys-8 immunofluorescence staining on skeletal muscle (the tibialis anterior muscle) and the heart from BL10, mdx and transgenic mdx mice. Dystrophin-positive myofibers show sarcolemma staining. Dys-3 is a human dystrophin-specific antibody that only recognizes human gene-derived micro-dystrophin. Mandys-8 binds to sprectrin-like repeat 11 in dystropin. This repeat is deleted in the micro-dystrophin gene. Hence, positive Mandys-8 staining indicates endogenous mouse dystrophin. Bar, 100 µm. (B) Representative dystrophin western blots of BL10, mdx, and trangenic mdx heart (left panel) and skeletal muscle (right panel). Dystrophin is detected with the DysB antibody which recognizes both human micro-dystrophin and full-lengh mouse dystrophin. Arrow denotes full-length dystrophin; arrowhead denotes micro-dystrophin. (C) Densitomitry quantification of the dystrophin western blot (N = 3 for each group). Asterisk denotes that the result is significantly different from that of the other groups in the same panel.
Dystrophic fibrosis is reduced in skeletal but not cardiac muscle in transgenic mdx mice
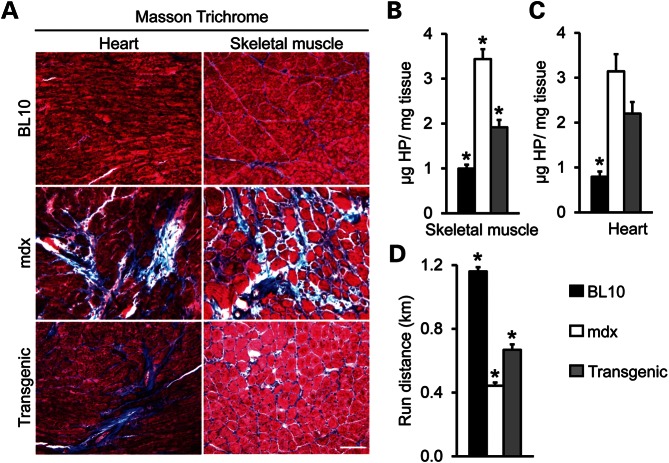
To determine whether skeletal muscle correction reduced heart disease in transgenic mice, we examined fibrosis by Masson trichrome staining and hydroxyproline quantification. Consistent with our previous reports (15,22,23), micro-dystrophin expression significantly reduced skeletal muscle fibrosis in transgenic mice (Fig. 2A and B). In contrast to skeletal muscle, the heart of transgenic mice showed fibrotic staining similar to that of mdx mice (Fig. 2A). Measurement of the cardiac hydroxyproline content suggests that there was no statistically significant difference in cardiac fibrosis between transgenic and non-transgenic mdx mice (Fig. 2C).
Figure 2.
Skeletal muscle correction does not reduce myocardial fibrosis in transgenic mdx mice. (A) Representative photomicrographs of Masson trichrome staining of skeletal muscle (the tibialis anterior muscle) and the heart in BL10, mdx and transgenic mdx mice. Fibrotic tissue stains in blue color. Bar, 100 µm. (B) Quantification of the hydroxyproline content in skeletal muscle (the extensor digitorium longus muscle, N = 7 for all strains). The P-values of the Bonferroni post hoc analysis were 0.000, 0.003 and 0.000 for BL10/non-transgenic mdx comparison, BL10/ transgenic mdx comparison and transgenic/non-transgenic mdx comparison, respectively. (C) Quantification of the hydroxyproline content in the heart (N = 5 each for BL10 and mdx, and N = 6 for transgenic mdx). The P-values of the Bonferroni post hoc analysis were 0.000, 0.003, 0.226 for BL10/non-transgenic mdx comparison, BL10/ transgenic mdx comparison and transgenic/non-transgenic mdx comparison, respectively. (D) Quantification of the treadmill running distance in BL10, mdx and transgenic mdx mice. N = 17 for BL10, N = 9 for mdx and N = 6 for transgenic mdx mice. The P-values of the Bonferroni post hoc analysis were 0.000 for all two group comparisons. Asterisk denotes that the result is significantly different from that of the other groups in the same panel.
Enhancing skeletal muscle activity neither impairs nor improves heart function in aged mdx mice
Skeletal muscle rescue has been suggested to increase physical activity of mdx mice (13,15). We compared treadmill running in 20-month-old mice. Although not reaching the level of normal mice, skeletal muscle rescued transgenic mdx mice significantly outperformed non-transgenic mdx mice (Fig. 2D).
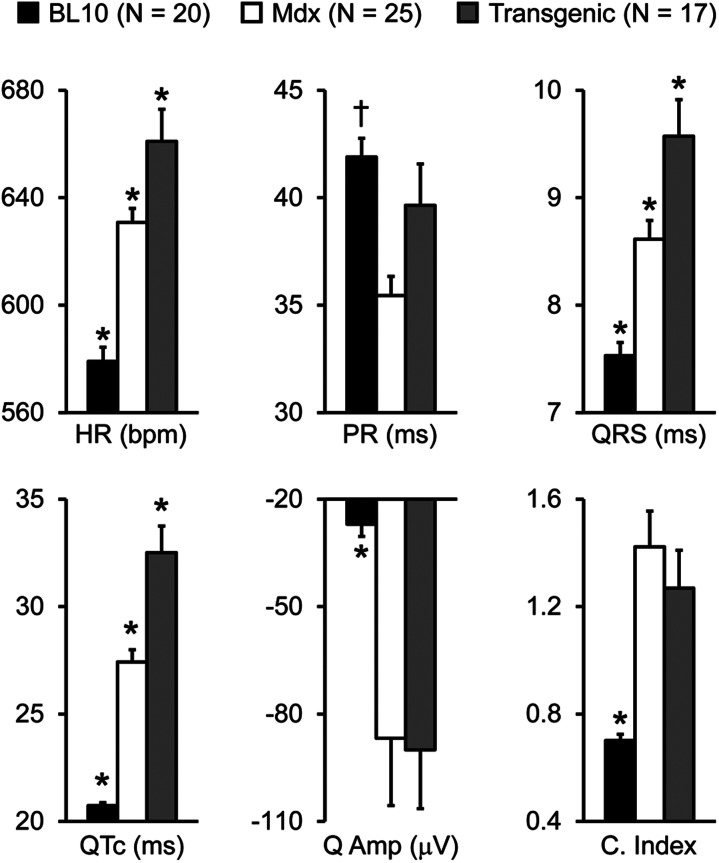
To test whether improved skeletal muscle function influences heart performance, we examined the heart weight and weight ratios and we also measured whole heart electrophysiology and hemodynamics (Table 1 and 2, Figs 3 and 4, Supplementary Material, Fig. S2) (24). As expected, mdx mice were significantly emaciated (Table 1). Interestingly, the heart weight (HW), ventricular weight (VW), HW/tibialis muscle weight (TW), and VW/TW were significantly higher in transgenic mdx mice than those of BL10 and non-transgenic mdx mice (Table 1). Similar to our previous reports, electrocardiogram tracing showed significant difference between BL10 and mdx mice in the heart rate (HR), PR interval, QRS duration, Mitchell's corrected QT (QTc) interval, Q wave amplitude and cardiomyopathy index (Fig. 3) (18,21). An interesting pattern was observed in skeletal muscle rescued transgenic mdx mice. Compared with non-transgenic mdx mice, several ECG parameters (HR, QRS duration and QTc interval) were significantly increased in micro-dystrophin transgenic mdx mice. For other ECG parameters (PR interval, Q wave amplitude and cardiomyopathy index), there was no significant difference between transgenic and non-transgenic mdx mice, although the PR interval of transgenic mice showed a trend toward normalization (Fig. 3).
Table 1.
Weights and weight ratios
| BL10 | Mdx | Transgenic | |
|---|---|---|---|
| Sample size (n) | 18 | 18 | 12 |
| Age (m) | 23.73 ± 0.80 | 23.74 ± 0.33 | 23.61 ± 0.47 |
| BW (g) | 35.72 ± 1.09 | 28.83 ± 0.71a | 35.99 ± 1.48 |
| TW (mg) | 40.26 ± 1.67 | 40.95 ± 1.32 | 31.06 ± 2.32a |
| HW (mg) | 140.91 ± 3.32 | 137.27 ± 4.13 | 159.70 ± 3.09a |
| VW (mg) | 132.97 ± 3.17 | 127.78 ± 4.00 | 149.27 ± 3.23a |
| TW/BW (mg/g) | 1.13 ± 0.04a | 1.43 ± 0.06a | 0.87 ± 0.06a |
| HW/BW (mg/g) | 3.97 ± 0.09a | 4.79 ± 0.16 | 4.49 ± 0.14 |
| HW/TW (mg/g) | 3.58 ± 0.13 | 3.40 ± 0.13 | 5.41 ± 0.35a |
| VW/BW (mg/g) | 3.75 ± 0.08a | 4.46 ± 0.15 | 4.23 ± 0.14 |
| VW/TW (mg/g) | 3.37 ± 0.12 | 3.16 ± 0.12 | 5.16 ± 0.34a |
BW, body weight; TW, anterior tibialis muscle weight; HW, heart weight; VW, ventricle weight.
aSignificantly different from those of other two groups.
Table 2.
Quantitative evaluation of left ventricular systolic and diastolic function
| BL10 | Mdx | Transgenic | |
|---|---|---|---|
| Sample size (n) | 17 | 17 | 16 |
| Age (m) | 23.7 ± 0.5 | 23.9 ± 0.4 | 22.8 ± 0.6 |
| End systolic volume (µl) | 7.94 ± 0.9a | 18.1 ± 3.0 | 20.0 ± 2.5 |
| Max pressure (mmHg) | 104.6 ± 1.8a | 80.0 ± 3.5 | 77.0 ± 3.2 |
| dP/dt max (KmmHg/s) | 13.0 ± 0.4a | 7.35 ± 0.5 | 6.45 ± 0.3 |
| EDV (µl) | 29.9 ± 1.5 | 24.8 ± 2.5 | 27.2 ± 2.2 |
| dP/dt min (KmmHg/s) | −11.4 ± 0.4a | −6.60 ± 0.4 | −6.30 ± 0.5 |
| Tau (ms) | 6.50 ± 0.3b | 9.8 ± 1.1 | 9.4 ± 0.5 |
Max, maximum; Min, minimum, P, pressure.
aSignificantly different from those of other two groups.
bSignificantly different from that of mdx only.
Figure 3.
Skeletal muscle rescued transgenic mdx mice display minor ECG alterations. Quantitative evaluation of the HR, PR interval, QRS duration, QTc interval, Q wave amplitude (Q amp) and cardiomyopathy index (C. index). Asterisk denotes significantly different from other two strains. Cross denotes significantly different from mdx only.
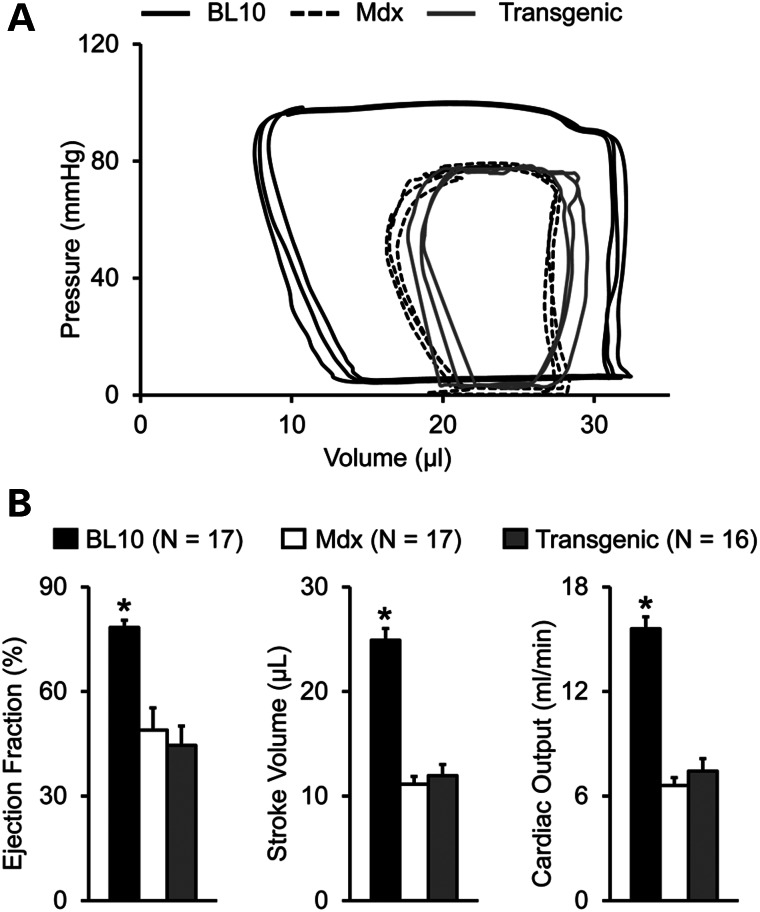
Figure 4.
Skeletal muscle correction does not improve left ventricular function in aged mdx mice. (A) Representative pressure–volume loops of BL10, mdx and transgenic mdx mice. (B) Quantitative hemodynamic evaluation of overall left ventricular performance in BL10, mdx and transgenic mdx mice. Asterisk denotes significantly different from other two strains.
Left ventricular catheterization revealed significant deterioration of cardiac function in transgenic mdx mice (Fig. 4). Representative tracings of pressure–volume (PV) loops in transgenic mdx mice showed a downward and rightward shift identical to those of non-transgenic mdx mice (Fig. 4A). There was also no difference in individual systolic and diastolic hemodynamic parameters between transgenic and non-transgenic mdx mice (Table 2). Indices of overall heart function (ejection fraction, stroke volume and cardiac output) were equally compromised in both mdx strains irrespective of skeletal muscle rescue (Fig. 4B).
Crisp et al. found that right ventricular function was compromised in 6- to 9-month-old mdx mice (12). This defect was corrected in Fiona mice (12). To study the function of the right ventricle, we adopted a catheter assay similar to our established left ventricle catheterization technique (24–26). Interestingly, we did not detect any statistically significant difference in right ventricular function (maximal pressure, dP/dt max, dP/dt min and ejection fraction) among BL10, non-transgenic and transgenic mdx mice at the age of 7.5 months (Supplementary Material, Fig. S2). Nevertheless, mdx mice (with or without transgenic micro-dystrophin expression) showed a trend of reduced dP/dt max and ejection fraction (Supplementary Material, Fig. S2).
DISCUSSION
In this study, we examined the cardiac outcome of life-long skeletal muscle rescue in the aged mdx model of Duchenne cardiomyopathy. We found an increased heart size and HR, and prolonged QRS duration and QTc interval in skeletal muscle transgenic mdx mice. However, compared with non-transgenic mdx mice, there was no significant difference in hemodynamic function nor was there a change in heart fibrosis. These findings suggest that treating skeletal muscle alone does not dramatically alter the outcome of dystrophic heart disease.
Understanding cardiac repercussion of targeted skeletal muscle rescue has become a pressing issue in light of the emerging novel therapeutic modalities such as antisense oligonucleotide (AON)-mediated exon skipping and adeno-associated virus (AAV)-mediated micro-dystrophin gene therapy. In the case of exon skipping, efficient myocardial correction has only been achieved in the murine model not long ago using the newly developed second generation AON (such as peptide-modified AON) (27,28). It remains a great challenge to achieve effective exon skipping in the heart of DMD patients (29). AAV micro-dystrophin gene therapy has been shown to alleviate skeletal muscle disease in dystrophic dog muscle (30). Surprisingly, systemic delivery of AAV-9, the so-called ‘cardiotropic’ AAV serotype, has failed to transduce the dog heart despite widespread skeletal muscles transduction (31). To determine the best treatment strategy, it is crucial to have a definitive answer on how a skeletal muscle-centered therapy may influence the course of heart disease. If cardiomyopathy is induced by skeletal muscle disease, one may envision a concomitant recovery of heart function following targeted repair of skeletal muscle damage. On the other hand, if exclusive skeletal muscle repair worsens heart disease, extreme precautions should be taken in moving forward with skeletal muscle-centered therapy.
There are currently two conflicting hypotheses on how skeletal muscle injury may affect dystrophin-deficient cardiomyopathy. One theory proposes that normalizing skeletal muscle function will accelerate heart disease progression (13,14). This theory is based on the rationale that correction of skeletal muscle disease enables more locomotor activity and hence a higher workload for the heart. The increased demand on the heart then triggers pathologic myocardial remodeling and dilated cardiomyopathy. The support for this theory mainly comes from studying skeletal muscle-specific micro-dystrophin transgenic mice by Townsend et al. (13). By quantifying cytosolic immunoglobulin G accumulation in the heart section, the authors found that targeted repair of skeletal muscle resulted in a 5-fold increase in myocardial damage of young mdx mice. The authors also studied left heart function using cardiac catheterization. They found that the PV loops were shifted toward the right and the end-diastolic volume (EDV) was increased in transgenic mice. The authors concluded that correction of skeletal muscle disease resulted in dilated cardiomyopathy in skeletal muscle-specific micro-dystrophin transgenic mdx mice. While the morphology data are convincing, the authors’ interpretation on the results of the catheter assay may require additional discussion. First, wild type control was not included in the study. This makes it difficult to determine whether the rightward shift of the PV loop represents functional amelioration or deterioration. Two prior studies from the same group of authors suggest that a rightward shift of the PV loop indicates cardiac function improvement in young mdx mice (32,33). Second, the authors reached the conclusion of dilated cardiomyopathy because the EDV of skeletal muscle-rescued transgenic mdx mice was larger than that of non-transgenic mdx mice. An increase in the EDV suggests that the left ventricle has a bigger size (chamber dilation). However this does not necessarily mean a pathological change. Studies from many groups, including those from Townsend et al., have provided compelling evidence that the chamber size of normal mice is significantly larger than that of mdx mice when mice are young (≤6-month-old) (12,32,33). As a matter of fact, the dystrophin-null heart first undergoes a hypertrophic stage (chamber size reduction) before it enters the phase of dilated cardiomyopathy (34,35). Collectively, the rightward PV loop shift and a high left ventricular EDV may suggest functional improvement rather than decline in young adult mdx mice.
Based on the hypothesis of Townsend et al., one would expect skeletal muscle transgenic mdx mice to have a shorter life span because aggravated cardiomyopathy will lead to early death from heart failure. Although our study was not designed to quantify the life span, all microgene transgenic mdx mice (N = 16) survived to the time of cardiac catheter assay (23 months of age), an indication of a life span that is at least comparable with that of mdx mice (the average life span of mdx mice is ∼22 months) (36,37). Taken together, the notion that targeted skeletal muscle repair causes emergent dilated cardiomyopathy appears not to be fully supported by the existing evidence. Finally, it is worth pointing out that exercise training is often prescribed to patients suffering from dilated cardiomyopathy (38). In this case, an appropriate increase of skeletal muscle activity actually enhances heart function.
A contradictory but quite enticing hypothesis was introduced by Crisp et al. (12). The authors proposed that skeletal muscle rescue, in particular respiratory muscle (such as the diaphragm) rescue, was sufficient to restore heart function in dystrophic mice. The logic behind this reasoning involves diaphragm dystrophy-induced respiratory failure and pulmonary hypertension. In DMD patients, a loss of respiratory muscle contractility results in lung dysfunction. This causes secondary pulmonary hypertension and subsequent right ventricular failure and eventually left ventricular dysfunction and whole heart failure. To test this hypothesis, Crisp et al. examined mouse heart function using magnetic resonance imaging (MRI) (12). They found that right ventricular dysfunction precedes left ventricular dysfunction in mdx mice. Using the same MRI method, the authors further showed that cardiac function was improved after repairing skeletal muscle damage by either transgenic utrophin expression in Fiona mice or exon-skipping in mdx and dystrophin/utrophin double knockout mice (12). The results of Crisp et al. are in line with the findings from myoD/dystrophin double knockout (m-dko) mice (11). MyoD elimination impairs skeletal muscle regeneration. Hence, m-dko mice display much severer skeletal myopathy. Interestingly, when Megeney et al. examined the heart of 5-month-old m-dko mice, they observed pronounced cardiac dilation and myocardial fibrosis suggesting a potential causal link between skeletal muscle disease and cardiomyopathy (11). In other words, dilated cardiomyopathy seen in m-dko mice is due to accelerated skeletal muscle deterioration and treating skeletal muscle may alleviate heart disease (11).
Our results here have revealed a third possibility, i.e. the loss of dystrophin in the heart can cause dystrophic cardiomyopathy independent of skeletal muscle condition. Studies on X-linked dilated cardiomyopathy (XLDC) offer compelling support for this hypothesis (reviewed in 39). In XLDC patients, dystrophin expression is selectively eliminated in the heart, but not skeletal muscle (40–42). These patients develop severe dilated cardiomyopathy and congestive heart failure yet no apparent skeletal muscle symptoms (39). Consistent with our results and the findings from XLDC patients, it has been shown that cardiomyopathy caused by deficiency of other dystrophin-associated proteins (such as γ-sarcoglycan) is also independent of skeletal muscle disease (43).
In this study, we have intentionally focused on very old mice because aged mdx mice display dystrophic cardiomyopathy similar to what has been described in human patients (17–21). Further, this experimental design also allows us to study the cumulative effect of life-long skeletal muscle correction. In skeletal muscle-specific micro-dystrophin transgenic mice, we observed robust micro-dystrophin expression in limb muscle and respiratory muscle (Fig. 1, Supplementary Material, Fig. S1). As expected, skeletal muscle-rescued transgenic mice showed an increased exercise capacity (Fig. 2D). Further their HR was significantly elevated, a sign of intensified sympathetic activity (consistent with increased exercise capacity in these mice) (Fig. 3). However, in contrast to the reports of Townsend et al. and Crisp et al. (12,13), we found that skeletal muscle correction neither reduced myocardial fibrosis nor improved left ventricular function in aged mdx mice (Figs 2 and 4). Interestingly, increased ventricular weight suggested that skeletal muscle transgenic mdx mice might have developed ventricular hypertrophy (Table 1). This notion was further supported by the findings of increased QRS duration (in other words, longer conduction through the ventricles) and prolonged QTc interval (an indication of delayed ventricular repolarization) (Fig. 3).
Crisp et al. evaluated right ventricular function by high-resolution cine MRI (12). They found that right ventricular dysfunction preceded left ventricular failure in young adult mdx mice (12). Further, skeletal muscle rescue significantly preserved right heart function (12). We performed the right ventricular catheter assay (24–26). However, we did not detect any statistically significant difference among BL10, mdx and transgenic mdx mice in the right ventricular hemodynamic parameters (maximal pressure, dP/dt max, dP/dt min and ejection fraction of the RV) (Supplementary Material, Fig. S2). Although BL10 mice showed a trend of better dP/dt max and ejection fraction, there was no difference between transgenic and non-transgenic mdx mice in the right ventricular catheter assay (Supplementary Material, Fig. S2).
In summary, we have demonstrated that exclusive correction of skeletal muscle disease neither exacerbates nor ameliorates dystrophic cardiomyopathy in aged mdx mice. Our result has important practical implications in designing/conducting DMD therapy. First, it reduces worries that skeletal muscle-centered therapy may aggravate dystrophic cardiomyopathy. Based on our data, strategies that only show promise in skeletal muscle (such as first-generation AON-mediated exon-skipping) should be encouraged rather than withheld. Second, it emphasizes the need for treating both skeletal muscle and heart in order to achieve a full recovery of DMD.
MATERIALS AND METHODS
Experimental animals
All animal experiments were approved by the institutional animal care and use committee and were in accordance with NIH guidelines. Experimental BL10 and mdx mice were generated in a barrier facility using founders from The Jackson Laboratory (Bar Harbor, ME, USA). The founder lines of human micro-dystrophin transgenic mice were generated at the University of Missouri transgenic core and have been published before (22,23). These mice express the ΔR4-23/ΔC microgene under the transcriptional control of the skeletal muscle-specific human skeletal α-actin promoter and the simian virus 40 polyadenylation sequence. The experimental microgene transgenic mice were obtained after backcrossing with mdx mice for at least five generations. Only male mice were used in the study. All mice were maintained in a specific-pathogen-free animal care facility on a 12-h light (25 lux):12-h dark cycle with access to food and water ad libitum. Mice were euthanized following the functional assays to harvest the tissues.
Morphological studies
Micro-dystrophin expression was evaluated by immunofluorescence staining using a human dystrophin-specific antibody (Dys-3, diluted 1:20, clone Dy10/12B2, IgG2a; Novocastra, Newcastle, UK). Additional immunofluorescence staining was performed using a dystrophin antibody specific to spectrin-like repeat 11 that is absent in the micro-dystrophin gene (Mandys-8, diluted 1:200; Sigma, St Louis, MO, USA). Slides were viewed at the identical exposure setting predetermined for each specific antibody. Masson trichrome staining was performed as described before (17,19).
Western blot
The frozen heart was ground to fine powder in liquid nitrogen. A whole heart muscle lysate was prepared as described before (19,20). Dystrophin was detected with the DysB antibody (1:100, clone 34C5, IgG1; Novocastra). This antibody recognizes an epitope located between hinge 1 and spectrin-like repeat 2. Since the region of hinge 1 to spectrin-like repeat 2 is not deleted in ΔR4-23/ΔC micro-dystrophin, the DysB antibody can detect both endogenous full-length mouse dystrophin and transgenic micro-dystrophin. Western blot quantification was performed with the gel analyzer unit of ImageJ (http://rsbweb.nih.gov/ij/). The relative intensity of the dystrophin band was normalized to the corresponding α-tubulin (loading control) band in the same blot. The relative band intensity in transgenic and non-transgenic mdx mice was normalized to that of BL10.
Hydroxyproline assay
Myocardial and skeletal muscle fibrosis were quantified using lyophilized heart and extensor digitorum longus muscle, respectively. Tissue was hydrolyzed in 6 N HCl for 3 h at 115°C. After pH neutralization, the hydroxyproline content was determined as described before (20). Briefly, a 1 ml tissue lysate was oxidized with1 ml chloramine-T at room temperature for 20 min. One milliliter of p-dimethylaminobenzaldehyde/perchloric acid was then added to the mixture and incubated for 15 min at 60°C. The hydroxyproline content was determined by measuring the absorbance using a Beckman Coulter DU640 spectrophotometer at 558 nm of the samples and a standard series.
Treadmill running
A treadmill endurance assay was performed as described before (24). Briefly, the mice were subjected to 5-day treadmill acclimation at a 7° uphill treadmill. The running distance was measured on day 6. The mouse was placed on an unmoving treadmill for 2 min and then run at 5 m/min for 5 min. The treadmill speed was increased by 1 m/min every 5 min. The total running distance was calculated after the mouse became exhausted. Exhaustion is diagnosed when the animal gives up running and ends up in contact with the shocker (at the minimal setting) for typically 1–3 s without attempting to re-enter the treadmill.
ECG and hemodynamic assay
A 12-lead ECG assay was performed using a commercial system from AD Instruments (Colorado Springs, CO, USA) according to our previously published protocol (24). The Q wave amplitude was determined using the lead I tracing. Other ECG parameters were analyzed using the lead II tracing. The QTc interval was determined by correcting the QT interval with the HR as described by Mitchell et al. (44). The cardiomyopathy index was calculated by dividing the QT interval by the PQ segment (45). Left ventricular hemodynamics was evaluated using a closed chest approach as we had previously described (24). The resulting PV loops were analyzed with the PVAN software (Millar Instruments, Houston, TX, USA).
Statistical analysis
Data are presented as mean ± standard error of the mean (s.e.m.). The SPSS software (SPSS, Chicago, IL, USA) was used for statistical analysis. One-way ANOVA analysis and Bonferroni post hoc analysis were used for multiple group comparisons. A P < 0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by grants from the National Institutes of Health HL-91883 (D.D.) and AR-49419 (D.D.), the Muscular Dystrophy Association (D.D.) and the Parent Project Muscular Dystrophy (D.D.).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Mr. Nick Marschalk, Ms. Chun Long, Lakmini Wasala and Keqing Zhang for excellent technical assistance. We thank the University of Missouri Transgenic Animal Core for the help with generating the founder transgenic mice.
Conflict of Interest statement. None declared.
References
- 1.American Academy of Pediatrics. Cardiovascular health supervision for individuals affected by Duchenne or Becker muscular dystrophy. Pediatrics. 2005;116:1569–1573. doi: 10.1542/peds.2005-2448. [DOI] [PubMed] [Google Scholar]
- 2.Cox G.F., Kunkel L.M. Dystrophies and heart disease. Curr. Opin. Cardiol. 1997;12:329–343. [PubMed] [Google Scholar]
- 3.Baxter P. Treatment of the heart in Duchenne muscular dystrophy. Dev. Med. Child Neurol. 2006;48:163. doi: 10.1017/S0012162206000351. [DOI] [PubMed] [Google Scholar]
- 4.Kaspar R.W., Allen H.D., Montanaro F. Current understanding and management of dilated cardiomyopathy in Duchenne and Becker muscular dystrophy. J. Am. Acad. Nurse Pract. 2009;21:241–249. doi: 10.1111/j.1745-7599.2009.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heydemann A., McNally E.M. Consequences of disrupting the dystrophin-sarcoglycan complex in cardiac and skeletal myopathy. Trends Cardiovasc. Med. 2007;17:55–59. doi: 10.1016/j.tcm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Burelle Y., Khairallah M., Ascah A., Allen B.G., Deschepper C.F., Petrof B.J., Des Rosiers C. Alterations in mitochondrial function as a harbinger of cardiomyopathy: lessons from the dystrophic heart. J. Mol. Cell. Cardiol. 2010;48:310–321. doi: 10.1016/j.yjmcc.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duan D. Challenges and opportunities in dystrophin-deficient cardiomyopathy Gene Therapy. Hum. Mol. Genet. 2006;15:R253–R261. doi: 10.1093/hmg/ddl180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin J.-H., Bostick B., Yue Y., Duan D. In: Muscle Gene Therapy. Duan D, editor. New York: Springer Science + Business Media, LLC; 2010. pp. 141–162. [Google Scholar]
- 9.Lai Y., Duan D. Progress in gene therapy of dystrophic heart disease. Gene Ther. 2012;19:678–685. doi: 10.1038/gt.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNally E.M., Goldstein J.A. Interplay between heart and skeletal muscle disease in heart failure: the 2011 George E. Brown Memorial Lecture. Circ. Res. 2012;110:749–754. doi: 10.1161/CIRCRESAHA.111.256776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Megeney L.A., Kablar B., Perry R.L., Ying C., May L., Rudnicki M.A. Severe cardiomyopathy in mice lacking dystrophin and MyoD. Proc. Natl. Acad. Sci. USA. 1999;96:220–225. doi: 10.1073/pnas.96.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crisp A., Yin H., Goyenvalle A., Betts C., Moulton H.M., Seow Y., Babbs A., Merritt T., Saleh A.F., Gait M.J., et al. Diaphragm rescue alone prevents heart dysfunction in dystrophic mice. Hum. Mol. Genet. 2011;20:413–421. doi: 10.1093/hmg/ddq477. [DOI] [PubMed] [Google Scholar]
- 13.Townsend D., Yasuda S., Li S., Chamberlain J.S., Metzger J.M. Emergent dilated cardiomyopathy caused by targeted repair of dystrophic skeletal muscle. Mol. Ther. 2008;16:832–835. doi: 10.1038/mt.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malerba A., Boldrin L., Dickson G. Long-term systemic administration of unconjugated morpholino oligomers for therapeutic expression of dystrophin by exon skipping in skeletal muscle: implications for cardiac muscle integrity. Nucleic Acid Ther. 2011;21:293–298. doi: 10.1089/nat.2011.0306. [DOI] [PubMed] [Google Scholar]
- 15.Harper S.Q., Hauser M.A., DelloRusso C., Duan D., Crawford R.W., Phelps S.F., Harper H.A., Robinson A.S., Engelhardt J.F., Brooks S.V., et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat. Med. 2002;8:253–261. doi: 10.1038/nm0302-253. [DOI] [PubMed] [Google Scholar]
- 16.Tinsley J., Deconinck N., Fisher R., Kahn D., Phelps S., Gillis J.M., Davies K. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat. Med. 1998;4:1441–1444. doi: 10.1038/4033. [DOI] [PubMed] [Google Scholar]
- 17.Bostick B., Shin J.H., Yue Y., Wasala N.B., Lai Y., Duan D. AAV micro-dystrophin gene therapy alleviates stress-induced cardiac death but not myocardial fibrosis in >21-m-old mdx mice, an end-stage model of Duchenne muscular dystrophy cardiomyopathy. J. Mol. Cell. Cardiol. 2012;53:217–222. doi: 10.1016/j.yjmcc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bostick B., Yue Y., Long C., Marschalk N., Fine D.M., Chen J., Duan D. Cardiac expression of a mini-dystrophin that normalizes skeletal muscle force only partially restores heart function in aged mdx mice. Mol. Ther. 2009;17:253–261. doi: 10.1038/mt.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bostick B., Yue Y., Long C., Duan D. Prevention of dystrophin-deficient cardiomyopathy in twenty-one-month-old carrier mice by mosaic dystrophin expression or complementary dystrophin/utrophin expression. Circ. Res. 2008;102:121–130. doi: 10.1161/CIRCRESAHA.107.162982. [DOI] [PubMed] [Google Scholar]
- 20.Bostick B., Shin J.-H., Yue Y., Duan D. AAV-microdystrophin therapy improves cardiac performance in aged female mdx mice. Mol. Ther. 2011;19:1826–1832. doi: 10.1038/mt.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bostick B., Yue Y., Duan D. Gender influences cardiac function in the mdx model of Duchenne cardiomyopathy. Muscle Nerve. 2010;42:600–603. doi: 10.1002/mus.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D., Yue Y., Lai Y., Hakim C.H., Duan D. Nitrosative stress elicited by nNOSmu delocalization inhibits muscle force in dystrophin-null mice. J. Pathol. 2011;223:88–98. doi: 10.1002/path.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hakim C.H., Duan D. Truncated dystrophins reduce muscle stiffness in the extensor digitorium longus muscle of mdx mice. J. Appl. Physiol. 2013;114:482–489. doi: 10.1152/japplphysiol.00866.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bostick B., Yue Y., Duan D. Phenotyping cardiac gene therapy in mice. Methods Mol. Biol. 2011;709:91–104. doi: 10.1007/978-1-61737-982-6_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yue Y., Skimming J.W., Liu M., Strawn T., Duan D. Full-length dystrophin expression in half of the heart cells ameliorates beta-isoproterenol-induced cardiomyopathy in mdx mice. Hum. Mol. Genet. 2004;13:1669–1675. doi: 10.1093/hmg/ddh174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li N., Wehrens X.H. Programmed electrical stimulation in mice. J. Vis. Exp. 2010:pii 1730. doi: 10.3791/1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin H., Saleh A.F., Betts C., Camelliti P., Seow Y., Ashraf S., Arzumanov A., Hammond S., Merritt T., Gait M.J., et al. Pip5 transduction peptides direct high efficiency oligonucleotide-mediated dystrophin exon skipping in heart and phenotypic correction in mdx mice. Mol. Ther. 2011;19:1295–1303. doi: 10.1038/mt.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Betts C., Saleh A.F., Arzumanov A.A., Hammond S.M., Godfrey C., Coursindel T., Gait M.J., Wood M.J. Pip6-PMO, a new generation of peptide-oligonucleotide conjugates with improved cardiac exon skipping activity for DMD treatment. Mol. Ther. Nucleic Acids. 2012;1:e38. doi: 10.1038/mtna.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arechavala-Gomeza V., Anthony K., Morgan J., Muntoni F. Antisense oligonucleotide-mediated exon skipping for Duchenne muscular dystrophy: progress and challenges. Curr. Gene Ther. 2012;12:152–160. doi: 10.2174/156652312800840621. [DOI] [PubMed] [Google Scholar]
- 30.Shin J.H., Pan X., Hakim C.H., Yang H.T., Yue Y., Zhang K., Terjung R.L., Duan D. Microdystrophin ameliorates muscular dystrophy in the canine model of Duchenne muscular dstrophy. Mol. Ther. 2013 doi: 10.1038/mt.2012.283. doi:10.1038/mt.2012.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yue Y., Ghosh A., Long C., Bostick B., Smith B.F., Kornegay J.N., Duan D. A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs. Mol. Ther. 2008;16:1944–1952. doi: 10.1038/mt.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yasuda S., Townsend D., Michele D.E., Favre E.G., Day S.M., Metzger J.M. Dystrophic heart failure blocked by membrane sealant poloxamer. Nature. 2005;436:1025–1029. doi: 10.1038/nature03844. [DOI] [PubMed] [Google Scholar]
- 33.Townsend D., Blankinship M.J., Allen J.M., Gregorevic P., Chamberlain J.S., Metzger J.M. Systemic administration of micro-dystrophin restores cardiac geometry and prevents dobutamine-inducedc cardiac pump failure. Mol. Ther. 2007;15:1086–1092. doi: 10.1038/sj.mt.6300144. [DOI] [PubMed] [Google Scholar]
- 34.Park O.Y., Ahn Y., Park W.S., Lim J.H., Park H.W., Kim J.H., Hong Y.J., Kim W., Jeong M.H., Cho J.G., et al. Rapid progression from hypertrophic cardiomyopathy to heart failure in a patient with Becker's muscular dystrophy. Eur. J. Heart Fail. 2005;7:684–688. doi: 10.1016/j.ejheart.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Nigro G., Comi L.I., Politano L., Bain R.J. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int. J. Cardiol. 1990;26:271–277. doi: 10.1016/0167-5273(90)90082-g. [DOI] [PubMed] [Google Scholar]
- 36.Li D., Long C., Yue Y., Duan D. Sub-physiological sarcoglycan expression contributes to compensatory muscle protection in mdx mice. Hum. Mol. Genet. 2009;18:1209–1220. doi: 10.1093/hmg/ddp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chamberlain J.S., Metzger J., Reyes M., Townsend D., Faulkner J.A. Dystrophin-deficient mdx mice display a reduced life span and are susceptible to spontaneous rhabdomyosarcoma. FASEB J. 2007;21:2195–2204. doi: 10.1096/fj.06-7353com. [DOI] [PubMed] [Google Scholar]
- 38.Beer M., Wagner D., Myers J., Sandstede J., Kostler H., Hahn D., Neubauer S., Dubach P. Effects of exercise training on myocardial energy metabolism and ventricular function assessed by quantitative phosphorus-31 magnetic resonance spectroscopy and magnetic resonance imaging in dilated cardiomyopathy. J. Am. Coll. Cardiol. 2008;51:1883–1891. doi: 10.1016/j.jacc.2007.09.075. [DOI] [PubMed] [Google Scholar]
- 39.Cohen N., Muntoni F. Multiple pathogenetic mechanisms in X linked dilated cardiomyopathy. Heart. 2004;90:835–841. doi: 10.1136/hrt.2003.023390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muntoni F., Cau M., Ganau A., Congiu R., Arvedi G., Mateddu A., Marrosu M.G., Cianchetti C., Realdi G., Cao A., et al. Brief report: deletion of the dystrophin muscle-promoter region associated with X-linked dilated cardiomyopathy. N. Engl. J. Med. 1993;329:921–925. doi: 10.1056/NEJM199309233291304. [DOI] [PubMed] [Google Scholar]
- 41.Towbin J.A., Hejtmancik J.F., Brink P., Gelb B., Zhu X.M., Chamberlain J.S., McCabe E.R., Swift M. X-linked dilated cardiomyopathy. Molecular genetic evidence of linkage to the Duchenne muscular dystrophy (dystrophin) gene at the Xp21 locus. Circulation. 1993;87:1854–1865. doi: 10.1161/01.cir.87.6.1854. [DOI] [PubMed] [Google Scholar]
- 42.Neri M., Valli E., Alfano G., Bovolenta M., Spitali P., Rapezzi C., Muntoni F., Banfi S., Perini G., Gualandi F., et al. The absence of dystrophin brain isoform expression in healthy human heart ventricles explains the pathogenesis of 5’ X-linked dilated cardiomyopathy. BMC Med. Genet. 2012;13:20. doi: 10.1186/1471-2350-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu X., Wheeler M.T., Hadhazy M., Lam M.Y., McNally E.M. Cardiomyopathy is independent of skeletal muscle disease in muscular dystrophy. FASEB J. 2002;16:1096–1098. doi: 10.1096/fj.01-0954fje. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell G.F., Jeron A., Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am. J. Physiol. 1998;274:H747–H751. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]
- 45.Nigro G., Comi L.I., Politano L., Nigro G. In: Myology: Basic and Clinical. Engel A., Franzini-Armstrong C, editors. Vol. 2. New York: McGraw-Hill, Medical Pub. Division; 2004. pp. 1239–1256. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.