Summary
The cyanobacterial circadian pacemaker consists of a three-protein clock – KaiA, KaiB and KaiC – that generates oscillations in the phosphorylation state of KaiC. Here we investigate how temporal information encoded in KaiC phosphorylation is transduced to RpaA, a transcription factor required for circadian gene expression. We show that phosphorylation of RpaA is regulated by two antagonistic histidine kinases, SasA and CikA, which are sequentially activated at distinct times by the Kai clock complex. SasA acts as a kinase toward RpaA, whereas CikA, previously implicated in clock input, acts as a phosphatase that dephosphorylates RpaA. CikA and SasA cooperate to generate an oscillation of RpaA activity that is distinct from that generated by either enzyme alone and offset from the rhythm of KaiC phosphorylation. Our observations reveal how circadian clocks can precisely control the timing of output pathways via the concerted action of two oppositely acting enzymes.
Introduction
Circadian clocks are endogenous oscillators found in a wide range of organisms that coordinate physiology and behavior with the diurnal cycle of the environment (Dunlap, 2004). Despite the lack of conservation of their underlying components, circadian clocks share a common set of fundamental properties – the ability to keep time with ~24 hour periodicity even in a constant environment (free run), the ability to adjust the phase to match that of environmental variation (entrainment), and a relative insensitivity of the period to temperature (temperature compensation) (Bell-Pedersen et al., 2005; Rosbash, 2009). In one of the simplest model systems known to possess a circadian clock, the cyanobacterium Synechococcus elongatus PCC 7942, the core pacemaker is made of the KaiA, KaiB, and KaiC proteins that interact to generate circadian oscillations in the phosphorylation state of KaiC (Nishiwaki et al., 2007; Qin et al., 2010; Rust et al., 2007). This phosphorylation cycle, which can be recapitulated in vitro, proceeds through sequential KaiA-promoted autophosphorylation of KaiC at two residues (threonine – 432 T-KaiC and serine 431 – S-KaiC). As more S-KaiC accumulates, KaiB binds KaiC and inhibits KaiA, switching KaiC to auto-dephosphorylation mode, and brings the system to unphosphorylated state (Rust et al., 2007). This pacemaker receives input from the environment, tuning it to the external day/night cycle, and also orchestrates output, controlling physiological processes such as gene expression (Ito et al., 2009), chromosome compaction (Smith and Williams, 2006) and the onset of cell division (Dong et al., 2010). The majority of genes in cyanobacteria are under circadian control (Ito et al., 2009; Liu et al., 1995; Vijayan et al., 2009). This control extends to regulation of kaiBC operon, forming a transcription-translation feedback loop (Ishiura et al., 1998; Taniguchi et al., 2007; Taniguchi et al., 2010).
The histidine kinase SasA and the DNA-binding domain containing response regulator RpaA were shown to be essential for clock-controlled gene expression (including kaiBC) (Iwasaki et al., 2000; Takai et al., 2006; Taniguchi et al., 2010). SasA is capable of phosphorylating RpaA in vitro at a conserved aspartate residue (a process referred to as phosphotransfer), and thus these proteins were proposed to form a cognate histidine kinase-response regulator pair (Takai et al., 2006). Furthermore, SasA is bound and stimulated by KaiC (Iwasaki et al., 2000; Smith and Williams, 2006), thereby coupling the phase of the oscillator with gene expression. CikA, another clock-associated histidine kinase (Ivleva et al., 2006), also regulates kaiBC expression, and is thus proposed to form another clock output pathway (Taniguchi et al., 2010). However, the cognate response regulator controlled by CikA is not known. Intriguingly, CikA also has a role in clock input, as cikA mutants fail to entrain the phase of their clock in response to a 5 h-dark pulse (Schmitz et al., 2000).
Here we show that RpaA is the cognate response of both SasA and CikA. However, these histidine kinases exert antagonistic effects on RpaA, with SasA acting as a kinase and CikA as a phosphatase. Furthermore, we demonstrate that the Kai oscillator stimulates the activity of CikA with timing distinct from its activation of SasA. This temporal separation of two oppositely acting enzymes that converge onto the same substrate creates an RpaA~P oscillation that is phase-advanced relative to that of KaiC~P, a phenomenon that is likely important for the accurate onset of gene expression.
Results and Discussion
RpaA is the cognate response regulator of both SasA and CikA
To identify candidate cognate response regulators for CikA, we used phosphotransfer profiling (Laub and Goulian, 2007; Skerker et al., 2005) to determine which of the predicted response regulators encoded by the S. elongatus genome (Table S1) is preferentially phosphorylated in vitro by CikA~P (phosphorylated CikA). This method relies on the ability of a given phosphorylated histidine kinase to transfer a radiolabeled phosphoryl group most rapidly to its cognate response regulator(s) when incubated in vitro with each receiver domain-containing protein from a genome of interest (Skerker et al., 2005). We find that the only response regulator phosphorylated by CikA~P after a short incubation time is RpaA (Fig. 1A). We applied the same approach to SasA and find that RpaA is the most preferred substrate of SasA~P phosphotransfer (Fig. 1A and S1B). The response regulator synPCC7942_1860 is also phosphorylated, but ~32-fold less efficiently than is RpaA (Fig. S1A). At longer incubation times other, likely non-cognate (Skerker et al., 2005), response regulators are phosphorylated (Fig. S1B). These results suggest that RpaA is the cognate response regulator of both SasA and CikA, indicating that it may be a key output node that integrates different circadian inputs.
Figure 1. In vitro phosphorylation of RpaA by CikA and SasA.
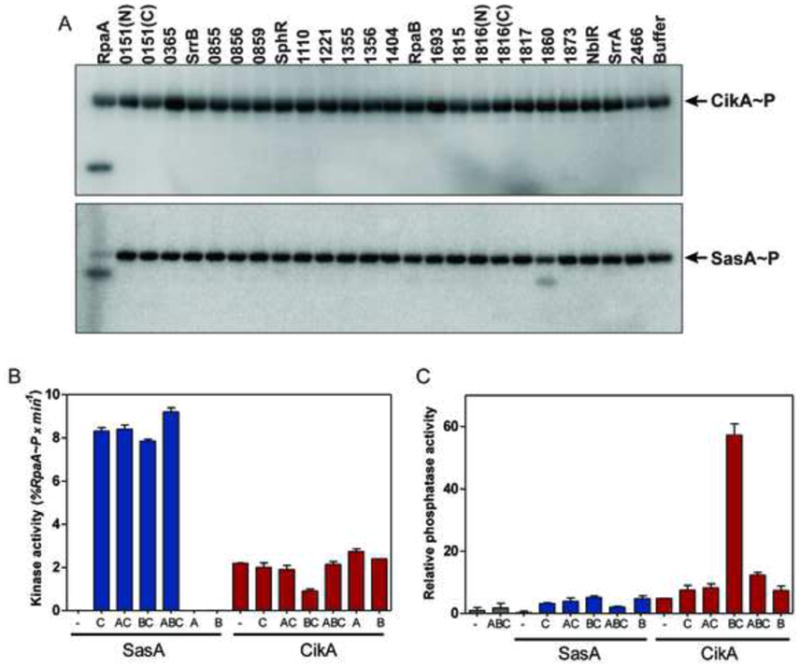
(A) Phosphotransfer profiling of CikA and SasA against each putative receiver domain-containing protein of S. elongatus PCC 7942 (see also Table S1). A five-minute time point (short time) is shown. (B) Kinase activity of SasA and CikA (initial rate of %RpaA~P accumulated) in the presence or absence of recombinant clock proteins (KaiA, KaiB and KaiC). Bars show the standard errors of the linear slopes. (C) Phosphatase activity of SasA and CikA toward RpaA~P in the absence or presence of clock proteins (KaiA, KaiB and KaiC). The rate of phosphatase activity is expressed relative to the rate of RpaA~P dephosphorylation in the absence of any proteins added. Bars correspond to the standard error of the rates extracted from non-linear fits of RpaA~P dephosphorylation. See also Fig. S1.
Kai proteins control SasA kinase and CikA phosphatase activities
Bacterial histidine kinases can act either as kinases (through the combined processes of autophosphorylation, in which they transfer the γ-phosphoryl group of ATP to their conserved histidine, and phosphotransfer) or as phosphatases (through dephosphorylation) toward their cognate response regulators (Casino et al., 2010; Gao and Stock, 2009; Russo and Silhavy, 1991). Either of these activities can be regulated by input stimuli to generate changes in the cellular level of phosphorylated response regulator. As both SasA and CikA have been shown to associate in vivo with the Kai protein complexes (Ivleva et al., 2006; Iwasaki et al., 2000) we hypothesized that the Kai oscillator may regulate the biochemical activities of these histidine kinases. We assayed SasA and CikA kinase and phosphatase activities towards RpaA and RpaA~P, using full-length proteins not modified by any tags, in the presence of different combinations of recombinant KaiA, KaiB and KaiC (Fig. 1B and Fig 1C). The kinase activity of SasA is greatly enhanced in the presence KaiC, as reported previously (Smith and Williams, 2006; Takai et al., 2006), whereas the kinase activity of CikA is modest and is largely unaffected by the presence of Kai proteins (Fig. 1B). CikA dephosphorylates RpaA~P, and this activity is greatly enhanced by KaiC and KaiB (Fig. 1C, S1C and S1D). By contrast, the phosphatase activity of SasA is modest and only slightly stimulated by the Kai proteins (Fig. 1C, S1C and S1D). Thus, CikA and SasA have opposing effects on RpaA phosphorylation that are modulated by Kai proteins.
In vivo, SasA promotes RpaA phosphorylation and CikA promotes dephosphorylation
To investigate the in vivo relevance of these biochemical effects, we analyzed RpaA phosphorylation in strains lacking CikA or SasA. To avoid secondary effects due to the defect in kaiBC expression in sasA and cikA mutants (Ivleva et al., 2006; Takai et al., 2006; Taniguchi et al., 2010), we analyzed RpaA phosphorylation in an engineered strain (referred to as the “clock-rescue”) in which kaiBC expression is under the control of the IPTG-inducible Ptrc promoter (Murayama et al., 2008). As previously reported (Murayama et al., 2008), addition of IPTG to the “clock-rescue” strain grown in constant light elevates KaiB and KaiC production to a level that is sufficient to restore circadian regulation of KaiC phosphorylation (Fig. 2A and S2A). In this “clock-rescue” strain we observe circadian oscillations in RpaA~P that peak 4 hours prior to the peak of KaiC~P. We then deleted cikA (Fig. 2B) or sasA (Fig. 2C) in this “clock-rescue” strain background, demonstrated that oscillations in KaiC phosphorylation are restored, and analyzed RpaA phosphorylation. In the absence of CikA, RpaA~P levels are high (Fig. 2B), and conversely, in the absence of SasA, RpaA~P is practically undetectable (Fig. 2C). Thus, in agreement with our biochemical observations, CikA and SasA have opposing actions on RpaA~P in vivo, with SasA promoting RpaA phosphorylation and CikA promoting accumulation of unphosphorylated RpaA. This observation is consistent with the high and low overall bioluminescence of a circadian gene reporter in cikA- or sasA-deficient strains, respectively (Taniguchi et al., 2010; Zhang et al., 2006).
Figure 2. Effect of cikA and sasA deletion on the level of RpaA~P in vivo.
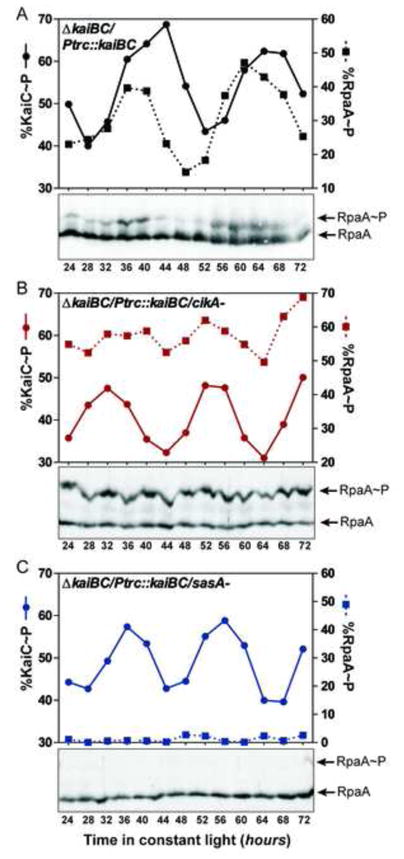
(A) RpaA~P and KaiC~P profiles measured by immunoblotting of extracts from the “clock-rescue” strain grown in the presence of 6 μM IPTG in continuous light. Lower panel – immunoblot of RpaA; graph above – quantification of RpaA~P and KaiC~P fractions. RpaA~P and KaiC~P profiles in the same genetic background but in the absence of cikA or sasA are shown in (B) and (C), respectively. See also Fig. S2A. Specific detection of RpaA and RpaA~P by immunoblotting with anti-RpaA polyclonal antibodies is shown in Fig. S2B.
ST-KaiC activates SasA kinase activity, and S-KaiC/KaiB complex stimulates CikA phosphatase activity
To determine which phosphorylation state of the KaiC oscillator is most potent in activating SasA and CikA, we measured the effect of adding partial clock reactions containing subsets of the Kai proteins on SasA kinase or CikA phosphatase activity. In partial clock reactions KaiC transits through four phosphorylation states: unphosphorylated KaiC (U-KaiC); KaiC phosphorylated only on threonine 432 (T-KaiC); KaiC phosphorylated on both serine 431 and threonine 432 (ST-KaiC); and KaiC phosphorylated only on serine 431 (S-KaiC) (Fig. 3A) (Rust et al., 2007). When we mix a partial clock reaction in which KaiC is phosphorylating (KaiC mixed with KaiA) with SasA and RpaA, we find that SasA kinase activity (reflected as RpaA~P accumulation) mirrors the abundance of the ST-KaiC phosphoform (Fig. 3A), suggesting that this state of KaiC is the most potent activator of SasA kinase activity. A similar result was obtained when autophosphorylation of SasA was measured (Fig. S3A), indicating that autophosphorylation is the activity regulated by the ST-KaiC phosphoform. When we mix CikA and RpaA~P with aliquots from a partial clock reaction in which KaiC is dephosphorylating (phosphorylated KaiC mixed with KaiB), we find that CikA phosphatase activity (reflected as a reduction in RpaA~P) correlates with the abundance of S-KaiC (Fig. 3B), and this correlation is dependent on the presence of KaiB (Fig. S3B). S-KaiC and KaiB form a complex during the clock cycle, which plays a critical role in feedback that maintains clock synchrony (Kageyama et al., 2006; Nishiwaki et al., 2007; Rust et al., 2007) and in this case also functions as an activator of CikA phosphatase activity.
Figure 3. Modulation of CikA and SasA activities by Kai proteins.
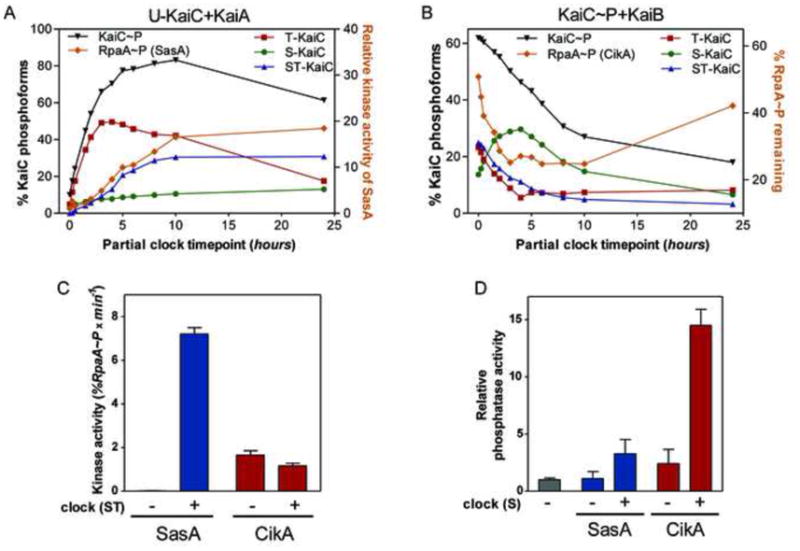
(A). Relative kinase activity of SasA in the presence of aliquots taken from partial clock reactions (U-KaiC+KaiA). Activity is expressed as fold RpaA~P change relative to the fraction of RpaA~P accumulated at time 0 h. (B) Dephosphorylation of RpaA~P by CikA in the presence of aliquots taken from KaiC auto-dephosphorylation partial clock reactions (KaiC-P+KaiB). %RpaA~P remaining is relative to the fraction of RpaA~P remaining when no clock was added. (C) Initial rates of kinase activity of SasA and CikA in the presence of a KaiA/KaiB/KaiC mix obtained from an oscillating clock reaction at the time when the ST-KaiC fraction was maximal. (D) Relative phosphatase activity of CikA and SasA in the presence of a mix obtained from the same oscillating clock reaction as in (C) at the time when S-KaiC fraction was maximal. The activity is expressed relative to the rate of RpaA~P dephosphorylation when no enzyme is added. Bars correspond to the standard error of linear (C) or non-linear rates (D). See also Fig. S3.
We then quantified the effects of the ST- and S-forms of KaiC on the kinase and phosphatase activities of SasA and CikA by adding aliquots from complete clock reactions (KaiA+KaiB+KaiC) collected when ST-KaiC and S-KaiC are at their peak levels (Rust et al., 2007). Addition of a clock reaction aliquot withdrawn when ST-KaiC is at its peak greatly enhances the initial rate of SasA kinase and autophosphorylation activity but not that of CikA (Fig. 3C and S3C). Enhancement of SasA activity by ST-KaiC was also observed in a study of KaiC phosphomimetic variants from a related cyanobacterium, Thermosynechococcus elongatus (Valencia S et al., 2012). Addition of a clock reaction aliquot enriched in S-KaiC significantly increases the phosphatase activity of CikA but not that of SasA (Fig. 3D).
Clock-controlled regulation of SasA and CikA determines the timing of RpaA phosphorylation
The timing of accumulation and decay of RpaA~P in cells is likely determined by the concerted action of SasA and CikA. Our in vivo measurements indicate that the peak of RpaA~P precedes the peak of KaiC~P by ~4 hours (Fig. 2A, Fig. 4A and S4A). Intriguingly, the phase of RpaA~P oscillation that would be directed by SasA kinase activity alone, as measured in vitro in the presence of time-resolved clock aliquots, is coincident with the phase of KaiC~P oscillation (that coincides with ST-KaiC accumulation) (Fig. 4B and S4B). On the other hand, the RpaA~P oscillation produced in vitro by the phosphatase action of CikA alone in the presence of clock aliquots peaks 8 h prior to the peak of KaiC~P (Fig. 4C), and its trough coincides with the increase in S-KaiC that occurs 6 h after the KaiC~P peak (Fig. S4C). The profiles of these separate activities suggest that their combined action has the potential to produce a novel phase of oscillation of RpaA~P that peaks prior to the KaiC~P peak (Fig. 4D) and would mirror the in vivo observations (Fig. 4A). A phase advanced-peak of RpaA~P accumulation relative to that of KaiC~P is consistent with the peak of gene expression as observed in bioluminescence and RNA abundance assays, which occurs ~4 hours prior to the peak of KaiC~P (Takai et al., 2006; Tomita et al., 2005). Such sequential convergence of two inputs that have opposite effects on a key regulator may also enhance the accuracy of transduction of temporal information in the face of cellular and environmental fluctuations.
Figure 4. Clock-mediated changes in SasA and CikA activities coordinate to generate a distinct oscillation of RpaA~P.
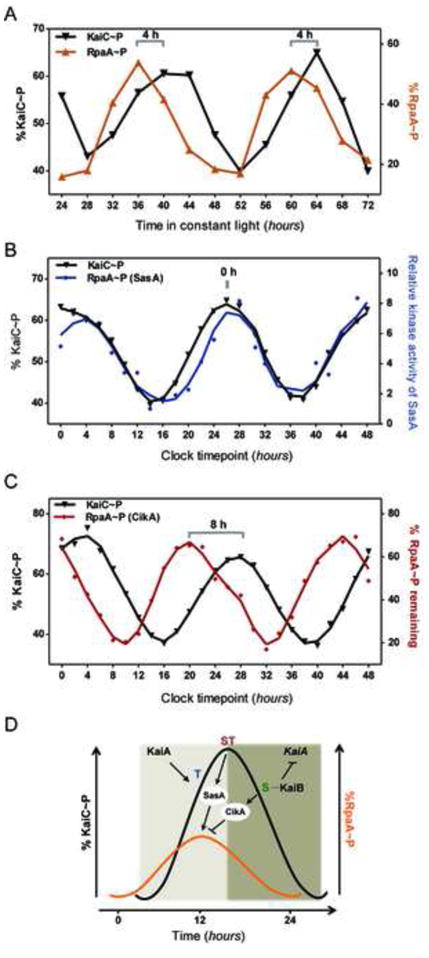
(A) Circadian profile of KaiC~P and RpaA~P in synchronized wild-type cells grown in continuous light, as measured by immunoblotting (see also Fig. S4A). (B) Relative kinase activity of SasA in the presence of clock aliquots obtained from an in vitro oscillating reaction. Activity (fold change) is expressed relative to the trough of RpaA~P accumulation (14 h clock timepoint) (see also Fig. S4B) (C) Effect of CikA on 32P-labeled-RpaA~P in the presence of protein aliquots obtained from an oscillating clock reaction. %RpaA-P is relative to the amount present when no clock reaction mix was added (see also Fig. S4C). (D) Model of the differential activation and convergence of SasA and CikA activities that create phase-advanced rhythms of RpaA~P relative to the core oscillator (KaiC~P). KaiA activates the autokinase activity of KaiC which leads to ordered phosphorylation of its two sites: threonine 432 (T) and serine 431 (S) (Nishiwaki et al., 2007; Rust et al., 2007). SasA kinase activity is enhanced by ST-KaiC (doubly phosphorylated). As more S-KaiC accumulates, KaiB binds KaiC and inhibits the phosphorylation-promoting activity of KaiA, switching KaiC in auto-dephosphorylation mode. The S-KaiC-KaiB complex enhances the phosphatase activity of CikA. The integration of SasA and CikA activities generate on oscillation of RpaA~P that is phase-advanced relative to KaiC~P.
Our biochemical observations provide insight into the functions attributed to CikA by genetic studies. Recent work implicated CikA in a clock output pathway – CikA acts as a negative regulator of kaiBC expression and was proposed to operate during late subjective night (Taniguchi et al., 2010), corresponding to the time when KaiC is dephosphorylating and the S-KaiC level is high. Our results enable mechanistic understanding of this role, as CikA inhibits RpaA activity (i.e. promotes its dephosphorylation), which leads to reduced kaiBC expression. CikA was originally identified as a component of the clock input pathway, required for entrainment of the cyanobacterial clock to changes in light availability (Schmitz et al., 2000). It is possible that CikA has a role in input unrelated to regulation of RpaA activity. Alternatively, the entrainment defect in the cikA mutant may be a consequence of misregulation of RpaA activity that disrupts transcriptional feedback of kaiBC expression. In addition to a defect in entrainment, the cikA- mutant also displays a cell elongation phenotype, due to its inability to properly gate the timing of cell division (Dong et al., 2010). We reason that this clock-dependent cell elongation phenotype displayed by cikA- and certain kai mutants (Dong et al., 2010) may be due to RpaA~P misregulation. For example, our observations predict that the cell elongation phenotypes of cikA- and kaiB- are due to an increased level of RpaA~P; in cikA- caused by the absence of CikA phosphatase activity and in kaiB- caused by lack of CikA activation.
Circadian clocks enable organisms to time the regulation of physiological processes to exploit the predictable variation in the earth’s light/dark cycle. For example, cyanobacteria time the production of the photosynthetic apparatus to anticipate daylight (Stal and Krumbein, 1987; Vijayan et al., 2009) and also use the clock to gate the cell division (Dong et al., 2010; Mori et al., 1996). In some cases, the appropriate timing of regulation of physiological processes may not coincide with the phase of the core oscillator. We have shown how an organism can use differentially regulated and opposing enzymes converging on a single output protein to generate a phase of output distinct from that of the clock itself, a phenomenon that is likely to be important for all circadian clocks.
Experimental procedures
Growth conditions
Wild-type, “clock-rescue” and sasA- and cikA- derivative strains of S. elongatus were grown in standard BG11M medium, at 30 °C, in white light with CO2-enriched-air bubbled through cultures that were repeatedly diluted with fresh medium to maintain an OD750 of ~0.3. Under these conditions a doubling time of 6.9 h was recorded. For circadian experiments, the cultures grown in 6 μM IPTG, were synchronized by two 12 h dark periods, spaced by 12 h in light. Sampling began 24 h after release into constant light.
Western blotting
Cells from 20 ml of culture were harvested by filtration on Whatman cellulose acetate filters and quickly frozen in liquid nitrogen. Lysates were obtained by bead-beating at 4 °C in lysis buffer (8 M urea, 20 mM Hepes-KOH pH 8, 1 mM β-mercaptoethanol (β-ME)). Total protein content was determined by Bradford assay against a BSA standard curve. KaiC immunobloting was performed as previously described (Rust et al., 2011). For detection of RpaA~P, lysates were run at 4°C on 7% polyacrylamide gel (Hoefer SE 600 system) containing 50 μM of Phos-tag AAL-107 (Wako Chemicals) and 100 μM MnCl2 and then transferred to a nitrocellulose membrane. Custom-made rabbit anti-RpaA polyclonal antibodies were used to detect phosphorylated and unphosphorylated RpaA (see Supplemental Information). Quantification of the Western blot bands was performed using AlphaImager EP software (Alpha Innotech). Within each lane the lower and the upper (retarded) bands were delineated using two identically sized non-overlapping boxes, which allowed the extraction of the sum of pixel intensities corresponding to each band. In addition, a similarly sized box positioned above each band set was used for background subtraction. The extent of phosphorylation at each timepoint (lane) was estimated by calculating the upper band signal as a fraction of the sum of the intensities of both bands (100*[upper band signal]/[total signal of both bands]).
In vitro assays
Purification of Kai proteins and clock reactions were largely performed as described previously (Rust et al., 2007), with the exception that the clock buffer contained an ATP-regeneration system (phosphoenolpyruvate and recombinant pyruvate kinase of Bacillus stearothermophilus (Sigma)). Full-length recombinant SasA, CikA and RpaA lacking a tag were prepared as described in the Supplemental Information. Except for phosphotransfer profiling, which was performed as previously described (Skerker et al., 2005), the ratios and the concentrations of the recombinant proteins used in assays were based on quantitative western blotting (Fig. S2C–S2G) and standard oscillating clock reactions (Rust et al., 2011): 3.5 μM KaiC, 3.5 μM KaiB, 1.5 μM KaiA, 2.5 μM RpaA, 0.65 μM SasA and 0.65 μM CikA. The kinase buffer used was: 20 mM Hepes-KOH pH 8, 150 mM KCl, 10% glycerol and 5 mM MgCl2. Unless indicated otherwise, kinase reactions were initiated with addition of ATP-containing Kai protein mixes or ATP-containing buffer (1 mM ATP final concentration) and incubated at 30°C. Aliquots from the reaction mix were quenched with the β-ME based Laemmli buffer and run on 7% polyacrylamide gels made with the Phos-tag reagent (Wako Chemicals) as described in the Western blotting section. For estimation of kinase rates, the linear slopes of %RpaA~P accumulation over time was measured. The sampling for Fig. 2B was done for all combinations of Kai proteins in parallel at 0.5, 2 and 10 min after addition of SasA or CikA (for CikA and KaiB combination only 0.5 and 10 min time points were obtained). To capture SasA kinase activity, in Fig. 3A and Fig. 4B the reactions were done in parallel for all the partial clock and complete clock aliquots with 2 min incubation with SasA and RpaA. For Fig. 3C, the times sampled were 0.5, 2, 5, 7 and 10 min after addition of ST-KaiC enriched clock aliquot to SasA/RpaA or CikA/RpaA mixes. After electrophoresis, the gels were stained with SyproRuby (Invitrogen) and imaged with the Typhoon Trio System (GE Healthcare). Densitometry analysis was performed with ImageQuant TL7.0 (GE Healthcare).
For phosphatase assays, radiolabelled RpaA~P that was independently prepared before each experiment by utilizing a CikA-coupled resin (See Supplemental Information). Reaction conditions were similar to the ones used for kinase assays. Aliquots from reactions were stopped with the addition of Laemmli loading dye and analyzed by SDS-PAGE and autoradiography. The time points sampled in Fig. 1C and 3D were 0, 15, 30 and 60 min following addition of Kai protein mixes. Background subtracted signal intensity of each band was normalized against the band signal at time 0 (which was actually ~30 s after addition of the Kai protein mixes) in order to obtain exponential decay rates from the non-linear fits of each time series (as exemplified in Fig. S1C). As dephosphorylation of RpaA~P appears to proceed through reverse phosphotransfer (Fig. S1C), we also included in the calculations the signal present in the histidine kinase bands, summing it with the signal of the corresponding RpaA~P band. All regressions were performed with Prism (GraphPad). The relative phosphatase activity represents the fold increase in the rate of dephosphorylation relative to the rate of RpaA~P dephosphorylation alone. In cases where the phosphatase activity was estimated from a single time point (15 min – Fig. 3B, Fig. S3B; 30 min – Fig. 4C, Fig. S4C), we reported only the fraction of RpaA~P remaining (sum of CikA~P and RpaA~P signal) relative to the signal present in the control lane (B – buffer added only). Each assay was independently performed at least twice, with similar results.
Supplementary Material
Highlights.
RpaA is the cognate response regulator of both SasA and CikA
SasA is the primary kinase for RpaA and is regulated by phosphorylated KaiC
CikA acts as phosphatase toward RpaA, and is regulated by the KaiC/KaiB complex
RpaA phosphorylation oscillates in vivo, phase-advanced to KaiC phosphorylation
Acknowledgments
We thank J. S. Markson for assistance with protein purification, for providing rpaA- and “clock rescue” strains as well as for insightful discussions. We thank M. Laub for providing MBP- and TRX-based destination vectors and for input on the project and manuscript and V. Vijayan, J. Piechura, C. Chidley, E. Czeko, R. Alvey and V. Denic for valuable comments on the paper. This work was supported by the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bell-Pedersen D, Cassone V, Earnest D, Golden S, Hardin P, Thomas T, Zoran M. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nature Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casino P, Rubio V, Marina A. The mechanism of signal transduction by two-component systems. Curr Opinion Struct Biol. 2010;20:763–771. doi: 10.1016/j.sbi.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Dong G, Yang Q, Wang Q, Kim YI, Wood TL, Osteryoung KW, van OA, Golden SS. Elevated ATPase activity of KaiC applies a circadian checkpoint on cell division in Synechococcus elongatus. Cell. 2010;140:529–539. doi: 10.1016/j.cell.2009.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap JC, Loros JJ, DeCoursey PJ. Chronobiology: Biological Timekeeping. Sunderland, MA: Sinauer; 2004. [Google Scholar]
- Gao R, Stock AM. Biological insights from structures of two-component proteins. Annu Rev Microbiol. 2009;63:133–154. doi: 10.1146/annurev.micro.091208.073214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiura M, Kutsuna S, Aoki S, Iwasaki H, Andersson C, Tanabe A, Golden S, Johnson C, Kondo T. Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria. Science. 1998;281:1519–1523. doi: 10.1126/science.281.5382.1519. [DOI] [PubMed] [Google Scholar]
- Ito H, Mutsuda M, Murayama Y, Tomita J, Hosokawa N, Terauchi K, Sugita C, Sugita M, Kondo T, Iwasaki H. Cyanobacterial daily life with Kai-based circadian and diurnal genome-wide transcriptional control in Synechococcus elongatus. Proc Natl Acad Sci USA. 2009;106:14168–14173. doi: 10.1073/pnas.0902587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivleva NB, Gao T, LiWang AC, Golden SS. Quinone sensing by the circadian input kinase of the cyanobacterial circadian clock. Proc Natl Acad Sci USA. 2006;103:17468–17473. doi: 10.1073/pnas.0606639103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H, Williams SB, Kitayama Y, Ishiura M, Golden SS, Kondo T. A KaiC-interacting sensory histidine kinase, SasA, necessary to sustain robust circadian oscillation in cyanobacteria. Cell. 2000;101:223–233. doi: 10.1016/S0092-8674(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Kageyama H, Nishiwaki T, Nakajima M, Iwasaki H, Oyama T, Kondo T. Cyanobacterial circadian pacemaker: Kai protein complex dynamics in the KaiC phosphorylation cycle in vitro. Molecular cell. 2006;23:161–171. doi: 10.1016/j.molcel.2006.05.039. [DOI] [PubMed] [Google Scholar]
- Laub MT, Goulian M. Specificity in two-component signal transduction pathways. Annu Rev Genet. 2007;41:121–145. doi: 10.1146/annurev.genet.41.042007.170548. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tsinoremas N, Johnson C, Lebedeva N, Golden S, Ishiura M, Kondo T. Circadian orchestration of gene expression in cyanobacteria. Genes Dev. 1995;9:1469–1478. doi: 10.1101/gad.9.12.1469. [DOI] [PubMed] [Google Scholar]
- Mori T, Binder B, Johnson C. Circadian gating of cell division in cyanobacteria growing with average doubling times of less than 24 hours. Proc Natl Acad Sci USA. 1996;93:10183–10188. doi: 10.1073/pnas.93.19.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama Y, Oyama T, Kondo T. Regulation of circadian clock gene expression by phosphorylation states of KaiC in cyanobacteria. J Bacteriol. 2008;190:1691–1698. doi: 10.1128/JB.01693-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki T, Satomi Y, Kitayama Y, Terauchi K, Kiyohara R, Takao T, Kondo T. A sequential program of dual phosphorylation of KaiC as a basis for circadian rhythm in cyanobacteria. EMBO J. 2007;26:4029–4037. doi: 10.1038/sj.emboj.7601832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Byrne M, Mori T, Zou P, Williams D, McHaourab H, Johnson C. Intermolecular associations determine the dynamics of the circadian KaiABC oscillator. Proc Natl Acad Sci USA. 2010;107:14805–14810. doi: 10.1073/pnas.1002119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbash M. The implications of multiple circadian clock origins. PLoS biology. 2009;7 doi: 10.1371/journal.pbio.1000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo F, Silhavy T. EnvZ controls the concentration of phosphorylated OmpR to mediate osmoregulation of the porin genes. J Mol Biol. 1991;222:567–580. doi: 10.1016/0022-2836(91)90497-t. [DOI] [PubMed] [Google Scholar]
- Rust MJ, Golden SS, O’Shea EK. Light-driven changes in energy metabolism directly entrain the cyanobacterial circadian oscillator. Science. 2011;331:220–223. doi: 10.1126/science.1197243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust MJ, Markson JS, Lane WS, Fisher DS, O’Shea EK. Ordered phosphorylation governs oscillation of a three-protein circadian clock. Science. 2007;318:809–812. doi: 10.1126/science.1148596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz O, Katayama M, Williams SB, Kondo T, Golden SS. CikA, a bacteriophytochrome that resets the cyanobacterial circadian clock. Science. 2000;289:765–768. doi: 10.1126/science.289.5480.765. [DOI] [PubMed] [Google Scholar]
- Skerker JM, Prasol MS, Perchuk BS, Biondi EG, Laub MT. Two-component signal transduction pathways regulating growth and cell cycle progression in a bacterium: a system-level analysis. PLoS Biol. 2005;3:e334. doi: 10.1371/journal.pbio.0030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RM, Williams SB. Circadian rhythms in gene transcription imparted by chromosome compaction in the cyanobacterium Synechococcus elongatus Proc. Natl Acad Sci USA. 2006;103:8564–8569. doi: 10.1073/pnas.0508696103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stal L, Krumbein W. Temporal separation of nitrogen fixation and photosynthesis in the filamentous, non-heterocystous cyanobacterium Oscillatoria sp. Arch Microbiol. 1987;149:76–80. [Google Scholar]
- Takai N, Nakajima M, Oyama T, Kito R, Sugita C, Sugita M, Kondo T, Iwasaki H. A KaiC-associating SasA-RpaA two-component regulatory system as a major circadian timing mediator in cyanobacteria. Proc Natl Acad Sci USA. 2006;103:12109–12114. doi: 10.1073/pnas.0602955103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi Y, Katayama M, Ito R, Takai N, Kondo T, Oyama T. labA: a novel gene required for negative feedback regulation of the cyanobacterial circadian clock protein KaiC. Genes Dev. 2007;21:60–70. doi: 10.1101/gad.1488107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi Y, Takai N, Katayama M, Kondo T, Oyama T. Three major output pathways from the KaiABC-based oscillator cooperate to generate robust circadian kaiBC expression in cyanobacteria. Proc Natl Acad Sci USA. 2010;107:3263–3268. doi: 10.1073/pnas.0909924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita J, Nakajima M, Kondo T, Iwasaki H. No transcription-translation feedback in circadian rhythm of KaiC phosphorylation. Science. 2005;307:251–254. doi: 10.1126/science.1102540. [DOI] [PubMed] [Google Scholar]
- Valencia SJ, Bitou K, Ishii K, Murakami R, Morishita M, Onai K, Furukawa Y, Imada K, Namba K, Ishiura M. Phase-dependent generation and transmission of time information by the KaiABC circadian clock oscillator through SasA-KaiC interaction in cyanobacteria. Genes cells. 2012;17:398–419. doi: 10.1111/j.1365-2443.2012.01597.x. [DOI] [PubMed] [Google Scholar]
- Vijayan V, Zuzow R, O’Shea EK. Oscillations in supercoiling drive circadian gene expression in cyanobacteria. Proc Natl Acad Sci USA. 2009;106:22564–22568. doi: 10.1073/pnas.0912673106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Dong G, Golden S. The pseudo-receiver domain of CikA regulates the cyanobacterial circadian input pathway. Mol Microbiol. 2006;60:658–668. doi: 10.1111/j.1365-2958.2006.05138.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


