Abstract
One of the greatest examples of integrated signal transduction is revealed by examination of effects mediated by AKT kinase in myocardial biology. Positioned at the intersection of multiple afferent and efferent signals, AKT exemplifies a molecular sensing node that coordinates dynamic responses of the cell in literally every aspect of biological responses. The balanced and nuanced nature of homeostatic signaling is particularly essential within the myocardial context, where regulation of survival, energy production, contractility, and response to pathological stress all flow through the nexus of AKT activation or repression. Equally important, the loss of regulated AKT activity is primarily the cause or consequence of pathological conditions leading to remodeling of the heart and eventual decompensation. This review presents an overview compendium of the complex world of myocardial AKT biology gleaned from more than a decade of research. Summarization of the widespread influence that AKT exerts upon myocardial responses leaves no doubt that the participation of AKT in molecular signaling will need to be reckoned with as a seemingly omnipresent regulator of myocardial molecular biological responses.
I. INTRODUCTION/BASICS OF AKT BIOLOGY
After decades of research, three vexing issues of cellular regulation continue to challenge cardiovascular biologists: growth, proliferation, and survival. In this respect, cardiovascular researchers share a similar obsession with cancer biologists who seek to influence the phenotypic behavior of transformed cells, with advances in understanding of oncogenic transformation repeatedly leading to profound in-sights regarding myocardial cell biology. Such was the case over three decades ago when the gene first identified in association with rodent T-cell lymphoma as the product of transforming retrovirus AKT8 (603, 604) possessing homology with protein kinases A and C (PKA and PKC, respectively) (8) dubbed protein kinase B (PKB) that has come to be known as AKT kinase. Retrospectively, it is refreshing to look back at the relatively limited perspective of AKT functional activities in cell survival and proliferation from those early days of important discovery (13, 56, 71, 107, 351, 378, 450) and, with the benefit of hindsight, recognize that these scientists had found the proverbial “tip of the iceberg” with these initial studies. Subsequent years have produced a literal explosion of intellectual and practical understanding of molecular signal transduction in both normal and pathological conditions, with AKT serving as a canonical example of the complexity that lies beneath integration of signals for maintenance of homeostasis. However, the cancer and cardiovascular disciplines have adopted diametrically opposed perspectives on how to exploit the regulatory functions of AKT: whereas persistent cell survival and proliferation are the antitheses of what is needed to treat cancer, these same properties have often been the holy grail of cardiovascular biologists searching for ways to limit damage and promote repair in the wake of myocardial insults.
The trifecta of cellular growth, proliferation, and survival lies at the crux of most, if not all, therapeutic interventional strategies to treat cardiovascular disease. Although manipulating these processes seems conceptually straightforward, this hypothetical goal has proven to be remarkably elusive in the myocardium. The challenges involved with this endeavor are readily illustrated by examining the legacy of literature documenting the relationship between AKT signal transduction and the myocardium. AKT serves as a critical nexus of integration between cellular stimuli and subsequent adaptive responses, and this pervasiveness of AKT participation had made it one of the most extensively characterized kinases in the myocardium. The substrates of AKT influence every aspect of cellular functions including not only growth, survival, and proliferation, but also metabolism, glucose uptake, gene expression, and cell-cell communication via initiation of paracrine and autocrine factor production. Owing to the enormity of information available for consideration, literature reviews typically concentrate on functional aspects of AKT biology in the context of a specific subtopic. With many such precedents providing excellent perspectives for additional information, readers of this review will be directed to those resources whenever possible. The distinguishing viewpoint of this treatise is an examination of AKT in the myocardial context by integrating a plethora of observations into a coherent perspective that will clarify how and why AKT has attained both celebrity and notoriety as a seemingly omnipresent node at the crossroads of myocardial cell biology.
II. AKT IN THE MYOCARDIAL CONTEXT
A. Survival
Evidence that serine/threonine kinases promote cell survival would seem indisputable at this point (132) as activation of these kinases is associated with pathogenesis of malignancies as well as resistance to apoptotic challenge that would otherwise limit dysregulated cell proliferation. Aside from oncogenic transformation, inhibition of these kinases also leads to increased damage in the wake of pathological challenge, indicating their role in normal cell persistence (25). Cellular survival induced by a plethora of cardioprotective agents converges on AKT activation. Subsequently, AKT activation leads to blockade of pro-apoptotic protein function and initiation of protective signaling cascades.
In the myocardial context, there is abundant evidence to support a cardioprotective role for AKT activation (138, 379, 458–460, 467). Preservation of cardiomyocytes and function is necessary for the heart. Several lines of evidence have shown the necessity of AKT signaling for cardiomyocyte, cardiac fibroblast, vascular smooth muscle cells (VSMCs), and endothelial cell survival (522). Insulin-like growth factor I (IGF-I) activates upstream phosphatidylinositol 3-kinase (PI3K), resulting in the activation of AKT and multiple downstream effectors. AKT activation reduced apoptotic cardiomyocyte death in response to ischemia-reperfusion injury (26, 32), pressure overload challenge (84), and oxidative stress (12). Declining AKT activity is also linked to increased apoptosis in pacing-induced heart failure (18). However, viral myocarditis may diverge from the generally cardioprotective role for AKT, as inhibition of AKT activity seems to improve protective effects (182–184).
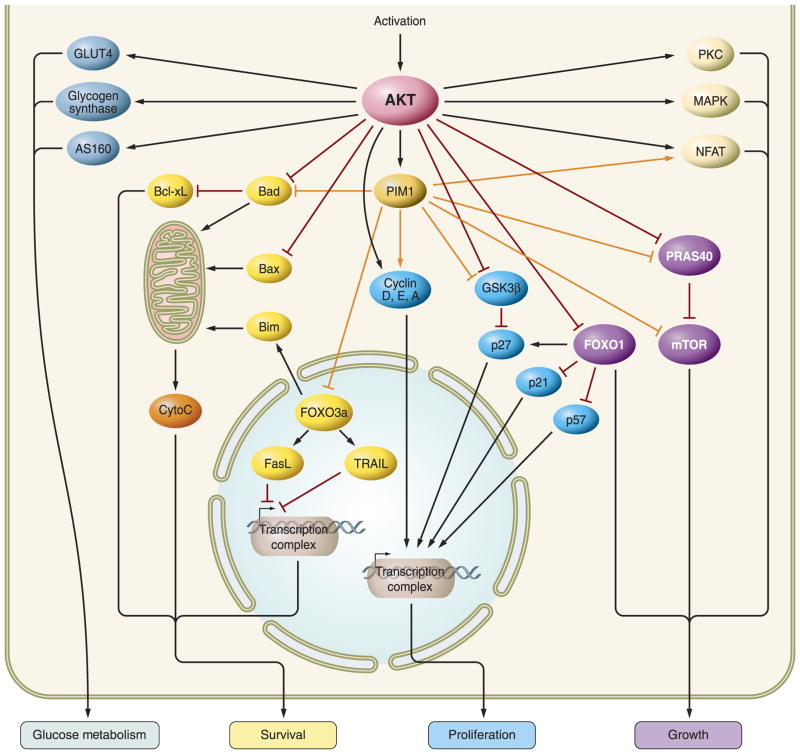
Many downstream targets of AKT have been shown to contribute to its pro-survival effects such as phosphorylation of BCL-2 family members (251, 301, 324), activation of Forkhead transcription factors (242, 406, 619), increase in nitric oxide (NO) (155, 275, 276, 518), regulation of Ca2+ cycling (103, 119, 349), and cardiac stem cell survival (632, 643). Activation of AKT has been shown to modulate pro-apoptotic proteins through the phosphorylation of BCL-2 family members BAX and BAD. During stress or injury, BAX will translocate to the mitochondria and permeabilize the membrane-forming pores, thus allowing for cytochrome c release, and jeopardizing the stability of the mitochondria. Phosphorylation of BAX, at serine 184, by AKT prevents BAX translocation to the mitochondria through a conformational change (642). Phosphorylation of BAD at serine 136 releases BCL-xL from BAD, allowing it to perform its anti-apoptotic effects (12, 334).
Forkhead transcription factor, FOXO3a, is involved in the regulation of the cell cycle by upregulating the transcription of death receptor ligands, including the regulation of FasL and TRAIL gene expression. Furthermore, Forkhead transcription factors have recently been shown to upregulate the expression of BIM. BIM is a BCL-2 family member that initiates mitochondrial dysfunction leading to apoptosis. Phosphorylation of FOXO3a by AKT in the nucleus results in FOXO3a nuclear exclusion and transport into the cytosol in an inactive state, resulting in a reduction of apoptosis (78).
Endothelial NO synthase (eNOS) is responsible for the production of NO. eNOS-derived NO serves important functions within the heart including ventricular relaxation, myocardial remodeling, regulation of VMSC proliferation, etc. The release of NO has been shown to be mediated through the PI3K/AKT pathway through engagement of membrane estrogen receptors and without an increase in intracellular Ca2+ to keep cardiac homeostasis (276). Activation of AKT during preconditioning leads to phosphorylation of eNOS and is essential for cardioprotection (270, 660).
B. Proliferation
As an oncogenic protein, it is no surprise that AKT promotes proliferation in the context of cancer. On the other hand, cardiomyocytes are notoriously resistant to oncogenic transformation and mitotic activity. Cardiomyocyte proliferation occuring primarily during prenatal and early postnatal development decreases shortly after birth. Neonatal cardiomyocytes can grow by increases in both cell number (proliferation) as well as cell size (hypertrophy), but adult cardiomyocytes grow predominantly by hypertrophy, with proliferation being identified at very low levels (44, 45, 338). Within the past decade, genetic manipulation has been utilized to induce cardiomyocyte proliferation and DNA synthesis by overexpressing cell cycle mediators (cyclin D, cyclin A, cyclin B, Cdk2), growth factors (IGF-I, FGF2), transcription factors (c-Myc, E2F2), and knockout of cell cycle inhibitors (p27, Rb; reviewed in Refs. 11, 53, 529). Around this time, factors that influence cardiomyocyte cell cycle reentry were also being identified. IGF-I, a potent activator of AKT, increases kinase activity of cyclin D/E/A and induces DNA synthesis in adult cardiomyocytes (549, 550). Transgenic overexpression of IGF-I results in a progressive increase in the number of cells in the heart without influencing myocyte volume (547). FGF1 stimulation and p38 inhibition promote cytokinesis in adult cardiomyocytes through a PI3K/AKT-dependent pathway (178). Combined administration of FGF1 and p38 mitogen-activated protein (MAP) kinase inhibitor increases cardiomyocyte mitosis and improves cardiac function after myocardial infarction (177). Platelet-derived growth factor (PDGF)-induced neonatal cardiomyocyte proliferation correlates with AKT activation leading to inactivation of glycogen synthase kinase 3β (GSK-3β) and downregulation of p27 (287). Periostin, a component of the extracellular matrix associated with epithelial-mesenchymal transition during cardiac development, induces cell-cycle reentry of adult cardiomyocytes by activation of AKT but not ERK1/2 (376). Over-expression of the phosphatase PTEN or treatment with LY294002 abrogates periostin-induced DNA synthesis and cell cycle reentry. Conversely, other studies show periostin is critical for regulation of hypertrophic responses (516) rather than proliferation (431) following pressure overload and myocardial infarction. Neuregulin1 induces adult mononucleated cardiomyocytes to divide by signaling through tyrosine kinase receptor ErbB4 to activate the PI3K/AKT pathway (47).
Several downstream targets of AKT regulate cardiomyocyte proliferation during development. Deletion of GSK-3β induces cardiomyocyte hyperproliferation associated with increased expression of GATA4, cyclin D1, and c-Myc (353). Myocardial specific transgenic expression of FOXO1 decreases myocyte proliferation during heart development by premature activation of p21, p27, and p57 (186). IGF-I stimulation/AKT overexpression promotes embryonic cardiomyocyte proliferation and cytoplasmic localization of FOXO (186, 503, 595). Nuclear-targeted AKT expression also produces a hypercellular phenotype (558) characterized by increased cardiomyocyte cycling and expansion of the cardiac progenitor cell (CPC) population (244). Consistent with observations of myocardial hyperplasia, in the presence of periostin, nuclear targeted AKT doubles the number of BrdU-positive cardiomyocytes (376). These findings are in agreement with observations of increased AKT activity correlating with proliferation of cardiomyocytes (287, 465), downregulation of AKT upon differentiation (337), and requirement of PI3K-dependent signaling in proliferation (342). Overexpression of NOTCH induces phosphorylation of AKT and proliferative signaling in neonatal and adult cardiomyocytes (75, 110, 245). During pathological challenge, upregulated levels of AKT (15, 60) correlate with increased abundance of c-KIT-positive CPCs (201, 373). These CPCs are maintained through AKT/GSK-3β signaling, because inhibition of AKT impairs CPC proliferation, whereas inhibition of GSK-3β enhances their growth (624). Collectively these results support the premise that PI3K/AKT signaling plays a critical role in proliferation of both cardiomyocytes and CPCs.
C. Metabolism
Metabolism and AKT are inextricably linked even through diet, as shown by multiple and divergent threads of investigation. Stimulation of glucose uptake triggers activation of AKT downstream of PI3K (161). AKT activity is tied to glycolytic metabolism, with reduced glycolysis prompting reduction of AKT phosphorylation and cardiomyopathic consequences (162). Impaired AKT activity is also a common feature of altered signaling associated with diabetic cardiomyopathy (167). In comparison, undernutrition results in compensatory increases in AKT activity associated with hyperinsulinemia (224). High cholesterol-fructose alters induction of AKT signaling through enhanced insulin resistance and provokes cardiomyopathic disease (146). Along similar lines, AKT activity is stimulated in response to a high-fat diet resulting in obesity and increased stress (159). Peroxisome proliferator-activated receptor (PPAR)-γ is one member of a family of nuclear receptor transcription factors regulating metabolism at the gene expression level that influences AKT activity with ties to hypertrophic remodeling, hypertension, and diabetes (168, 185, 309–311, 412, 440, 527, 685). Supplementation of diet with omega-3 polyunsaturated fatty acids (omega-3 PUFA) purported to reduce the risk of heart failure leads to increased AKT expression, although activity was maintained at constant levels (169). Dietary supplementation with red palm oil improves recovery from ischemia-reperfusion injury in rats associated with increased AKT phosphorylation (179).
AKT exerts this central role in regulating heart metabolism by direct or indirect interaction with key regulatory molecules controlling glucose transporter 4 (GLUT4) (62), FOXO proteins transcriptional activity (636), mTOR pathway (523), GSK-3β (recently reviewed in Ref. 449), and mitochondrial function (483, 617) as discussed later in this review.
1. AKT and GLUT4
The heart normally derives energy from oxidation of fatty acids (FA) (60–70%), glucose (30–40%), and lactate (10%) (430). However, glucose oxidation has a central role in energy metabolism of the heart. Obesity and diabetes, two of the most important risk factors for development of cardiomyopathy, are associated with reduced utilization of glucose and increased oxidation of FA and lactate (64, 89, 659), concomitantly to impaired insulin-dependent AKT activation (646). In cardiomyocytes, glucose metabolism is triggered by transport through the membrane mediated through GLUT1 and GLUT4 glucose transporters localized in the sarcolemma and intracellular membrane compartments, respectively. GLUT1 is implicated in maintenance of glucose homeostasis under basal conditions, whereas GLUT4 translocates to the sarcolemma and transverse tubule membranes in response to normal and pathological stimuli (134, 190). Decreased glucose utilization and increased fatty acid consumption caused by diet-induced obesity correlates with reduced expression of GLUT4, which precedes the impairment of insulin-dependent AKT activation (667). Impairment of insulin-stimulated AKT/GLUT4 signaling parallels ventricular contractile dysfunction and increased mortality rate of streptozotocin-induced diabetic rats subjected to ischemia-reperfusion treatment (307). AKT also drives GLUT4 translocation to the sarcolemma under oxidative stress condition in cardiomyocytes, and also following ischemia in conjunction with AMP-activated protein kinase (AMPK; Ref. 297). AKT promotes GLUT4 translocation to the sarcolemma by phosphorylating and inactivating AKT substrate 160 (AS160), thereby inhibiting Rab function and favoring GLUT4 translocation in adipocytes and muscle (663). Importance of GLUT4 translocation under pathological conditions is demonstrated by the fact that its activation is the major mechanism by which the heart increases glucose uptake during ischemia (612). So too, chronic cardiac-specific overexpression of activated AKT increases basal glucose uptake and glycogen deposition while inhibiting the response to insulin (457). Cardiac-selective GLUT4 deficiency leads to profound and irreversible systolic and diastolic dysfunction after ischemia and reperfusion in mice (629). In summary, the antiapoptotic effect of insulin following ischemic reperfusion injury is mostly mediated by PI3K/AKT pathway (213), pointing directly toward the protective effect of AKT being influenced by and inextricably tied to glucose metabolism (670).
2. AKT and FOXO
Another emerging pathway through which AKT influences metabolism is by regulating translocation and activity of the forkhead transcription factors (FOXO) subfamily that includes FOXO1, FOXO3a, and FOXO4, which are directly phosphorylated by AKT. FOXO transcription factors participate in control of energy metabolism by regulating insulin signaling and glucose and lipid metabolism (242), although most of the literature regarding FOXO proteins is based on experiments performed on noncardiac cells. For example, in the liver, AKT inhibits gluconeogenesis by blocking FOXO-mediated transcription of gluco-neogenic enzymes, such as phosphenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) (181). However, new experimental evidence proves a central role for the FOXO protein family in the cardiac context as well (556). FOXO1 and FOXO3a expression increases and accumulates in the nucleus during heart development concomitant with cyclin kinase inhibitors (CKIs), p21CIP1, and p27KIP1, inducing cell cycle withdrawal in cardiomyocytes after birth (186). Cardiac-restricted overexpression of wild-type or dominant negative FOXO1 induces embryonic lethality at E10.5 and abnormal morphology of the myocardium by embryonic day 18.5, respectively. These phenotypes are related to premature induction or prolonged suppression of CKIs in the heart and intimates that the PI3K/AKT/FOXO pathway has a central role in heart development (186). In postnatal heart the PI3K/AKT/FOXO axis regulates cardiomyocyte size, with increased phosphorylation levels of AKT and FOXO3a associated with cardiac hypertrophy in vivo, while FOXO3a overexpression reduces IGF-I-mediated hypertrophic effects and decreases cardiomyocyte size in vivo (595). Sustained FOXO proteins overexpression in cardiomyocytes leads to increased AKT phosphorylation and kinase activity without influencing other signaling pathways such as p38, ERK, or JNK (503). AKT and FOXO proteins are also apparently connected through atrogin-1 (a direct target gene of FOXO3a) and the phosphatases PP2A and calcineurin, the latter proposed to target AKT directly (503). Transcriptional induction of atrogin-1 proteosomal factor reduces PP2A and calcineurin phosphatase activity as well as interaction with AKT. The physiological result of this sustained activation is an attenuated insulin response in cardiomyocytes (503).
3. AKT and mTOR
AKT influences protein synthesis through acting upon several translation factors and ribosomal proteins. AKT phosphorylates and inactivates tuberous sclerosis factor 2 (TSC2), thereby inducing formation of active Rheb which, in turn, phosphorylates and activates the mammalian target of rapamycin (mTOR) (318, 542), which is central to protein synthesis and cell growth. Activated mTOR targets 4E-binding protein-1 (4E-BP1) and p70 ribosomal S6 protein kinase (p70S6K) (543). Phosphorylation of 4E-BP1 by mTOR is necessary to ablate its inhibitory function on the eukaryotic initiation factor 4E (eIF-4E), thus promoting the initiation step of protein synthesis. Concurrent activation of p70S6K phosphorylates S6 ribosomal protein that is involved in the regulation of protein translation. Downstream of p70S6K is the eukaryotic elongation factor-2 (eEF2), which upon phosphorylation is inactivated, promoting protein elongation (543).
The relationship between insulin or IGF-I-mediated AKT activation and cardiac cell growth (119, 471) depends on mTOR activation. Specifically, insulin induces TSC2 phosphorylation in adult ventricular cardiomyocytes (555), and the physiological hypertrophic response of NRCMs to T3 thyroid hormone is associated with mTOR activation mediated by AKT (350). In addition, rapamycin attenuates heart overgrowth in transgenic mice overexpressing constitutively activated AKT specifically in the heart (584). Another mechanism by which AKT influences mTOR pathway is associated with the phopshorylation of the proline-rich AKT substrate of 40 kDa (PRAS40), a recently identified mTOR regulator. Once phosphorylated by AKT, PRAS40 binds to 14-3-3, thereby relieving PRAS40-induced inhibition of mTOR and allowing its action on p70S6K (565, 652). In the heart, insulin activates mTOR through the AKT/PRAS40 pathway, while leucine, another strong inducer of mTOR, elicits PRAS40 phosphorylation by a pathway directly dependent on PDK1 activation (543). Although still poorly characterized, the connection between the AKT and mTOR pathways represents a novel entry point for molecular intervention to regulate myocardial hypertrophy and remodeling.
D. Growth/Hypertrophy
By virtue of participation as a nodal kinase in facilitating cellular metabolism and remodeling, AKT has long been recognized as a pivotal participant in hypertrophic signaling (163, 254, 278, 396, 434). Interestingly, AKT expression decreases during pregnancy and normalizes during the post-partum period, suggesting AKT plays an antihypertrophic role in physiological hypertrophy (237). The developmental growth and physiological hypertrophy mediated by AKT signaling stem from upstream induction via class I(A) PI3Ks (435). AKT phosphorylation levels show temporal changes in exercised rats, decreasing at 1 wk and increasing selective phosphorylation of Ser-473 at 3 wk (239). AKT activity is induced by treatment of neonatal rat cardiomyocytes with TNF-α, leading to increased protein synthesis and cellular hypertrophy (290).
Thyroid hormones regulate physiological cardiac hypertrophy acting both as transcriptionally active proteins while also participating in cytoplasmic-initiated signaling processes (210). Thyroid hormones activate PI3K/AKT in cardiomyocytes, which in turn induces the mTOR pathway and increases protein translation (350). Activation/inactivation of AKT/mTOR pathway seems to be related to development of physiological adaptative versus pathological cardiac hypertrophy. Mice subjected to either treadmill training for 6 wk or transverse aortic constriction (TAC) developed physiological versus pathological cardiac hypertrophy associated with activation versus inhibition of the AKT/mTOR signaling pathway (348, 589). Thus the PI3K/AKT axis seems more linked to physiological hypertrophy, whereas MAPK signaling, in collaboration with the PKC and calcineurin/NFAT pathways, participates in the development of the pathological hypertrophy typically induced by angiotensin II (469). AKT also controls cardiomyocyte size by inactivating the FOXO transcription factors that promote the expression of atrophic genes (595). Importantly, recent data suggest that the deregulation of the AKT/FOXO axis can be associated with the development of pathological hypertrophy (407). These results confirm the idea that AKT-dependent hypertrophic heart in vivo is associated with hyperphysiological levels of kinase activity in the cytoplasm resulting in a deregulation of AKT upstream and downstream targets (114, 455, 498, 584). Interestingly, our group demonstrated that AKT localization is crucial to regulating function (347, 562). Overexpression of nuclear targeted AKT enhances cardioprotection and antagonizes cardiac hypertophy (590, 641).
E. Remodeling/Regeneration/Repair
Alterations in AKT activity level are linked to the “reverse remodeling” observed following initiation of left ventricular assist device (LVAD) support in patients suffering from heart failure (27). Decreases in the PI3K/AKT pathway are likely to contribute to molecular changes in aging myocardium associated with enhanced susceptibility to cell death (85). Increased AKT phosphorylation is also associated with exercise (367, 368). Collectively, these observations indicate the central role AKT plays in cardiac remodeling.
Within the last few decades, research into cardiac regeneration has gained traction and paved the way for development of potential therapies targeting cardiac repair following pathological insult. As a prosurvival and proliferative cardiac signal, not surprisingly, the PI3K/AKT pathway participates in almost every aspect of cardiac regeneration. The following sections present various roles of AKT in angiogenesis, myocyte renewal, stem cell activation, and cell based therapies.
1. AKT role in vasculogenesis
As a downstream effector of various angiogenic cytokines and growth factors, AKT is frequently identified as the mechanism underlying cytoprotection and neovascularization conferred by these agents. For example, AKT is thought to mediate the beneficial effects of statins applied to a model of hindlimb ischemia, enhancing proliferation, migration, and survival of bone marrow-derived EPCs (381, 426). Conversely, knockdown of PI3Kγ results in impaired neovascularization and endothelial progenitor function in ischemic hindlimb muscles (439). Cardioprotective effects of the traditional Chinese medicine shu-mai-tang include angiogenesis and arteriogenesis and are thought to be mediated via PI3K/AKT signaling (682). Exogenous nerve growth factor supports angiogenesis and myocyte survival in infarcted murine hearts via the AKT/FOXO pathway (78, 475). CD151 induces endothelial cell proliferation, migration, and neovascularization in infarcted hearts via PI3K/AKT activation (695, 696), while periostin signals through FAK and AKT to mediate recruitment of activated cardiac fibroblasts to sites of cardiac injury following acute myocardial infarction (581). Intracardiac injection of SDF-1a into infarcted mouse heart improves cardiomyocyte survival and increases neoangiogenesis, potentially via activation of AKT (572). VEGF2-treated EPCs have enhanced AKT activation, and infarcted hearts receiving these VEGF-2 treated EPCs exhibit improved angiogenesis and cardiac function compared with control treated hearts (582), while inhibition of AKT by Ox-LDL impairs endothelial differentiation in bone marrow stem cells (102). Collectively, these studies indicate a pivotal role for PI3K/AKT signaling in vasculogenesis following cardiac injury.
2. AKT role in myocyte renewal and stem cell activation
Cardiac stem cells express IGF-I receptor and the IGF-I ligand, rendering them responsive to growth factor treatment in the infarcted myocardium. Stimulation with IGF-I activates AKT in these cells, promoting proliferation and survival, and thereby enhancing cardiac repair (644). IGF-I overexpression in murine heart increases activation of AKT, improves cardiomyocyte survival and renewal, and boosts the population of cardiogenic c-KIT+ progenitor cells. Additionally, studies applying nanofibers coated with IGF-I to infarcted myocardium alone or in combination with adoptively transferred cardiac progenitor cells demonstrate improved survival and regeneration of myocytes and vessels in conjunction with AKT activation (136, 524, 633). Postnatal cardiac myocyte proliferation is extended and progenitor cell cycling enhanced in hearts of mice engineered to overexpress cardiac specific nuclear-targeted AKT (244). Similarly, PIM1, identified as a mediator of cardiac protection downstream of AKT, also promotes cardiac myocyte and progenitor proliferation in hearts of mice engineered to overexpress cardiac specific PIM1 (117).
Cardiac c-KIT+ precursor cells expressing AT2 receptors may trigger AKT and STAT3 survival signaling in damaged myocardium (15). Likewise, cultured rat postinfarct cardiac c-KIT+/estrogen receptor (ER)α cells exhibit increased gene expression of AKT and enhance myocyte survival in coculture with adult rat cardiomyocytes (60).
3. AKT cross-talk with developmental/stem cell signaling pathways
AKT has been shown to activate and be activated by stem cell signaling proteins such as NOTCH and sonic hedgehog (19) and may contribute to the cardioprotective mechanism underlying their regenerative activity in the heart (245). Treatment of infarcted hearts with SHH gene therapy improves cardiac function and upregulates expression of cytokines upstream of PI3K/AKT signaling, notably IGF-I and VEGF, in cardiac fibroblasts (382). PI3K/AKT may also mediate cardiomyocyte differentiation by canonical WNT by suppressing GSK-3β activity. Conversely, AKT counteracts profibrotic canonical WNT signaling during cardiomyogenesis and postinjury repair (482, 499).
4. Paracrine effects of exogenous progenitor cells
Cell-based therapy has emerged as an exciting frontier for the treatment of heart disease. Numerous laboratories are now investigating the reparative potential of various cells types, such as mesenchymal stem cells (MSCs), CPCs, or embryonic stem cells (ESCs). Varying degrees of functional benefit are documented depending on the model system and cell type used, and a key question remains as to whether cell engraftment or paracrine effects of the adoptively transferred cells are responsible for the improvement in cardiac function over control treated hearts.
Adoptive transfer of cardiosphere-derived human cardiac progenitor cells increases AKT protein levels in the infarct region and border zone of recipient mouse hearts. The authors measure the proportion of cardioprotection derived from paracrine effects versus direct regeneration and conclude that both mechanisms contribute to the cardiac improvement observed (98).
Bone marrow-derived MSCs engineered to overexpress AKT repair infarcted myocardium better than lacZ expressing control cells. Subsequent studies claim that paracrine effects, namely, secretion of growth factors and cytokines that promote survival and proliferation, account for cardiac benefits bestowed by these cells. Most recently, secreted frizzled related protein 2 (SFRP2) has been identified as a specific paracrine factor generated by AKT-overexpressing MSCs. SFRP2 acts by inhibiting the pro-apoptotic actions of canonical WNT3a signaling in cardiac myocytes subjected to hypoxia/reoxygenation injury (231–233, 446, 482, 509, 694). Interestingly, IGF-I overexpressing MSCs exhibit paracrine activity and enhanced engraftment when adoptively transferred into infarcted rat myocardium. IGF-I MSCs stimulate activation of AKT in recipient hearts as well as secretion of SDF-1a, which mobilizes and attracts endogenous bone marrow stem cells. Levels of phosphorylated AKT are increased in SDF-1a-treated MSCs, while inhibition of PI3K/AKT prevents SDF-1a/CXCR4-dependent migration of MSCs (257, 684).
Conversely, c-KIT+ bone marrow-derived stem cells lacking AKT1 perform poorly compared with their wild-type counterparts; intravenously injected “armed” wild-type stem cells restore ventricular function, promote angiogenesis, and are retained for at least 2 wk in infarcted mouse hearts, whereas application of “armed” AKT-deficient stem cells confer nominal cardiac benefit if any (639).
F. Aging
Cellular senescence contributes to the decline of cell function during aging. The loss of pro-survival signaling and increased cellular senescence leads to declining function of the heart in old age. The connection between aging and diminution of IGF-I signaling eventually led to examination of AKT-mediated signaling as the critical hub of age-related heart disease (340). Loss of AKT activity correlates with diminished proliferation and development of a senescent phenotype in cardiac fibroblasts (153). Correlates of the aging phenotype are reduced insulin sensitivity and cardiac dysfunction associated with reductions in AKT expression and phosphorylation levels (188, 189).
Oxidative stress contributes a great deal to age-related diseases due to the accumulation of reactive oxygen species. The decrease in survival signaling through AKT leads to sensitivity in ROS-induced apoptosis in the heart, along with many other cell types (313, 316). Decrease in IGF-I signaling decreases CSC division leading to the decrease of functionally competent CSC reserves and the potential of regenerating new myocytes (633). Antagonizing IGF-I and blunted AKT expression leads to the upregulation of pleiotrophin during myocardial infarction as well as dilated cardiomyopathy resulting in an increase of apoptosis (410). Furthermore, exacerbated reperfusion injury in aged female hearts is correlated with blunted AKT activation (313).
AKT-mediated signaling has been shown to act upon different mediators of senescence. Specific cellular proteins correlated with the induction of senescence include p16, p21, p27, and p53. Accumulation of p16 in aged mice is representative of cellular senescence. However, in IGF-I transgenic mice, which have a consistent activation of PI3K/AKT, expression of p16 is blunted in older ages, allowing the assembly of cyclin D and CDK4/6 complexes to form uninterrupted for G1 to S phase transition. AKT has been shown to inhibit p21 through phosphorylation on two sites and allowing for sustained cellular proliferation (413, 697). p27 has been shown to inhibit G1 phase cyclins and CDKs causing cell cycle arrest. The presence of AKT has been shown to phosphorylate p27 on multiple sites, including Thr-198 (205). Phosphorylation of p27 on Thr-198 by AKT promotes binding of 14-3-3 and its cytoplasmic localization and eventually degradation of p27 (205). AKT has been shown to phosphorylate and activate MDM2 ubiquitination activity. Levels of p53 protein are decreased with the presence of AKT through increased ubiquitination of p53 by MDM2 (511).
Aging that prompts downregulation of VEGF, and presumably downstream AKT signaling as well, is blunted by exercise training (313). Aerobic exercise leads to an increase in insulin signaling which activates the AKT/mTOR pathway and enhances muscle protein synthesis (206). AKT has a phosphorylation consensus target sequence within mouse telomerase, and increasing amounts of nuclear AKT increase telomerase activity (633). Furthermore, age-related alterations in AKT expression affect eNOS phosphorylation which, in turn, increases risk of age-associated hypertension (596). Multiple lines of evidence show an increase in AKT phosphorylation on a caloric restricted diet in the heart as well as hepatocytes (1, 316, 428).
G. AKT Isoforms: AKT1 Versus AKT2
Mammalian cells contain three genes that encode for three isoforms of AKT, termed AKT1 (PKBα), AKT2 (PKBβ), and AKT3 (PKBγ). The three isoforms are highly related to each other and are activated by shared pathways via PI3K. All isoforms are expressed in the heart, but AKT1 and AKT2 are the most abundant isoform in the myocardium (459). The distinctions of effects mediated between AKT isoforms add layers of complexity to the delineation of AKT-mediated effects in the myocardium. The advent of genetically engineered AKT knockout models has empowered assessment of the roles played by AKT isoforms in myocardial biology and revealed distinct functions for each protein. In mouse models of global deficiency of AKT1, diminished somatic growth is observed, while AKT2 deficiency causes insulin resistance and diabetes mellitus (100, 101), indicating that AKT2 plays a key role in glucose metabolism. The latter is further confirmed by the existence of a family with an inherited missense mutation in the AKT2 gene, the phenotype of which is associated with severe insulin resistance and diabetes (226). Knockout of AKT3 reduces brain size but has no effects on growth or metabolism (171), whereas cardiac specific transgenic overexpression of AKT3 in the heart leads to maladaptive hypertrophy (622). Phenotypes of mice with ablation of AKT isoforms are summarized in TABLE 1. Studies comparing AKT1 and AKT2 (as the most abundant physiological isoforms in the heart) reveal the split personality of AKT isoforms participating in “physiological” versus “pathological” hypertrophic remodeling (140, 141, 496). Several studies have proven the cardioprotective role of AKT1 in response to pathological challenges, and the impact of AKT1 activity is predominantly in the realm of physiological cardiac growth and antagonized pathological remodeling. Conversly, the loss of AKT1 in this context leads to exacerbated hypertrophic responses consistent with a role for AKT blunting hypertrophy, similar to effects noted for nuclear accumulation of AKT (641). In contrast, AKT2 is dispensable in the development of cardiac hypertrophy in response to physiological or pathological stimuli, but is primarily involved in insulin-stimulated glucose uptake and metabolism as well as cellular survival in response to ischemic injury.
Table 1.
Phenotypic effects on the heart for genetic manipulation of AKT
| Genotype | Phenotype | Reference Nos. |
|---|---|---|
| AKT1 GKO | Viable, reduced size of organs, decreased survival after cardiomyopathic injury. | 91, 141 |
| AKT2 GKO | Normal cardiac phenotype. Insulin resistance, diabetes, pancreatic β-cell failure. | 100, 222 |
| AKT3 GKO | Neurological phenotype, reduced brain size. | 173 |
| Cardiac AKT1 TG | Cardiac hypertrophy. Increased cardiomyocyte cell size. | 114, 455, 584 |
| Nuclear AKT1 TG | Increased cell number, decreased cell size, increased contractile function. | 558, 590 |
| Cardiac AKT3 TG | Maladaptive hypertrophy. | 622 |
III. AKT SIGNALING IN THE MYOCARDIUM
A. Upstream Inductive Signals: Hormones, Cytokines, Drugs, Dietary Agents, Enzymes, Integrins, and Others
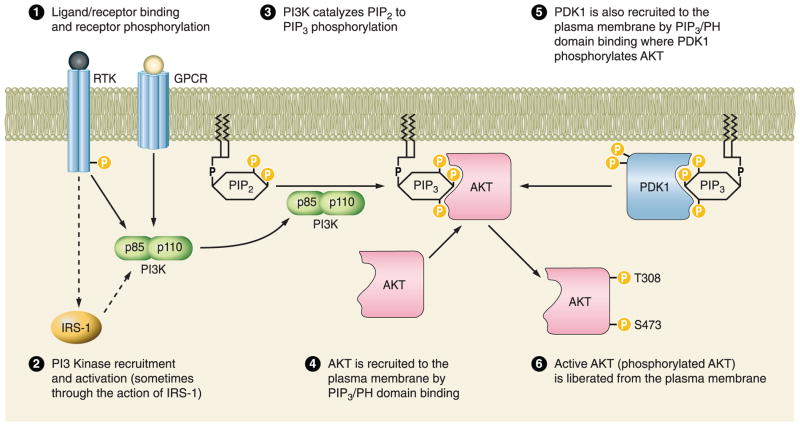
The mechanism of AKT activation in the heart and other systems has been well reviewed in several publications (459, 610). The binding of a ligand (hormone, cytokine, integrin, peptide, or small molecule) causes cell surface receptor intracellular domain phosphorylation (receptor tyrosine kinase, RTK) or receptor conformational change (G protein-coupled receptor, GPCR). The SH2 domain of the p85 subunit of PI3K binds to the activated receptor, bringing the complex into close association with the cell membrane or cardiomyocyte sarcolemma. The p110 catalytic subunit of PI3K catalyzes the phosphorylation of phosphatidylinositol 4,5-bisphosphate (PIP2), which is embedded in the cell membrane, to phosphatidylinositol 3,4,5-trisphosphate (PIP3). Both AKT and 3-phosphoinositide-dependent protein kinase [PDK1, (or PDPK1)] contain a pleckstrin homology (PH) domain which will bind to PIP3 at the cell membrane, bringing the two kinases into close association with the plasma membrane and, hence, each other. AKT is a substrate of the constitutively active kinase PDK1 and will be phosphorylated at serine-473 and tyrosine-308 as well as other sites when the two proteins interact. Active, phosphorylated AKT is then liberated from the sarcolemma and can migrate to different cellular compartments to phosphorylate substrate molecules (FIGURE 1).
FIGURE 1.
Upstream AKT signaling. Schematic diagram representing the receptor-mediated phosphorylation and activation steps required for the ultimate phosphorylation and activation of AKT. GPCR, G protein-coupled receptor; RTK, receptor tyrosine kinase; IRS-1, insulin receptor substrate 1; PI3K, phosphoinositide 3-kinase; PDK1, phosphoinositide-dependent protein kinase-1, PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; PH, plekstrin homology.
A multitude of cardioprotective factors exert their anti-apoptotic action, at least in part, in conjunction with AKT activation. These factors are diverse in nature and can be categorized as hormones, cytokines, integrins, drugs/small molecules, nutrients, as well as others. These categories may overlap and are subject to interpretation. However, here, for the sake of clarity, hormones are generally systemic actors (endocrine) and cytokines are local actors (paracrine/autocrine). The list of AKT activators described below is representative but by no means exhaustive. See TABLE 2 for a summary of the upstream AKT activators, receptors, and their respective reported effects.
Table 2.
Summary of AKT upstream inductive signaling
| Category | Name | Receptor/Target | Receptor Type | Effect | Reference Nos. |
|---|---|---|---|---|---|
| Endocrine | Adrenomedullin | Calcitonin receptor-like | GPCR | Anti-apoptotic, multiple cardioprotective mechanisms |
675 |
| Endocrine | Angiotensin II | Angiotensin II receptor | GPCR | Anti-apoptotic, hypertrophic, vasoactive |
128, 130, 228, 285 |
| Endocrine | Atrial natriuretic peptide (ANP) | Natriuretic peptide receptor A/B/C |
Guanylyl cyclase | Anti-apoptotic | 347 |
| Endocrine | Erythropoietin | EPO receptor | Hematopoietin receptor superfamily |
359, 515, 635 | |
| Endocrine | Estrogen | Estrogen G protein-coupled receptor |
GPCR | Anti-hypertrophic, anti-apoptotic |
306, 532, 534 |
| Endocrine | Ghrelin | NR | N/A | Anti-apoptotic | 33 |
| Endocrine | Growth hormone (GH) | GH receptor | RTK | Hypertrophic | 432 |
| Endocrine | Insulin | Insulin receptor, IGF1 receptor |
RTK | Anti-apoptotic | 12, 48, 213 |
| Endocrine | Resistin | Unknown | N/A | Anti-apoptotic | 214 |
| Endocrine | Thyroid hormone | Thyroid receptorα1 | Intracellular | Hypertrophic, anti-apototic |
350, 386, 388 |
| Cytokine | Angiopoetin | Integrins, Tie2 | Anti-hypertrphic | 122 | |
| Cytokine | Cardiotrophin | gp130/LIFR | Anti-apoptotic | 59, 385 | |
| Cytokine | Granulocyte colony-stimulating factor (G-CSF) |
G-CSF receptor | Hematopoietin receptor superfamily | Anti-autophagic, myocardial regeneration |
415, 486 |
| Cytokine | Insulin-like growth factor I (IGF-I) | IGF1 receptor | RTK | Anti-apotpotic, hypertrophic | 158, 298, 411, 672 |
| Cytokine | Interleukin-18 | IL-18 receptor | Immunoglobulin superfamily | Not antiapoptotic, hypertrophic |
88, 112 |
| Cytokine | Leukemia inhibitory factor (LIF) | LIF receptor (CD118)/gp130 | RTK | Anti-apoptotic, hypertrophic | 289, 502 |
| Cytokine | Neuregulin-1 | ErbB3/4 | RTK | Anti-apoptotic, hypertrophic, contractility | 207, 404, 631 |
| Cytokine | Platelet-derived growth factor (PDGF) | PDGF receptor | RTK | Anti-apoptotic, hypertrophic, pro-proliferative | 286, 300 |
| Cytokine | Stromal cell-derived factor 1 | CXCR4 | GPCR | Anti-apoptotic | 302, 572 |
| Cytokine | Urocortin | CRF-R2 | GPCR | Anti-apoptotic | 58 |
| Cytokine | WNT1-induced Secreted protein-1 | Hypertrophic, pro-fibrotic | 113 | ||
| Other: peptide | Bradykinin | Via epidermal growth factor receptor | RTK | Anti-apoptotic | 108, 478 |
| Other: peptide | Endothelin (ET) | ET-(A, B1, B2) | GPCR | Anti-apoptotic, vasoactive | 577 |
| Other: peptide | Thymosin β4 | Integrin linked kinase | Integrin linked kinase | Anti-apoptotic | 54 |
| Drug/small molecule | Acetylcholine | Muscarinic Acetylcholine receptor | GPCR | Anti-apoptotic | 339, 371 |
| Drug/small molecule | Adenosine/Adenosine-like agonists | Adenosine A1/A3 receptor | GPCR | Anti-apoptotic, vasoactive | 227 |
| Drug/small molecule | β2-Adrenergic agonists, zinterol | β2 Adrenergic receptor | GPCR | Anti-apoptotic | 96 |
| Drug/small molecule | Cannabinoids | Cannabinoidβ1 receptor | GPCR | NR | 284 |
| Drug/small molecule | Ceramide | NR | N/A | Anti-apoptotic | 127 |
| Drug/small molecule | Cruzipain | NR | N/A | Anti-apoptotic | 24 |
| Drug/small molecule | Eplerenone | Mineralocorticoid receptor antagonist | Intracellular | NR | 363 |
| Drug/small molecule | Glucan phosphate | NR | N/A | Anti-apoptotic | 252 |
| Drug/small molecule | Isofluorane and related compounds | Via adenosine A1 receptor | GPCR | Anti-apoptotic | 324, 557, 698 |
| Drug/small molecule | Lipopolysaccharide | Toll-like receptor 4 | Toll-like receptor | Anti-apoptotic | 253, 282 |
| Drug/small molecule | Morphine | Opioid receptor | GPCR | NR | 243 |
| Drug/small molecule | N,N-dimethylsphingosine (DMS) | Via epidermal growth Factor receptor | RTK | NR | 329 |
| Drug/small molecule | Ouabain | Na+-K+-ATPase | Ion Pump | Hypertrophic | 424 |
| Drug/small molecule | Phenylephrine | α1 Adrenergic receptor | GPCR | Hypertrophic | 106 |
| Drug/small molecule | Rosiglitazone | PPAR gamma | Intracellular | Anti-apoptotic | 356, 691 |
| Drug/small molecule | S-nitroso-N-acetylpenicillamine (SNAP) | NR | N/A | NR | 384 |
| Drug/small molecule | Statins | RhoA inhibition | N/A | Anti-hypertrophic, anti-apoptotic | 268, 417 |
| Drug/small molecule | VO(OPT), bis(1-oxy-2-pyridinethiolato) oxovanadium(IV) | Tyrosine phosphatase inhibitor | N/A | Anti-apoptotic | 50, 51 |
| Dietary agent | Ginsenoside | NR | NR | Anti-necrotic | 615 |
| Dietary agent | Myricetin | NR | N/A | Vasoactive | 20 |
| Dietary agent | Phytoestrogen | Estrogen receptorα | Intracellular | Anti-hypertrophic | 221 |
| Dietary agent | Polyphenols | NR | N/A | Angiogenic, free radical scavenger | 36, 164 |
| Dietary agent | Resveratrol | Adenosine A3 receptor | GPCR | Anti-apoptotic, anti-hypertrophic | 129, 131, 234 |
| Enzyme: phosphatase | Calcineurin | N/A | N/A | Anti-apoptotic, hypertrophic | 139, 279 |
| Enzyme: kinase | cGMP-dependent protein kinase G | N/A | N/A | Anti-apoptotic, anti-necrotic | 125 |
| Enzyme: kinase, Heat shock protein | H11 kinase | N/A | N/A | Hypertrophic | 149, 267 |
| Enzyme | Heme oxygenase-1 | N/A, ANG II required | N/A | Anti-apoptotic | 198 |
| Enzyme | Kallekrein-kinin | ACE/kinin B2 receptor | Transmembrane Zinc Metallopeptidase | Anti-apoptotic, anti-hypertrophic | 5, 408 |
GPCR, G protein-coupled receptor, RTK, receptor tyrosine kinase, NR, not reported, N/A, not applicable.
Hormones and cytokines are the classically described activators of AKT signaling including the following: adrenomedullin (675), angiotensin II (ANG II) (128, 130, 228, 285), atrial natriuretic peptide (ANP) (347), erythropoietin (359, 515, 635), estrogen (306, 532, 534), ghrelin (33), growth hormone (GH) (432), insulin (12, 48, 213), resistin (214), thyroid hormone (350, 386, 387), angiopoetin (122), cardiotrophin (59, 385, 486), granulocyte colony-stimulating factor (G-CSF) (415, 486), IGF-I (158, 298, 411, 672), interleukin-18 (88, 112), leukemia inhibitory factor (LIF) (289, 502), neuregulin-1 (207, 404, 631), PDGF (286, 300), stromal cell-derived factor 1 (SDF-1α) (302, 572), urocortin (58), and WNT1-induced secreted protein-1 (WISP1) (113). Small signaling peptides bradykinin (108, 478), endothelin (577), and secreted thymosin β4 activate (54) AKT via indirect mechanisms. A majority of the hormone and cytokine factors act through AKT to induce hypertrophy or repress apoptosis.
Ingestion of compounds such as pharmacological agents and nutritional supplements can also activate AKT. Some small molecules that are available on the legitimate or not-so-legitimate market that have been shown to activate AKT in the context of the heart are as follows: acetylcholine (339, 371), adenosine or adenosine-like agonists (227), β2 adrenergic agonists (96), cannabinoids (284), eplerenone (363), phenylephrine (106), rosiglitazone (356, 692), and the statin-class molecules (268, 417). Isofluorane and related compounds (324, 557, 698) as well as morphine (243) have been shown to activate AKT in conjunction with anesthesia-induced cardioprotection. There is an abundance of research on investigational new classes of compounds, some of which are from unexpected sources. Interestingly, two compounds from pathogenic agents mediate cardiomyocyte survival in part via activation of AKT, a Trypanosoma cruzi glycoprotein known as cruzipain (24) and lipopolysaccaride (253, 282). An immunomodulatory agent, glucan phosphate, has also been show to preserve myocardium and activates AKT (252). Low dose N,N-dimethyl-sphingosine (DMS), a sphingosine kinase inhibitor, enhances epidermal growth factor receptor signaling leading to an increase in AKT activity (329). Exogenous treatment with ceramide, a sphingomyelin breakdown product, has also been show to be cardioprotective in a manner similar to ischemic preconditioning (127). The positive ionotrope oubain, which is toxic in high doses, induces hypertrophy via AKT in low doses (424). Treatment with the NO donor S-nitroso-N-acetylpenicillamine (SNAP) induces phospho-AKT along with vasoactive effects (384). VO(OPT), bis(1-oxy-2-pyridinethiolato)oxovanadium(IV), is a tyrosine phosphatase inhibitor that increases phospho-AKT related to an increase in insulin receptor phosphorylation (50, 51). Vanadyl sulfate, a VO (OPT) precursor, is currently marketed as an insulin mimetic and sports-nutritional supplement. Natural chemical compounds ingested nutritionally such as the flavinoid myricetin (20), polyphenols found in wine (36) and green tea (164) as well as phytoestrogens from soy (221) or ginsenoside Re (615), derived from ginseng root, induce AKT activation. Resveratrol, found naturally in red wine, activates AKT and is reported to be both anti-apoptotic and anti-hypertrophic in the myocardium (129, 131, 234).
Exogenous overexpression of certain enzymes also results in cardioprotection associated with an increase in phospho-AKT. Calcineurin, a calcium/calmodulin regulated phosphatase, is pro-hypertrophic but also anti-apoptotic when adenovirally or genetically overexpressed in the myocardium (139, 279). Cardiomyocytes transduced with cGMP-dependent protein kinase G show increased phospho-AKT as well as resistance to both necrosis and apoptosis (125). H11 kinase mediates hypertophy via AKT but is reportedly toxic at high doses (149, 267). Exogenous overexpression of heme oxygenase-1 can augment activated AKT levels induced by other agents (198). The enzyme kallikrein cleaves kininogen to the protective peptide kinin. Overexpression of kallekrein increases phospho-AKT levels and is anti-apoptotic in myocardial infarction models (5, 408).
Extracellular stimuli influence AKT activation by nonparacrine mechanisms such as mechanotransduction (57) or cell-cell contact. Mechanical stress induced by regional ischemia, inflation of an intraventicular balloon, or creation of an aortocaval shunt all lead to increased AKT activation (357). A muscle specific β1-integrin interacting protein named melusin appears to exert cardioprotective properties linked to AKT activation (137). Myocardial hypoxia followed by reperfusion is a powerful trigger for AKT activation (90). However, not all cells in the myocardium will respond comparably, as stimuli mediating AKT activity (234) are likely to show context-dependent cell type differences such as those observed in cardiac fibroblasts (111). Osmotic stress can also activate stress kinases including AKT such as hyperosmolarity induced by sorbitol or mannitol (212). In congruence with AKT’s role as a central mediator of growth and survival signaling, it is to be expected that the wide variety of signals described above would act as upstream inductive signals to AKT activation.
B. Antagonists: GSK3β, PTEN
The dependence of AKT activity on upstream regulation by PI3K had been demonstrated in numerous studies. The production of phosphoinositides by PI3K is reversed by phosphoinositide phosphatases. Protein phosphatase and tensin homolog (PTEN) deleted on chromosome 10 possesses phosphoinositide phosphatase activity, and activation of PTEN results in inactivation of AKT. PTEN protein levels are decreased in conjunction with preconditioning concomitant with increased AKT activation, supporting reciprocity in the PTEN antagonism of AKT activity (74). PTEN also participates in regulation of hypertrophic remodeling and influences contractility via effects on PI3K signaling that lies upstream of AKT activity (119). In PTEN null hearts, there is an increased level of phospho-AKT/PKB (serine-473), and the inactivation of PTEN in cardiomyocytes PTEN results in hypertrophy. The hypertrophy found in PTEN-deficient hearts displayed features characteristic of physiological hypertrophy, such as increase in both the length and width of the myocytes, no fibrotic changes, and no decompensation into dilated cardiomyopathy. Recently, it was shown that loss of PTEN prevents the development of mal-adaptive ventricular remodeling with preservation of angiogenesis and metabolic gene expression in response to pressure overload (521). Consistent with the critical role for AKT in cell survival, gain of PTEN activity leads to enhanced apoptosis, and increased expression of PTEN induces an expected increase of apoptosis in neonatal cardiomyocytes (578).
GSK-3 is a serine/threonine kinase that phosphorylates and inactivates glycogen synthase, and the ability of AKT to inhibit GSK-3β via phosphorylation and repressive effects of GSK-3β upon AKT actions is a classic study in reciprocal molecular antagonism (264). GSK-3 has two mammalian isoforms: GSK-3α and -β, which are both expressed in heart. GSK-3β is constitutively active in unstimulated cells where it phosphorylates several targets (in addition to glycogen synthase) including cyclin D, c-Jun, NFAT proteins, and β-catenin leading to their inactivation and/or degradation. Phosphorylation of serine-9 residue in NH2-terminal region of GSK-3β by AKT inhibits GSK-3β, thereby leading to diverse effects including improved cell survival and hypertrophy, and improves contractile function in pressure-overloaded hearts, implying the activity of AKT as a cardio-protective mechanism (35, 261).
Direct dephosphorylation and inactivation of AKT is mediated by other phosphatases and inhibitory interactions. Another negative regulator of AKT activity in cardiomyocytes are 14-3-3 proteins and poly (ADP-ribose) polymerase 1 (PARP). 14-3-3 proteins are a family of regulatory molecules that are found ubiquitously in eukaryotes. 14-3-3 proteins inhibit cardiomyocyte hypertrophic responses, and AKT activity is also blunted by the 14-3-3 proteins that inhibit hypertrophy (418). The PARP family of enzymes has many intracellular functions, including transcriptional regulation, detection of DNA strand breaks and initiation of repair to damaged DNA. Inhibition of PARP resulted in a significant increase in phospho-AKT, and inhibition of PARP helps protect the cardiomyocyte from impaired function following ischemia (215, 370). Pleotrophin is a developmentally regulated cytokine and AKT antagonist. Pleiotrophin antagonizes IGF-I associated Ser-473 phosphorylation of AKT/PKB, and it concomitantly decreases phosphorylation of downstream AKT targets such as BAD and GSK-3 (410). Protein-tyrosine-phosphatase-1B overexpression (PTP1B) negatively regulates insulin signaling leading to inhibition of AKT phosphorylation (175). However, the exact role of PTP1B in cardiomyocytes remains to be defined. Three less characterized pathways that alter AKT activity are TNF-α, MyD88, and Toll-like receptor4 (TLR4). Growth factor TNF-α overexpression results in inhibition of AKT that was dependent on upregulation of NFκB (283). Inhibition of the MyD88 pathway also protects the myocardium from ischemia reperfusion injury via activation of AKT (304) and deletion of TLR4 results in enhanced AKT-dependent cardioprotection (305). The exact role of these pathways and their contribution to altered AKT signaling in disease states also remain to be defined.
C. Downstream Target Molecules: GSK, TORC, FOXO, BCL, BAD, etc
The most widely studied downstream target of AKT is GSK-3, a proline-directed serine/threonine kinase that regulates a wide range of cellular processes including glycogen metabolism, gene transcription, protein translation, and cell apoptosis. GSK-3 has two isoforms in mammalian cells, GSK-3α (51 kDa) and GSK-3β (47 kDa). AKT phosphorylates both GSK-3α (Ser-21) and GSK-3β (Ser-9) to inhibit their activity. Overexpression of a constitutively active phosphomimetic mutant of AKT (E40K) induces cardiac hypertrophy by phosphorylation of GSK-3β and upregulation of GATA4 (114), although adenoviral injection (460) or transgenic overexpression (455, 584) of another constitutively active AKT, AKT-myr, enhances kinase activity without phosphorylation of GSK-3β. Both GSK-3α and GSK-3β are expressed in mammalian heart and negatively regulate cardiac hypertrophy, but most studies are focused on GSK-3β. GSK-3β localizes predominantly in the cytosol but is also found in the nucleus and mitochondria. Under basal unstimulated conditions, GSK-3 is highly active and inhibits glycogen synthesis by phosphorylation of glycogen synthase. GSK-3 negatively regulates gene transcription and protein translation by phosphorylation of a range of transcription regulators (NFAT, GATA4, myocardin, c-Myc, c-Jun, β-catenin) and translation initiation factor eIF2B. GSK-3 phosphorylates NFAT and promotes nuclear export (39, 647) as well as proteasomal degradation of NFAT (683). Cardiac-specific expression of GSK-3β attenuates pressure overload-induced hypertrophy by inhibiting the increase of nuclear NFAT (22). Cardiac transcription factor GATA4 is also exported from the nucleus after phosphorylation by GSK3β (490). Myocardin, another cardiac-specific transcription factor, is also phosphorylated by GSK3β, which reduces intrinsic myocardin transcriptional activity and related hypertrophy (30). Inhibition of PI3K/AKT signaling activates GSK-3, which accumulates in the nucleus (40), where GSK-3 phosphorylates c-Myc on Thr-58, thereby promoting its ubiquitination and degradation (241, 308). Similarly, phosphorylation of c-Jun by GSK3 resulted in binding of E3 ligase Fbw7, which targets c-Jun to proteasomal degradation (665). Inhibition of GSK-3 activity by AKT is critical to hypertrophic stimulus-induced stabilization of the transcriptional activator β-catenin (263). Eukaryotic initiation factor eIF2B, which regulates the initiation of mRNA translation, can be phosphorylated and inactivated by GSK-3 (666). Phosphorylation of eIF2B inhibits protein function and, in turn, accounts for the anti-hypertrophic effect of GSK-3β (265). The result of eliminating GSK-3β is hypertrophic cardiomyopathy in knock-out mice associated with increased expression of GATA4, cyclin D1, and c-Myc (353). In addition to inhibition of cardiomyocyte hypertrophy, GSK-3β also promotes apoptosis by the intrinsic mitochondrial pathway (464, 662) in cardiomyocytes (477). Cardiac-specific overexpression of dominant negative GSK-3β induces compensatory hypertrophy and inhibits apoptosis by myeloid cell leukemia-1 (291). Similarly, GSK-3α is also antihypertrophic and pro-apoptotic, but apparently through a different mechanism, i.e., inhibition of ERK activity (687). During zebrafish cardiogenesis, the deletion of GSK-3α increases cardiomyocyte apoptosis, whereas deletion of GSK-3β disrupts left-right asymmetry and heart positioning (400). While phosphorylation of GSK-3β (S9) mediates pathological hypertrophy, phosphorylation of GSK-3α (S21; predominantly in nucleus) negatively regulates hypertrophy during pressure overload (453). Differential remodeling responses occur following mutation of GSK-3α or GSK-3β, resulting from altered phosphorylation at AKT target residues of GSK-3α (S21A) or GSK-3β (S9A) when expressed in mice. As research progresses, more differences between GSK-3α and GSK-3β are likely to be revealed.
The Forkhead (FOXO) family of transcription factors are well-known AKT targets. FOXO factors regulate transcription of several genes possessing the 5′-TTGTTTAC-3′ sequence in their promoter region (211). Cell cycle regulators p27kip (cyclin dependent kinase inhibitor) and p130 are influenced by FOXO, along with proapoptotic molecules BIM and Fas ligand. AKT phosphorylates FOXO1, FOXO3a, and FOX4, resulting in export from the nucleus and attenuation of FOXO-mediated apoptosis (664). Phosphorylation of FOXO factors by AKT creates docking sites for subsequent interaction with 14-3-3 proteins, leading to cytosolic sequestration as a mechanism to inhibit proapoptotic function. Nuclear targeted AKT increases cytosolic FOXO levels, potentially facilitating protection against ischemic injury in mice overexpresssing nuclear AKT (76, 620).
Telomere maintenance is influenced by AKT via phosphorylation of telomere repeat binding factor 1 (TRF1) (94) and telomerase (TERT) (256). These phosphorylation events seem to have opposing effects depending on cell type. Telomeres shorten when AKT phosphorylates TRF1 in HEK293T cells (94), whereas TERT phosphorylation increases enzyme activity and has been shown to be protective in cardiac cells (513, 514). Further studies will need to elucidate the role of AKT in relation to genomic stability, specifically telomere preservation.
The BCL-2 family member BAD (BCL-xL/BCL-2 associated death promoter) contributes to cellular apoptosis by heterodimerizing with BCL-xL/BCL-2 and neutralizing their protective effect (676). AKT phosphorylation of BAD at Ser-136 disrupts the dimerization between BAD and BCL-xL (133, 144) and inhibits apoptosis (438). Phosphorylated BAD is sequestered in the cytosol through binding to 14-3-3 (686). In cultured cardiomyocytes, cardiotrophin-1 promotes survival by phosphorylation of BAD through a PI3K/AKT-dependent pathway (385). Leukemia inhibitory factor (LIF) prevents doxorubicin-induced cardiomyocyte apoptosis by PI3K-mediated phosphorylation of BAD, disrupting heterodimerization of BAD with BCL-xL (502). In adult heart, doxorubicin upregulates phosphatase 1, which dephosphorylates AKT and its downstream target S136-BAD (187). Cardiac resynchronization of dogs with dyssynchronous heart failure is accompanied by increased AKT activity, marked BAD phosphorylation, and enhanced BAD/14-3-3 interaction (87). Kallikrein gene delivery attenuates ischemia/reperfusion-induced cardiomyocyte apoptosis through increased phosphorylation of AKT and BAD (S136) (681). Thus antagonism of BAD by AKT-mediated phosphorylation plays a central role in regulating cell survival.
TOR (target of rapamycin), a serine/threonine kinase, was originally discovered by Heitman and colleagues in a genetic screen of yeast mutants whereby resistance to growth was conferred via inhibition of the immunosuppressant complex FKBP (FK506 binding protein)-rapamycin (280). The corresponding 289-kDa mammalian homolog mTOR was then identified (61, 99, 563) and confirmed as a novel downstream target of AKT (501).
mTOR serves as a central node in multiple tissue types, including the heart, for cellular signaling particularly in terms of “sensing” environmental stimuli including, but not limited to, nutrient availability (such as insulin, glucose, and amino acids), growth factors (such as PDGF and EGF), and hypoxia (as observed within infarction after heart attack) (reviewed in Ref. 395). Upon examination of these stimuli and their effects on mTOR, researchers have elucidated a more complex signaling mechanism between AKT and mTOR. First, the tuberin (TSC2)/hamartin (TSC1) tumor suppressor protein complex was identified as a key modulator between AKT and its activation of mTOR. Upon activation of AKT, TSC2 is phosphorylated, thereby disrupting its association with TSC1. Disruption of this complex is accompanied by activation (i.e., phosphorylation) of mTOR (218, 319, 541). Further research into the TSC2/TSC1 protein complex has led to the discovery of another intermediary between AKT and mTOR: the small GTPase, Rheb (Ras homologue enriched in brain). Once the TSC2/TSC1 complex is dissociated, phosphorylated TSC2 activates the GTP form of Rheb, thereby allowing Rheb to directly bind to and activate mTOR (414, 429, 626, 693).
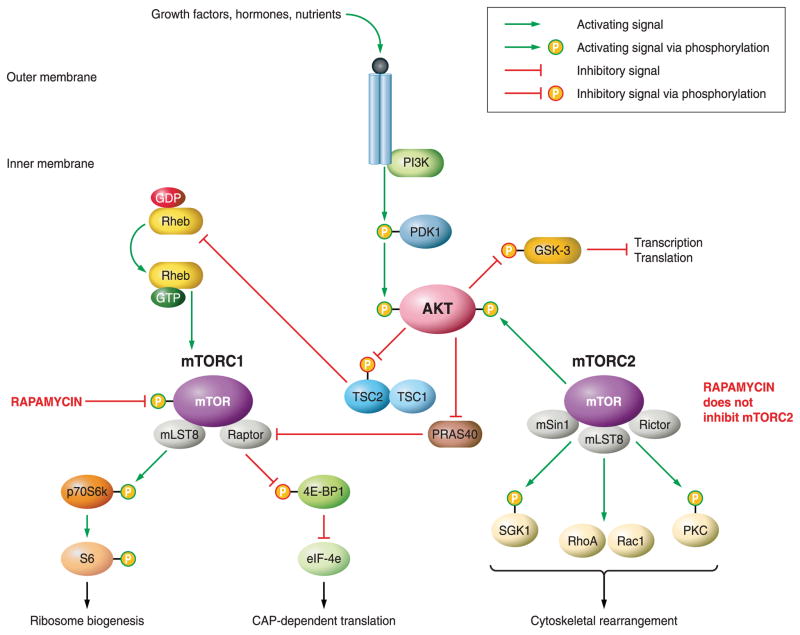
Signaling through PI3K-AKT-TSC1/2-Rheb was considered to be the main avenue through which most activating stimuli are transduced to mTOR. However, recent studies have indicated another novel mechanism by which AKT signaling bypasses the TSC2-Rheb portion to directly activate mTOR. PRAS40 (proline-rich AKT/PKB substrate of 40 kDa) was identified through coimmunoprecipitation experiments as a negative regulator of mTOR. Researchers illustrated that under basal conditions PRAS40 binds to mTOR to inactivate it, and that mTOR inactivation is relieved when insulin stimulation activates AKT to phosphorylate PRAS40, thereby initiating release from mTOR (566, 651). Furthermore, coimmunoprecipitation studies have revealed two functionally distinct mTOR complexes: mTORC1 and mTORC2 (273). mTORC1 associates with Raptor and mLST8, creating a complex that is sensitive to the mTOR inhibitor rapamycin (668). mTORC2 binds to Rictor, mSIN1, and mLST8 to form a complex that is considered rapamycin insensitive (568) unless treated chronically (569). Most of the signaling in the myocardium between AKT and mTOR has been observed through mTORC1, and this portion of the review will focus on those interactions. However, it is important to note that full activation of AKT to signal to mTORC1 is necessitated through phosphorylation via mTORC2 (FIGURE 2).
FIGURE 2.
Pathways of AKT influencing the mTOR protein complexes. Schematic diagram representing the regulatory functions of the mTORC1 and mTORC2 complexes in relation to AKT signaling and cellular outcomes. Induction of AKT activity by extracellular signals results in the activation of mTORC1. mTORC2 activity positively regulates AKT activity. Green arrows represent positive regulation. Green arrows leading to phosphorylation represent activation via phosphorylation. Red arrows represent negative regulation. Red arrows leading to phosphorylation represent repression via phosphorylation.
Activation of mTORC1 has been linked to numerous cancers and proliferative cell disorders including myocardial hypertrophy (reviewed in Ref. 246). Regulation of mTORC1 via AKT is central to coordinating the regulation of two important cellular processes: 1) cell size and mass and 2) cellular proliferation/cell cycle progression. Upon activation of mTORC1, two downstream targets are dually affected with opposing end-target effects. One downstream target, p70S6k, is phosphorylated and directly activates the ribosomal protein S6, a component of the 40S ribosomal subunit. Activation of S6 ultimately leads to increased ribosomal biogenesis and activated metabolism (reviewed in Ref. 325). Decreased cell size, as observed in Drosophila (690) and mammalian models (472, 535, 580), has been associated with inactivating mutations in p70S6k. Another downstream target, 4E-BP1, is inactivated by phosphorylation via mTOR. 4E-BP1, when hypophosphorylated, binds to and inactivates elongation initiation factor 4E (eIF4E), thereby inhibiting CAP-dependent translation. Inactivation/phosphorylation of 4E-BP1 therefore allows for activation of protein translation and ultimately cellular proliferation (reviewed in Ref. 325). Consequently, regulation of mTOR is central to the coordinated regulation of both cellular proliferation (via p70S6k and 4E-BP1) and cell size (via p70S6k).
The role of mTOR in the myocardium, particularly with regard to cardiac hypertrophy, has attracted increasing interest within the last 10 years. Several studies have indicated a crucial role for AKT-mTORC1 signaling in the heart. Initial studies of insulin growth factor (IGF-I) overexpression in the heart proved that PI3K/AKT signaling is crucial in the development of cardiac hypertrophy (548). Research into heart-specific (under the control of the α-myosin heavy chain promoter) murine models of either overexpressed (584), constitutively activated (116), or membrane localized (via myristoylation) (456) AKT further confirmed the role of PI3K/AKT in cardiac hypertrophy. However, the link between AKT/mTORC1 signaling and cardiac hypertrophy was first established by observation of AKT/mTORC1 pathway activation in cultured cardiac myocytes (512). Subsequent studies confirmed AKT signaling through mTOR produces myocardial hypertrophic growth (TABLE 3). Even now, current models of cardiac hypertrophy demonstrate increased AKT/mTORC1 signaling [i.e., hypercholesterolemia (387), spontaneous hypertension (230)].
Table 3.
Cardiac hypertrophy models targeting AKT/mTOR signaling
| Hypertrophy Model | Model System | Protein Studied | Effect on Myocardium | Reference Nos. |
|---|---|---|---|---|
| Cardiac IGF-I | Murine | AKT; p70S6k | Hypertrophy; increased cardiomyocyte proliferation | 548 |
| Whole body OE-AKT | Murine | AKT | Hypertrophy | 585 |
| Whole body AKT KO | Murine | AKT | Pathological hypertrophy; reduced body size | 142 |
| Cardiac CA-AKT | Murine | AKT; p70S6k | Hypertrophy; reduced contractility; increased cardiomyocyte size | 116 |
| Cardiac Myr-AKT | Murine | AKT; p70S6k | Hypertrophy; contractility not affected | 116; 456 |
| Cardiac DN-AKT | Murine | AKT; p70S6k | Reduced heart and cardiomyocyte size; contractility not affected | 585 |
| Whole body p70S6k KO | Drosophila; murine | p70S6k | No hypertrophy; reduced body size and cell size | 690; 535, 580* |
| Cardiac CA-GSK3β | Murine | GSK-3β | Hypertrophy | 21 |
| Cardiac inducible GSK3β | Murine | GSK-3β | Reversal of hypertrophy | 564 |
| Thyroid hormone | Rat | AKT; mTOR | Hypertrophy | 387 |
| Hypercholesterolemia | Swine | mTOR | Hypertrophy | 230 |
| Hypertension | Rat | mTOR | Hypertrophy | 598 |
OE, overexpressed; CA, constitutively active; Myr, myristoylated; DN, dominant-negative, KO, knock-out.
Rapamycin has been touted in the cardiology field as an important therapeutic strategy for preventing restenosis (194). From favorable responses observed in treating patients with rapamycin-treated stents (272), several studies have validated that pharmacological inhibition of mTOR (via rapamycin and/or rapamycin analogs) reduces hypertrophic remodeling observed in cardiovascular disease (such as hypertensive-, diabetic-, or hypercholesteremic-induced cardiac hypertrophy) (73, 355, 470, 586). Preclinical trial research continues to assess feasibility of rapamycin as a treatment for cardiovascular disease.
Signaling through AKT inevitably leads to changes in both gene expression as well as metabolism that are inextricably linked (reviewed in Ref. 105). Intracellular NO production depends on AKT activity (143). Control of metabolic signaling is also involved, as AKT activity regulates insulin-induced regulation of 6-phosphofructo-2 kinase in the heart (150). AKT activity has been linked to antagonizing β1-adrenergic receptor activity by promoting internalization (225). AKT activity phosphorylates and inhibits the action of GSK-3β, thereby allowing for stabilization of β-catenin signaling (263). Inhibition of AKT signaling blocks induction of VEGF gene expression in cardiomyocytes (277). AKT also blunts activation of AMP-activated protein kinase (AMPK) α by phosphorylation (299), and AKT activation can lead to decreased AMPK activity (369). AKT also phosphorylates and activates p70S6 kinase, resulting in cardioprotection (333, 334).
D. Consequences for Protein Expression/Repression
Consequences of AKT activation for gene expression have been studied in transgenic mice engineered with cardiac-specific expression of myristoylated AKT resulting in a broad range of effects on genes controlling cardiomyocyte survival, metabolism, and growth (115). Cataloging these effects of altered AKT expression provides interesting insights into consequences of aberrant activity, with the caveat that the resultant listing of target genes is likely to be skewed by the nonphysiological timing, level of induction, and profound remodeling of the myocardium resulting from chronic AKT activity. Activation of cardiac AKT increases the anti-apoptotic protein FSTL1 (517)and insulin-like growth factor-binding protein-5 (IGFBP-5) and decreases PPARα/PGC-1α transcripts, which plays a critical role in myocardial energy metabolism (115). Physiological cardiac growth, which is accompanied by increased PPARα/PGC-1, is associated with increased fatty acid and oxygen consumption. Conversely, pathological hypertrophy is related to decreased PPARα-PGC-1α expression and a shift towards glycolysis that allows continued ATP production with less oxygen consumption (172). Activation of AKT increases sarcolemmal expression of GLUT4, leading to higher levels of glucose uptake and cardiac metabolism (460). By using an inducible AKT transgenic mouse model, Schiekofer et al. (576) showed that acute AKT1 activation (2 wk) that changes expression of 826 transcripts results in reversible hypertrophy with maintained contractility. In comparison, chronic AKT1 activation (6 wk) that changed expression of 1,611 transcripts leads to severe cardiac hypertrophy and dysfunction (576). In another report, chronic AKT activation induces dramatically larger infarcts in response to ischemia-reperfusion through feedback inhibition of PI3K activity by decreasing insulin receptor substrate-1 (IRS-1) (498). Administration of insulin (213, 334) or IGF-I (136, 203) reduces postischemic myocardial apoptotic death and infarct size by activating the PI3K/AKT signaling pathway. Loss-of-function experiments have also been utilized to study the physiological effects of AKT. Knockout of AKT1 gene results in growth retardation and increased spontaneous apoptosis in mice (92, 101). Knockout of AKT2 leads to insulin resistance (100, 222) and enhanced apoptosis in response to myocardial ischemia (140). Double knockout of AKT1/AKT2 causes severe deficiency in development of skin, bone, and skeletal muscle and mice die shortly after birth (536). Combined deletion of AKT1/AKT3 leads to embryonic lethality with severe developmental defects in the cardiovascular and nervous systems (679). The survival of single knockout mice suggests functional redundancy among the three AKT isoforms. PI3K activates AKT through phosphorylation of PIP2 to form PIP3. Class IA PI3Ks (PI3Kα, -β, and -δ), which are activated by receptor tyrosine kinases (RTKs) in response to cytokines/growth factors (insulin, IGF-I, etc.), regulate physiological growth during development. In contrast, class IB PI3K (PI3Kγ), which is activated by G protein-coupled receptor (GPCR) agonists (endothelin-1, ANG II, α-AR and β-AR agonists) and pressure overload, leads to pathological hypertrophy (522). At basal conditions, cardiac-specific expression of constitutively active PI3Kα results in larger hearts, while dominant-negative PI3K results in smaller hearts (583). However, mice expressing a dominant-negative PI3K (p110α) mutant display significant hypertrophy in response to pressure overload but not exercise training (473). Subsequent studies using PI3K (p110α) overexpressing transgenic mice have shown that PI3Kα blunts cardiomyocyte hypertrophy induced by pressure overload but not exercise training (468), indicating PI3Kα is critical for the induction of physiological cardiac growth but not pathological growth. Cardiac-specific deletion of the PI3K p85α/β regulatory subunits attenuates AKT signaling and exercise-induced cardiac hypertrophy (435). PI3Kγ-deficient mice exhibit less activation of AKT/ERK1/2 and attenuated hypertrophy in response to isoproterenol (520) and transverse aortic constriction (530). Consistent with this paradigm, AKT1 null mice are resistant to swimming-induced cardiac hypertrophy. Unexpectedly, when subjected to pressure overload, the AKT1 null mice develop an exacerbated form of cardiac hypertrophy (141). Based on these findings, the authors propose that AKT1 promotes physiological hypertrophy and suppresses pathological cardiac hypertrophy.
Studies with altered myocardial AKT activity reveal a cornucopia of phenotypic outcomes. PI3K inhibitors wortmannin and LY294002 attenuate the protection of insulin (213, 334), IGF-I (203), NRG-1 (207), ischemic preconditioning (270, 362, 487), postconditioning (637) against cardiomyocyte apoptosis, and ischemia-reperfusion injury by preventing AKT phosphorylation. The lipid phosphatase PTEN (phosphatase and tensin homologue deleted on chromosome 10) negatively regulates the PI3K/AKT signaling pathway by dephosphorylating PIP3. Overexpression of PTEN causes cardiomyocyte apoptosis through inhibition of PI3K signaling (578). In contrast, inactivation of PTEN induces cardiomyocyte hypertrophy through PI3Kα and decreases myocardial contractility through PI3Kγ (119, 578). Mice deficient in PTEN display basal hypertrophy and mild reduction in systolic function, yet exhibit reduced pathological hypertrophy and apoptosis with preserved left ventricular function in response to pressure overload (521). Consistently, inducible cardiac-specific deletion of PTEN activates AKT and protects the heart from ischemia/reperfusion injury (561). Activation of PI3K leads to AKT phosphorylation at Thr-308 by phosphoinositide-dependent kinase 1 (PDK1) (14) and Ser-473 by the rictor-mTOR complex (570). Cardiac-specific knockout of PDK1 abolishes the activation of AKT by insulin and results in heart failure through reduced cardiomyocyte volume (489) and increased apoptosis (320). Administration of IGF-I or deletion of PTEN increases the density of L-type Ca2+ channel (LTCC) through the PI3K-AKT pathway, leading to increased Ca2+ influx and cardiac contractility (613, 654). Recently, by using PDK1-deficient mice, AKT has also been shown to increase LTCC protein density and improve sarcoplasmic reticulum (SR) Ca2+ handling through phosphorylation of Cavβ2 (82) and phospholamban (81).
Another facet of AKT activity is the potentiation and enhancement of stem cell-mediated regeneration and repair, whether by direct or indirect mechanisms (231, 234, 364, 390, 420, 446). Transplantation of mesenchymal stem cells overexpressing AKT reduces infarct size and prevents remodeling due to decreased stem cell apoptosis (420, 446). AKT increases secretion of paracrine factors (VEGF, IGF, SFRP2) (231, 233, 482) but not differentiation (509) and plays a critical role in these reports of cardioprotection. In cardiac stem cells, overexpression of AKT promotes proliferation (244), whereas inhibition of AKT activity impairs proliferation and induces apoptosis (624). On the basis of these findings, it seems reasonable to propose that alterations of AKT activity will influence the reparative and regenerative potential of the myocardium.
IV. ALTERING AKT SIGNALING
Physiological regulation of AKT occurs via triggering of membrane receptors and subsequent regulation of downstream activity by phosphatases such as PTEN (401, 441) or PHLPP2 (216) which depress AKT kinase activity via de-phosphorylation. The dynamic and transient nature of receptor-driven activation of kinase signaling makes determination of AKT functional effects more challenging. Therefore, overexpression systems using molecular biology tools have created a variety of altered AKT signaling constructs with activities that are heightened, impaired, or targeted to specific subcellular compartments. These tools have yielded much of the literature dedicated to AKT function in myocardial contexts with important insights into regulation of signaling and remodeling by manipulation of AKT activity in aberrant ways that may reflect pathophysiological conditions. However, it is important to remember that all such endeavors take a decidedly nonphysiological approach to examining AKT function and that understanding the normal physiological role of AKT is best served by models that mimic the consequences of AKT activity observed under physiological stimulation by the inductive signals detailed in the previous section. Thus the following molecularly engineered forms of AKT have been essential in elucidating its many functions to varying degrees.
A. Wild-Type AKT Expression
AKT normally exists in the cellular milieu with activity regulated by posttranslational phosphorylation. A significant effect of overexpression of wild-type AKT has not been reported in the literature. The available data indicate that AKT activity in cardiomyocytes is tightly controlled by signaling events originating at the membrane. Knock-out of AKT1 in mice results in an impaired growth phenotype (101), but cardiac-specific data have yet to be reported.
B. Myristoylated AKT Expression
Modification of AKT with a myristoylation moiety (myr-AKT) results in enhanced plasma membrane association that encourages proximity to the constitutively active PDK enzyme which leads to AKT phosphorylation and activity (365). Adenoviral-mediated expression of myr-AKT protects cultured neonatal cardiomyocytes from hypoxia-induced apoptosis (454). In vivo gene transfer utilizing adenoviral expression vectors of myr-AKT in the setting of acute ischemia-reperfusion challenge results in smaller infarct size and preservation of cardiac function in rat models (460). Transgenic mice expressing cardiac-specific myr-AKT exhibit cardiomyocyte hypertrophy along with an increase in heart size, although cardiac function is preserved (455). These mice are also protected from ischemic injury and show reduced scar size after myocardial infarction (455).
C. Dominant Negative Expression
As might be expected from the protective effects of AKT activation, the inhibition of AKT via dominant negative constructs with impaired phosphorylation sites leads to increased susceptibility to apoptotic challenge and can block the protective effects of agents such as IGF-I or neuregulin-1 that normally act to prevent apoptosis via induction of AKT activity (203, 207). To produce these phenotypes, site-specific mutagenesis has been utilized to produce nonactivated forms of AKT by replacing PDK1 phosphorylation sites threonine 308 and serine 473 with either alanine (328) or asparatate (23). In addition to becoming susceptible to apoptotic stimuli, inactive AKT promotes mitochondrial electrochemical dysfunction (391), reduces protein synthesis, impairs calcium transients (460), and results in diminished cardiomyocyte size (114). Mutations in the PH domain render the kinase unable to bind to phospholipids or proteins sequestering it away from the membrane and unable to be activated by PDK (459). These dominant negative mutations mimic phenotypes seen in AKT-specific phosphatase overexpressing transgenics including PTEN (578, 592) and PP2A (553, 636). Mutations to AKT in the ATP binding site (K179M) produced an inactive form of the kinase with some lethality 2 and 11 wk after birth (584). These kinase dead transgenics have blunted downstream target activity, but not all activity is completely attenuated consistent with the dominant negative phenotype. Morphometric and hemodynamic analysis revealed no statistical difference in hearts from these mutants versus control (584), suggesting endogenous compensatory signaling.
D. Phosphomimetic Expression
Another modification of AKT allowing for increased activity is the substitution of charged residues at selected sites, thereby creating a phosphomimetic mutant that is purported to possess enhanced affinity for PI3K-generated phospholipids by a substitution of glutamic acid (E) for a lysine residue (K) at position 40 in the pleckstrin homology domain (43). Thus the activity of the E40K mutant is higher than that of wild-type nonphosphorylated AKT but much lower than myristoylated AKT. Cardiac-specific transgenesis with the E40K mutant leads to cardiomyocyte hypertrophy, cardiac remodeling, and increased contractility (114). Post mortem analysis revealed that transgenic mice have increased total heart mass, right and left chamber mass, and heart weight-to-body weight ratios (114). Histo-chemical analysis revealed increased concentric myocyte hypertrophy without fibrosis (114). Upon further analysis increased hemodynamic function was subsequently associated with increased expression and activity of SR Ca2+-ATPase 2a (SERCA2A) (361). The increased SERCA2A activity in these transgenics was attributed to the ability of AKT to directly phosphorylate phospholamban, a regulatory protein associated with SERCA at Thr-17 (81). GSK-3β is another downstream target hyper-phosphorylated particularly in E40K transgenic hearts and not myristoylated and wild-type mutants (114, 584). Phosphorylation of GSK-3β by AKT is known to inhibit the activity of GSK-3β and is essential for both physiological and pathological hypertrophy (163). These signals in concert contribute to the ability of these E40K transgenic hearts to withstand pathological challenge by pressure overload with reduced apoptotic cell death (84). Interestingly, mice with phosphomimetic over-expression of AKT do not have elevated levels of phosphorylated p44/42 MAPK signals commonly associated with physiological hypertrophy (104). Phosphorylation of AKT on Thr-308 and Ser-473 is essential for activation; mutations on these residues to aspartic acid imitate the negative charge of phosphorylation and produce a phosphomimetic phenotype. Mice with myocyte specific overexpression of this mutation have elevated S6 kinase activity, suggesting increased protein synthesis and hypertrophy (584). Chronic AKT activation has produced both beneficial and deleterious results (455, 633, 672). Perhaps this is due to the levels of AKT overexpression; the transgenics that survive have increased concentric hypertrophy, enhanced cardiomyocyte glucose uptake, and tend to be functionally normal (457).
E. Nuclear Targeted Expression
The concept of nuclear AKT accumulation playing a critical role in myocardial biology was originally championed in seminal studies demonstrating nuclear localization of AKT in the myocardium (76, 611). In the first of a series of nuclear-targeted AKT-related publications, a wild-type AKT was used to maintain near-physiological levels of kinase activity with targeting mediated by a concatameric nuclear localization sequence. Nuclear accumulation of AKT produced profound anti-apoptotic activity without evidence of hypertrophic growth in either cultured cardiomyocytes or genetically engineered mice that specifically expressed nuclear targeted AKT (590). Inhibition of apoptosis met or exceeded that of myristoylated AKT, and prevention of ischemia/reperfusion damage in vivo was comparable to the potent effect of preconditioning. Striking similarities between cardiac-specific expression of nuclear-targeted AKT or IGF indicated the identification of a pivotal requirement for AKT activation, allowing for beneficial characteristics of IGF-mediated protection without mal-adaptive hypertrophy or undesirable paracrine-signaling side effects. Indeed, subsequent publications have demonstrated that nuclear accumulation of AKT is actually anti-hypertrophic (641), in agreement with findings obtained with AKT knockout mice (141). Furthermore, the proliferation of myocardial stem and progenitor cell populations is enhanced by myocardial-specific nuclear AKT expression, casting new light on the implementation of AKT activity as a molecular interventional approach for treatment of cardiomyopathic damage resulting from acute injury, chronic stress, or the debilitating changes of aging (244, 616).
V. RELATIONSHIP OF AKT TO PIM-1 KINASE
A. PIM-1 Biology
Pro-survival and proliferative effects of AKT activity in the myocardium are well documented (203, 590, 656). However, recent evidence indicates that these actions previously ascribed to AKT are actually mediated by a downstream kinase called PIM-1, one of a three member family of serine/threonine kinases belonging to the calmodulin-dependent protein kinase (CAMK) related group (69, 295). Similar to AKT in several respects, PIM-1 is also a serine/threonine kinase originally identified as a cellular oncogene that inhibits apoptosis and promotes proliferation (29, 661). PIM-1 is expressed in various hematopoetic sites including thymus, spleen, bone marrow, and fetal liver, but can also be found in oral epithelia, prostate, hippocampus (in response to seizures), vascular smooth muscle (in response to injury), and many tumorigenic cell types (176, 344, 399, 416, 445, 474, 497, 605). In comparison, adult myocardium exhibits relatively low PIM-1 expression under normal conditions. Induction of PIM-1 is mediated through a variety of growth factors that can involve JAK/STAT pathway signaling with rapid accumulation of protein reminiscent of an early response gene (451, 533, 608). AKT signaling has also been linked to induction of PIM-1 expression resulting from prolactin treatment (372).
B. Cross-Talk Between AKT and PIM-1
Similar to AKT, PIM-1 has many substrate targets that participate in gene transcription, cell cycle regulation, signal transduction, and antagonizing apoptosis. For example, both AKT and PIM-1 both directly phosphorylate and inactivate BAD, a proapoptotic BCL-2 family member (10, 133, 673). Additional intersections exist at targets controlling the IκB/NFκB transcription factor complex, regulation of protein synthesis via mTOR, and GSK-3β phosphorylation (17). Overlapping roles for AKT and PIM-1 as regulators of cellular proliferation and survival were found in studies of nontransformed hematopoetic stem cells (260). Furthermore, the pharmacological compound LY294002 previously thought to specifically inhibit PI3K and subsequent AKT activation also directly inhibits PIM-1, suggesting that effects previously ascribed to blockade of PI3K/AKT need to be reinterpreted for potential consequences of concurrent PIM-1 inhibition (323). Collectively, these observations point to a close interrelationship between AKT and PIM-1 in cellular signaling. However, mechanistically there is a pivotal distinction between the two kinases: whereas AKT is activated by posttranslational phosphorylation, PIM-1 is constitutively active and therefore must be controlled by protein turnover involving regulation at transcriptional, posttranscriptional, translational, and post-translational levels. Thus, while AKT may be present but inactive, the only way to decrease PIM-1 activity is rapid turnover through proteosomal degradation (661).
C. Implications for Myocardial Biology
Our group extended observations of PIM-1 expression to include the myocardium, where PIM-1 expression is found in cardiomyocytes of the postnatal heart and is downregulated within a few weeks after birth (492). Induction of PIM-1 occurs after pathological challenge to the adult heart, with accumulation and persistence of PIM-1 in surviving myocytes that border areas of infarction. Cardiac-specific expression of PIM-1 was highly protective in response to infarction challenge, whereas genetic deletion of PIM-1 rendered mice more susceptible to infarction damage despite significant compensatory increases in AKT expression and phosphorylation. These findings point to PIM-1 as a critical downstream participant in AKT-mediated cardio-protection, with implications for PIM-1 as a participant in survival, proliferative, and reparative processes previously associated with AKT activity. Future studies expanding on the role of myocardial PIM-1 may lead to more focused avenues for intervening in cellular processes rather than AKT, since PIM-1 activity can be directly regulated by expression level and may not have the widespread and often deleterious (114) impact of altered AKT signaling previously observed in the heart (455, 457, 498, 584, 587, 656).
VI. MITOCHONDRIA
A. Mitochondrial Integrity and Survival Kinases
The critical role of mitochondria as arbiters of cell survival is widely recognized and well documented in the myocardial context (4, 126, 145, 271, 317, 326, 466, 495, 500, 688). Since mitochondria act as integrators of multiple cellular conditions reflecting physiological and genomic stresses, it is reasonable to expect that kinase signaling mechanisms influencing cell survival impinge either directly or indirectly on mitochondrial integrity. Indeed, a cornucopia of studies have documented the influence of each major kinase signaling pathway on mitochondrial activity including PKA, PKC, ERK, JNK, p38, and AKT (296). AKT protective signaling has been shown to act on many levels of mitochondrial function. Outer mitochondrial membrane integrity, predominatly controlled by BCL-2 family proteins, is both directly and indirectly affected by AKT activity. Moreover, certain cardioprotective effects of AKT have been suggested to depend on translocation from the cytosol to mitochondria (7), where it inhibits opening of the permeability transition pore (mPTP) to maintain mitochondrial integrity (135, 326, 336). Connections between insulin, IGF, and cardiotrophin on AKT signaling point to the interplay of AKT with mitochondria (391). As the role of AKT in protection of mitochondrial integrity has recently been reviewed in detail (525), this section summarizes basic principles of intrinsic cell death and focuses on recent advances regarding hexokinases in the context of the myocardium.
B. Intrinsic Apoptotic Cascades
Mitochondrial-dependent apoptosis, also referred to as programmed cell death, is activated in response to a variety of extracellular or intracellular insults initiating a multiplicity of downstream cascades. Intrinsic apoptotic events predominantly center on mitochondrial integrity which acts as a cumulative breaking point upon which apoptosis hinges (259, 463, 546). Therefore, mitochondria act as cellular “executioners” by releasing pro-apoptotic molecules normally held within the intermembrane space such as cytochrome c, apoptosis inducing factor (AIF), Smac/Diablo, HtrA2/Omi, and endonuclease G (545). Once mitochondrial membrane integrity is breached, these activators of apoptotic cascades lead to cell death via multiple independent mechanisms. Thus a critical facet of inhibiting apoptosis is prevention of mitochondrial membrane permeabilization.
The stability of mitochondrial membranes is largely dictated by the BCL-2 proteins, a large family of both pro- and anti-apoptotic members that exist in a dynamic balance. Interaction of AKT with two of these BCL-2 members BAD and BAX has been the focus of considerable attention. BAD promotes apoptosis by forming heterodimers with anti-apoptotic BCL-2 or BCL-xL proteins, thereby inhibiting their protective effects. In comparison, BAX undergoes a conformational shift that allows for its insertion into mitochondrial membranes and oligomerization with cytochrome c to promote membrane permeabilization (251). AKT antagonizes pro-apoptotic actions of these BCL-2 family members by kinase activity, phosphorylating both BAD (Ser-136) (133) as well as BAX (Ser-184) (671). Phosphorylation dissociates BAD from complexes with BCL-2/BCL-xL proteins and promotes association with 14-3-3 to sequester BAD in the cytosol, thereby negating interference of BAD with protective signaling (301). Recently, new lines of evidence have indicated that BAD may play a more direct role in cell homeostasis in addition to its well-known action of inhibiting anti-apoptotic BCL-2 proteins. One such target is mPTP. Dephosphorylation of BAD by PP2A or by inhibition of PKA and PKC sensitizes PTP to Ca2+ by ceramide, an effect that is independent of BAX and BAK (560). As ceramide treatment increased BAD/BCL-xL interaction, PTP sensitization may be due in part to BCL-xL activation, although its putative activity is drawn into question as BAX and BAK are thought to be targets of BCL-xL. Recent evidence also suggests that phosphorylation of the BH3 domain of BAD, responsible for the anti-apoptotic activity of BAD, has an additional role in glucokinase activity and glucose-stimulated insulin secretion (124), again promoting the observation that metabolic and survival/apoptotic signaling not only interact but share many common substrates. Although these additional roles have not been shown in the heart, glucose metabolism in the heart is a likely intersection of the known roles of AKT, BAD phosphorylation, and hexokinase regulation. This interaction might be especially prevalent as ischemic myocytes are believed to upregulate glycolysis in response to increased ADP/ATP ratios and that further stimulation of glycolysis protects against myocyte failure during ischemia and reperfusion (331, 332).
The consequence of BAX phosphorylation by AKT is to promote heterodimerization with BCL-xL or MCL-1 (a BCL-2 related protein), thereby sequestering BAX away from mitochondrial membranes (219). Alternatively, AKT may directly interfere with molecules that promote conformational change of BAX, such as BID or BIF-1. Through modulation of cytosolic BCL-2 family members via phosphorylation, AKT regulates the initiation of mitochondrial membrane permeabilization that leads to apoptosis. Multiple studies have ferreted out the relationship between AKT activation, BCL-2 family member regulation, and inhibition of cardiomyopathic damage (334, 345, 385, 502, 528, 643). Although inhibition of BAX translocation via phosphorylation by AKT has not been shown in the heart, BAX−/− mice are protected against ischemia-reperfusion injury (292). AKT has also been suggested to suppress activities of pro-apoptotic molecules released from compromised mitochondria such as AIF and HtrA2/Omi (93, 678), but these observations in nonmyocardial contexts will require further studies to validate their role in the heart. Additionally, mitochondrial integrity is impacted by effects of AKT activity via altered gene transcription of forkhead family members, MDM2, NFkB, CREB, and YAP (38, 63, 123, 165). Thus AKT controls a multifaceted array of downstream mediators that are directly or indirectly responsible for regulating mitochondrial integrity.
C. Hexokinase: Targets of AKT in Mitochondria
The intertwined relationship linking AKT to preservation of mitochondria creates the mechanistic basis for a sensing mechanism to regulate cellular energy metabolism and survival. Consider that most stimuli for cellular growth and proliferation operate via AKT-associated signaling to promote energy utilization derived from mitochondrial function. As such, preservation of mitochondrial integrity is a synergistic consequence of AKT function that enhances growth and survival processes via kinase activity that consumes ATP to phosphorylate target molecules. Availability of energy substrates is critical for growth and survival, and AKT also has a dependence on glucose to antagonize apoptotic signaling. Current speculation posits that metabolic functions of AKT preceded and eventually evolved into additional roles in preservation of cell survival as well, with glucose-dependent antiapoptotic signaling of AKT interfacing through phosphorylation of hexokinases to protect mitochondria.
Fueling this model of AKT/mitochondrial symbiosis, a strong correlation also exists for preservation of mitochondrial integrity by AKT action to promote localization and stabilization of mitochondrial hexokinase on the outer membrane. Hexokinases (HKs) regulate glucose uptake and metabolism chiefly by phosphorylating free intracellular glucose. The product of this reaction, glucose-6-phosphate, cannot pass back through the plasma membrane and thus maintains a positive glucose gradient from the bloodstream (601). Under basal conditions, hexokinase activity is regulated positively by insulin, and negatively by its product. There are four isoforms of hexokinase, with the heart expressing mainly variants I and II (602). Many tissues, including the heart, respond to metabolic stress such as hypoxia or ischemia by upregulating hexokinase activity in an effort to maintain critical ATP levels. Studies over the last 15 years have shown that HK plays a protective role at least in part through glycolytic signaling. Recently, the association of HK with mitochondrian has been implicated as an important mechanism of HK-mediated protection. HKs I and II contain NH2-terminal mitochondrial-binding motifs, and overexpression of truncated forms resulted in reduce protection against H2O2-induced MPT pore opening in neonatal cardiomyocytes (614). Treatment with volatile anesthetics or ischemic preconditioning (IPC), both known to be cardioprotective following ischemia/reperfusion insult, promote HK association with the mitochondria, corroborating in vitro data (248, 700). Mitochondrial HK may also support mitochondrial membrane integrity by occupying VDAC binding sites, making them unavailable to BAX/BAK recruitment or by reducing oxidative stress (554, 567). Recent studies of AKT in HK-dependent survival have revealed the interdependence of these pathways in protecting the heart. Several lines of evidence support HK as the facilitator of AKT-mediated protective signaling: 1) ectopic expression of HK mimics the effects of AKT activation to inhibit apoptosis (443), 2) both AKT and HK require glucose for antiapoptotic activity (544), 3) targeted disruption of HK interactions with mitochondria impairs the protective actions of AKT (442), 4) association of HK with mitochondria is impaired in AKT-deficient cells following growth factor stimulation, and 5) glucose deprivation reduces HK association with mitochondria (436). More recently it has been shown that AKT can act directly on mitochondria to phosphorylate HK-II, resulting in protection from oxidant or calcium-stimulated permeability transition pore opening in cardiomyocytes (484). In conclusion, although we are at the tip of the proverbial iceberg with regard to assessing this new facet of AKT-mediated signaling in the myocardium, the increasingly apparent codependence of AKT and mitochondria for mutual functional activity points to an inexorably linked partnership through HK that may be the evolutionary interface designed to balance energy conditions, cell metabolism, growth, and survival under stress.
VII. CONTRACTILITY AND CALCIUM SIGNALING
A. Functional Effects
Gain- and loss-of-function studies comprehensively characterized AKT1 as a regulator of contractility and calcium cycling in cardiac myocytes, and enhanced contractility can be observed in a variety of settings associated with enhanced AKT activity both in vitro and in vivo. Conversely, impairment of this signaling pathway is an important determinant of cardiac malfunction. For example, cardiac specific overexpression of IGF-I receptor and knockout of insulin receptor resulted in enhanced and reduced cardiac contractility, respectively (523, 654). Of clinical importance and in accordance with results from animal models, it is known that IGF-I treatment of the failing human heart leads to enhanced contractility (156, 157).
For the physiological stimulus of endurance exercise (e.g., swim training), hemodynamic stress results in adaptive myocyte growth with preserved contractile function (physiological hypertrophy). In contrast, pathological stimuli such as pressure overload lead to hypertrophy, which often progresses to heart failure (278). The differences between physiological or pathological cardiac hypertrophy are most likely due to differences in proximal signaling pathways. Whereas activation of G protein-coupled receptors is necessary to induce pathological hypertrophy, insulin or IGF-I coupled to the PI3K/AKT1 pathway has been associated with physiological growth of the heart (588). In line with this, studies have shown that inhibition of PI3K or genetic ablation of AKT1 prevents exercise-induced hypertrophy (140, 141, 473), which indicates that PI3K/AKT is required for compensatory growth in the heart. In line with this, increased inotropism, lusitropism, and improved calcium dynamics were observed following physiological adaptive hypertrophy following exercise training and in experimental models of elevated activity of the IGF-PI3K-AKT signaling cascade (103, 349, 699).
Multiple studies have proven that the molecular mechanisms of AKT regarding the enhanced contractility are conveyed by direct consequences for calcium handling by either directly or indirectly modifying the function of proteins responsible for calcium cycling (103, 114, 119, 361, 396, 558, 590, 613).
The process of excitation-contraction coupling (ECC) in skeletal and cardiac muscle cells requires membrane depolarization. After membrane depolarization Ca2+ influx is activated via voltage-gated L-type Ca2+ channels into the cytosol of both skeletal muscle cells and cardiac myocytes (46). This rise in cytoplasmic Ca2+concentration leads to Ca2+ release from the SR (Ca2+-induced Ca2+release; CICR) by activation of ryanodine receptors (RyR). After Ca2+release of the RyR, Ca2+ molecules subsequently bind to the contractile proteins such as troponin c, which causes contraction of the myocytes. Thereafter, Ca2+ is cleared from the cytosol by reuptake of Ca2+ into the SR by the action of a SERCA. As discussed in detail below, both plasma membrane and SR calcium fluxes required for contraction are regulated by AKT activity.
B. Contractile Effects
Associations between AKT1 activity and calcium handling proteins were initially observed in experimental models of cardiomyopathy wherein decreased AKT1 activation was concurrent with diminished SERCA, NCX, and PLB phosphorylation (167). Conversely, in transgenic mice with cardiac specific overexpression of AKT, it was shown that the amplitude of Ca2+current (ICa) was enhanced in AKT myocytes compared with that in wild-type myocytes, which may be at least in part responsible for the enhanced cellular Ca2+ transients (114, 361). Second, an increased protein expression of the SERCA could be identified as another molecular mechanism in transgenic mice expressing cardiac-specific constitutively active AKT. Adenoviral gene transfer of the transgene into rat myocardium (81, 103) recapitulates this phenotype. Recently, another study showed that activated AKT phosphorylates PLN at Thr-17, providing a new mechanism whereby the preferential translocation of AKT to the SR is responsible for enhancement of contractility without stimulation of hypertrophy (81).
Similarly, mice created with cardiac-specific expression of nuclear-targeted AKT also showed enhanced contractility and supraphysiological ventricular dynamics, but the molecular mechanisms responsible for the increased cardiac performance were distinctly different and were related to increased loading of the SR due to increased phosphorylation of phospholamban (Ser-16 PLB) (558). In addition, it was shown that phosphatase PP1, which dephosphorylates PLB and thereby inhibits SERCA, is downregulated in TG myocytes, providing an additional pathway for increased contractility.
Taken together, these studies indicate that cardiac specific AKT1, whether constitutively active or nuclear targeted, improves contractility through elevated Ca2+ handling via increases in ICa,L amplitudes or increased SERCA activity.
VIII. ANGIOGENESIS
AKT/PKB is a pivotal regulatory kinase with various roles in growth, metabolism, and survival (80, 115, 244, 493, 590). More specifically, roles for AKT in cardiac growth as well as cardioprotection after pathological injury have been extensively documented (15, 115, 232, 244, 335, 420, 446, 462, 493, 558, 562, 590, 591, 593, 641). To date, a variety of studies attribute short-term AKT activation to the profound protective effects seen in postischemic injury models by which AKT increases cell cycling and inhibits apoptosis (364, 590, 641). AKT activation has also been demonstrated to stimulate neoangiogenesis and vasculogenesis (3, 84, 439, 491, 575, 579, 588, 621), in part accounting for the dramatic improvements and survival of myocardial tissue.
During embryonic development as well as after ischemic injury, vascular endothelial growth factor (VEGF) is secreted to initiate blood vessel formation (619). VEGF expression activates AKT through initiation of phosphorylation (2). Particularly in the heart, secretion of VEGF results in an increase in AKT phosphorylation and subsequent activation of the downstream target eNOS (154, 208). The production of NO has numerous protective effects on the vasculature including vasodilation and inhibition of intimal formation within damaged vessels (155, 519). Studies have shown deletion of eNOS and reduction of NO results in increased damage after ischemic injury. Interestingly, in transgenic mice deficient for eNOS, female mice have reduced intimal formation and better recovery after ischemic insult attributed to the AKT activator estrogen. Additional studies supporting a role for AKT in angiogenesis are demonstrated in animal models that specifically overexpress AKT in the endothelial cell lineage. Activation of AKT in endothelial cells promotes cardiac angiogenesis, increases NO production, decreases neointima formation, and results in attenuated lesion formation during ischemic injury (2, 3, 84, 154, 204, 491, 519, 575). Inhibition of PI3K or use of dominant negative AKT results in profound angiogenic and vascular defects including decreased capillarization and arteriogenesis, decreased eNOS phosphorylation, decreased endothelial cell proliferation, and reduction of NO production (439). Further studies demonstrate that AKT knockout mice possess leaky vasculature as well as impaired mobilization of endothelial progenitor cells in response to VEGF stimulation (91).
Potentiation of angiogenesis by AKT is increasingly apparent, particularly in the heart after ischemic injury. Animal models detailing effects of cardiac specific overexpression of AKT indicate a decrease in infarct size with an accompanying increase in vessel and capillary density. Cardiac specific overexpression of AKT also induces a potent release of cytokines, many of which have specific roles in mediating the growth and induction of vasculogenesis. A recent molecular profiling study revealed that in hearts of mice with conditionally activated AKT, a 33% increase in vascular density was observed along with increased secretion of angiogenic paracrine factors: VEGF receptor 2, neuropillin, and connective tissue growth factor (Ctgf) compared with nontransgenic control hearts (575). Additionally, similar studies indicated AKT activation led to the release of follistatin-1 (517). Taken together, these data implicate a substantial and potentially clinically relevant role for AKT in the regulation of angiogenesis.
To date, both stem and progenitor cell types have been used to treat pathological injury after ischemic injury. Thus far, pro-angiogenic molecules have been used to modify various types of stem and progenitor cells in attempts to mitigate damage after ischemic injury. Delivery of mesenchymal stem cells modified with AKT and ANG II (591) as well as embryonic stem cells modified with VEGF (669) to areas of ischemic damage have resulted in increased AKT phosphorylation and increased angiogenesis in the surrounding tissues. These types of therapies have short-term success in attenuating damage by potentiating angiogenesis through AKT activation. However, long-term AKT overexpression has also been demonstrated to have detrimental effects, including abnormal vascular remodeling and lethal vascular defects (91, 539). While hope exists for using AKT as a novel therapeutic target to induce angiogenesis and reduce ischemic injury, a thorough understanding of effects with regard to timing and expression level is critical before implementation can be expected in the clinical arena.
IX. RELATIONSHIPS WITH MicroRNA
The link between AKT activity and hypertrophic remodeling is indisputable, as is the recent incorporation of microRNAs (miRNA) as critical regulators of cardiac hypertrophy and failure (37, 79, 95, 448, 573, 625, 627, 649, 650). These small noncoding RNA molecules regulate gene expression and control cell growth, differentiation, and apoptosis. As these processes are central to cardiac biology, it is inevitable that miRNAs are either influenced by or participate in cardiac remodeling and pathogenesis, as borne out by studies in both experimental models and human heart samples (TABLE 4). Constitutive activation of AKT by cardiac-specific transgenesis leads to downregulation of miRNA-133 and miRNA-1, similar to changes observed following experimentally induced pressure overload or physiological hypertrophy from exercise (79). Cardiac remodeling can be stimulated or inhibited by manipulating miRNA activity. For example, in cultured cardiomyocytes, over-expression of miRNA-1 leads to inhibition of hypertrophy (573), whereas forced expression of several stress-induced miRNAs results in hypertrophic enlargement (649). Correlations of these changes have yet to be mapped to changes in AKT expression or activity level. However, it is reasonable to speculate that the miRNAs that regulate remodeling, cell survival, and proliferation will also influence AKT, and these relationships are likely to exist in reciprocal fashion, with AKT activity influencing expression of miRNAs.
Table 4.
Examples of microRNAs altered in myocardial diseases
| MicroRNAs Altered in Heart Diseases: (315) | Function | MicroRNAs Altered in Heart Diseases:(315) | Function |
|---|---|---|---|
| Let7b | Developmental timing (343) | miR100 | Stem cell differentiation (623), β-adrenergic signaling (609) |
| Let7c | Developmental timing (377) | miR101 | Epigenetic regulation (77, 653) |
| Let7e | Developmental timing (660) | miR103 | Mesenchymal stem cell signaling (425) |
| mir1 | Anti-hypertrophic (508) | miR106a | Cell cycle arrest and senescence (255, 405) |
| miR10a | Smooth muscle cell differentiation (642) | miR125b | Inhibitor of endothelin-1 (537) |
| miR15b | Pro-apoptotic, reduces cellular ATP (506) | miR126 | Angiogenesis (55, 196, 341, 505) |
| miR17-5p | Both anti-and pro-proliferative (452, 674) | miR140 | Reduced AKT and ERK activation (angiogenesis) (322) |
| miR19a, b | Target PTEN, anti-aging (255, 538) | miR145 | Vascular smooth muscle cell differentiation (55, 193) |
| miR20a, b | Pro-proliferative, anti-aging (255, 510) | miR181a | p27 repression (121) |
| miR23a, b | Early hypertrophic growth, regulate metabolism (72, 421, 658) | miR195 | Hypertrophic signaling (72, 649) |
| miR24 | Cell cycle arrest (394) | miR199a | Cardiomyocyte size maintenance (600) |
| miR26b | Cell proliferation (504) | miR214 | Cell survival (677) |
| miR27a, b | Pro-apoptotic, pro-differentiation (360, 689) | miR222 | Neovascularization (148, 374, 375, 540, 645) |
| miR30 | Myocardial matrix remodeling (170) | miR320 | Neovascularization (552, 660) |
| miR93 | Target VEGF (427) |
With regard to specific miRNA actions in the myocardial context, examples abound in the published literature. miR-133 has been shown to be downregulated in a cardiac hypertrophy mouse model, and overexpression of miR-133 leads to an inhibition of protein synthesis and downregulation of AKT-dependent genes. miR-133 targets the small GTPases Cdc42 and RhoA, which are implicated in cardiac hypertrophy (79). miR-126 is highly expressed in murine heart and lungs (266), and recently, miR-126 was shown to modulate VEGF-induced ERK and AKT pathways during neovascularization. Blocking miR-126 by antisense RNA in HUVECs reduces the phosphorylation of AKT and ERK activation upon VEGF stimulation. miR-126 was found to inhibit the p85β regulatory subunit of PI3K (PIK3R2) and Sprouty-related EVH1 domain-containing protein 1 (SPRED1) (195). These proteins negatively regulate AKT downstream of the PI3K and MAPK pathways. Hence, by negatively regulating suppressors of the AKT/ERK pathway, miR-126 acts as a positive regulator of VEGF signaling (195). A retrospective study identifies miRs differentially expressed in the heart during ischemic reperfusion and myocardial infarction (314). miR-21 was found to be highly expressed in cardiofibroblast, specifically in the infarcted zone at 7 days post myocardial infarction (MI). miR-21 targets and downregulates the AKT suppressor PTEN. miR-21 suppression of PTEN activates AKT and increases MMP-2 expression, which is implicated in cardiac remodeling post-MI (559). miR-21 also regulates Sprouty homolog 1 (Spry1), which downregulates the ERK/MAPK pathway, thereby modulating cardiac dysfunction after pressure-induced hypertrophy and MI (628). Another study demonstrated the therapeutic potential of miR-21 by reducing infarct size (680). miR-210 is upregulated in the heart during development and when the heart begins to fail. Ischemic preconditioning is known to be protective and has been shown to induce phosphorylation and activation of AKT and ERK proteins, followed by nuclear translocation of hypoxia inducible factor alpha (HIFα). In response to AKT and ERK activation, miR-210 is expressed and thought to contribute to cardiomyocyte survival. The cytoprotective mechanism of ischemic preconditioning has been attributed to miR-210 suppression of caspase-8-associated protein 2 (CASP8AP2), but a direct correlation between miR-210, CASP8AP2, and AKT needs to be established (358).
Of the many miRs differentially expressed during heart diseases, some of them regulate AKT as shown in cancer studies. miR-214 targets the AKT/PTEN pathway in ovarian cancer (677), and miR-126 reduces p85β of PI3K and phospho-AKT levels in colon cancer (247). miR-216a and -217 directly inhibit the PTEN and enhance the AKT activation by TGF-β signaling (346). miR-216 induced AKT activation led to glomerular mesangial cell survival and hypertrophy in diabetic nephropathy (346). Overexpression of miR-330 in prostate cancer cell line PC3 reduced the phosphorylation of AKT by targeting the E2F1 transcription factor. Overexpression of miR-330 induced apoptosis of PC3 cells through downregulation of AKT and activation of apoptotic factors like BAD and caspase-9 (402). Although it seems various miRs interact with AKT in cancer lines, these studies need to be examined in the context of myocardium.
X. GENDER DIFFERENCES
Differences in cardiac phenotypes between the sexes have been observed using surgically or genetically engineered experimental animal models (166). Estrogenic stimulation promotes AKT activation. Over the last several years, a number of studies have identified a link between estrogen, AKT, and cardiac remodeling or protection from failure (32, 76, 86, 152, 209, 274, 306, 367, 532, 571, 611, 638). Studies in mouse models have demonstrated the ability of estrogen to attenuate cardiac remodeling in response to pressure overload (648), and subsequent studies extended this idea to include the protective effects of estrogen following MI as well as deleterious effects of testosterone (83). Additional studies reinforced this hormone-linked impact upon cardiac remodeling in response to pathological challenge (9, 28, 70, 166, 217, 534, 594, 655), whereas other studies established correlations with estrogenic stimulation from dietary sources (220, 281, 288, 327, 574, 632) or documented gender-specific distinctions in cardiac remodeling (32, 52, 152, 166, 306, 367, 403, 494, 655). Hypertrophic remodeling dependent on AKT is influenced by p38 MAPK activity in vivo in a gender-dependent fashion, perhaps because inhibition of p38 signaling leads to enhanced estrogen-induced activation of AKT (423). The connection between estrogenic stimulation, gender, and AKT activation in the myocardium was identified by our group in studies of mouse models that documented the nuclear accumulation of AKT in response to estradiol or phytoestrogen treatment, as well as establishing differences in basal levels between males and females (76, 611). Subsequent studies documented the participation of the PI3K/AKT signaling axis in cardioprotective effects mediated by estrogenic treatment (32, 532). Since estrogen promotes nuclear accumulation of AKT (76) that is both anti-apoptotic (590) as well as antihypertrophic (640), it is reasonable to conclude that AKT activation plays a critical role in estrogen-mediated cardioprotective effects. The relevance for these connections as they relate to issues of women’s health and postmenopausal hormone replacement therapy continues to be an area of active research and debate (312, 531, 551, 607).
Estrogen activation of AKT is known to influence events such as metabolism, cell cycle, and cell survival (76). Several murine models allude to the beneficial effects of estrogen and active AKT after pathological injury such as ischemia/reperfusion (I/R) injury (32). In age-matched rats, gender disparity was evident in response to I/R in vivo, where females showed an increased propensity to activate AKT and its downstream effector PKC-ε (32). Phenotypically female rats exhibited reduced infarct size and increased postrecovery left ventricular function after I/R injury compared with males in vivo (32). This was corroborated with the use of ovariectomized female rats and estrogen replacement by administration of 17β-estradiol. The proposed mechanism, which increased female resiliency to heart injury in this study, correlates with upregulated p-AKT in the nucleus (31, 76, 611). Furthermore, AKT is essential for activation of PKCs; PKC-ε in this system was shown to inhibit apoptosis in adverse cardiac events, and both AKT and PKC-ε were highly upregulated in female rats compared with males, indicative of cardioprotection (32). I/R injury is also detrimental to contractility, affecting intracellular Ca2+ loading in isolated cardiomyocytes (86). Female derived cardiomyocytes exhibit less SR Ca2+ loading compared with males, where altered calcium handling often leads to pro-apoptotic cascades (86). AKT downstream of PI3K has been described to influence cellular function and contractility, yet the mechanisms are still unclear.
Chronic AKT activation and localization to the nucleus is well known in the heart mediated by estrogen, but mechanistically the subcellular localization of active AKT is still being characterized in relation to sex hormones. Chronic activation of AKT and its localization in the nucleus has various physiological effects for the cell. AKT is a well-known antagonist of pro-apoptotic pathways and promotes proliferation in the cell (31). Studies that focus on the anti-apoptotic role of AKT show that with administration of estrogen, there would not only be a consistent increase in p-AKT levels in the nucleus, but an AKT-dependent inactivation and removal of the pro-apoptotic protein forkhead from the nucleus to the cytoplasm where it will be degraded (76, 611). In addition, a breast cancer model shows that women with functional estrogen receptors (ERα) have more active AKT in the nucleus of their cells, leading to increased cell survival and progression of the disease (31). This ERα-regulated response was found to be dependent on TCL1 family members that are known to regulate the nuclear localization of AKT in different cell types (31), therefore regulating apoptotic signals.
In vitro studies have shown that 17β-estradiol treatments of cardiomyoctyes decrease chemical-induced apoptosis. In addition, activation of AKT affects metabolic features of the cell particularly by increasing cardiac glycogen synthesis by phosphorylation and inhibition of GSK-3, which deactivates its activity (476, 657). Increase in glycogen synthesis is beneficial in the myocardial setting because it not only increases the cells resistance to ischemic events by allowing beneficial sensitization and prolonged activity during anaerobic respiration events (657). In the effect that 17β-estradiol is introduced to cardiomyocytes, there appears to be a substantial increase in glycogen synthesis in cardiomyocytes indicative of AKT activation (476, 657).
Sex hormones such as estrogen have been studied as potential mediators of the inflammatory response, especially in incidences of I/R in various organs (223, 526). Estrogen in particular has been shown to influence the migration and activation of leukocytes that often progress to inflammation and vessel occlusion during pathology (526). For example, estrogen administration reduced the incidence of atherosclerotic plaques, which is often worsened by chronic inflammation leading to irreversible vasoconstriction (41). Circulating cytokines after a critical inflammatory event are reduced after administration of 17β-estradiol in burn victims, which correlated with increased p-ERK and p-AKT levels and lower apoptotic incidences (223). Sexual dimorphism becomes apparent during various ischemic events where organ remodeling facilitated by reperfusion through the vasculature is inherently “gender biased.” In premenopausal woman, estrogen acts as an upstream regulator of eNOS, which by a PI3K-dependent pathway activates AKT to become phosphorylated. Activation of eNOS allows for release of NO, which has been described to facilitate vasodilation, maintain vascular tone, reduce inflammation, and support AKT dependent anti-apoptotic events via BCL-2 (275, 397, 480, 606). Contrasting evidence shows that in the renal system, when testosterone was administered to female mice there was an apparent increase in damage to the kidney assessed by inflammation and functional vasculature discrepancies in the organ (526). This deterioration of function in the kidney to eNOS and AKT was attributed to downregulation and alterations in MAPK-mediated protective pathways (526).
The sexual dichotomy of cardiovascular diseases is difficult to ignore. Various studies mentioned here are referenced to describe the apparent effects of sex hormones on pro-survival pathways most notably estrogen activation of PI3K/AKT. Ongoing studies that correlate the susceptibility of disease and morbidity of the sexes are not only limited to the heart but have reached out to studying patterns of sex hormone regulation of regeneration in the central nervous system or in easing systemic inflammation (223, 526). This study may give insight to certain physiological phenomena as to why men have higher incidences of cardiovascular diseases and decreased survival after onset of pathological insult compared with females. Similarly, women who are pregnant and have higher circulating androgens run the risk of pregnancy-induced hypertension leading to systemic organ damage (229, 330). Overall, the discrepancy between the sexes seems rooted at least in part with estrogen as a mechanism for activating survival kinases such as AKT to blunt cardiovascular diseases and progression of systemic diseases.
XI. EFFECTS OF CARDIOMYOPATHIC INJURY
A. Ischemia, Reperfusion, and Pre/Postconditioning
The “reperfusion injury signaling kinase” (RISK) pathway is an initial line of defense for cardiomyocytes attempting to stave off the damaging consequences of reperfusion (419). Reperfusion activates AKT that leads to stimulation of mTOR signaling and subsequent protein synthesis (120). The PI3K-AKT and MEK1-ERK1/2 pathways cross-talk to each other and act in concert to mediate protection (269–271). The effect of AKT activity in prevention of reperfusion damage is likely connected to protection of mitochondrial integrity (258). Simulated ischemia using cultured cardiomyocytes reveals increased AKT activtion, but differential timing for increased phosphorylation of the key S473 versus T308 residues (180). Human fetal cardiomyocytes are also highly resistant to hypoxic stress that may be mediated, in part, by heightened AKT activation (109). Over-expression of constitutively activated AKT protected cardiomyocytes from apoptosis in response to ischemia-reperfusion injury in vivo (203).
Different treatments before (preconditioning) or immediately with the onset of reperfusion (postconditioning) have a powerful protective effect on the myocardium to subsequent infarction challenge by activating a variety of survival pathways including AKT (118, 362, 366, 444, 637). Preconditioning the myocardium by brief alternating cycles of ischemia and reperfusion leads to reduction in infarct size that is dependent on 3′ phosphoinositide-dependent kinase-1 (PDK1) (65), which presumably leads to downstream activation of AKT.
Alternative to ischemic conditioning, pharmacological conditioning would likely be more relevant to the clinical setting, e.g., ANG II mediates cardioprotective effects by enhancing reperfusion-initiated AKT phosphorylation (42). Preconditioning can be influenced by pathways that impinge on AKT-mediated effects, as shown in experiments using FRZ/sFRP-1, a secreted antagonist of the WNT/FRIZZLED pathway that inhibits phosphorylation of GSK-3β (34) or the transcription factor Ref-1 (249). Pharmacological preconditioning agents such as acetylcholine, bradykinin, the synthetic δ-opioid agonist DADLE, and the anti-ischemic metabolite drug Trimetazidine increase AKT phosphorylation (108, 199, 383). Analogously, a novel member of the calcitonin/calcitonin gene-related peptide family named intermedin, the volatile anesthetic isoflurane, or phosphodiesterase inhibitors olprinone or tadalafil reduce myocardial damage by AKT activation in the myocardial I/R model (6, 461, 599). Administration of insulin in reperfusion enhances AKT-mediated cardioprotection and leads to cross-talk between AKT and JNK pathways (422). Furthermore, different ligands of PPAR, such as the glucose-sensitizing drug Rosiglithazone or WY-14643, reduced infarct size in an AKT-dependent manner (68, 356, 692). Similarly, activation of a membrane-bound estrogen receptor or inhibition of the non-long terminal repeat retrotransposon long interspersed nuclear element 1 (LINE-1, L1) in the ischemic heart increases AKT expression and phosphorylation and functional recovery following reperfusion (151, 433). Besides steroids, peptido-hormones including adiponectin, adrenomedullin, or growth hormone releasing hormone reduce myocardial injury in an AKT-dependent manner (235, 240, 634). Stimulation of GPCRs, e.g., by SDF-1α, also mediates AKT phosphorylation and prevents myocardial cell death (572). In contrast, hypercholesterolemia augmented myocardial necrosis in a pig I/R model correlating with reduced AKT phosphorylation (518).
Chemical agents are another approach to achieve preconditioning. AKT activation prompted by either carbon monoxide exposure or xenon inhalation prior to I/R challenge preconditions against injury (202, 481). Preconditioning with cobalt chloride, a hypoxia mimetic agent, or hydrogen sulfide can effectively confer cardioprotective effects prior to deep hypothermic circulatory arrest (352, 479). Preconditioning mediated by hydrogen sulfide also indicates AKT as a contributor to cardioprotection (303).
Postconditioning stimulation is more complex and AKT activity apparently depends on the duration of ischemia. Analogous to preconditioning, ischemic postconditioning is based on repeated cycles of intermittent ischemia, although treatment is applied with the onset of reperfusion following the index ischemic event. Application of ischemic postconditioning at late time points after index ischemia (more than 45 min) results in AKT activation and protective effects on cardiac injury that is attenuated by PI3K inhibition. In contrast, postconditioning performed early after index ischemia had no functional protective effect consistent with lack of AKT activation (447). Ischemic postconditioning resulting in AKT activation is initiated via JAK-STAT3 upstream of the RISK pathway (238) that can be stimulated by TNF-α (389). Conversely, downstream of the RISK pathway, protective effects involve alterations to GSK-3β and mitochondrial permeability transition (192). As an alternative approach to ischemic postconditioning, pharmacological postconditioning involves application of active components with the onset of reperfusion. As reported for preconditioning, isofluorane induces AKT activity during the process of postconditioning (97, 191, 192). Protective effects could also be achieved by postconditioning with levosimendane, sphingosine-1-phosphate receptor agonists like FTY720, the peptido-hormone apelin, or the phytoestrogen genistein in a AKT-dependent manner (293, 294, 597, 632). Treatment with the antidiabetic drugs glimepiride (a sulfonyl urea) or metformin (a biguanide) at the onset of reperfusion limited myocardial injury in perfused Langendorff rabbit hearts, correlating with AKT phosphorylation that was antagonized by PI3K inhibitors (49, 507). Also, treatment with exenatide (a glucagon like peptide-1 agonist) limits myocardial injury with concomitant AKT phosphorylation (630). RISK survival signaling also appears augmented by administration of statins during reperfusion (174).
B. Heart Failure, Pressure, or Volume Overload
Heart failure is the common final destination of different pathological conditions such as ischemia, pressure, or volume overload. Initially, pressure-challenged hearts undergo hypertrophy, which can be beneficial by compensation in the early phase of remodeling in the stressed heart. However, ongoing hypertrophy and remodeling result secondarily in diastolic dysfunction and consequently heart failure. For this response several signaling and transcription pathways are activated including (but by no means limited to) NFAT, ERK1/2, and PI3K/AKT (66, 67, 455, 488, 618). Mechanical overload stimulates AKT activation, possibly through or in conjunction with induction of focal adhesion kinase signaling (200). AKT activation takes place after pressure as well as volume overload in rabbit models with different time courses for each (485). Heart failure resulting from volume overload is characterized by early increases in AKT activity (160) followed by decreased phosphorylation of AKT that may contribute to decompensation (147). Transgenic animals expressing cardiac-specific constitutively active AKT show a spectrum of phenotypes from cardiac hypertrophy with preserved systolic function and cardioprotection to massive cardiac dilatation and sudden death (455). In the analysis of the human specimen, AKT activation can be found in failing hearts, whereas in hypertrophic samples no AKT activation may be found (262). Depression of the gp130 survival pathway and associated depression of AKT activity are linked to human heart failure (236, 262). Chronic increases in LIF found in failing hearts may promote inhibition of AKT phosphorylation, thereby exacerbating deterioration of cardiac function (197, 236). Heart failure due to infarction damage can be blunted by postoperative treatment with G-CSF that correlates with increased VEGF and AKT activity in the ischemic region (321, 415). Under conditions of hypertrophic remodeling, dysregulation of AKT activity leads to increased susceptibility to I/R injury due to increased GSK-3β activity and concomitant alterations in glycolysis (35). AKT phosphorylation is downregulated in hearts of spontaneously hypertensive rats (SHR) (380) but can be normalized by exercise (393). In Dalt salt-sensitive rats with heart failure, treatment with the aldosterone inhibitor eplerenone induced AKT activation correlating with improved cardiac function (363).
Diabetic cardiomyopathy is associated with impaired activation of AKT in streptozotocin-treated rats (250, 398) as well as diabetic rats stimulated by cardioprotective opioid treatment (243), although AKT phosphorylation may initially be increased in early stages of diabetic cardiomyopathy (437). Altered AKT phosphorylation occurs in the Zucker-diabetic-fatty (ZDF) rat model (392) and can be normalized with exercise (393). Altered AKT signaling is also present in the mdx mouse model of dystrophin-deficient cardiomyopathy (354).
Collectively, the picture of AKT activity in the pathologically challenged heart is predictably complex, more so because there are also differences in AKT isoforms and the roles they play in prompting physiological versus pathological remodeling. However, in the end-stage failing heart where normal signal transduction has given way to dysregulated and desperate compensatory activity, the role of AKT is probably less about a particular phenotypic outcome and more indicative of the overall decline in coordinated signal transduction that depends heavily on cross-talk through AKT.
XII. CONCLUSION: AKT AS A NODAL REGULATORY KINASE IN THE MYOCARDIUM
Despite ongoing frustration in moving toward a clinically relevant outcome for heart failure, research into the relationships between signal transduction and molecular interventional strategies to deal with cardiomyopathic disease maintain their relentless pace. The logic is pure and simple: signaling molecules regulate the biological processes of cells, so a focused and regulated approach to the right target will have the desired outcome of increasing salutary effects or blunting maladaptive consequences. But the devil is in the details, as evidenced by this review of the multifaceted nature of AKT signaling. Since most critical modulators of cell biology are present and maintained in homeostasis with concurrent environmental conditions, any targeted alteration of molecular signaling is likely to produce shrapnel effects that will compromise other aspects of cell adaptation to stress. Of course, this does not mean that the wealth of understanding related to signal transduction and myocardial biology is irrelevant to clinical treatment. On the contrary, this review shows that manipulation of AKT-dependent signaling mechanisms has the power to control a multitude of important aspects of myocardial biological processes. The limitation lies in our technical capabilities. Despite our best efforts, to date we still lack the ability to influence AKT signaling with the multifaceted and nuanced networking that typifies normal cell biology, and AKT in particular (FIGURE 3). As we delve deeper into mechanisms that regulate AKT, we will undoubtedly discover even more ways to direct, influence, and control the outcome of activation, localization, and target substrate phosphorylation. AKT is a critical nodal kinase in the cell that integrates a host of ambient information into powerful phenotypic responses. The challenge ahead is to harness this powerful signaling molecule and take advantage of the staggering spectrum of intracellular processes under the regulation of AKT.
FIGURE 3.
Schematic overview of selected AKT targets, many of which are highlighted in this review. Cellular signaling around AKT and AKT substrates regulates major cellular processes in the myocardium. Activated AKT increases protein translation, cellular growth, metabolism, and cell cycle activity through regulation of downstream mediators.
Acknowledgments
GRANTS
M. A. Sussman is supported by National Heart, Lung, and Blood Institute Grants 1R21HL102714-01, 2 R01 HL067245, IR37 HL091102-01, P01HL085577-05, RC1HL100891-02, R21 HL102613-01, and 1 R21 HL104544-01. K. Fischer and N. Gude are Fellows of the Rees-Stealy Research Foundation and the San Diego State University Heart Institute. C.T. Cottage is supported by the Rees-Stealy Research Foundation, the San Diego ARCS Foundation, American Heart Association Predoctoral Fellowship 10PRE3060046, and an Inamori Foundation Fellowship. M. Völkers and M. H. Konstandin are supported by DFG Grants MV 1659 1/1 and KO 3900/1-1.
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Abete P, Testa G, Ferrara N, De Santis D, Capaccio P, Viati L, Calabrese C, Cacciatore F, Longobardi G, Condorelli M, Napoli C, Rengo F. Cardioprotective effect of ischemic preconditioning is preserved in food-restricted senescent rats. Am J Physiol Heart Circ Physiol. 2002;282:H1978–H1987. doi: 10.1152/ajpheart.00929.2001. [DOI] [PubMed] [Google Scholar]
- 2.Abid MR, Guo S, Minami T, Spokes KC, Ueki K, Skurk C, Walsh K, Aird WC. Vascular endothelial growth factor activates PI3K/Akt/forkhead signaling in endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:294–300. doi: 10.1161/01.ATV.0000110502.10593.06. [DOI] [PubMed] [Google Scholar]
- 3.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams JW, Pagel AL, Means CK, Oksenberg D, Armstrong RC, Brown JH. Cardiomyocyte apoptosis induced by Galphaq signaling is mediated by permeability transition pore formation and activation of the mitochondrial death pathway. Circ Res. 2000;87:1180–1187. doi: 10.1161/01.res.87.12.1180. [DOI] [PubMed] [Google Scholar]
- 5.Agata J, Chao L, Chao J. Kallikrein gene delivery improves cardiac reserve and attenuates remodeling after myocardial infarction. Hypertension. 2002;40:653–659. doi: 10.1161/01.hyp.0000036035.41122.99. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad N, Wang Y, Ali AK, Ashraf M. Long-acting phosphodiesterase-5 inhibitor, tadalafil, induces sustained cardioprotection against lethal ischemic injury. Am J Physiol Heart Circ Physiol. 2009;297:H387–H391. doi: 10.1152/ajpheart.00169.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad N, Wang Y, Haider KH, Wang B, Pasha Z, Uzun O, Ashraf M. Cardiac protection by mitoKATP channels is dependent on Akt translocation from cytosol to mitochondria during late preconditioning. Am J Physiol Heart Circ Physiol. 2006;290:H2402–H2408. doi: 10.1152/ajpheart.00737.2005. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed NN, Franke TF, Bellacosa A, Datta K, Gonzalez-Portal ME, Taguchi T, Testa JR, Tsichlis PN. The proteins encoded by c-akt and v-akt differ in post-translational modification, subcellular localization and oncogenic potential. Oncogene. 1993;8:1957–1963. [PubMed] [Google Scholar]
- 9.Ahn BH, Park HK, Cho HG, Lee HA, Lee YM, Yang EK, Lee WJ. Estrogen and enalapril attenuate the development of right ventricular hypertrophy induced by monocrota-line in ovariectomized rats. J Korean Med Sci. 2003;18:641–648. doi: 10.3346/jkms.2003.18.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aho TL, Sandholm J, Peltola KJ, Mankonen HP, Lilly M, Koskinen PJ. Pim-1 kinase promotes inactivation of the pro-apoptotic Bad protein by phosphorylating it on the Ser112 gatekeeper site. FEBS Lett. 2004;571:43–49. doi: 10.1016/j.febslet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 11.Ahuja P, Sdek P, MacLellan WR. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol Rev. 2007;87:521–544. doi: 10.1152/physrev.00032.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aikawa R, Nawano M, Gu Y, Katagiri H, Asano T, Zhu W, Nagai R, Komuro I. Insulin prevents cardiomyocytes from oxidative stress-induced apoptosis through activation of PI3 kinase/Akt. Circulation. 2000;102:2873–2879. doi: 10.1161/01.cir.102.23.2873. [DOI] [PubMed] [Google Scholar]
- 13.Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 14.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 15.Altarche-Xifro W, Curato C, Kaschina E, Grzesiak A, Slavic S, Dong J, Kappert K, Steckelings M, Imboden H, Unger T, Li J. Cardiac c-kit+AT2+ cell population is increased in response to ischemic injury and supports cardiomyocyte performance. Stem Cells. 2009;27:2488–2497. doi: 10.1002/stem.171. [DOI] [PubMed] [Google Scholar]
- 17.Amaravadi R, Thompson CB. The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest. 2005;115:2618–2624. doi: 10.1172/JCI26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ananthakrishnan R, Moe GW, Goldenthal MJ, Marin-Garcia J. Akt signaling pathway in pacing-induced heart failure. Mol Cell Biochem. 2005;268:103–110. doi: 10.1007/s11010-005-3699-3. [DOI] [PubMed] [Google Scholar]
- 19.Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- 20.Angelone T, Pasqua T, Di Majo D, Quintieri AM, Filice E, Amodio N, Tota B, Giammanco M, Cerra MC. Distinct signalling mechanisms are involved in the dissimilar myocardial and coronary effects elicited by quercetin and myricetin, two red wine flavonols. Nutr Metab Cardiovasc Dis. 2011;21:362–371. doi: 10.1016/j.numecd.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, Richardson JA, Hill JA, Olson EN. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci USA. 2002;99:907–912. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, Richardson JA, Hill JA, Olson EN. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci USA. 2002;99:907–912. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt PK. The akt kinase: molecular determinants of oncogenicity. Proc Natl Acad Sci USA. 1998;95:14950–14955. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.del Aoki MP, Cano RC, Pellegrini AV, Tanos T, Guinazu NL, Coso OA, Gea S. Different signaling pathways are involved in cardiomyocyte survival induced by a Trypanosoma cruzi glycoprotein. Microbes Infect. 2006;8:1723–1731. doi: 10.1016/j.micinf.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Aoyama T, Matsui T, Novikov M, Park J, Hemmings B, Rosenzweig A. Serum and glucocorticoid-responsive kinase-1 regulates cardiomyocyte survival and hypertrophic response. Circulation. 2005;111:1652–1659. doi: 10.1161/01.CIR.0000160352.58142.06. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong SC. Protein kinase activation and myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;61:427–436. doi: 10.1016/j.cardiores.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 27.Baba HA, Stypmann J, Grabellus F, Kirchhof P, Sokoll A, Schafers M, Takeda A, Wilhelm MJ, Scheld HH, Takeda N, Breithardt G, Levkau B. Dynamic regulation of MEK/Erks and Akt/GSK-3beta in human end-stage heart failure after left ventricular mechanical support: myocardial mechanotransduction-sensitivity as a possible molecular mechanism. Cardiovasc Res. 2003;59:390–399. doi: 10.1016/s0008-6363(03)00393-6. [DOI] [PubMed] [Google Scholar]
- 28.Babiker FA, De Windt LJ, van Eickels M, Thijssen V, Bronsaer RJ, Grohe C, van Bilsen M, Doevendans PA. 17Beta-estradiol antagonizes cardiomyocyte hypertrophy by autocrine/paracrine stimulation of a guanylyl cyclase A receptor-cyclic guanosine monophosphate-dependent protein kinase pathway. Circulation. 2004;109:269–276. doi: 10.1161/01.CIR.0000105682.85732.BD. [DOI] [PubMed] [Google Scholar]
- 29.Bachmann M, Moroy T. The serine/threonine kinase Pim-1. Int J Biochem Cell Biol. 2005;37:726–730. doi: 10.1016/j.biocel.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Badorff C, Seeger FH, Zeiher AM, Dimmeler S. Glycogen synthase kinase 3beta inhibits myocardin-dependent transcription and hypertrophy induction through site-specific phosphorylation. Circ Res. 2005;97:645–654. doi: 10.1161/01.RES.0000184684.88750.FE. [DOI] [PubMed] [Google Scholar]
- 31.Badve S, Collins NR, Bhat-Nakshatri P, Turbin D, Leung S, Thorat M, Dunn SE, Geistlinger TR, Carroll JS, Brown M, Bose S, Teitell MA, Nakshatri H. Subcellular localization of activated AKT in estrogen receptor- and progesterone receptor-expressing breast cancers: potential clinical implications. Am J Pathol. 2010;176:2139–2149. doi: 10.2353/ajpath.2010.090477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bae S, Zhang L. Gender differences in cardioprotection against ischemia/reperfusion injury in adult rat hearts: focus on Akt and protein kinase C signaling. J Pharmacol Exp Ther. 2005;315:1125–1135. doi: 10.1124/jpet.105.090803. [DOI] [PubMed] [Google Scholar]
- 33.Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F, Papotti M, Surico N, Bussolino F, Isgaard J, Deghenghi R, Sinigaglia F, Prat M, Muccioli G, Ghigo E, Graziani A. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002;159:1029–1037. doi: 10.1083/jcb.200207165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barandon L, Dufourcq P, Costet P, Moreau C, Allieres C, Daret D, Dos Santos P, Daniel Lamaziere JM, Couffinhal T, Duplaa C. Involvement of FrzA/sFRP-1 and the Wnt/frizzled pathway in ischemic preconditioning. Circ Res. 2005;96:1299–1306. doi: 10.1161/01.RES.0000171895.06914.2c. [DOI] [PubMed] [Google Scholar]
- 35.Barillas R, Friehs I, Cao-Danh H, Martinez JF, del Nido PJ. Inhibition of glycogen synthase kinase-3beta improves tolerance to ischemia in hypertrophied hearts. Ann Thorac Surg. 2007;84:126–133. doi: 10.1016/j.athoracsur.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baron-Menguy C, Bocquet A, Guihot AL, Chappard D, Amiot MJ, Andriantsitohaina R, Loufrani L, Henrion D. Effects of red wine polyphenols on postischemic neovascularization model in rats: low doses are proangiogenic, high doses anti-angiogenic. FASEB J. 2007;21:3511–3521. doi: 10.1096/fj.06-7782com. [DOI] [PubMed] [Google Scholar]
- 37.Basson M. MicroRNAs loom large in the heart. Nat Med. 2007;13:541. doi: 10.1038/nm0507-541. [DOI] [PubMed] [Google Scholar]
- 38.Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 39.Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1934. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- 40.Bechard M, Dalton S. Subcellular localization of glycogen synthase kinase 3beta controls embryonic stem cell self-renewal. Mol Cell Biol. 2009;29:2092–2104. doi: 10.1128/MCB.01405-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bechlioulis A, Naka KK, Calis KA, Makrigiannakis A, Michalis L, Kalantaridou SN. Cardiovascular effects of endogenous estrogen and hormone therapy. Curr Vasc Pharmacol. 8:249–258. doi: 10.2174/157016110790886974. [DOI] [PubMed] [Google Scholar]
- 42.Bell RM, Clark JE, Hearse DJ, Shattock MJ. Reperfusion kinase phosphorylation is essential but not sufficient in the mediation of pharmacological preconditioning: characterisation in the bi-phasic profile of early and late protection. Cardiovasc Res. 2007;73:153–163. doi: 10.1016/j.cardiores.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 44.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 45.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 47.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 48.Bertrand L, Horman S, Beauloye C, Vanoverschelde JL. Insulin signalling in the heart. Cardiovasc Res. 2008;79:238–248. doi: 10.1093/cvr/cvn093. [DOI] [PubMed] [Google Scholar]
- 49.Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, Mocanu MM, Yellon DM. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol. 2008;103:274–284. doi: 10.1007/s00395-007-0691-y. [DOI] [PubMed] [Google Scholar]
- 50.Bhuiyan MS, Fukunaga K. Cardioprotection by vanadium compounds targeting Akt-mediated signaling. J Pharmacol Sci. 2009;110:1–13. doi: 10.1254/jphs.09r01cr. [DOI] [PubMed] [Google Scholar]
- 51.Bhuiyan MS, Shibuya M, Shioda N, Moriguchi S, Kasahara J, Iwabuchi Y, Fukunaga K. Cytoprotective effect of bis(1-oxy-2-pyridinethiolato)oxovanadiun(IV) on myocardial ischemia/reperfusion injury elicits inhibition of Fas ligand and Bim expression and elevation of FLIP expression. Eur J Pharmacol. 2007;571:180–188. doi: 10.1016/j.ejphar.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 52.Bhuiyan MS, Shioda N, Fukunaga K. Ovariectomy augments pressure overload-induced hypertrophy associated with changes in Akt and nitric oxide synthase signaling pathways in female rats. Am J Physiol Endocrinol Metab. 2007;293:E1606–E1614. doi: 10.1152/ajpendo.00246.2007. [DOI] [PubMed] [Google Scholar]
- 53.Bicknell KA, Coxon CH, Brooks G. Can the cardiomyocyte cell cycle be reprogrammed? J Mol Cell Cardiol. 2007;42:706–721. doi: 10.1016/j.yjmcc.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 55.Bonauer A, Boon RA, Dimmeler S. Vascular microRNAs. Curr Drug Targets. 11:943–949. doi: 10.2174/138945010791591313. [DOI] [PubMed] [Google Scholar]
- 56.Bos JL. A target for phosphoinositide 3-kinase: Akt/PKB. Trends Biochem Sci. 1995;20:441–442. doi: 10.1016/s0968-0004(00)89097-0. [DOI] [PubMed] [Google Scholar]
- 57.Brancaccio M, Hirsch E, Notte A, Selvetella G, Lembo G, Tarone G. Integrin signalling: the tug-of-war in heart hypertrophy. Cardiovasc Res. 2006;70:422–433. doi: 10.1016/j.cardiores.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 58.Brar BK, Stephanou A, Knight R, Latchman DS. Activation of protein kinase B/Akt by urocortin is essential for its ability to protect cardiac cells against hypoxia/reoxygenation-induced cell death. J Mol Cell Cardiol. 2002;34:483–492. doi: 10.1006/jmcc.2002.1529. [DOI] [PubMed] [Google Scholar]
- 59.Brar BK, Stephanou A, Pennica D, Latchman DS. CT-1 mediated cardioprotection against ischaemic re-oxygenation injury is mediated by PI3 kinase, Akt and MEK1/2 pathways. Cytokine. 2001;16:93–96. doi: 10.1006/cyto.2001.0951. [DOI] [PubMed] [Google Scholar]
- 60.Brinckmann M, Kaschina E, Altarche-Xifro W, Curato C, Timm M, Grzesiak A, Dong J, Kappert K, Kintscher U, Unger T, Li J. Estrogen receptor alpha supports cardiomyocytes indirectly through post-infarct cardiac c-kit+ cells. J Mol Cell Cardiol. 2009;47:66–75. doi: 10.1016/j.yjmcc.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 61.Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 62.Brownsey RW, Boone AN, Allard MF. Actions of insulin on the mammalian heart: metabolism, pathology and biochemical mechanisms. Cardiovasc Res. 1997;34:3–24. doi: 10.1016/s0008-6363(97)00051-5. [DOI] [PubMed] [Google Scholar]
- 63.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 64.Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE, Abel ED. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–5349. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- 65.Budas GR, Sukhodub A, Alessi DR, Jovanovic A. 3′ Phosphoinositide-dependent kinase-1 is essential for ischemic preconditioning of the myocardium. FASEB J. 2006;20:2556–2558. doi: 10.1096/fj.06-6252fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res. 2002;91:776–781. doi: 10.1161/01.res.0000038488.38975.1a. [DOI] [PubMed] [Google Scholar]
- 68.Bulhak AA, Jung C, Ostenson CG, Lundberg JO, Sjoquist PO, Pernow J. PPAR-alpha activation protects the type 2 diabetic myocardium against ischemia-reperfusion injury: involvement of the PI3-Kinase/Akt and NO pathway. Am J Physiol Heart Circ Physiol. 2009;296:H719–H727. doi: 10.1152/ajpheart.00394.2008. [DOI] [PubMed] [Google Scholar]
- 69.Bullock AN, Debreczeni J, Amos AL, Knapp S, Turk BE. Structure and substrate specificity of the Pim-1 kinase. J Biol Chem. 2005;280:41675–41682. doi: 10.1074/jbc.M510711200. [DOI] [PubMed] [Google Scholar]
- 70.Bureau I, Gueux E, Mazur A, Rock E, Roussel AM, Rayssiguier Y. Female rats are protected against oxidative stress during copper deficiency. J Am Coll Nutr. 2003;22:239–246. doi: 10.1080/07315724.2003.10719299. [DOI] [PubMed] [Google Scholar]
- 71.Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 72.Busk PK, Cirera S. MicroRNA profiling in early hypertrophic growth of the left ventricle in rats. Biochem Biophys Res Commun. 396:989–993. doi: 10.1016/j.bbrc.2010.05.039. [DOI] [PubMed] [Google Scholar]
- 73.Buss SJ, Muenz S, Riffel JH, Malekar P, Hagenmueller M, Weiss CS, Bea F, Bekeredjian R, Schinke-Braun M, Izumo S, Katus HA, Hardt SE. Beneficial effects of mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol. 2009;54:2435–2446. doi: 10.1016/j.jacc.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 74.Cai Z, Semenza GL. PTEN activity is modulated during ischemia and reperfusion: involvement in the induction and decay of preconditioning. Circ Res. 2005;97:1351–1359. doi: 10.1161/01.RES.0000195656.52760.30. [DOI] [PubMed] [Google Scholar]
- 75.Campa VM, Gutierrez-Lanza R, Cerignoli F, Diaz-Trelles R, Nelson B, Tsuji T, Barcova M, Jiang W, Mercola M. Notch activates cell cycle reentry and progression in quiescent cardiomyocytes. J Cell Biol. 2008;183:129–141. doi: 10.1083/jcb.200806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Camper-Kirby D, Welch S, Walker A, Shiraishi I, Setchell KD, Schaefer E, Kajstura J, Anversa P, Sussman MA. Myocardial Akt activation and gender: increased nuclear activity in females versus males. Circ Res. 2001;88:1020–1027. doi: 10.1161/hh1001.090858. [DOI] [PubMed] [Google Scholar]
- 77.Cao P, Deng Z, Wan M, Huang W, Cramer SD, Xu J, Lei M, Sui G. MicroRNA-101 negatively regulates Ezh2 and its expression is modulated by androgen receptor and HIF-1alpha/HIF-1beta. Mol Cancer. 9:108. doi: 10.1186/1476-4598-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Caporali A, Sala-Newby GB, Meloni M, Graiani G, Pani E, Cristofaro B, Newby AC, Madeddu P, Emanueli C. Identification of the prosurvival activity of nerve growth factor on cardiac myocytes. Cell Death Differ. 2008;15:299–311. doi: 10.1038/sj.cdd.4402263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Hoydal M, Autore C, Russo MA, Dorn GW, 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 80.Catalucci D, Condorelli G. Effects of Akt on cardiac myocytes: location counts. Circ Res. 2006;99:339–341. doi: 10.1161/01.RES.0000239409.90634.a9. [DOI] [PubMed] [Google Scholar]
- 81.Catalucci D, Latronico MV, Ceci M, Rusconi F, Young HS, Gallo P, Santonastasi M, Bellacosa A, Brown JH, Condorelli G. Akt increases sarcoplasmic reticulum Ca2+ cycling by direct phosphorylation of phospholamban at Thr17. J Biol Chem. 2009;284:28180–28187. doi: 10.1074/jbc.M109.036566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Catalucci D, Zhang DH, DeSantiago J, Aimond F, Barbara G, Chemin J, Bonci D, Picht E, Rusconi F, Dalton ND, Peterson KL, Richard S, Bers DM, Brown JH, Condorelli G. Akt regulates L-type Ca2+ channel activity by modulating Cavalpha1 protein stability. J Cell Biol. 2009;184:923–933. doi: 10.1083/jcb.200805063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cavasin MA, Sankey SS, Yu AL, Menon S, Yang XP. Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;284:H1560–H1569. doi: 10.1152/ajpheart.01087.2002. [DOI] [PubMed] [Google Scholar]
- 84.Ceci M, Gallo P, Santonastasi M, Grimaldi S, Latronico MV, Pitisci A, Missol-Kolka E, Scimia MC, Catalucci D, Hilfiker-Kleiner D, Condorelli G. Cardiac-specific overexpression of E40K active Akt prevents pressure overload-induced heart failure in mice by increasing angiogenesis and reducing apoptosis. Cell Death Differ. 2007;14:1060–1062. doi: 10.1038/sj.cdd.4402095. [DOI] [PubMed] [Google Scholar]
- 85.Centurione L, Antonucci A, Miscia S, Grilli A, Rapino M, Grifone G, Di Giacomo V, Di Giulio C, Falconi M, Cataldi A. Age-related death-survival balance in myocardium: an immunohistochemical and biochemical study. Mech Ageing Dev. 2002;123:341–350. doi: 10.1016/s0047-6374(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 86.Ceylan-Isik AF, LaCour KH, Ren J. Gender disparity of streptozotocin-induced intrinsic contractile dysfunction in murine ventricular myocytes: role of chronic activation of Akt. Clin Exp Pharmacol Physiol. 2006;33:102–108. doi: 10.1111/j.1440-1681.2006.04331.x. [DOI] [PubMed] [Google Scholar]
- 87.Chakir K, Daya SK, Tunin RS, Helm RH, Byrne MJ, Dimaano VL, Lardo AC, Abraham TP, Tomaselli GF, Kass DA. Reversal of global apoptosis and regional stress kinase activation by cardiac resynchronization. Circulation. 2008;117:1369–1377. doi: 10.1161/CIRCULATIONAHA.107.706291. [DOI] [PubMed] [Google Scholar]
- 88.Chandrasekar B, Mummidi S, Claycomb WC, Mestril R, Nemer M. Interleukin-18 is a pro-hypertrophic cytokine that acts through a phosphatidylinositol 3-kinase-phosphoinositide-dependent kinase-1-Akt-GATA4 signaling pathway in cardiomyocytes. J Biol Chem. 2005;280:4553–4567. doi: 10.1074/jbc.M411787200. [DOI] [PubMed] [Google Scholar]
- 89.Chatham JC, Seymour AM. Cardiac carbohydrate metabolism in Zucker diabetic fatty rats. Cardiovasc Res. 2002;55:104–112. doi: 10.1016/s0008-6363(02)00399-1. [DOI] [PubMed] [Google Scholar]
- 90.Chen H, Li D, Saldeen T, Mehta JL. TGF-beta(1) modulates NOS expression and phosphorylation of Akt/PKB in rat myocytes exposed to hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 2001;281:H1035–H1039. doi: 10.1152/ajpheart.2001.281.3.H1035. [DOI] [PubMed] [Google Scholar]
- 91.Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen X, Thakkar H, Tyan F, Gim S, Robinson H, Lee C, Pandey SK, Nwokorie C, Onwudiwe N, Srivastava RK. Constitutively active Akt is an important regulator of TRAIL sensitivity in prostate cancer. Oncogene. 2001;20:6073–6083. doi: 10.1038/sj.onc.1204736. [DOI] [PubMed] [Google Scholar]
- 94.Chen YC, Teng SC, Wu KJ. Phosphorylation of telomeric repeat binding factor 1 (TRF1) by Akt causes telomere shortening. Cancer Invest. 2009;27:24–28. doi: 10.1080/07357900802027081. [DOI] [PubMed] [Google Scholar]
- 95.Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol. 2007;170:1831–1840. doi: 10.2353/ajpath.2007.061170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3′-kinase. Circ Res. 2000;87:1172–1179. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- 97.Chiari PC, Bienengraeber MW, Pagel PS, Krolikowski JG, Kersten JR, Warltier DC. Isoflurane protects against myocardial infarction during early reperfusion by activation of phosphatidylinositol-3-kinase signal transduction: evidence for anesthetic-induced postconditioning in rabbits. Anesthesiology. 2005;102:102–109. doi: 10.1097/00000542-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 98.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, Marban E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chiu MI, Katz H, Berlin V. RAPT1, a mammalian homolog of yeast Tor, interacts with the FKBP12/rapamycin complex. Proc Natl Acad Sci USA. 1994;91:12574–12578. doi: 10.1073/pnas.91.26.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 101.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 102.Chu L, Hao H, Luo M, Huang Y, Chen Z, Lu T, Zhao X, Verfaillie CM, Zweier JL, Liu Z. Ox-LDL modifies the behavior of bone marrow stem cells and impairs their endothelial differentiation via inhibition of Akt phosphorylation. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cittadini A, Monti MG, Iaccarino G, Di Rella F, Tsichlis PN, Di Gianni A, Stromer H, Sorriento D, Peschle C, Trimarco B, Sacca L, Condorelli G. Adenoviral gene transfer of Akt enhances myocardial contractility and intracellular calcium handling. Gene Ther. 2006;13:8–19. doi: 10.1038/sj.gt.3302589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clerk A, Bogoyevitch MA, Anderson MB, Sugden PH. Differential activation of protein kinase C isoforms by endothelin-1 and phenylephrine and subsequent stimulation of p42 and p44 mitogen-activated protein kinases in ventricular myocytes cultured from neonatal rat hearts. J Biol Chem. 1994;269:32848–32857. [PubMed] [Google Scholar]
- 105.Clerk A, Cullingford TE, Fuller SJ, Giraldo A, Markou T, Pikkarainen S, Sugden PH. Signaling pathways mediating cardiac myocyte gene expression in physiological and stress responses. J Cell Physiol. 2007;212:311–322. doi: 10.1002/jcp.21094. [DOI] [PubMed] [Google Scholar]
- 106.Clerk A, Sugden PH. Activation of protein kinase cascades in the heart by hypertrophic G protein-coupled receptor agonists. Am J Cardiol. 1999;83:64H–69H. doi: 10.1016/s0002-9149(99)00261-1. [DOI] [PubMed] [Google Scholar]
- 107.Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cohen MV, Philipp S, Krieg T, Cui L, Kuno A, Solodushko V, Downey JM. Preconditioning-mimetics bradykinin and DADLE activate PI3-kinase through divergent pathways. J Mol Cell Cardiol. 2007;42:842–851. doi: 10.1016/j.yjmcc.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coles JG, Boscarino C, Takahashi M, Grant D, Chang A, Ritter J, Dai X, Du C, Musso G, Yamabi H, Goncalves J, Kumar AS, Woodgett J, Lu H, Hannigan G. Cardioprotective stress response in the human fetal heart. J Thorac Cardiovasc Surg. 2005;129:1128–1136. doi: 10.1016/j.jtcvs.2004.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Collesi C, Zentilin L, Sinagra G, Giacca M. Notch1 signaling stimulates proliferation of immature cardiomyocytes. J Cell Biol. 2008;183:117–128. doi: 10.1083/jcb.200806091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Colombo F, Gosselin H, El-Helou V, Calderone A. Beta-adrenergic receptor-mediated DNA synthesis in neonatal rat cardiac fibroblasts proceeds via a phosphatidylinositol 3-kinase dependent pathway refractory to the antiproliferative action of cyclic AMP. J Cell Physiol. 2003;195:322–330. doi: 10.1002/jcp.10251. [DOI] [PubMed] [Google Scholar]
- 112.Colston JT, Boylston WH, Feldman MD, Jenkinson CP, de la Rosa SD, Barton A, Trevino RJ, Freeman GL, Chandrasekar B. Interleukin-18 knockout mice display mal-adaptive cardiac hypertrophy in response to pressure overload. Biochem Biophys Res Commun. 2007;354:552–558. doi: 10.1016/j.bbrc.2007.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Colston JT, de la Rosa SD, Koehler M, Gonzales K, Mestril R, Freeman GL, Bailey SR, Chandrasekar B. Wnt-induced secreted protein-1 is a prohypertrophic and profibrotic growth factor. Am J Physiol Heart Circ Physiol. 2007;293:H1839–H1846. doi: 10.1152/ajpheart.00428.2007. [DOI] [PubMed] [Google Scholar]
- 114.Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, Latronico MV, Napoli C, Sadoshima J, Croce CM, Ross J., Jr Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci USA. 2002;99:12333–12338. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cook SA, Matsui T, Li L, Rosenzweig A. Transcriptional effects of chronic Akt activation in the heart. J Biol Chem. 2002;277:22528–22533. doi: 10.1074/jbc.M201462200. [DOI] [PubMed] [Google Scholar]
- 116.Cook SA, Matsui T, Li L, Rosenzweig A. Transcriptional effects of chronic Akt activation in the heart. J Biol Chem. 2002;277:22528–22533. doi: 10.1074/jbc.M201462200. [DOI] [PubMed] [Google Scholar]
- 117.Cottage CT, Bailey B, Fischer KM, Avitable D, Collins B, Tuck S, Quijada P, Gude N, Alvarez R, Muraski J, Sussman MA. Cardiac progenitor cell cycling stimulated by pim-1 kinase. Circ Res. 106:891–901. doi: 10.1161/CIRCRESAHA.109.208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Couvreur N, Lucats L, Tissier R, Bize A, Berdeaux A, Ghaleh B. Differential effects of postconditioning on myocardial stunning and infarction: a study in conscious dogs and anesthetized rabbits. Am J Physiol Heart Circ Physiol. 2006;291:H1345–H1350. doi: 10.1152/ajpheart.00124.2006. [DOI] [PubMed] [Google Scholar]
- 119.Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, Sah R, Cheng HY, Rybin VO, Lembo G, Fratta L, Oliveirados-Santos AJ, Benovic JL, Kahn CR, Izumo S, Steinberg SF, Wymann MP, Backx PH, Penninger JM. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell. 2002;110:737–749. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 120.Crozier SJ, Zhang X, Wang J, Cheung J, Kimball SR, Jefferson LS. Activation of signaling pathways and regulatory mechanisms of mRNA translation following myocardial ischemia-reperfusion. J Appl Physiol. 2006;101:576–582. doi: 10.1152/japplphysiol.01122.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cuesta R, Martinez-Sanchez A, Gebauer F. miR-181a regulates cap-dependent translation of p27(kip1) mRNA in myeloid cells. Mol Cell Biol. 2009;29:2841–2851. doi: 10.1128/MCB.01971-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dallabrida SM, Ismail NS, Pravda EA, Parodi EM, Dickie R, Durand EM, Lai J, Cassiola F, Rogers RA, Rupnick MA. Integrin binding angiopoietin-1 monomers reduce cardiac hypertrophy. FASEB J. 2008;22:3010–3023. doi: 10.1096/fj.07-100966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-κB is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Danial NN, Walensky LD, Zhang CY, Choi CS, Fisher JK, Molina AJ, Datta SR, Pitter KL, Bird GH, Wikstrom JD, Deeney JT, Robertson K, Morash J, Kulkarni A, Neschen S, Kim S, Greenberg ME, Corkey BE, Shirihai OS, Shulman GI, Lowell BB, Korsmeyer SJ. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med. 2008;14:144–153. doi: 10.1038/nm1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Das A, Smolenski A, Lohmann SM, Kukreja RC. Cyclic GMP-dependent protein kinase Ialpha attenuates necrosis and apoptosis following ischemia/reoxygenation in adult cardiomyocyte. J Biol Chem. 2006;281:38644–38652. doi: 10.1074/jbc.M606142200. [DOI] [PubMed] [Google Scholar]
- 126.Das DK, Maulik N. Mitochondrial function in cardiomyocytes: target for cardioprotection. Curr Opin Anaesthesiol. 2005;18:77–82. doi: 10.1097/00001503-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 127.Das M, Cui J, Das DK. Generation of survival signal by differential interaction of p38MAPKalpha and p38MAPKbeta with caveolin-1 and caveolin-3 in the adapted heart. J Mol Cell Cardiol. 2007;42:206–213. doi: 10.1016/j.yjmcc.2006.08.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Das M, Das S, Das DK. Caveolin and MAP kinase interaction in angiotensin II preconditioning of the myocardium. J Cell Mol Med. 2007;11:788–797. doi: 10.1111/j.1582-4934.2007.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 129.Das S, Cordis GA, Maulik N, Das DK. Pharmacological preconditioning with resveratrol: role of CREB-dependent Bcl-2 signaling via adenosine A3 receptor activation. Am J Physiol Heart Circ Physiol. 2005;288:H328–H335. doi: 10.1152/ajpheart.00453.2004. [DOI] [PubMed] [Google Scholar]
- 130.Das S, Otani H, Maulik N, Das DK. Redox regulation of angiotensin II preconditioning of the myocardium requires MAP kinase signaling. J Mol Cell Cardiol. 2006;41:248–255. doi: 10.1016/j.yjmcc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 131.Das S, Tosaki A, Bagchi D, Maulik N, Das DK. Resveratrol-mediated activation of cAMP response element-binding protein through adenosine A3 receptor by Akt-dependent and -independent pathways. J Pharmacol Exp Ther. 2005;314:762–769. doi: 10.1124/jpet.105.084285. [DOI] [PubMed] [Google Scholar]
- 132.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 133.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 134.Davey KA, Garlick PB, Warley A, Southworth R. Immunogold labeling study of the distribution of GLUT-1 and GLUT-4 in cardiac tissue following stimulation by insulin or ischemia. Am J Physiol Heart Circ Physiol. 2007;292:H2009–H2019. doi: 10.1152/ajpheart.00663.2006. [DOI] [PubMed] [Google Scholar]
- 135.Davidson SM, Hausenloy D, Duchen MR, Yellon DM. Signalling via the reperfusion injury signalling kinase (RISK) pathway links closure of the mitochondrial permeability transition pore to cardioprotection. Int J Biochem Cell Biol. 2006;38:414–419. doi: 10.1016/j.biocel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 136.Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci USA. 2006;103:8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.De Acetis M, Notte A, Accornero F, Selvetella G, Brancaccio M, Vecchione C, Sbroggio M, Collino F, Pacchioni B, Lanfranchi G, Aretini A, Ferretti R, Maffei A, Altruda F, Silengo L, Tarone G, Lembo G. Cardiac overexpression of melusin protects from dilated cardiomyopathy due to long-standing pressure overload. Circ Res. 2005;96:1087–1094. doi: 10.1161/01.RES.0000168028.36081.e0. [DOI] [PubMed] [Google Scholar]
- 138.De Jonge N, Goumans MJ, Lips D, Hassink R, Vlug EJ, van der Meel R, Emmerson CD, Nijman J, de Windt L, Doevendans PA. Controlling cardiomyocyte survival. Novartis Found Symp. 2006;274:41–51. [PubMed] [Google Scholar]
- 139.De Windt LJ, Lim HW, Taigen T, Wencker D, Condorelli G, Dorn GW, 2nd, Kitsis RN, Molkentin JD. Calcineurin-mediated hypertrophy protects cardiomyocytes from apoptosis in vitro and in vivo: an apoptosis-independent model of dilated heart failure. Circ Res. 2000;86:255–263. doi: 10.1161/01.res.86.3.255. [DOI] [PubMed] [Google Scholar]
- 140.DeBosch B, Sambandam N, Weinheimer C, Courtois M, Muslin AJ. Akt2 regulates cardiac metabolism and cardiomyocyte survival. J Biol Chem. 2006;281:32841–32851. doi: 10.1074/jbc.M513087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 142.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 143.Dedkova EN, Wang YG, Ji X, Blatter LA, Samarel AM, Lipsius SL. Signalling mechanisms in contraction-mediated stimulation of intracellular NO production in cat ventricular myocytes. J Physiol. 2007;580:327–345. doi: 10.1113/jphysiol.2006.126805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 145.Del Re DP, Miyamoto S, Brown JH. RhoA/Rho kinase up-regulate Bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis. J Biol Chem. 2007;282:8069–8078. doi: 10.1074/jbc.M604298200. [DOI] [PubMed] [Google Scholar]
- 146.Deng JY, Huang JP, Lu LS, Hung LM. Impairment of cardiac insulin signaling and myocardial contractile performance in high-cholesterol/fructose-fed rats. Am J Physiol Heart Circ Physiol. 2007;293:H978–H987. doi: 10.1152/ajpheart.01002.2006. [DOI] [PubMed] [Google Scholar]
- 147.Dent MR, Das S, Dhalla NS. Alterations in both death and survival signals for apoptosis in heart failure due to volume overload. J Mol Cell Cardiol. 2007;43:726–732. doi: 10.1016/j.yjmcc.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 148.Dentelli P, Rosso A, Orso F, Olgasi C, Taverna D, Brizzi MF. microRNA-222 controls neovascularization by regulating signal transducer and activator of transcription 5A expression. Arterioscler Thromb Vasc Biol. 30:1562–1568. doi: 10.1161/ATVBAHA.110.206201. [DOI] [PubMed] [Google Scholar]
- 149.Depre C, Hase M, Gaussin V, Zajac A, Wang L, Hittinger L, Ghaleh B, Yu X, Kudej RK, Wagner T, Sadoshima J, Vatner SF. H11 kinase is a novel mediator of myocardial hypertrophy in vivo. Circ Res. 2002;91:1007–1014. doi: 10.1161/01.res.0000044380.54893.4b. [DOI] [PubMed] [Google Scholar]
- 150.Deprez J, Vertommen D, Alessi DR, Hue L, Rider MH. Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades. J Biol Chem. 1997;272:17269–17275. doi: 10.1074/jbc.272.28.17269. [DOI] [PubMed] [Google Scholar]
- 151.Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardio-protective in male and female rats. Am J Physiol Heart Circ Physiol. 2009;297:H1806–H1813. doi: 10.1152/ajpheart.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Desrois M, Sidell RJ, Gauguier D, Davey CL, Radda GK, Clarke K. Gender differences in hypertrophy, insulin resistance and ischemic injury in the aging type 2 diabetic rat heart. J Mol Cell Cardiol. 2004;37:547–555. doi: 10.1016/j.yjmcc.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 153.Diez C, Nestler M, Friedrich U, Vieth M, Stolte M, Hu K, Hoppe J, Simm A. Down-regulation of Akt/PKB in senescent cardiac fibroblasts impairs PDGF-induced cell proliferation. Cardiovasc Res. 2001;49:731–740. doi: 10.1016/s0008-6363(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 154.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 155.Dimmeler S, Zeiher AM. Exercise and cardiovascular health: get active to “AKTivate” your endothelial nitric oxide synthase. Circulation. 2003;107:3118–3120. doi: 10.1161/01.CIR.0000074244.82874.A0. [DOI] [PubMed] [Google Scholar]
- 156.Donath MY, Jenni R, Brunner HP, Anrig M, Kohli S, Glatz Y, Froesch ER. Cardiovascular and metabolic effects of insulin-like growth factor I at rest and during exercise in humans. J Clin Endocrinol Metab. 1996;81:4089–4094. doi: 10.1210/jcem.81.11.8923865. [DOI] [PubMed] [Google Scholar]
- 157.Donath MY, Sutsch G, Yan XW, Piva B, Brunner HP, Glatz Y, Zapf J, Follath F, Froesch ER, Kiowski W. Acute cardiovascular effects of insulin-like growth factor I in patients with chronic heart failure. J Clin Endocrinol Metab. 1998;83:3177–3183. doi: 10.1210/jcem.83.9.5122. [DOI] [PubMed] [Google Scholar]
- 158.Donath MY, Zapf J, Eppenberger-Eberhardt M, Froesch ER, Eppenberger HM. Insulin-like growth factor I stimulates myofibril development and decreases smooth muscle alpha-actin of adult cardiomyocytes. Proc Natl Acad Sci USA. 1994;91:1686–1690. doi: 10.1073/pnas.91.5.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dong F, Li Q, Sreejayan N, Nunn JM, Ren J. Metallothionein prevents high-fat diet induced cardiac contractile dysfunction: role of peroxisome proliferator activated receptor gamma coactivator 1alpha and mitochondrial biogenesis. Diabetes. 2007;56:2201–2212. doi: 10.2337/db06-1596. [DOI] [PubMed] [Google Scholar]
- 160.Donker DW, Maessen JG, Verheyen F, Ramaekers FC, Spatjens RL, Kuijpers H, Ramakers C, Schiffers PM, Vos MA, Crijns HJ, Volders PG. Impact of acute and enduring volume overload on mechanotransduction and cytoskeletal integrity of canine left ventricular myocardium. Am J Physiol Heart Circ Physiol. 2007;292:H2324–H2332. doi: 10.1152/ajpheart.00392.2006. [DOI] [PubMed] [Google Scholar]
- 161.Donthi RV, Huisamen B, Lochner A. Effect of vanadate and insulin on glucose transport in isolated adult rat cardiomyocytes. Cardiovasc Drugs Ther. 2000;14:463–470. doi: 10.1023/a:1007876703644. [DOI] [PubMed] [Google Scholar]
- 162.Donthi RV, Ye G, Wu C, McClain DA, Lange AJ, Epstein PN. Cardiac expression of kinase-deficient 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase inhibits glycolysis, promotes hypertrophy, impairs myocyte function, and reduces insulin sensitivity. J Biol Chem. 2004;279:48085–48090. doi: 10.1074/jbc.M405510200. [DOI] [PubMed] [Google Scholar]
- 163.Dorn GW, 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dreger H, Lorenz M, Kehrer A, Baumann G, Stangl K, Stangl V. Characteristics of catechin- and theaflavin-mediated cardioprotection. Exp Biol Med. 2008;233:427–433. doi: 10.3181/0710-RM-292. [DOI] [PubMed] [Google Scholar]
- 165.Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 166.Du XJ. Gender modulates cardiac phenotype development in genetically modified mice. Cardiovasc Res. 2004;63:510–519. doi: 10.1016/j.cardiores.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 167.Duan J, Zhang HY, Adkins SD, Ren BH, Norby FL, Zhang X, Benoit JN, Epstein PN, Ren J. Impaired cardiac function and IGF-I response in myocytes from calmodulin-diabetic mice: role of Akt and RhoA. Am J Physiol Endocrinol Metab. 2003;284:E366–E376. doi: 10.1152/ajpendo.00254.2002. [DOI] [PubMed] [Google Scholar]
- 168.Duan SZ, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circ Res. 2005;97:372–379. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- 169.Duda MK, O’Shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, Hoit BD, Kop WJ, Stanley WC. Dietary supplementation with omega-3 PUFA increases adiponectin and attenuates ventricular remodeling and dysfunction with pressure overload. Cardiovasc Res. 2007;76:303–310. doi: 10.1016/j.cardiores.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, Herias V, van Leeuwen RE, Schellings MW, Barenbrug P, Maessen JG, Heymans S, Pinto YM, Creemers EE. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. 2009;104:170–178. doi: 10.1161/CIRCRESAHA.108.182535. [DOI] [PubMed] [Google Scholar]
- 171.Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans. 2007;35:231–235. doi: 10.1042/BST0350231. [DOI] [PubMed] [Google Scholar]
- 172.Duncan JG, Finck BN. The PPARalpha-PGC-1alpha axis controls cardiac energy metabolism in healthy and diseased myocardium. PPAR Res. 2008;2008:253817. doi: 10.1155/2008/253817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum MJ. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Efthymiou CA, Mocanu MM, Yellon DM. Atorvastatin and myocardial reperfusion injury: new pleiotropic effect implicating multiple prosurvival signaling. J Cardiovasc Pharmacol. 2005;45:247–252. doi: 10.1097/01.fjc.0000154376.82445.06. [DOI] [PubMed] [Google Scholar]
- 175.Egawa K, Maegawa H, Shimizu S, Morino K, Nishio Y, Bryer-Ash M, Cheung AT, Kolls JK, Kikkawa R, Kashiwagi A. Protein-tyrosine phosphatase-1B negatively regulates insulin signaling in l6 myocytes and Fao hepatoma cells. J Biol Chem. 2001;276:10207–10211. doi: 10.1074/jbc.M009489200. [DOI] [PubMed] [Google Scholar]
- 176.Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–238. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 177.Engel FB, Hsieh PC, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci USA. 2006;103:15546–15551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Engelbrecht AM, Esterhuyse J, du Toit EF, Lochner A, van Rooyen J. p38-MAPK and PKB/Akt, possible role players in red palm oil-induced protection of the isolated perfused rat heart? J Nutr Biochem. 2006;17:265–271. doi: 10.1016/j.jnutbio.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 180.Engelbrecht AM, Niesler C, Page C, Lochner A. p38 and JNK have distinct regulatory functions on the development of apoptosis during simulated ischaemia and reperfusion in neonatal cardiomyocytes. Basic Res Cardiol. 2004;99:338–350. doi: 10.1007/s00395-004-0478-3. [DOI] [PubMed] [Google Scholar]
- 181.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 182.Esfandiarei M, Boroomand S, Suarez A, Si X, Rahmani M, McManus B. Coxsackievirus B3 activates nuclear factor kappa B transcription factor via a phosphatidylinositol-3 kinase/protein kinase B-dependent pathway to improve host cell viability. Cell Microbiol. 2007;9:2358–2371. doi: 10.1111/j.1462-5822.2007.00964.x. [DOI] [PubMed] [Google Scholar]
- 183.Esfandiarei M, Luo H, Yanagawa B, Suarez A, Dabiri D, Zhang J, McManus BM. Protein kinase B/Akt regulates coxsackievirus B3 replication through a mechanism which is not caspase dependent. J Virol. 2004;78:4289–4298. doi: 10.1128/JVI.78.8.4289-4298.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Esfandiarei M, Suarez A, Amaral A, Si X, Rahmani M, Dedhar S, McManus BM. Novel role for integrin-linked kinase in modulation of coxsackievirus B3 replication and virus-induced cardiomyocyte injury. Circ Res. 2006;99:354–361. doi: 10.1161/01.RES.0000237022.72726.01. [DOI] [PubMed] [Google Scholar]
- 185.Essop MF, Chan WY, Taegtmeyer H. Metabolic gene switching in the murine female heart parallels enhanced mitochondrial respiratory function in response to oxidative stress. FEBS Lett. 2007;274:5278–5284. doi: 10.1111/j.1742-4658.2007.06051.x. [DOI] [PubMed] [Google Scholar]
- 186.Evans-Anderson HJ, Alfieri CM, Yutzey KE. Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ Res. 2008;102:686–694. doi: 10.1161/CIRCRESAHA.107.163428. [DOI] [PubMed] [Google Scholar]
- 187.Fan GC, Zhou X, Wang X, Song G, Qian J, Nicolaou P, Chen G, Ren X, Kranias EG. Heat shock protein 20 interacting with phosphorylated Akt reduces doxorubicin-triggered oxidative stress and cardiotoxicity. Circ Res. 2008;103:1270–1279. doi: 10.1161/CIRCRESAHA.108.182832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fang CX, Dong F, Ren BH, Epstein PN, Ren J. Metallothionein alleviates cardiac contractile dysfunction induced by insulin resistance: role of Akt phosphorylation, PTB1B, PPARgamma and c-Jun. Diabetologia. 2005;48:2412–2421. doi: 10.1007/s00125-005-1940-y. [DOI] [PubMed] [Google Scholar]
- 189.Fang CX, Doser TA, Yang X, Sreejayan N, Ren J. Metallothionein antagonizes aging-induced cardiac contractile dysfunction: role of PTP1B, insulin receptor tyrosine phosphorylation and Akt. Aging Cell. 2006;5:177–185. doi: 10.1111/j.1474-9726.2006.00201.x. [DOI] [PubMed] [Google Scholar]
- 190.Fazakerley DJ, Lawrence SP, Lizunov VA, Cushman SW, Holman GD. A common trafficking route for GLUT4 in cardiomyocytes in response to insulin, contraction and energy-status signalling. J Cell Sci. 2009;122:727–734. doi: 10.1242/jcs.041178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Feng J, Fischer G, Lucchinetti E, Zhu M, Bestmann L, Jegger D, Arras M, Pasch T, Perriard JC, Schaub MC, Zaugg M. Infarct-remodeled myocardium is receptive to protection by isoflurane postconditioning: role of protein kinase B/Akt signaling. Anesthesiology. 2006;104:1004–1014. doi: 10.1097/00000542-200605000-00017. [DOI] [PubMed] [Google Scholar]
- 192.Feng J, Lucchinetti E, Ahuja P, Pasch T, Perriard JC, Zaugg M. Isoflurane postconditioning prevents opening of the mitochondrial permeability transition pore through inhibition of glycogen synthase kinase 3beta. Anesthesiology. 2005;103:987–995. doi: 10.1097/00000542-200511000-00013. [DOI] [PubMed] [Google Scholar]
- 193.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Roxe T, Muller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 194.Finn AV, John M, Nakazawa G, Polavarapu R, Karmali V, Xu X, Cheng Q, Davis T, Raghunathan C, Acampado E, Ezell T, Lajoie S, Eppihimer M, Kolodgie FD, Virmani R, Gold HK. Differential healing after sirolimus, paclitaxel, bare metal stent placement in combination with peroxisome proliferator-activator receptor gamma agonists requirement for mTOR/Akt2 in PPAR gamma activation. Circ Res. 2009;105:1003–1012. doi: 10.1161/CIRCRESAHA.109.200519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fish JE, Srivastava D. MicroRNAs: opening a new vein in angiogenesis research. Sci Signal. 2009;2:pe1. doi: 10.1126/scisignal.252pe1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Florholmen G, Thoresen GH, Rustan AC, Jensen J, Christensen G, Aas V. Leukaemia inhibitory factor stimulates glucose transport in isolated cardiomyocytes and induces insulin resistance after chronic exposure. Diabetologia. 2006;49:724–731. doi: 10.1007/s00125-006-0150-6. [DOI] [PubMed] [Google Scholar]
- 198.Foo RS, Siow RC, Brown MJ, Bennett MR. Heme oxygenase-1 gene transfer inhibits angiotensin II-mediated rat cardiac myocyte apoptosis but not hypertrophy. J Cell Physiol. 2006;209:1–7. doi: 10.1002/jcp.20723. [DOI] [PubMed] [Google Scholar]
- 199.Forster K, Kuno A, Solenkova N, Felix SB, Krieg T. The delta-opioid receptor agonist DADLE at reperfusion protects the heart through activation of pro-survival kinases via EGF receptor transactivation. Am J Physiol Heart Circ Physiol. 2007;293:H1604–H1608. doi: 10.1152/ajpheart.00418.2007. [DOI] [PubMed] [Google Scholar]
- 200.Franchini KG, Torsoni AS, Soares PH, Saad MJ. Early activation of the multicomponent signaling complex associated with focal adhesion kinase induced by pressure overload in the rat heart. Circ Res. 2000;87:558–565. doi: 10.1161/01.res.87.7.558. [DOI] [PubMed] [Google Scholar]
- 201.Fransioli J, Bailey B, Gude NA, Cottage CT, Muraski JA, Emmanuel G, Wu W, Alvarez R, Rubio M, Ottolenghi S, Schaefer E, Sussman MA. Evolution of the c-kit-positive cell response to pathological challenge in the myocardium. Stem Cells. 2008;26:1315–1324. doi: 10.1634/stemcells.2007-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fujimoto H, Ohno M, Ayabe S, Kobayashi H, Ishizaka N, Kimura H, Yoshida K, Nagai R. Carbon monoxide protects against cardiac ischemia-reperfusion injury in vivo via MAPK and Akt-eNOS pathways. Arterioscler Thromb Vasc Biol. 2004;24:1848–1853. doi: 10.1161/01.ATV.0000142364.85911.0e. [DOI] [PubMed] [Google Scholar]
- 203.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fujita N, Sato S, Katayama K, Tsuruo T. Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J Biol Chem. 2002;277:28706–28713. doi: 10.1074/jbc.M203668200. [DOI] [PubMed] [Google Scholar]
- 206.Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, Volpi E. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes. 2007;56:1615–1622. doi: 10.2337/db06-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol. 2003;35:1473–1479. doi: 10.1016/j.yjmcc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 208.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Furukawa T, Kurokawa J. Regulation of cardiac ion channels via non-genomic action of sex steroid hormones: implication for the gender difference in cardiac arrhythmias. Pharmacol Ther. 2007;115:106–115. doi: 10.1016/j.pharmthera.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 210.Furuya F, Lu C, Guigon CJ, Cheng SY. Nongenomic activation of phosphatidylinositol 3-kinase signaling by thyroid hormone receptors. Steroids. 2009;74:628–634. doi: 10.1016/j.steroids.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J. 2000;349:629–634. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Galvez AS, Ulloa JA, Chiong M, Criollo A, Eisner V, Barros LF, Lavandero S. Aldose reductase induced by hyperosmotic stress mediates cardiomyocyte apoptosis: differential effects of sorbitol and mannitol. J Biol Chem. 2003;278:38484–38494. doi: 10.1074/jbc.M211824200. [DOI] [PubMed] [Google Scholar]
- 213.Gao F, Gao E, Yue TL, Ohlstein EH, Lopez BL, Christopher TA, Ma XL. Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation. 2002;105:1497–1502. doi: 10.1161/01.cir.0000012529.00367.0f. [DOI] [PubMed] [Google Scholar]
- 214.Gao J, Chang ChuaChen C, Wang Z, Xu H, RCHX, McMullen JR, Shioi T, Izumo S, Chua BH. Resistin, an adipocytokine, offers protection against acute myocardial infarction. J Mol Cell Cardiol. 2007;43:601–609. doi: 10.1016/j.yjmcc.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gao L, Kwan JC, Macdonald PS, Yang L, Preiss T, Hicks M. Improved poststorage cardiac function by poly (ADP-ribose) polymerase inhibition: role of phosphatidylinositol 3-kinase Akt pathway. Transplantation. 2007;84:380–386. doi: 10.1097/01.tp.0000276924.08343.78. [DOI] [PubMed] [Google Scholar]
- 216.Gao MH, Miyanohara A, Feramisco JR, Tang T. Activation of PH-domain leucine-rich protein phosphatase 2 (PHLPP2) by agonist stimulation in cardiac myocytes expressing adenylyl cyclase type 6. Biochem Biophys Res Commun. 2009;384:193–198. doi: 10.1016/j.bbrc.2009.04.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gao XM, Agrotis A, Autelitano DJ, Percy E, Woodcock EA, Jennings GL, Dart AM, Du XJ. Sex hormones and cardiomyopathic phenotype induced by cardiac beta 2-adrenergic receptor overexpression. Endocrinology. 2003;144:4097–4105. doi: 10.1210/en.2002-0214. [DOI] [PubMed] [Google Scholar]
- 218.Gao XS, Zhang Y, Arrazola P, Hino O, Kobayashi T, Yeung RS, Ru BG, Pan DJ. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nature Cell Biol. 2002;4:699–704. doi: 10.1038/ncb847. [DOI] [PubMed] [Google Scholar]
- 219.Gardai SJ, Hildeman DA, Frankel SK, Whitlock BB, Frasch SC, Borregaard N, Marrack P, Bratton DL, Henson PM. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem. 2004;279:21085–21095. doi: 10.1074/jbc.M400063200. [DOI] [PubMed] [Google Scholar]
- 220.Gardner JD, Brower GL, Voloshenyuk TG, Janicki JS. Cardioprotection in female rats subjected to chronic volume overload: synergistic interaction of estrogen and phytoestrogens. Am J Physiol Heart Circ Physiol. 2008;294:H198–H204. doi: 10.1152/ajpheart.00281.2007. [DOI] [PubMed] [Google Scholar]
- 221.Gardner JD, Brower GL, Voloshenyuk TG, Janicki JS. Cardioprotection in female rats subjected to chronic volume overload: synergistic interaction of estrogen and phytoestrogens. Am J Physiol Heart Circ Physiol. 2008;294:H198–H204. doi: 10.1152/ajpheart.00281.2007. [DOI] [PubMed] [Google Scholar]
- 222.Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, McNeish JD, Coleman KG. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gatson JW, Maass DL, Simpkins JW, Idris AH, Minei JP, Wigginton JG. Estrogen treatment following severe burn injury reduces brain inflammation and apoptotic signaling. J Neuroinflammation. 2009;6:30. doi: 10.1186/1742-2094-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gavete ML, Agote M, Martin MA, Alvarez C, Escriva F. Effects of chronic undernutrition on glucose uptake and glucose transporter proteins in rat heart. Endocrinology. 2002;143:4295–4303. doi: 10.1210/en.2002-220258. [DOI] [PubMed] [Google Scholar]
- 225.Gavi S, Yin D, Shumay E, Wang HY, Malbon CC. Insulin-like growth factor-I provokes functional antagonism and internalization of beta1-adrenergic receptors. Endocrinology. 2007;148:2653–2662. doi: 10.1210/en.2006-1569. [DOI] [PubMed] [Google Scholar]
- 226.George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC, Soos MA, Murgatroyd PR, Williams RM, Acerini CL, Dunger DB, Barford D, Umpleby AM, Wareham NJ, Davies HA, Schafer AJ, Stoffel M, O’Rahilly S, Barroso I. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304:1325–1328. doi: 10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Germack R, Griffin M, Dickenson JM. Activation of protein kinase B by adenosine A1 and A3 receptors in newborn rat cardiomyocytes. J Mol Cell Cardiol. 2004;37:989–999. doi: 10.1016/j.yjmcc.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 228.Giani JF, Gironacci MM, Munoz MC, Pena C, Turyn D, Dominici FP. Angiotensin-(1–7) stimulates the phosphorylation of JAK2, IRS-1 and Akt in rat heart in vivo: role of the AT1 and Mas receptors. Am J Physiol Heart Circ Physiol. 2007;293:H1154–H1163. doi: 10.1152/ajpheart.01395.2006. [DOI] [PubMed] [Google Scholar]
- 229.Gilbert JS, Nijland MJ. Sex differences in the developmental origins of hypertension and cardiorenal disease. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1941–R1952. doi: 10.1152/ajpregu.90724.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Glazer HP, Osipov RM, Clements RT, Sellke FW, Bianchi C. Hypercholesterolemia is associated with hyperactive cardiac mTORC1 and mTORC2 signaling. Cell Cycle. 2009;8:1738–1746. doi: 10.4161/cc.8.11.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 232.Gnecchi M, He H, Melo LG, Noiseaux N, Morello F, de Boer RA, Zhang L, Pratt RE, Dzau VJ, Ingwall JS. Early beneficial effects of bone marrow-derived mesenchymal stem cells overexpressing Akt on cardiac metabolism after myocardial infarction. Stem Cells. 2009;27:971–979. doi: 10.1002/stem.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 234.Goh SS, Woodman OL, Pepe S, Cao AH, Qin C, Ritchie RH. The red wine antioxidant resveratrol prevents cardiomyocyte injury following ischemia-reperfusion via multiple sites and mechanisms. Antioxid Redox Signal. 2007;9:101–113. doi: 10.1089/ars.2007.9.101. [DOI] [PubMed] [Google Scholar]
- 235.Gonon AT, Widegren U, Bulhak A, Salehzadeh F, Persson J, Sjoquist PO, Pernow J. Adiponectin protects against myocardial ischaemia-reperfusion injury via AMP-activated protein kinase, Akt, and nitric oxide. Cardiovasc Res. 2008;78:116–122. doi: 10.1093/cvr/cvn017. [DOI] [PubMed] [Google Scholar]
- 236.Gonzalez A, Ravassa S, Loperena I, Lopez B, Beaumont J, Querejeta R, Larman M, Diez J. Association of depressed cardiac gp130-mediated antiapoptotic pathways with stimulated cardiomyocyte apoptosis in hypertensive patients with heart failure. J Hypertens. 2007;25:2148–2157. doi: 10.1097/HJH.0b013e32828626e2. [DOI] [PubMed] [Google Scholar]
- 237.Gonzalez AM, Osorio JC, Manlhiot C, Gruber D, Homma S, Mital S. Hypertrophy signaling during peri-partum cardiac remodeling. Am J Physiol Heart Circ Physiol. 2007;293:H3008–H3013. doi: 10.1152/ajpheart.00401.2007. [DOI] [PubMed] [Google Scholar]
- 238.Goodman MD, Koch SE, Fuller-Bicer GA, Butler KL. Regulating RISK: a role for JAK-STAT signaling in postconditioning? Am J Physiol Heart Circ Physiol. 2008;295:H1649–H1656. doi: 10.1152/ajpheart.00692.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gosselin H, Beliveau L, Burelle Y, Clement R, Lajoie C, El-Helou V, Calderone A. Disparate regulation of signaling proteins after exercise and myocardial infarction. Med Sci Sports Exercise. 2006;38:455–462. doi: 10.1249/01.mss.0000205138.02440.79. [DOI] [PubMed] [Google Scholar]
- 240.Granata R, Trovato L, Gallo MP, Destefanis S, Settanni F, Scarlatti F, Brero A, Ramella R, Volante M, Isgaard J, Levi R, Papotti M, Alloatti G, Ghigo E. Growth hormone-releasing hormone promotes survival of cardiac myocytes in vitro and protects against ischaemia-reperfusion injury in rat heart. Cardiovasc Res. 2009;83:303–312. doi: 10.1093/cvr/cvp090. [DOI] [PubMed] [Google Scholar]
- 241.Gregory MA, Qi Y, Hann SR. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J Biol Chem. 2003;278:51606–51612. doi: 10.1074/jbc.M310722200. [DOI] [PubMed] [Google Scholar]
- 242.Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 243.Gross ER, Hsu AK, Gross GJ. The JAK/STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt, and GSK-3 beta. Am J Physiol Heart Circ Physiol. 2006;291:H827–H834. doi: 10.1152/ajpheart.00003.2006. [DOI] [PubMed] [Google Scholar]
- 244.Gude N, Muraski J, Rubio M, Kajstura J, Schaefer E, Anversa P, Sussman MA. Akt promotes increased cardiomyocyte cycling and expansion of the cardiac progenitor cell population. Circ Res. 2006;99:381–388. doi: 10.1161/01.RES.0000236754.21499.1c. [DOI] [PubMed] [Google Scholar]
- 245.Gude NA, Emmanuel G, Wu W, Cottage CT, Fischer K, Quijada P, Muraski JA, Alvarez R, Rubio M, Schaefer E, Sussman MA. Activation of Notch-mediated protective signaling in the myocardium. Circ Res. 2008;102:1025–1035. doi: 10.1161/CIRCRESAHA.107.164749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11:353–361. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 247.Guo C, Sah JF, Beard L, Willson JK, Markowitz SD, Guda K. The noncoding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Genes Chromosomes Cancer. 2008;47:939–946. doi: 10.1002/gcc.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gurel E, Smeele KM, Eerbeek O, Koeman A, Demirci C, Hollmann MW, Zuurbier CJ. Ischemic preconditioning affects hexokinase activity and HKII in different subcellular compartments throughout cardiac ischemia-reperfusion. J Appl Physiol. 2009;106:1909–1916. doi: 10.1152/japplphysiol.90537.2008. [DOI] [PubMed] [Google Scholar]
- 249.Gurusamy N, Malik G, Gorbunov NV, Das DK. Redox activation of Ref-1 potentiates cell survival following myocardial ischemia reperfusion injury. Free Radic Biol Med. 2007;43:397–407. doi: 10.1016/j.freeradbiomed.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 250.Gurusamy N, Watanabe K, Ma M, Prakash P, Hirabayashi K, Zhang S, Muslin AJ, Kodama M, Aizawa Y. Glycogen synthase kinase 3beta together with 14-3-3 protein regulates diabetic cardiomyopathy: effect of losartan and tempol. FEBS Lett. 2006;580:1932–1940. doi: 10.1016/j.febslet.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 251.Gustafsson AB, Gottlieb RA. Bcl-2 family members and apoptosis, taken to heart. Am J Physiol Cell Physiol. 2007;292:C45–C51. doi: 10.1152/ajpcell.00229.2006. [DOI] [PubMed] [Google Scholar]
- 252.Ha T, Hua F, Grant D, Xia Y, Ma J, Gao X, Kelley J, Williams DL, Kalbfleisch J, Browder IW, Kao RL, Li C. Glucan phosphate attenuates cardiac dysfunction and inhibits cardiac MIF expression and apoptosis in septic mice. Am J Physiol Heart Circ Physiol. 2006;291:H1910–H1918. doi: 10.1152/ajpheart.01264.2005. [DOI] [PubMed] [Google Scholar]
- 253.Ha T, Hua F, Liu X, Ma J, McMullen JR, Shioi T, Izumo S, Kelley J, Gao X, Browder W, Williams DL, Kao RL, Li C. Lipopolysaccharide-induced myocardial protection against ischaemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovasc Res. 2008;78:546–553. doi: 10.1093/cvr/cvn037. [DOI] [PubMed] [Google Scholar]
- 254.Ha T, Li Y, Gao X, McMullen JR, Shioi T, Izumo S, Kelley JL, Zhao A, Haddad GE, Williams DL, Browder IW, Kao RL, Li C. Attenuation of cardiac hypertrophy by inhibiting both mTOR and NFkappaB activation in vivo. Free Radic Biol Med. 2005;39:1570–1580. doi: 10.1016/j.freeradbiomed.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 255.Hackl M, Brunner S, Fortschegger K, Schreiner C, Micutkova L, Muck C, Laschober GT, Lepperdinger G, Sampson N, Berger P, Herndler-Brandstetter D, Wieser M, Kuhnel H, Strasser A, Rinnerthaler M, Breitenbach M, Mildner M, Eckhart L, Tschachler E, Trost A, Bauer JW, Papak C, Trajanoski Z, Scheideler M, Grillari-Voglauer R, Grubeck-Loebenstein B, Jansen-Durr P, Grillari J. miR-17, miR-19b, miR-20a, and miR-106a are down-regulated in human aging. Aging Cell. 9:291–296. doi: 10.1111/j.1474-9726.2010.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Haendeler J, Hoffmann J, Rahman S, Zeiher AM, Dimmeler S. Regulation of telomerase activity and anti-apoptotic function by protein-protein interaction and phosphorylation. FEBS Lett. 2003;536:180–186. doi: 10.1016/s0014-5793(03)00058-9. [DOI] [PubMed] [Google Scholar]
- 257.Haider H, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res. 2008;103:1300–1308. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 258.Halestrap AP, Clarke SJ, Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta. 2007;1767:1007–1031. doi: 10.1016/j.bbabio.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Halestrap AP, Doran E, Gillespie JP, O’Toole A. Mitochondria and cell death. Biochem Soc Trans. 2000;28:170–177. doi: 10.1042/bst0280170. [DOI] [PubMed] [Google Scholar]
- 260.Hammerman PS, Fox CJ, Birnbaum MJ, Thompson CB. Pim and Akt oncogenes are independent regulators of hematopoietic cell growth and survival. Blood. 2005;105:4477–4483. doi: 10.1182/blood-2004-09-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Haq S, Choukroun G, Kang ZB, Ranu H, Matsui T, Rosenzweig A, Molkentin JD, Alessandrini A, Woodgett J, Hajjar R, Michael A, Force T. Glycogen synthase kinase-3beta is a negative regulator of cardiomyocyte hypertrophy. J Cell Biol. 2000;151:117–130. doi: 10.1083/jcb.151.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Haq S, Choukroun G, Lim H, Tymitz KM, del Monte F, Gwathmey J, Grazette L, Michael A, Hajjar R, Force T, Molkentin JD. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- 263.Haq S, Michael A, Andreucci M, Bhattacharya K, Dotto P, Walters B, Woodgett J, Kilter H, Force T. Stabilization of beta-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth. Proc Natl Acad Sci USA. 2003;100:4610–4615. doi: 10.1073/pnas.0835895100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Hardt SE, Sadoshima J. Glycogen synthase kinase-3beta: a novel regulator of cardiac hypertrophy and development. Circ Res. 2002;90:1055–1063. doi: 10.1161/01.res.0000018952.70505.f1. [DOI] [PubMed] [Google Scholar]
- 265.Hardt SE, Tomita H, Katus HA, Sadoshima J. Phosphorylation of eukaryotic translation initiation factor 2Bepsilon by glycogen synthase kinase-3beta regulates beta-adrenergic cardiac myocyte hypertrophy. Circ Res. 2004;94:926–935. doi: 10.1161/01.RES.0000124977.59827.80. [DOI] [PubMed] [Google Scholar]
- 266.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hase M, Depre C, Vatner SF, Sadoshima J. H11 has dose-dependent and dual hypertrophic and proapoptotic functions in cardiac myocytes. Biochem J. 2005;388:475–483. doi: 10.1042/BJ20041314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hauck L, Harms C, Grothe D, An J, Gertz K, Kronenberg G, Dietz R, Endres M, von Harsdorf R. Critical role for FoxO3a-dependent regulation of p21CIP1/WAF1 in response to statin signaling in cardiac myocytes. Circ Res. 2007;100:50–60. doi: 10.1161/01.RES.0000254704.92532.b9. [DOI] [PubMed] [Google Scholar]
- 269.Hausenloy DJ, Mocanu MM, Yellon DM. Cross-talk between the survival kinases during early reperfusion: its contribution to ischemic preconditioning. Cardiovasc Res. 2004;63:305–312. doi: 10.1016/j.cardiores.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 270.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–H976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 271.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 272.Hausleiter J, Kastrati A, Mehilli J, Vogeser M, Zohlnhofer D, Schuhlen H, Goos C, Pache J, Dotzer F, Pogatsa-Murray G, Dirschinger J, Heemann U, Schomig A. Randomized, double-blind, placebo-controlled trial of oral sirolimus for restenosis prevention in patients with instent restenosis: the oral sirolimus to inhibit recurrent instent stenosis (OSIRIS) trial. Circulation. 2004;110:790–795. doi: 10.1161/01.CIR.0000138935.17503.35. [DOI] [PubMed] [Google Scholar]
- 273.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 274.Haynes MP, Li L, Russell KS, Bender JR. Rapid vascular cell responses to estrogen and membrane receptors. Vasc Pharmacol. 2002;38:99–108. doi: 10.1016/s0306-3623(02)00133-7. [DOI] [PubMed] [Google Scholar]
- 275.Haynes MP, Li L, Sinha D, Russell KS, Hisamoto K, Baron R, Collinge M, Sessa WC, Bender JR. Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J Biol Chem. 2003;278:2118–2123. doi: 10.1074/jbc.M210828200. [DOI] [PubMed] [Google Scholar]
- 276.Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res. 2000;87:677–682. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- 277.He Z, Opland DM, Way KJ, Ueki K, Bodyak N, Kang PM, Izumo S, Kulkarni RN, Wang B, Liao R, Kahn CR, King GL. Regulation of vascular endothelial growth factor expression and vascularization in the myocardium by insulin receptor and PI3K/Akt pathways in insulin resistance and ischemia. Arterioscler Thromb Vasc Biol. 2006;26:787–793. doi: 10.1161/01.ATV.0000209500.15801.4e. [DOI] [PubMed] [Google Scholar]
- 278.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 279.Heineke J, Wollert KC, Osinska H, Sargent MA, York AJ, Robbins J, Molkentin JD. Calcineurin protects the heart in a murine model of dilated cardiomyopathy. J Mol Cell Cardiol. 48:1080–1087. doi: 10.1016/j.yjmcc.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 281.Herrington D. Role of estrogens, selective estrogen receptor modulators and phytoestrogens in cardiovascular protection. Can J Cardiol. 2000;16 (Suppl E):5E–9E. [PubMed] [Google Scholar]
- 282.Hickson-Bick DL, Jones C, Buja LM. The response of neonatal rat ventricular myocytes to lipopolysaccharide-induced stress. Shock. 2006;25:546–552. doi: 10.1097/01.shk.0000209549.03463.91. [DOI] [PubMed] [Google Scholar]
- 283.Higuchi Y, Chan TO, Brown MA, Zhang J, DeGeorge BR, Jr, Funakoshi H, Gibson G, McTiernan CF, Kubota T, Jones WK, Feldman AM. Cardioprotection afforded by NF-kappaB ablation is associated with activation of Akt in mice overexpressing TNF-alpha. Am J Physiol Heart Circ Physiol. 2006;290:H590–H598. doi: 10.1152/ajpheart.00379.2005. [DOI] [PubMed] [Google Scholar]
- 284.Hiley CR, Ford WR. Cannabinoid pharmacology in the cardiovascular system: potential protective mechanisms through lipid signalling. Biol Rev Camb Philos Soc. 2004;79:187–205. doi: 10.1017/s1464793103006201. [DOI] [PubMed] [Google Scholar]
- 285.Hingtgen SD, Tian X, Yang J, Dunlay SM, Peek AS, Wu Y, Sharma RV, Engelhardt JF, Davisson RL. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol Genomics. 2006;26:180–191. doi: 10.1152/physiolgenomics.00029.2005. [DOI] [PubMed] [Google Scholar]
- 286.Hinrichsen R, Haunso S, Busk PK. Different regulation of p27 and Akt during cardiomyocyte proliferation and hypertrophy. Growth Factors. 2007;25:132–140. doi: 10.1080/08977190701549835. [DOI] [PubMed] [Google Scholar]
- 287.Hinrichsen R, Haunso S, Busk PK. Different regulation of p27 and Akt during cardiomyocyte proliferation and hypertrophy. Growth Factors. 2007;1 doi: 10.1080/08977190701549835. [DOI] [PubMed] [Google Scholar]
- 288.Hintz KK, Ren J. Phytoestrogenic isoflavones daidzein and genistein reduce glucose-toxicity-induced cardiac contractile dysfunction in ventricular myocytes. Endocr Res. 2004;30:215–223. doi: 10.1081/erc-120037730. [DOI] [PubMed] [Google Scholar]
- 289.Hiraoka E, Kawashima S, Takahashi T, Rikitake Y, Hirase T, Yokoyama M. PI 3-kinase-Akt-p70 S6 kinase in hypertrophic responses to leukemia inhibitory factor in cardiac myocytes. Kobe J Med Sci. 2003;49:25–37. [PubMed] [Google Scholar]
- 290.Hiraoka E, Kawashima S, Takahashi T, Rikitake Y, Kitamura T, Ogawa W, Yokoyama M. TNF-alpha induces protein synthesis through PI3-kinase-Akt/PKB pathway in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2001;280:H1861–H1868. doi: 10.1152/ajpheart.2001.280.4.H1861. [DOI] [PubMed] [Google Scholar]
- 291.Hirotani S, Zhai P, Tomita H, Galeotti J, Marquez JP, Gao S, Hong C, Yatani A, Avila J, Sadoshima J. Inhibition of glycogen synthase kinase 3beta during heart failure is protective. Circ Res. 2007;101:1164–1174. doi: 10.1161/CIRCRESAHA.107.160614. [DOI] [PubMed] [Google Scholar]
- 292.Hochhauser E, Kivity S, Offen D, Maulik N, Otani H, Barhum Y, Pannet H, Shneyvays V, Shainberg A, Goldshtaub V, Tobar A, Vidne BA. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;284:H2351–H2359. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]
- 293.Hofmann U, Burkard N, Vogt C, Thoma A, Frantz S, Ertl G, Ritter O, Bonz A. Protective effects of sphingosine-1-phosphate receptor agonist treatment after myocardial ischaemia-reperfusion. Cardiovasc Res. 2009;83:285–293. doi: 10.1093/cvr/cvp137. [DOI] [PubMed] [Google Scholar]
- 294.Honisch A, Theuring N, Ebner B, Wagner C, Strasser RH, Weinbrenner C. Postconditioning with levosimendan reduces the infarct size involving the PI3K pathway and KATP-channel activation but is independent of PDE-III inhibition. Basic Res Cardiol. 2010;105:155–167. doi: 10.1007/s00395-009-0064-9. [DOI] [PubMed] [Google Scholar]
- 295.Hoover D, Friedmann M, Reeves R, Magnuson NS. Recombinant human pim-1 protein exhibits serine/threonine kinase activity. J Biol Chem. 1991;266:14018–14023. [PubMed] [Google Scholar]
- 296.Horbinski C, Chu CT. Kinase signaling cascades in the mitochondrion: a matter of life or death. Free Radic Biol Med. 2005;38:2–11. doi: 10.1016/j.freeradbiomed.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 297.Horie T, Ono K, Nagao K, Nishi H, Kinoshita M, Kawamura T, Wada H, Shimatsu A, Kita T, Hasegawa K. Oxidative stress induces GLUT4 translocation by activation of PI3-K/Akt and dual AMPK kinase in cardiac myocytes. J Cell Physiol. 2008;215:733–742. doi: 10.1002/jcp.21353. [DOI] [PubMed] [Google Scholar]
- 298.Horio T, Maki T, Kishimoto I, Tokudome T, Okumura H, Yoshihara F, Suga S, Takeo S, Kawano Y, Kangawa K. Production and autocrine/paracrine effects of endogenous insulin-like growth factor-1 in rat cardiac fibroblasts. Regul Pept. 2005;124:65–72. doi: 10.1016/j.regpep.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 299.Horman S, Vertommen D, Heath R, Neumann D, Mouton V, Woods A, Schlattner U, Wallimann T, Carling D, Hue L, Rider MH. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006;281:5335–5340. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 300.Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Hsu SY, Kaipia A, Zhu L, Hsueh AJ. Interference of BAD (Bcl-xL/Bcl-2-associated death promoter)-induced apoptosis in mammalian cells by 14-3-3 isoforms and P11. Mol Endocrinol. 1997;11:1858–1867. doi: 10.1210/mend.11.12.0023. [DOI] [PubMed] [Google Scholar]
- 302.Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, Guo Y, Bolli R, Rokosh G. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116:654–663. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Hu Y, Chen X, Pan TT, Neo KL, Lee SW, Khin ES, Moore PK, Bian JS. Cardioprotection induced by hydrogen sulfide preconditioning involves activation of ERK and PI3K/Akt pathways. Pflügers Arch. 2007 doi: 10.1007/s00424-007-0321-4. [DOI] [PubMed] [Google Scholar]
- 304.Hua F, Ha T, Ma J, Gao X, Kelley J, Williams DL, Browder IW, Kao RL, Li C. Blocking the MyD88-dependent pathway protects the myocardium from ischemia/reperfusion injury in rat hearts. Biochem Biophys Res Commun. 2005;338:1118–1125. doi: 10.1016/j.bbrc.2005.10.068. [DOI] [PubMed] [Google Scholar]
- 305.Hua F, Ha T, Ma J, Li Y, Kelley J, Gao X, Browder IW, Kao RL, Williams DL, Li C. Protection against myocardial ischemia/reperfusion injury in TLR4-deficient mice is mediated through a phosphoinositide 3-kinase-dependent mechanism. J Immunol. 2007;178:7317–7324. doi: 10.4049/jimmunol.178.11.7317. [DOI] [PubMed] [Google Scholar]
- 306.Huang A, Kaley G. Gender-specific regulation of cardiovascular function: estrogen as key player. Microcirculation. 2004;11:9–38. doi: 10.1080/10739680490266162. [DOI] [PubMed] [Google Scholar]
- 307.Huang JP, Huang SS, Deng JY, Hung LM. Impairment of insulin-stimulated Akt/GLUT4 signaling is associated with cardiac contractile dysfunction and aggravates I/R injury in STZ-diabetic rats. J Biomed Sci. 2009;16:77. doi: 10.1186/1423-0127-16-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Huang M, Kamasani U, Prendergast GC. RhoB facilitates c-Myc turnover by supporting efficient nuclear accumulation of GSK-3. Oncogene. 2006;25:1281–1289. doi: 10.1038/sj.onc.1209174. [DOI] [PubMed] [Google Scholar]
- 309.Huisamen B. Protein kinase B in the diabetic heart. Mol Cell Biochem. 2003;249:31–38. [PubMed] [Google Scholar]
- 310.Huisamen B, Donthi RV, Lochner A. Insulin in combination with vanadate stimulates glucose transport in isolated cardiomyocytes from obese Zucker rats. Cardiovasc Drugs Ther. 2001;15:445–452. doi: 10.1023/a:1013358011019. [DOI] [PubMed] [Google Scholar]
- 311.Huisamen B, van Zyl M, Keyser A, Lochner A. The effects of insulin and beta-adrenergic stimulation on glucose transport, glut 4 and PKB activation in the myocardium of lean and obese non-insulin dependent diabetes mellitus rats. Mol Cell Biochem. 2001;223:15–25. doi: 10.1023/a:1017528402205. [DOI] [PubMed] [Google Scholar]
- 312.Hunter JC, Kostyak JC, Novotny JL, Simpson AM, Korzick DH. Estrogen deficiency decreases ischemic tolerance in the aged rat heart: roles of PKCdelta, PKCepsilon, Akt, and GSK3beta. Am J Physiol Regul Integr Comp Physiol. 2007;292:R800–R809. doi: 10.1152/ajpregu.00374.2006. [DOI] [PubMed] [Google Scholar]
- 313.Iemitsu M, Maeda S, Jesmin S, Otsuki T, Miyauchi T. Exercise training improves aging-induced downregulation of VEGF angiogenic signaling cascade in hearts. Am J Physiol Heart Circ Physiol. 2006;291:H1290–H1298. doi: 10.1152/ajpheart.00820.2005. [DOI] [PubMed] [Google Scholar]
- 314.Ikeda S, Kong SW, Lu J, Bisping E, Zhang H, Allen PD, Golub TR, Pieske B, Pu WT. Altered microRNA expression in human heart disease. Physiol Gen. 2007;31:367–373. doi: 10.1152/physiolgenomics.00144.2007. [DOI] [PubMed] [Google Scholar]
- 315.Ikeda S, Pu WT. Expression and function of microRNAs in heart disease. Curr Drug Targets. 11:913–925. doi: 10.2174/138945010791591304. [DOI] [PubMed] [Google Scholar]
- 316.Ikeyama S, Kokkonen G, Shack S, Wang XT, Holbrook NJ. Loss in oxidative stress tolerance with aging linked to reduced extracellular signal-regulated kinase and Akt kinase activities. FASEB J. 2002;16:114–116. doi: 10.1096/fj.01-0409fje. [DOI] [PubMed] [Google Scholar]
- 317.Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ Res. 2004;95:734–741. doi: 10.1161/01.RES.0000143898.67182.4c. [DOI] [PubMed] [Google Scholar]
- 318.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nature Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 319.Inoki K, Li Y, Zhu TQ, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nature Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 320.Ito K, Akazawa H, Tamagawa M, Furukawa K, Ogawa W, Yasuda N, Kudo Y, Liao CH, Yamamoto R, Sato T, Molkentin JD, Kasuga M, Noda T, Nakaya H, Komuro I. PDK1 coordinates survival pathways and beta-adrenergic response in the heart. Proc Natl Acad Sci USA. 2009;106:8689–8694. doi: 10.1073/pnas.0900064106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Iwanaga K, Takano H, Ohtsuka M, Hasegawa H, Zou Y, Qin Y, Odaka K, Hiroshima K, Tadokoro H, Komuro I. Effects of G-CSF on cardiac remodeling after acute myocardial infarction in swine. Biochem Biophys Res Commun. 2004;325:1353–1359. doi: 10.1016/j.bbrc.2004.10.149. [DOI] [PubMed] [Google Scholar]
- 322.Izzotti A, Calin GA, Arrigo P, Steele VE, Croce CM, De Flora S. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J. 2009;23:806–812. doi: 10.1096/fj.08-121384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Jacobs MD, Black J, Futer O, Swenson L, Hare B, Fleming M, Saxena K. Pim-1 ligand-bound structures reveal the mechanism of serine/threonine kinase inhibition by LY294002. J Biol Chem. 2005;280:13728–13734. doi: 10.1074/jbc.M413155200. [DOI] [PubMed] [Google Scholar]
- 324.Jamnicki-Abegg M, Weihrauch D, Pagel PS, Kersten JR, Bosnjak ZJ, Warltier DC, Bienengraeber MW. Isoflurane inhibits cardiac myocyte apoptosis during oxidative and inflammatory stress by activating Akt and enhancing Bcl-2 expression. Anesthesiology. 2005;103:1006–1014. doi: 10.1097/00000542-200511000-00015. [DOI] [PubMed] [Google Scholar]
- 325.Jastrzebski K, Hannan KM, Tchoubrieva EB, Hannan RD, Pearson RB. Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors. 2007;25:209–226. doi: 10.1080/08977190701779101. [DOI] [PubMed] [Google Scholar]
- 326.Javadov S, Karmazyn M. Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cell Physiol Biochem. 2007;20:1–22. doi: 10.1159/000103747. [DOI] [PubMed] [Google Scholar]
- 327.Ji ES, Yue H, Wu YM, He RR. Effects of phytoestrogen genistein on myocardial ischemia/reperfusion injury and apoptosis in rabbits. Acta Pharmacol Sin. 2004;25:306–312. [PubMed] [Google Scholar]
- 328.Jiang BH, Aoki M, Zheng JZ, Li J, Vogt PK. Myogenic signaling of phosphatidylinositol 3-kinase requires the serine-threonine kinase Akt/protein kinase B. Proc Natl Acad Sci USA. 1999;96:2077–2081. doi: 10.1073/pnas.96.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Jin ZQ, Karliner JS. Low dose N,N-dimethylsphingosine is cardioprotective and activates cytosolic sphingosine kinase by a PKCepsilon dependent mechanism. Cardiovasc Res. 2006;71:725–734. doi: 10.1016/j.cardiores.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 330.Jirecek S, Joura EA, Tempfer C, Knofler M, Husslein P, Zeisler H. Elevated serum concentrations of androgens in women with pregnancy-induced hypertension. Wien Klin Wochenschr. 2003;115:162–166. doi: 10.1007/BF03040303. [DOI] [PubMed] [Google Scholar]
- 331.Jonassen AK, Aasum E, Riemersma RA, Mjos OD, Larsen TS. Glucose-insulin-potassium reduces infarct size when administered during reperfusion. Cardiovasc Drugs Ther. 2000;14:615–623. doi: 10.1023/a:1007802630604. [DOI] [PubMed] [Google Scholar]
- 332.Jonassen AK, Brar BK, Mjos OD, Sack MN, Latchman DS, Yellon DM. Insulin administered at reoxygenation exerts a cardioprotective effect in myocytes by a possible anti-apoptotic mechanism. J Mol Cell Cardiol. 2000;32:757–764. doi: 10.1006/jmcc.2000.1118. [DOI] [PubMed] [Google Scholar]
- 333.Jonassen AK, Mjos OD, Sack MN. p70s6 kinase is a functional target of insulin activated Akt cell-survival signaling. Biochem Biophys Res Commun. 2004;315:160–165. doi: 10.1016/j.bbrc.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 334.Jonassen AK, Sack MN, Mjos OD, Yellon DM. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res. 2001;89:1191–1198. doi: 10.1161/hh2401.101385. [DOI] [PubMed] [Google Scholar]
- 335.Juhasz B, Thirunavukkarasu M, Pant R, Zhan L, Penumathsa SV, Secor ER, Jr, Srivastava S, Raychaudhuri U, Menon VP, Otani H, Thrall RS, Maulik N. Bromelain induces cardioprotection against ischemia-reperfusion injury through Akt/FOXO pathway in rat myocardium. Am J Physiol Heart Circ Physiol. 2008;294:H1365–H1370. doi: 10.1152/ajpheart.01005.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Kageyama K, Ihara Y, Goto S, Urata Y, Toda G, Yano K, Kondo T. Overexpression of calreticulin modulates protein kinase B/Akt signaling to promote apoptosis during cardiac differentiation of cardiomyoblast H9c2 cells. J Biol Chem. 2002;277:19255–19264. doi: 10.1074/jbc.M112377200. [DOI] [PubMed] [Google Scholar]
- 338.Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami CA, Anversa P. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci USA. 1998;95:8801–8805. doi: 10.1073/pnas.95.15.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Kakinuma Y, Ando M, Kuwabara M, Katare RG, Okudela K, Kobayashi M, Sato T. Acetylcholine from vagal stimulation protects cardiomyocytes against ischemia and hypoxia involving additive non-hypoxic induction of HIF-1alpha. FEBS Lett. 2005;579:2111–2118. doi: 10.1016/j.febslet.2005.02.065. [DOI] [PubMed] [Google Scholar]
- 340.Kaminker P. Is Akt the mastermind behind age-related heart disease? Sci Aging Knowledge Environ. 2004:pe8. doi: 10.1126/sageke.2004.8.pe8. [DOI] [PubMed] [Google Scholar]
- 341.Kane NM, Meloni M, Spencer HL, Craig MA, Strehl R, Milligan G, Houslay MD, Mountford JC, Emanueli C, Baker AH. Derivation of endothelial cells from human embryonic stem cells by directed differentiation: analysis of microRNA and angiogenesis in vitro and in vivo. Arterioscler Thromb Vasc Biol. 30:1389–1397. doi: 10.1161/ATVBAHA.110.204800. [DOI] [PubMed] [Google Scholar]
- 342.Kang JO, Sucov HM. Convergent proliferative response and divergent morphogenic pathways induced by epicardial and endocardial signaling in fetal heart development. Mech Dev. 2005;122:57–65. doi: 10.1016/j.mod.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 343.Kasinski AL, Slack FJ. Potential microRNA therapies targeting Ras, NFkappaB and p53 signaling. Curr Opin Mol Ther. 12:147–157. [PubMed] [Google Scholar]
- 344.Katakami N, Kaneto H, Hao H, Umayahara Y, Fujitani Y, Sakamoto K, Gorogawa S, Yasuda T, Kawamori D, Kajimoto Y, Matsuhisa M, Yutani C, Hori M, Yamasaki Y. Role of pim-1 in smooth muscle cell proliferation. J Biol Chem. 2004;279:54742–54749. doi: 10.1074/jbc.M409140200. [DOI] [PubMed] [Google Scholar]
- 345.Kato K, Yin H, Agata J, Yoshida H, Chao L, Chao J. Adrenomedullin gene delivery attenuates myocardial infarction and apoptosis after ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;285:H1506–H1514. doi: 10.1152/ajpheart.00270.2003. [DOI] [PubMed] [Google Scholar]
- 346.Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi JJ, Natarajan R. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol. 2009;11:881–889. doi: 10.1038/ncb1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Kato T, Muraski J, Chen Y, Tsujita Y, Wall J, Glembotski CC, Schaefer E, Beckerle M, Sussman MA. Atrial natriuretic peptide promotes cardiomyocyte survival by cGMP-dependent nuclear accumulation of zyxin and Akt. J Clin Invest. 2005;115:2716–2730. doi: 10.1172/JCI24280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Kemi OJ, Ceci M, Wisloff U, Grimaldi S, Gallo P, Smith GL, Condorelli G, Ellingsen O. Activation or inactivation of cardiac Akt/mTOR signaling diverges physiological from pathological hypertrophy. J Cell Physiol. 2008;214:316–321. doi: 10.1002/jcp.21197. [DOI] [PubMed] [Google Scholar]
- 349.Kemi OJ, Ellingsen O, Smith GL, Wisloff U. Exercise-induced changes in calcium handling in left ventricular cardiomyocytes. Front Biosci. 2008;13:356–368. doi: 10.2741/2685. [DOI] [PubMed] [Google Scholar]
- 350.Kenessey A, Ojamaa K. Thyroid hormone stimulates protein synthesis in the cardiomyocyte by activating the Akt-mTOR and p70S6K pathways. J Biol Chem. 2006;281:20666–20672. doi: 10.1074/jbc.M512671200. [DOI] [PubMed] [Google Scholar]
- 351.Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 352.Kerendi F, Kirshbom PM, Halkos ME, Wang NP, Kin H, Jiang R, Zhao ZQ, Kanter KR, Guyton RA, Vinten-Johansen J. Thoracic Surgery Directors Association Award Cobalt chloride pretreatment attenuates myocardial apoptosis after hypothermic circulatory arrest. Ann Thorac Surg. 2006;81:2055–2062. doi: 10.1016/j.athoracsur.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 353.Kerkela R, Kockeritz L, Macaulay K, Zhou J, Doble BW, Beahm C, Greytak S, Woulfe K, Trivedi CM, Woodgett JR, Epstein JA, Force T, Huggins GS. Deletion of GSK-3beta in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyper-proliferation. J Clin Invest. 2008;118:3609–3618. doi: 10.1172/JCI36245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Khairallah M, Khairallah R, Young ME, Dyck JR, Petrof BJ, Des Rosiers C. Metabolic and signaling alterations in dystrophin-deficient hearts precede overt cardiomyopathy. J Mol Cell Cardiol. 2007;43:119–129. doi: 10.1016/j.yjmcc.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 355.Khan SA, Salloum F, Das A, Xi L, Vetrovec GW, Kukreja RC. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J Mol Cell Cardiol. 2006;41:256–264. doi: 10.1016/j.yjmcc.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 356.Kilter H, Werner M, Roggia C, Reil JC, Schafers HJ, Kintscher U, Bohm M. The PPAR-gamma agonist rosiglitazone facilitates Akt rephosphorylation and inhibits apoptosis in cardiomyocytes during hypoxia/reoxygenation. Diabetes Obes Metab. 2009;11:1060–1067. doi: 10.1111/j.1463-1326.2009.01097.x. [DOI] [PubMed] [Google Scholar]
- 357.Kim CH, Cho YS, Chun YS, Park JW, Kim MS. Early expression of myocardial HIF-1alpha in response to mechanical stresses: regulation by stretch-activated channels and the phosphatidylinositol 3-kinase signaling pathway. Circ Res. 2002;90:E25–E33. doi: 10.1161/hh0202.104923. [DOI] [PubMed] [Google Scholar]
- 358.Kim HW, Haider HK, Jiang S, Ashraf M. Ischemic preconditioning augments survival of stem cells via MIR-210 expression by targeting caspase-8 associated protein 2. J Biol Chem. 2009;284:33161–33168. doi: 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Kim KH, Oudit GY, Backx PH. Erythropoietin protects against doxorubicin-induced cardiomyopathy via a phosphatidylinositol 3-kinase-dependent pathway. J Pharmacol Exp Ther. 2008;324:160–169. doi: 10.1124/jpet.107.125773. [DOI] [PubMed] [Google Scholar]
- 360.Kim SY, Kim AY, Lee HW, Son YH, Lee GY, Lee JW, Lee YS, Kim JB. miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARgamma expression. Biochem Biophys Res Commun. 392:323–328. doi: 10.1016/j.bbrc.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 361.Kim YK, Kim SJ, Yatani A, Huang Y, Castelli G, Vatner DE, Liu J, Zhang Q, Diaz G, Zieba R, Thaisz J, Drusco A, Croce C, Sadoshima J, Condorelli G, Vatner SF. Mechanism of enhanced cardiac function in mice with hypertrophy induced by overexpressed Akt. J Biol Chem. 2003;278:47622–47628. doi: 10.1074/jbc.M305909200. [DOI] [PubMed] [Google Scholar]
- 362.Kis A, Yellon DM, Baxter GF. Second window of protection following myocardial preconditioning: an essential role for PI3 kinase and p70S6 kinase. J Mol Cell Cardiol. 2003;35:1063–1071. doi: 10.1016/s0022-2828(03)00208-6. [DOI] [PubMed] [Google Scholar]
- 363.Kobayashi N, Yoshida K, Nakano S, Ohno T, Honda T, Tsubokou Y, Matsuoka H. Cardioprotective mechanisms of eplerenone on cardiac performance and remodeling in failing rat hearts. Hypertension. 2006;47:671–679. doi: 10.1161/01.HYP.0000203148.42892.7a. [DOI] [PubMed] [Google Scholar]
- 364.Koc ON, Gerson SL. Akt helps stem cells heal the heart. Nat Med. 2003;9:1109–1110. doi: 10.1038/nm0903-1109. [DOI] [PubMed] [Google Scholar]
- 365.Kohn AD, Takeuchi F, Roth RA. Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J Biol Chem. 1996;271:21920–21926. doi: 10.1074/jbc.271.36.21920. [DOI] [PubMed] [Google Scholar]
- 366.Koneru S, Penumathsa SV, Thirunavukkarasu M, Samuel SM, Zhan L, Han Z, Maulik G, Das DK, Maulik N. Redox regulation of ischemic preconditioning is mediated by the differential activation of caveolins and their association with eNOS and GLUT-4. Am J Physiol Heart Circ Physiol. 2007;292:H2060–H2072. doi: 10.1152/ajpheart.01169.2006. [DOI] [PubMed] [Google Scholar]
- 367.Konhilas JP, Maass AH, Luckey SW, Stauffer BL, Olson EN, Leinwand LA. Sex modifies exercise and cardiac adaptation in mice. Am J Physiol Heart Circ Physiol. 2004;287:H2768–H2776. doi: 10.1152/ajpheart.00292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Konhilas JP, Widegren U, Allen DL, Paul AC, Cleary A, Leinwand LA. Loaded wheel running and muscle adaptation in the mouse. Am J Physiol Heart Circ Physiol. 2005;289:H455–H465. doi: 10.1152/ajpheart.00085.2005. [DOI] [PubMed] [Google Scholar]
- 369.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 370.Kovacs K, Toth A, Deres P, Kalai T, Hideg K, Gallyas F, Jr, Sumegi B. Critical role of PI3-kinase/Akt activation in the PARP inhibitor induced heart function recovery during ischemia-reperfusion. Biochem Pharmacol. 2006;71:441–452. doi: 10.1016/j.bcp.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 371.Krieg T, Qin Q, McIntosh EC, Cohen MV, Downey JM. ACh and adenosine activate PI3-kinase in rabbit hearts through transactivation of receptor tyrosine kinases. Am J Physiol Heart Circ Physiol. 2002;283:H2322–H2330. doi: 10.1152/ajpheart.00474.2002. [DOI] [PubMed] [Google Scholar]
- 372.Krishnan N, Pan H, Buckley DJ, Buckley A. Prolactin-regulated pim-1 transcription: identification of critical promoter elements and Akt signaling. Endocrine. 2003;20:123–130. doi: 10.1385/endo:20:1-2:123. [DOI] [PubMed] [Google Scholar]
- 373.Kubo H, Jaleel N, Kumarapeli A, Berretta RM, Bratinov G, Shan X, Wang H, Houser SR, Margulies KB. Increased cardiac myocyte progenitors in failing human hearts. Circulation. 2008;118:649–657. doi: 10.1161/CIRCULATIONAHA.107.761031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Kuehbacher A, Urbich C, Dimmeler S. Targeting microRNA expression to regulate angiogenesis. Trends Pharmacol Sci. 2008;29:12–15. doi: 10.1016/j.tips.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 375.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 376.Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 377.Kuhn DE, Nuovo GJ, Martin MM, Malana GE, Pleister AP, Jiang J, Schmittgen TD, Terry AV, Jr, Gardiner K, Head E, Feldman DS, Elton TS. Human chromosome 21-derived miRNAs are overexpressed in down syndrome brains and hearts. Biochem Biophys Res Commun. 2008;370:473–477. doi: 10.1016/j.bbrc.2008.03.120. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 378.Kulik G, Klippel A, Weber MJ. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Kumar D, Lou H, Singal PK. Oxidative stress and apoptosis in heart dysfunction. Herz. 2002;27:662–668. doi: 10.1007/s00059-002-2430-3. [DOI] [PubMed] [Google Scholar]
- 380.Kuo WW, Chu CY, Wu CH, Lin JA, Liu JY, Hsieh YH, Ueng KC, Lee SD, Hsieh DJ, Hsu HH, Chen LM, Huang CY. Impaired IGF-I signalling of hypertrophic hearts in the developmental phase of hypertension in genetically hypertensive rats. Cell Biochem Funct. 2005;23:325–331. doi: 10.1002/cbf.1244. [DOI] [PubMed] [Google Scholar]
- 381.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Kusano KF, Pola R, Murayama T, Curry C, Kawamoto A, Iwakura A, Shintani S, Ii M, Asai J, Tkebuchava T, Thorne T, Takenaka H, Aikawa R, Goukassian D, von Samson P, Hamada H, Yoon YS, Silver M, Eaton E, Ma H, Heyd L, Kearney M, Munger W, Porter JA, Kishore R, Losordo DW. Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat Med. 2005;11:1197–1204. doi: 10.1038/nm1313. [DOI] [PubMed] [Google Scholar]
- 383.Kutala VK, Khan M, Mandal R, Ganesan LP, Tridandapani S, Kalai T, Hideg K, Kuppusamy P. Attenuation of myocardial ischemia-reperfusion injury by trimetazidine derivatives functionalized with antioxidant properties. J Pharmacol Exp Ther. 2006;317:921–928. doi: 10.1124/jpet.105.100834. [DOI] [PubMed] [Google Scholar]
- 384.Kuwabara M, Kakinuma Y, Ando M, Katare RG, Yamasaki F, Doi Y, Sato T. Nitric oxide stimulates vascular endothelial growth factor production in cardiomyocytes involved in angiogenesis. J Physiol Sci. 2006;56:95–101. doi: 10.2170/physiolsci.rp002305. [DOI] [PubMed] [Google Scholar]
- 385.Kuwahara K, Saito Y, Kishimoto I, Miyamoto Y, Harada M, Ogawa E, Hamanaka I, Kajiyama N, Takahashi N, Izumi T, Kawakami R, Nakao K. Cardiotrophin-1 phosphorylates akt and BAD, prolongs cell survival via a PI3K-dependent pathway in cardiac myocytes. J Mol Cell Cardiol. 2000;32:1385–1394. doi: 10.1006/jmcc.2000.1177. [DOI] [PubMed] [Google Scholar]
- 386.Kuzman JA, Gerdes AM, Kobayashi S, Liang Q. Thyroid hormone activates Akt and prevents serum starvation-induced cell death in neonatal rat cardiomyocytes. J Mol Cell Cardiol. 2005;39:841–844. doi: 10.1016/j.yjmcc.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 387.Kuzman JA, Vogelsang KA, Thomas TA, Gerdes AM. L-Thyroxine activates Akt signaling in the heart. J Mol Cell Cardiol. 2005;39:251–258. doi: 10.1016/j.yjmcc.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 389.Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201–208. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- 390.Ladage D, Brixius K, Steingen C, Mehlhorn U, Schwinger RH, Bloch W, Schmidt A. Mesenchymal stem cells induce endothelial activation via paracine mechanisms. Endothelium. 2007;14:53–63. doi: 10.1080/10623320701343319. [DOI] [PubMed] [Google Scholar]
- 391.Lai HC, Liu TJ, Ting CT, Sharma PM, Wang PH. Insulin-like growth factor-1 prevents loss of electrochemical gradient in cardiac muscle mitochondria via activation of PI 3 kinase/Akt pathway. Mol Cell Endocrinol. 2003;205:99–106. doi: 10.1016/s0303-7207(03)00200-4. [DOI] [PubMed] [Google Scholar]
- 392.Lajoie C, Beliveau L, Trudeau F, Lavoie N, Massicotte G, Gagnon S, Calderone A. The rapid onset of hyperglycaemia in ZDF rats was associated with a widespread alteration of metabolic proteins implicated in glucose metabolism in the heart. Can J Physiol Pharmacol. 2006;84:1205–1213. doi: 10.1139/y06-070. [DOI] [PubMed] [Google Scholar]
- 393.Lajoie C, Calderone A, Beliveau L. Exercise training enhanced the expression of myocardial proteins related to cell protection in spontaneously hypertensive rats. Pflügers Arch. 2004;449:26–32. doi: 10.1007/s00424-004-1307-0. [DOI] [PubMed] [Google Scholar]
- 394.Lal A, Navarro F, Maher CA, Maliszewski LE, Yan N, O’Day E, Chowdhury D, Dykxhoorn DM, Tsai P, Hofmann O, Becker KG, Gorospe M, Hide W, Lieberman J. miR-24 inhibits cell proliferation by targeting E2F2, MYC, and other cell-cycle genes via binding to “seedless” 3′UTR microRNA recognition elements. Mol Cell. 2009;35:610–625. doi: 10.1016/j.molcel.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Latronico MV, Costinean S, Lavitrano ML, Peschle C, Condorelli G. Regulation of cell size and contractile function by AKT in cardiomyocytes. Ann NY Acad Sci. 2004;1015:250–260. doi: 10.1196/annals.1302.021. [DOI] [PubMed] [Google Scholar]
- 397.Lau YT. Receptor-dependent and genomic-independent actions of estrogen in vascular protection. Chang Gung Med J. 2002;25:636–644. [PubMed] [Google Scholar]
- 398.Laviola L, Belsanti G, Davalli AM, Napoli R, Perrini S, Weir GC, Giorgino R, Giorgino F. Effects of streptozocin diabetes and diabetes treatment by islet transplantation on in vivo insulin signaling in rat heart. Diabetes. 2001;50:2709–2720. doi: 10.2337/diabetes.50.12.2709. [DOI] [PubMed] [Google Scholar]
- 399.Leduc I, Karsunky H, Mathieu N, Schmidt T, Verthuy C, Ferrier P, Moroy T. The Pim-1 kinase stimulates maturation of TCRbeta-deficient T cell progenitors: implications for the mechanism of Pim-1 action. Int Immunol. 2000;12:1389–1396. doi: 10.1093/intimm/12.10.1389. [DOI] [PubMed] [Google Scholar]
- 400.Lee HC, Tsai JN, Liao PY, Tsai WY, Lin KY, Chuang CC, Sun CK, Chang WC, Tsai HJ. Glycogen synthase kinase 3 alpha and 3 beta have distinct functions during cardiogenesis of zebrafish embryo. BMC Dev Biol. 2007;7:93. doi: 10.1186/1471-213X-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- 402.Lee KH, Chen YL, Yeh SD, Hsiao M, Lin JT, Goan YG, Lu PJ. MicroRNA-330 acts as tumor suppressor and induces apoptosis of prostate cancer cells through E2F1-mediated suppression of Akt phosphorylation. Oncogene. 2009;28:3360–3370. doi: 10.1038/onc.2009.192. [DOI] [PubMed] [Google Scholar]
- 403.Leinwand LA. Sex is a potent modifier of the cardiovascular system. J Clin Invest. 2003;112:302–307. doi: 10.1172/JCI19429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Lemmens K, Fransen P, Sys SU, Brutsaert DL, De Keulenaer GW. Neuregulin-1 induces a negative inotropic effect in cardiac muscle: role of nitric oxide synthase. Circulation. 2004;109:324–326. doi: 10.1161/01.CIR.0000114521.88547.5E. [DOI] [PubMed] [Google Scholar]
- 405.Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Alterations in microRNA expression in stress-induced cellular senescence. Mech Ageing Dev. 2009;130:731–741. doi: 10.1016/j.mad.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Li HH, Kedar V, Zhang C, McDonough H, Arya R, Wang DZ, Patterson C. Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J Clin Invest. 2004;114:1058–1071. doi: 10.1172/JCI22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Li HH, Willis MS, Lockyer P, Miller N, McDonough H, Glass DJ, Patterson C. Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of Forkhead proteins. J Clin Invest. 2007;117:3211–3223. doi: 10.1172/JCI31757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Li HJ, Yin H, Yao YY, Shen B, Bader M, Chao L, Chao J. Tissue kallikrein protects against pressure overload-induced cardiac hypertrophy through kinin B2 receptor and glycogen synthase kinase-3beta activation. Cardiovasc Res. 2007;73:130–142. doi: 10.1016/j.cardiores.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Li J, Wei H, Chesley A, Moon C, Krawczyk M, Volkova M, Ziman B, Margulies KB, Talan M, Crow MT, Boheler KR. The pro-angiogenic cytokine pleiotrophin potentiates cardiomyocyte apoptosis through inhibition of endogenous AKT/PKB activity. J Biol Chem. 2007;282:34984–34993. doi: 10.1074/jbc.M703513200. [DOI] [PubMed] [Google Scholar]
- 411.Li Q, Li B, Wang X, Leri A, Jana KP, Liu Y, Kajstura J, Baserga R, Anversa P. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest. 1997;100:1991–1999. doi: 10.1172/JCI119730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Li R, Zheng W, Pi R, Gao J, Zhang H, Wang P, Le K, Liu P. Activation of peroxisome proliferator-activated receptor-alpha prevents glycogen synthase 3beta phosphorylation and inhibits cardiac hypertrophy. FEBS Lett. 2007;581:3311–3316. doi: 10.1016/j.febslet.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 413.Li Y, Dowbenko D, Lasky LA. AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J Biol Chem. 2002;277:11352–11361. doi: 10.1074/jbc.M109062200. [DOI] [PubMed] [Google Scholar]
- 414.Li Y, Inoki K, Guan KL. Biochemical and functional characterizations of small GTPase Rheb and TSC2 GAP activity. Mol Cell Biol. 2004;24:7965–7975. doi: 10.1128/MCB.24.18.7965-7975.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Li Y, Takemura G, Okada H, Miyata S, Esaki M, Maruyama R, Kanamori H, Li L, Ogino A, Misao Y, Khai NC, Mikami A, Minatoguchi S, Fujiwara T, Fujiwara H. Treatment with granulocyte colony-stimulating factor ameliorates chronic heart failure. Lab Invest. 2006;86:32–44. doi: 10.1038/labinvest.3700367. [DOI] [PubMed] [Google Scholar]
- 416.Liang H, Hittelman W, Nagarajan L. Ubiquitous expression and cell cycle regulation of the protein kinase PIM-1. Arch Biochem Biophys. 1996;330:259–265. doi: 10.1006/abbi.1996.0251. [DOI] [PubMed] [Google Scholar]
- 417.Liao JK. Statin therapy for cardiac hypertrophy and heart failure. J Invest Med. 2004;52:248–253. doi: 10.1136/jim-52-04-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Liao W, Wang S, Han C, Zhang Y. 14-3-3 proteins regulate glycogen synthase 3beta phosphorylation and inhibit cardiomyocyte hypertrophy. FEBS Lett. 2005;272:1845–1854. doi: 10.1111/j.1742-4658.2005.04614.x. [DOI] [PubMed] [Google Scholar]
- 419.Liem DA, Honda HM, Zhang J, Woo DD, Ping P. Past and present course of cardioprotection against ischemia reperfusion injury. J Appl Physiol. 2007;103:2129–2136. doi: 10.1152/japplphysiol.00383.2007. [DOI] [PubMed] [Google Scholar]
- 420.Lim SY, Kim YS, Ahn Y, Jeong MH, Hong MH, Joo SY, Nam KI, Cho JG, Kang PM, Park JC. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc Res. 2006;70:530–542. doi: 10.1016/j.cardiores.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 421.Lin Z, Murtaza I, Wang K, Jiao J, Gao J, Li PF. miR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy. Proc Natl Acad Sci USA. 2009;106:12103–12108. doi: 10.1073/pnas.0811371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Liu HT, Zhang HF, Si R, Zhang QJ, Zhang KR, Guo WY, Wang HC, Gao F. Insulin protects isolated hearts from ischemia/reperfusion injury: cross-talk between PI3-K/Akt and JNKs. Sheng Li Xue Bao. 2007;59:651–659. [PubMed] [Google Scholar]
- 423.Liu J, Sadoshima J, Zhai P, Hong C, Yang G, Chen W, Yan L, Wang Y, Vatner SF, Vatner DE. Pressure overload induces greater hypertrophy and mortality in female mice with p38alpha MAPK inhibition. J Mol Cell Cardiol. 2006;41:680–688. doi: 10.1016/j.yjmcc.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 424.Liu L, Zhao X, Pierre SV, Askari A. Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am J Physiol Cell Physiol. 2007;293:C1489–C1497. doi: 10.1152/ajpcell.00158.2007. [DOI] [PubMed] [Google Scholar]
- 425.Liu SP, Fu RH, Yu HH, Li KW, Tsai CH, Shyu WC, Lin SZ. MicroRNAs regulation modulated self-renewal and lineage differentiation of stem cells. Cell Transplant. 2009;18:1039–1045. doi: 10.3727/096368909X471224. [DOI] [PubMed] [Google Scholar]
- 426.Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, Walsh K, Isner JM, Asahara T. HMG-CoA reductase inhibitor mobilizes bone marrow-derived endothelial progenitor cells. J Clin Invest. 2001;108:399–405. doi: 10.1172/JCI13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Long J, Wang Y, Wang W, Chang BH, Danesh FR. Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J Biol Chem. 285:23457–23465. doi: 10.1074/jbc.M110.136168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Long P, Nguyen Q, Thurow C, Broderick TL. Caloric restriction restores the cardioprotective effect of preconditioning in the rat heart. Mech Ageing Dev. 2002;123:1411–1413. doi: 10.1016/s0047-6374(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 429.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 430.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 431.Lorts A, Schwanekamp JA, Elrod JW, Sargent MA, Molkentin JD. Genetic manipulation of periostin expression in the heart does not affect myocyte content, cell cycle activity, or cardiac repair. Circ Res. 2009;104:e1–e7. doi: 10.1161/CIRCRESAHA.108.188649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Lu C, Schwartzbauer G, Sperling MA, Devaskar SU, Thamotharan S, Robbins PD, McTiernan CF, Liu JL, Jiang J, Frank SJ, Menon RK. Demonstration of direct effects of growth hormone on neonatal cardiomyocytes. J Biol Chem. 2001;276:22892–22900. doi: 10.1074/jbc.M011647200. [DOI] [PubMed] [Google Scholar]
- 433.Lucchinetti E, Feng J, Silva R, Tolstonog GV, Schaub MC, Schumann GG, Zaugg M. Inhibition of LINE-1 expression in the heart decreases ischemic damage by activation of Akt/PKB signaling. Physiol Genomics. 2006;25:314–324. doi: 10.1152/physiolgenomics.00251.2005. [DOI] [PubMed] [Google Scholar]
- 434.Luft FC. Harbingers of hypertrophy and heart failure. J Mol Med. 2004;82:635–637. doi: 10.1007/s00109-004-0577-5. [DOI] [PubMed] [Google Scholar]
- 435.Luo J, McMullen JR, Sobkiw CL, Zhang L, Dorfman AL, Sherwood MC, Logsdon MN, Horner JW, DePinho RA, Izumo S, Cantley LC. Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol Cell Biol. 2005;25:9491–9502. doi: 10.1128/MCB.25.21.9491-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Lynch RM, Carrington W, Fogarty KE, Fay FS. Metabolic modulation of hexokinase association with mitochondria in living smooth muscle cells. Am J Physiol Cell Physiol. 1996;270:C488–C499. doi: 10.1152/ajpcell.1996.270.2.C488. [DOI] [PubMed] [Google Scholar]
- 437.Ma G, Al-Shabrawey M, Johnson JA, Datar R, Tawfik HE, Guo D, Caldwell RB, Caldwell RW. Protection against myocardial ischemia/reperfusion injury by short-term diabetes: enhancement of VEGF formation, capillary density, and activation of cell survival signaling. Naunyn-Schmiedebergs Arch Pharmacol. 2006;373:415–427. doi: 10.1007/s00210-006-0102-1. [DOI] [PubMed] [Google Scholar]
- 438.Mabuchi S, Ohmichi M, Kimura A, Hisamoto K, Hayakawa J, Nishio Y, Adachi K, Takahashi K, Arimoto-Ishida E, Nakatsuji Y, Tasaka K, Murata Y. Inhibition of phosphorylation of BAD and Raf-1 by Akt sensitizes human ovarian cancer cells to paclitaxel. J Biol Chem. 2002;277:33490–33500. doi: 10.1074/jbc.M204042200. [DOI] [PubMed] [Google Scholar]
- 439.Madeddu P, Kraenkel N, Barcelos LS, Siragusa M, Campagnolo P, Oikawa A, Caporali A, Herman A, Azzolino O, Barberis L, Perino A, Damilano F, Emanueli C, Hirsch E. Phosphoinositide 3-kinase gamma gene knockout impairs postischemic neovascularization and endothelial progenitor cell functions. Arterioscler Thromb Vasc Biol. 2008;28:68–76. doi: 10.1161/ATVBAHA.107.145573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Madrazo JA, Kelly DP. The PPAR trio: regulators of myocardial energy metabolism in health and disease. J Mol Cell Cardiol. 2008;44:968–975. doi: 10.1016/j.yjmcc.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 441.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 442.Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, Gottlob K, Chandel NS, Thompson CB, Robey RB, Hay N. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 443.Majewski N, Nogueira V, Robey RB, Hay N. Akt inhibits apoptosis downstream of BID cleavage via a glucose-dependent mechanism involving mitochondrial hexokinases. Mol Cell Biol. 2004;24:730–740. doi: 10.1128/MCB.24.2.730-740.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Malik G, Gorbounov N, Das S, Gurusamy N, Otani H, Maulik N, Goswami S, Das DK. Ischemic preconditioning triggers nuclear translocation of thioredoxin and its interaction with Ref-1 potentiating a survival signal through the PI-3-kinase-Akt pathway. Antioxid Redox Signal. 2006;8:2101–2109. doi: 10.1089/ars.2006.8.2101. [DOI] [PubMed] [Google Scholar]
- 445.Mally MI, Vogt M, Swift SE, Haas M. Oncogene expression in murine splenic T cells and in murine T-cell neoplasms. Virology. 1985;144:115–126. doi: 10.1016/0042-6822(85)90310-1. [DOI] [PubMed] [Google Scholar]
- 446.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 447.Manintveld OC, Te Lintel Hekkert M, van den Bos EJ, Suurenbroek GM, Dekkers DH, Verdouw PD, Lamers JM, Duncker DJ. Cardiac effects of postconditioning depend critically on the duration of index ischemia. Am J Physiol Heart Circ Physiol. 2007;292:H1551–H1560. doi: 10.1152/ajpheart.00151.2006. [DOI] [PubMed] [Google Scholar]
- 448.Mann DL. MicroRNAs and the failing heart. N Engl J Med. 2007;356:2644–2645. doi: 10.1056/NEJMcibr072068. [DOI] [PubMed] [Google Scholar]
- 449.Markou T, Cullingford TE, Giraldo A, Weiss SC, Alsafi A, Fuller SJ, Clerk A, Sugden PH. Glycogen synthase kinases 3alpha and 3beta in cardiac myocytes: regulation and consequences of their inhibition. Cell Signal. 2008;20:206–218. doi: 10.1016/j.cellsig.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 450.Marte BM, Downward J. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 451.Matikainen S, Sareneva T, Ronni T, Lehtonen A, Koskinen PJ, Julkunen I. Interferon-alpha activates multiple STAT proteins and upregulates proliferation-associated IL-2Ralpha, c-myc, and pim-1 genes in human T cells. Blood. 1999;93:1980–1991. [PubMed] [Google Scholar]
- 452.Matsubara H, Takeuchi T, Nishikawa E, Yanagisawa K, Hayashita Y, Ebi H, Yamada H, Suzuki M, Nagino M, Nimura Y, Osada H, Takahashi T. Apoptosis induction by antisense oligonucleotides against miR-17–5p and miR-20a in lung cancers overexpressing miR-17–92. Oncogene. 2007;26:6099–6105. doi: 10.1038/sj.onc.1210425. [DOI] [PubMed] [Google Scholar]
- 453.Matsuda T, Zhai P, Maejima Y, Hong C, Gao S, Tian B, Goto K, Takagi H, Tamamori-Adachi M, Kitajima S, Sadoshima J. Distinct roles of GSK-3alpha and GSK-3beta phosphorylation in the heart under pressure overload. Proc Natl Acad Sci USA. 2008;105:20900–20905. doi: 10.1073/pnas.0808315106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral gene transfer of activated phosphatidylinositol 3′-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation. 1999;100:2373–2379. doi: 10.1161/01.cir.100.23.2373. [DOI] [PubMed] [Google Scholar]
- 455.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277:22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 456.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao RL, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277:22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 457.Matsui T, Nagoshi T, Hong EG, Luptak I, Hartil K, Li L, Gorovits N, Charron MJ, Kim JK, Tian R, Rosenzweig A. Effects of chronic Akt activation on glucose uptake in the heart. Am J Physiol Endocrinol Metab. 2006;290:E789–E797. doi: 10.1152/ajpendo.00564.2004. [DOI] [PubMed] [Google Scholar]
- 458.Matsui T, Nagoshi T, Rosenzweig A. Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival. Cell Cycle. 2003;2:220–223. [PubMed] [Google Scholar]
- 459.Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol. 2005;38:63–71. doi: 10.1016/j.yjmcc.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 460.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 461.Matsumoto S, Cho S, Tosaka S, Ureshino H, Maekawa T, Hara T, Sumikawa K. Pharmacological preconditioning in type 2 diabetic rat hearts: the roles of mitochondrial ATP-sensitive potassium channels and the phosphatidylinositol 3-kinase-Akt pathway. Cardiovasc Drugs Ther. 2009;23:263–270. doi: 10.1007/s10557-009-6184-5. [DOI] [PubMed] [Google Scholar]
- 462.Matsuura K, Honda A, Nagai T, Fukushima N, Iwanaga K, Tokunaga M, Shimizu T, Okano T, Kasanuki H, Hagiwara N, Komuro I. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest. 2009;119:2204–2217. doi: 10.1172/JCI37456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Mattson MP, Kroemer G. Mitochondria in cell death: novel targets for neuroprotection and cardioprotection. Trends Mol Med. 2003;9:196–205. doi: 10.1016/s1471-4914(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 464.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 465.McDevitt TC, Laflamme MA, Murry CE. Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI 3-kinase/Akt signaling pathway. J Mol Cell Cardiol. 2005;39:865–873. doi: 10.1016/j.yjmcc.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.McFalls EO, Liem D, Schoonderwoerd K, Lamers J, Sluiter W, Duncker D. Mitochondrial function: the heart of myocardial preservation. J Lab Clin Med. 2003;142:141–148. doi: 10.1016/S0022-2143(03)00109-4. [DOI] [PubMed] [Google Scholar]
- 467.McGowan BS, Ciccimaro EF, Chan TO, Feldman AM. The balance between pro-apoptotic and anti-apoptotic pathways in the failing myocardium. Cardiovasc Toxicol. 2003;3:191–206. doi: 10.1385/ct:3:3:191. [DOI] [PubMed] [Google Scholar]
- 468.McMullen JR, Amirahmadi F, Woodcock EA, Schinke-Braun M, Bouwman RD, Hewitt KA, Mollica JP, Zhang L, Zhang Y, Shioi T, Buerger A, Izumo S, Jay PY, Jennings GL. Protective effects of exercise and phosphoinositide 3-kinase (p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proc Natl Acad Sci USA. 2007;104:612–617. doi: 10.1073/pnas.0606663104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.McMullen JR, Jennings GL. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol. 2007;34:255–262. doi: 10.1111/j.1440-1681.2007.04585.x. [DOI] [PubMed] [Google Scholar]
- 470.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S. Inhibition of mammalian target of rapamycin signaling regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;110:604. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 471.McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, Schinke M, Kong S, Sherwood MC, Brown J, Riggi L, Kang PM, Izumo S. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase (p110alpha) pathway. J Biol Chem. 2004;279:4782–4793. doi: 10.1074/jbc.M310405200. [DOI] [PubMed] [Google Scholar]
- 472.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Dorfman AL, Longnus S, Pende M, Martin KA, Blenis J, Thomas G, Izumo S. Deletion of ribosomal S6 kinases does not attenuate pathological, physiological, or insulin-like growth factor 1 receptor-phosphoinositide 3-kinase-induced cardiac hypertrophy. Mol Cell Biol. 2004;24:6231–6240. doi: 10.1128/MCB.24.14.6231-6240.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase (p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci USA. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Meeker TC, Nagarajan L, ar-Rushdi A, Rovera G, Huebner K, Croce CM. Characterization of the human PIM-1 gene: a putative proto-oncogene coding for a tissue specific member of the protein kinase family. Oncogene Res. 1987;1:87–101. [PubMed] [Google Scholar]
- 475.Meloni M, Caporali A, Graiani G, Lagrasta C, Katare R, Van Linthout S, Spillmann F, Campesi I, Madeddu P, Quaini F, Emanueli C. Nerve growth factor promotes cardiac repair following myocardial infarction. Circ Res. 2010;106:1275–1284. doi: 10.1161/CIRCRESAHA.109.210088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Mendez P, Garcia-Segura LM. Phosphatidylinositol 3-kinase and glycogen synthase kinase 3 regulate estrogen receptor-mediated transcription in neuronal cells. Endocrinology. 2006;147:3027–3039. doi: 10.1210/en.2005-1224. [DOI] [PubMed] [Google Scholar]
- 477.Menon B, Johnson JN, Ross RS, Singh M, Singh K. Glycogen synthase kinase-3beta plays a pro-apoptotic role in beta-adrenergic receptor-stimulated apoptosis in adult rat ventricular myocytes: Role of beta1 integrins. J Mol Cell Cardiol. 2007;42:653–661. doi: 10.1016/j.yjmcc.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Methner C, Donat U, Felix SB, Krieg T. Cardioprotection of bradykinin at reperfusion involves transactivation of the epidermal growth factor receptor via matrix metallo-proteinase-8. Acta Physiol. 2009;197:265–271. doi: 10.1111/j.1748-1716.2009.02018.x. [DOI] [PubMed] [Google Scholar]
- 479.Minamishima S, Bougaki M, Sips PY, Yu JD, Minamishima YA, Elrod JW, Lefer DJ, Bloch KD, Ichinose F. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation. 2009;120:888–896. doi: 10.1161/CIRCULATIONAHA.108.833491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Mineo C, Shaul PW. HDL stimulation of endothelial nitric oxide synthase: a novel mechanism of HDL action. Trends Cardiovasc Med. 2003;13:226–231. doi: 10.1016/s1050-1738(03)00098-7. [DOI] [PubMed] [Google Scholar]
- 481.Mio Y, Shim YH, Richards E, Bosnjak ZJ, Pagel PS, Bienengraeber M. Xenon preconditioning: the role of prosurvival signaling, mitochondrial permeability transition and bioenergetics in rats. Anesth Analg. 2009;108:858–866. doi: 10.1213/ane.0b013e318192a520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Miyamoto S, Murphy AN, Brown JH. Akt mediated mitochondrial protection in the heart: metabolic and survival pathways to the rescue. J Bioenerget Biomembr. 2009;41:169–180. doi: 10.1007/s10863-009-9205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2007;15:521–529. doi: 10.1038/sj.cdd.4402285. [DOI] [PubMed] [Google Scholar]
- 485.Miyamoto T, Takeishi Y, Takahashi H, Shishido T, Arimoto T, Tomoike H, Kubota I. Activation of distinct signal transduction pathways in hypertrophied hearts by pressure and volume overload. Basic Res Cardiol. 2004;99:328–337. doi: 10.1007/s00395-004-0482-7. [DOI] [PubMed] [Google Scholar]
- 486.Miyata S, Takemura G, Kawase Y, Li Y, Okada H, Maruyama R, Ushikoshi H, Esaki M, Kanamori H, Li L, Misao Y, Tezuka A, Toyo-Oka T, Minatoguchi S, Fujiwara T, Fujiwara H. Autophagic cardiomyocyte death in cardiomyopathic hamsters and its prevention by granulocyte colony-stimulating factor. Am J Pathol. 2006;168:386–397. doi: 10.2353/ajpath.2006.050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Mocanu MM, Bell RM, Yellon DM. PI3 kinase and not p42/p44 appears to be implicated in the protection conferred by ischemic preconditioning. J Mol Cell Cardiol. 2002;34:661–668. doi: 10.1006/jmcc.2002.2006. [DOI] [PubMed] [Google Scholar]
- 488.Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 489.Mora A, Davies AM, Bertrand L, Sharif I, Budas GR, Jovanovic S, Mouton V, Kahn CR, Lucocq JM, Gray GA, Jovanovic A, Alessi DR. Deficiency of PDK1 in cardiac muscle results in heart failure and increased sensitivity to hypoxia. EMBO J. 2003;22:4666–4676. doi: 10.1093/emboj/cdg469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Morisco C, Seta K, Hardt SE, Lee Y, Vatner SF, Sadoshima J. Glycogen synthase kinase 3beta regulates GATA4 in cardiac myocytes. J Biol Chem. 2001;276:28586–28597. doi: 10.1074/jbc.M103166200. [DOI] [PubMed] [Google Scholar]
- 491.Mukai Y, Rikitake Y, Shiojima I, Wolfrum S, Satoh M, Takeshita K, Hiroi Y, Salomone S, Kim HH, Benjamin LE, Walsh K, Liao JK. Decreased vascular lesion formation in mice with inducible endothelial-specific expression of protein kinase Akt. J Clin Invest. 2006;116:334–343. doi: 10.1172/JCI26223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Muraski JAFJ, Gude N, Martindale J, Glembotski C, Magnuson N, Berns A, Sussman MA. Pim-1 regulates cardiomyocyte survival downstream of Akt. Circulation. 2006;114:294. [Google Scholar]
- 493.Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, Esposito G, Delucchi F, Arcarese M, Alvarez R, Siddiqi S, Emmanuel GN, Wu W, Fischer K, Martindale JJ, Glembotski CC, Leri A, Kajstura J, Magnuson N, Berns A, Beretta RM, Houser SR, Schaefer EM, Anversa P, Sussman MA. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13:1467–1475. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 494.Murphy E, Steenbergen C. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovasc Res. 2007;75:478–486. doi: 10.1016/j.cardiores.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 495.Murphy E, Steenbergen C. Preconditioning: the mitochondrial connection. Annu Rev Physiol. 2007;69:51–67. doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- 496.Muslin AJ, DeBosch B. Role of Akt in cardiac growth and metabolism. Novartis Found Symp. 2006;274:118–131. [PubMed] [Google Scholar]
- 497.Nagarajan L, Louie E, Tsujimoto Y, ar-Rushdi A, Huebner K, Croce CM. Localization of the human pim oncogene (PIM) to a region of chromosome 6 involved in translocations in acute leukemias. Proc Natl Acad Sci USA. 1986;83:2556–2560. doi: 10.1073/pnas.83.8.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, Ogawa W, del Monte F, Gwathmey JK, Grazette L, Hemmings BA, Kass DA, Champion HC, Rosenzweig A. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J Clin Invest. 2005;115:2128–2138. doi: 10.1172/JCI23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Naito AT, Akazawa H, Takano H, Minamino T, Nagai T, Aburatani H, Komuro I. Phosphatidylinositol 3-kinase-Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt signaling. Circ Res. 2005;97:144–151. doi: 10.1161/01.RES.0000175241.92285.f8. [DOI] [PubMed] [Google Scholar]
- 500.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Nave BT, Ouwens DM, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344:427–431. [PMC free article] [PubMed] [Google Scholar]
- 502.Negoro S, Oh H, Tone E, Kunisada K, Fujio Y, Walsh K, Kishimoto T, Yamauchi-Takihara K. Glycoprotein 130 regulates cardiac myocyte survival in doxorubicin-induced apoptosis through phosphatidylinositol 3-kinase/Akt phosphorylation and Bcl-xL/caspase-3 interaction. Circulation. 2001;103:555–561. doi: 10.1161/01.cir.103.4.555. [DOI] [PubMed] [Google Scholar]
- 503.Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, Gerard RD, Kuro OM, Rothermel BA, Hill JA. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci USA. 2007;104:20517–20522. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Niagara MI, Haider H, Jiang S, Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100:545–555. doi: 10.1161/01.RES.0000258460.41160.ef. [DOI] [PubMed] [Google Scholar]
- 505.Nikolic I, Plate KH, Schmidt MH. EGFL7 meets miRNA-126: an angiogenesis alliance. J Angiogenes Res. 2:9. doi: 10.1186/2040-2384-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Nishi H, Ono K, Iwanaga Y, Horie T, Nagao K, Takemura G, Kinoshita M, Kuwabara Y, Mori RT, Hasegawa K, Kita T, Kimura T. MicroRNA-15b modulates cellular ATP levels and degenerates mitochondria via Arl2 in neonatal rat cardiac myocytes. J Biol Chem. 285:4920–4930. doi: 10.1074/jbc.M109.082610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Nishida H, Sato T, Nomura M, Miyazaki M, Nakaya H. Glimepiride treatment upon reperfusion limits infarct size via the phosphatidylinositol 3-kinase/Akt pathway in rabbit hearts. J Pharmacol Sci. 2009;109:251–256. doi: 10.1254/jphs.08202fp. [DOI] [PubMed] [Google Scholar]
- 508.Niu Z, Iyer D, Conway SJ, Martin JF, Ivey K, Srivastava D, Nordheim A, Schwartz RJ. Serum response factor orchestrates nascent sarcomerogenesis and silences the biomineralization gene program in the heart. Proc Natl Acad Sci USA. 2008;105:17824–17829. doi: 10.1073/pnas.0805491105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 510.O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 511.Ogawara Y, Kishishita S, Obata T, Isazawa Y, Suzuki T, Tanaka K, Masuyama N, Gotoh Y. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J Biol Chem. 2002;277:21843–21850. doi: 10.1074/jbc.M109745200. [DOI] [PubMed] [Google Scholar]
- 512.Oh H, Fujio Y, Kunisada K, Hirota H, Matsui H, Kishimoto T, Yamauchi-Takihara K. Activation of phosphatidylinositol 3-kinase through glycoprotein 130 induces protein kinase B and p70 S6 kinase phosphorylation in cardiac myocytes. J Biol Chem. 1998;273:9703–9710. doi: 10.1074/jbc.273.16.9703. [DOI] [PubMed] [Google Scholar]
- 513.Oh H, Schneider MD. The emerging role of telomerase in cardiac muscle cell growth and survival. J Mol Cell Cardiol. 2002;34:717–724. doi: 10.1006/jmcc.2002.2018. [DOI] [PubMed] [Google Scholar]
- 514.Oh H, Taffet GE, Youker KA, Entman ML, Overbeek PA, Michael LH, Schneider MD. Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival. Proc Natl Acad Sci USA. 2001;98:10308–10313. doi: 10.1073/pnas.191169098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Ohori K, Miura T, Tanno M, Miki T, Sato T, Ishikawa S, Horio Y, Shimamoto K. Ser9 phosphorylation of mitochondrial GSK-3beta is a primary mechanism of cardiomyocyte protection by erythropoietin against oxidant-induced apoptosis. Am J Physiol Heart Circ Physiol. 2008;295:H2079–H2086. doi: 10.1152/ajpheart.00092.2008. [DOI] [PubMed] [Google Scholar]
- 516.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–321. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008;117:3099–3108. doi: 10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Osipov RM, Bianchi C, Feng J, Clements RT, Liu Y, Robich MP, Glazer HP, Sodha NR, Sellke FW. Effect of hypercholesterolemia on myocardial necrosis and apoptosis in the setting of ischemia-reperfusion. Circulation. 2009;120:S22–S30. doi: 10.1161/CIRCULATIONAHA.108.842724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, Walsh K. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem. 2008;283:32802–32811. doi: 10.1074/jbc.M803440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Oudit GY, Crackower MA, Eriksson U, Sarao R, Kozieradzki I, Sasaki T, Irie-Sasaki J, Gidrewicz D, Rybin VO, Wada T, Steinberg SF, Backx PH, Penninger JM. Phosphoinositide 3-kinase gamma-deficient mice are protected from isoproterenol-induced heart failure. Circulation. 2003;108:2147–2152. doi: 10.1161/01.CIR.0000091403.62293.2B. [DOI] [PubMed] [Google Scholar]
- 521.Oudit GY, Kassiri Z, Zhou J, Liu QC, Liu PP, Backx PH, Dawood F, Crackower MA, Scholey JW, Penninger JM. Loss of PTEN attenuates the development of pathological hypertrophy and heart failure in response to biomechanical stress. Cardiovasc Res. 2008;78:505–514. doi: 10.1093/cvr/cvn041. [DOI] [PubMed] [Google Scholar]
- 522.Oudit GY, Penninger JM. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res. 2009;82:250–260. doi: 10.1093/cvr/cvp014. [DOI] [PubMed] [Google Scholar]
- 523.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 524.Padin-Iruegas ME, Misao Y, Davis ME, Segers VF, Esposito G, Tokunou T, Urbanek K, Hosoda T, Rota M, Anversa P, Leri A, Lee RT, Kajstura J. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120:876–887. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Parcellier A, Tintignac LA, Zhuravleva E, Hemmings BA. PKB and the mitochondria: AKTing on apoptosis. Cell Signal. 2008;20:21–30. doi: 10.1016/j.cellsig.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 526.Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem. 2004;279:52282–52292. doi: 10.1074/jbc.M407629200. [DOI] [PubMed] [Google Scholar]
- 527.Park SY, Cho YR, Finck BN, Kim HJ, Higashimori T, Hong EG, Lee MK, Danton C, Deshmukh S, Cline GW, Wu JJ, Bennett AM, Rothermel B, Kalinowski A, Russell KS, Kim YB, Kelly DP, Kim JK. Cardiac-specific overexpression of peroxisome proliferator-activated receptor-alpha causes insulin resistance in heart and liver. Diabetes. 2005;54:2514–2524. doi: 10.2337/diabetes.54.9.2514. [DOI] [PubMed] [Google Scholar]
- 528.Pastukh V, Ricci C, Solodushko V, Mozaffari M, Schaffer SW. Contribution of the PI 3-kinase/Akt survival pathway toward osmotic preconditioning. Mol Cell Biochem. 2005;269:59–67. doi: 10.1007/s11010-005-2536-z. [DOI] [PubMed] [Google Scholar]
- 529.Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res. 2002;90:1044–1054. doi: 10.1161/01.res.0000020201.44772.67. [DOI] [PubMed] [Google Scholar]
- 530.Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, Silengo L, Altruda F, Wetzker R, Wymann MP, Lembo G, Hirsch E. PI3Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell. 2004;118:375–387. doi: 10.1016/j.cell.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 531.Patten RD, Karas RH. Estrogen replacement and cardiomyocyte protection. Trends Cardiovasc Med. 2006;16:69–75. doi: 10.1016/j.tcm.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 532.Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, Michael A, Haq S, Nuedling S, Grohe C, Force T, Mendelsohn ME, Karas RH. 17Beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phosphoinositide-3 kinase/Akt signaling. Circ Res. 2004;95:692–699. doi: 10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- 533.Paukku K, Silvennoinen O. STATs as critical mediators of signal transduction and transcription: lessons learned from STAT5. Cytokine Growth Factor Rev. 2004;15:435–455. doi: 10.1016/j.cytogfr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 534.Pedram A, Razandi M, Aitkenhead M, Levin ER. Estrogen inhibits cardiomyocyte hypertrophy in vitro. Antagonism of calcineurin-related hypertrophy through induction of MCIP1. J Biol Chem. 2005;280:26339–26348. doi: 10.1074/jbc.M414409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, Klumperman J, Thorens B, Thomas G. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature. 2000;408:994–997. doi: 10.1038/35050135. [DOI] [PubMed] [Google Scholar]
- 536.Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, Hay N. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Penumathsa SV, Thirunavukkarasu M, Koneru S, Juhasz B, Zhan L, Pant R, Menon VP, Otani H, Maulik N. Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. J Mol Cell Cardiol. 2007;42:508–516. doi: 10.1016/j.yjmcc.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Pezzolesi MG, Platzer P, Waite KA, Eng C. Differential expression of PTEN-targeting microRNAs miR-19a and miR-21 in Cowden syndrome. Am J Hum Genet. 2008;82:1141–1149. doi: 10.1016/j.ajhg.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I, Nagy JA, Lin MI, Walsh K, Dvorak AM, Briscoe DM, Neeman M, Sessa WC, Dvorak HF, Benjamin LE. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S, Rainaldi G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 541.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nature Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 542.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 543.Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J. 2007;403:217–234. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]
- 544.Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol. 2003;23:7315–7328. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Reed JC, Paternostro G. Postmitochondrial regulation of apoptosis during heart failure. Proc Natl Acad Sci USA. 1999;96:7614–7616. doi: 10.1073/pnas.96.14.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Regula KM, Ens K, Kirshenbaum LA. Mitochondria-assisted cell suicide: a license to kill. J Mol Cell Cardiol. 2003;35:559–567. doi: 10.1016/s0022-2828(03)00118-4. [DOI] [PubMed] [Google Scholar]
- 547.Reiss K, Cheng W, Ferber A, Kajstura J, Li P, Li B, Olivetti G, Homcy CJ, Baserga R, Anversa P. Overexpression of insulin-like growth factor-1 in the heart is coupled with myocyte proliferation in transgenic mice. Proc Natl Acad Sci USA. 1996;93:8630–8635. doi: 10.1073/pnas.93.16.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Reiss K, Cheng W, Ferber A, Kajstura J, Li P, Li BS, Olivetti G, Homcy CJ, Baserga R, Anversa P. Overexpression of insulin-like growth factor-1 in the heart is coupled with myocyte proliferation in transgenic mice. Proc Natl Acad Sci USA. 1996;93:8630–8635. doi: 10.1073/pnas.93.16.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Reiss K, Cheng W, Pierzchalski P, Kodali S, Li B, Wang S, Liu Y, Anversa P. Insulin-like growth factor-1 receptor and its ligand regulate the reentry of adult ventricular myocytes into the cell cycle. Exp Cell Res. 1997;235:198–209. doi: 10.1006/excr.1997.3669. [DOI] [PubMed] [Google Scholar]
- 550.Reiss K, Kajstura J, Zhang X, Li P, Szoke E, Olivetti G, Anversa P. Acute myocardial infarction leads to upregulation of the IGF-1 autocrine system, DNA replication, and nuclear mitotic division in the remaining viable cardiac myocytes. Exp Cell Res. 1994;213:463–472. doi: 10.1006/excr.1994.1224. [DOI] [PubMed] [Google Scholar]
- 551.Ren J, Hintz KK, Roughead ZK, Duan J, Colligan PB, Ren BH, Lee KJ, Zeng H. Impact of estrogen replacement on ventricular myocyte contractile function and protein kinase B/Akt activation. Am J Physiol Heart Circ Physiol. 2003;284:H1800–H1807. doi: 10.1152/ajpheart.00866.2002. [DOI] [PubMed] [Google Scholar]
- 552.Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, Nicolaou P, Pritchard TJ, Fan GC. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Resjo S, Goransson O, Harndahl L, Zolnierowicz S, Manganiello V, Degerman E. Protein phosphatase 2A is the main phosphatase involved in the regulation of protein kinase B in rat adipocytes. Cell Signal. 2002;14:231–238. doi: 10.1016/s0898-6568(01)00238-8. [DOI] [PubMed] [Google Scholar]
- 554.Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–4696. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- 555.Rolfe M, McLeod LE, Pratt PF, Proud CG. Activation of protein synthesis in cardiomyocytes by the hypertrophic agent phenylephrine requires the activation of ERK and involves phosphorylation of tuberous sclerosis complex 2 (TSC2) Biochem J. 2005;388:973–984. doi: 10.1042/BJ20041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Ronnebaum SM, Patterson C. The FoxO family in cardiac function and dysfunction. Annu Rev Physiol. 72:81–94. doi: 10.1146/annurev-physiol-021909-135931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Roscoe AK, Christensen JD, Lynch C., 3rd Isoflurane, but not halothane, induces protection of human myocardium via adenosine A1 receptors and adenosine triphosphate-sensitive potassium channels. Anesthesiology. 2000;92:1692–1701. doi: 10.1097/00000542-200006000-00029. [DOI] [PubMed] [Google Scholar]
- 558.Rota M, Boni A, Urbanek K, Padin-Iruegas ME, Kajstura TJ, Fiore G, Kubo H, Sonnenblick EH, Musso E, Houser SR, Leri A, Sussman MA, Anversa P. Nuclear targeting of Akt enhances ventricular function and myocyte contractility. Circ Res. 2005;97:1332–1341. doi: 10.1161/01.RES.0000196568.11624.ae. [DOI] [PubMed] [Google Scholar]
- 559.Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ, Sen CK. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Roy SS, Madesh M, Davies E, Antonsson B, Danial N, Hajnoczky G. Bad targets the permeability transition pore independent of Bax or Bak to switch between Ca2+-dependent cell survival and death. Mol Cell. 2009;33:377–388. doi: 10.1016/j.molcel.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Ruan H, Li J, Ren S, Gao J, Li G, Kim R, Wu H, Wang Y. Inducible and cardiac specific PTEN inactivation protects ischemia/reperfusion injury. J Mol Cell Cardiol. 2009;46:193–200. doi: 10.1016/j.yjmcc.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 562.Rubio M, Avitabile D, Fischer K, Emmanuel G, Gude N, Miyamoto S, Mishra S, Schaefer EM, Brown JH, Sussman MA. Cardioprotective stimuli mediate phosphoinositide 3-kinase and phosphoinositide dependent kinase 1 nuclear accumulation in cardiomyocytes. J Mol Cell Cardiol. 2009;47:96–103. doi: 10.1016/j.yjmcc.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Sabatini DM, Erdjumentbromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 564.Sanbe A, Gulick J, Hanks MC, Liang QR, Osinska H, Robbins J. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res. 2003;92:609–616. doi: 10.1161/01.RES.0000065442.64694.9F. [DOI] [PubMed] [Google Scholar]
- 565.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 566.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 567.Santiago AP, Chaves EA, Oliveira MF, Galina A. Reactive oxygen species generation is modulated by mitochondrial kinases: correlation with mitochondrial antioxidant per-oxidases in rat tissues. Biochimie. 2008;90:1566–1577. doi: 10.1016/j.biochi.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 568.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Current Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 569.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 570.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 571.Satoh M, Matter CM, Ogita H, Takeshita K, Wang CY, Dorn GW, 2nd, Liao JK. Inhibition of apoptosis-regulated signaling kinase-1 and prevention of congestive heart failure by estrogen. Circulation. 2007;115:3197–3204. doi: 10.1161/CIRCULATIONAHA.106.657981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Saxena A, Fish JE, White MD, Yu S, Smyth JW, Shaw RM, DiMaio JM, Srivastava D. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008;117:2224–2231. doi: 10.1161/CIRCULATIONAHA.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res. 2007;100:416–424. doi: 10.1161/01.RES.0000257913.42552.23. [DOI] [PubMed] [Google Scholar]
- 574.Sbarouni E, Iliodromitis EK, Zoga A, Vlachou G, Andreadou I, Kremastinos DT. The effect of the phytoestrogen genistein on myocardial protection, preconditioning and oxidative stress. Cardiovasc Drugs Ther. 2006;20:253–258. doi: 10.1007/s10557-006-8971-6. [DOI] [PubMed] [Google Scholar]
- 575.Schiekofer S, Belisle K, Galasso G, Schneider JG, Boehm BO, Burster T, Schmitz G, Walsh K. Angiogenic-regulatory network revealed by molecular profiling heart tissue following Akt1 induction in endothelial cells. Angiogenesis. 2008;11:289–299. doi: 10.1007/s10456-008-9112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Schiekofer S, Shiojima I, Sato K, Galasso G, Oshima Y, Walsh K. Microarray analysis of Akt1 activation in transgenic mouse hearts reveals transcript expression profiles associated with compensatory hypertrophy and failure. Physiol Genomics. 2006;27:156–170. doi: 10.1152/physiolgenomics.00234.2005. [DOI] [PubMed] [Google Scholar]
- 577.Schorlemmer A, Matter ML, Shohet RV. Cardioprotective signaling by endothelin. Trends Cardiovasc Med. 2008;18:233–239. doi: 10.1016/j.tcm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Schwartzbauer G, Robbins J. The tumor suppressor gene PTEN can regulate cardiac hypertrophy and survival. J Biol Chem. 2001;276:35786–35793. doi: 10.1074/jbc.M102479200. [DOI] [PubMed] [Google Scholar]
- 579.Shi RZ, Wang JC, Huang SH, Wang XJ, Li QP. Angiotensin II induces vascular endothelial growth factor synthesis in mesenchymal stem cells. Exp Cell Res. 2009;315:10–15. doi: 10.1016/j.yexcr.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 580.Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. Embo J. 1998;17:6649–6659. doi: 10.1093/emboj/17.22.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, Saito M, Fukuda K, Nishiyama T, Kitajima S, Saga Y, Fukayama M, Sata M, Kudo A. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med. 2008;205:295–303. doi: 10.1084/jem.20071297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Shintani S, Kusano K, Ii M, Iwakura A, Heyd L, Curry C, Wecker A, Gavin M, Ma H, Kearney M, Silver M, Thorne T, Murohara T, Losordo DW. Synergistic effect of combined intramyocardial CD34+ cells and VEGF2 gene therapy after MI. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S123–S128. doi: 10.1038/ncpcardio0430. [DOI] [PubMed] [Google Scholar]
- 583.Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 2000;19:2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 587.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–3365. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 589.Shiojima I, Yefremashvili M, Luo Z, Kureishi Y, Takahashi A, Tao J, Rosenzweig A, Kahn CR, Abel ED, Walsh K. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J Biol Chem. 2002;277:37670–37677. doi: 10.1074/jbc.M204572200. [DOI] [PubMed] [Google Scholar]
- 590.Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, Schaefer E, Walsh K, Rosenzweig A, Torella D, Nurzynska D, Kajstura J, Leri A, Anversa P, Sussman MA. Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ Res. 2004;94:884–891. doi: 10.1161/01.RES.0000124394.01180.BE. [DOI] [PubMed] [Google Scholar]
- 591.Shujia J, Haider HK, Idris NM, Lu G, Ashraf M. Stable therapeutic effects of mesenchymal stem cell-based multiple gene delivery for cardiac repair. Cardiovasc Res. 2008;77:525–533. doi: 10.1093/cvr/cvm077. [DOI] [PubMed] [Google Scholar]
- 592.Siddall HK, Warrell CE, Yellon DM, Mocanu MM. Ischemia-reperfusion injury and cardioprotection: investigating PTEN, the phosphatase that negatively regulates PI3K, using a congenital model of PTEN haploinsufficiency. Basic Res Cardiol. 2008;103:560–568. doi: 10.1007/s00395-008-0735-y. [DOI] [PubMed] [Google Scholar]
- 593.Siddiqi S, Gude N, Hosoda T, Muraski J, Rubio M, Emmanuel G, Fransioli J, Vitale S, Parolin C, D’Amario D, Schaefer E, Kajstura J, Leri A, Anversa P, Sussman MA. Myocardial induction of nucleostemin in response to postnatal growth and pathological challenge. Circ Res. 2008;103:89–97. doi: 10.1161/CIRCRESAHA.107.169334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Skavdahl M, Steenbergen C, Clark J, Myers P, Demianenko T, Mao L, Rockman HA, Korach KS, Murphy E. Estrogen receptor-beta mediates male-female differences in the development of pressure overload hypertrophy. Am J Physiol Heart Circ Physiol. 2005;288:H469–H476. doi: 10.1152/ajpheart.00723.2004. [DOI] [PubMed] [Google Scholar]
- 595.Skurk C, Izumiya Y, Maatz H, Razeghi P, Shiojima I, Sandri M, Sato K, Zeng L, Schiekofer S, Pimentel D, Lecker S, Taegtmeyer H, Goldberg AL, Walsh K. The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J Biol Chem. 2005;280:20814–20823. doi: 10.1074/jbc.M500528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Smith AR, Hagen TM. Vascular endothelial dysfunction in aging: loss of Akt-dependent endothelial nitric oxide synthase phosphorylation and partial restoration by (R)-alpha-lipoic acid. Biochem Soc Trans. 2003;31:1447–1449. doi: 10.1042/bst0311447. [DOI] [PubMed] [Google Scholar]
- 597.Smith CC, Mocanu MM, Bowen J, Wynne AM, Simpkin JC, Dixon RA, Cooper MB, Yellon DM. Temporal changes in myocardial salvage kinases during reperfusion following ischemia: studies involving the cardioprotective adipocytokine apelin. Cardiovasc Drugs Ther. 2007;21:409–414. doi: 10.1007/s10557-007-6054-y. [DOI] [PubMed] [Google Scholar]
- 598.Soesanto W, Lin HY, Hu E, Lefler S, Litwin SE, Sena S, Abel ED, Symons JD, Jalili T. Mammalian target of rapamycin is a critical regulator of cardiac hypertrophy in spontaneously hypertensive rats. Hypertension. 2009;54:1321-409–U1380. doi: 10.1161/HYPERTENSIONAHA.109.138818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Song JQ, Teng X, Cai Y, Tang CS, Qi YF. Activation of Akt/GSK-3beta signaling pathway is involved in intermedin(1–53) protection against myocardial apoptosis induced by ischemia/reperfusion. Apoptosis. 2009;14:1299–1307. doi: 10.1007/s10495-009-0398-7. [DOI] [PubMed] [Google Scholar]
- 600.Song XW, Li Q, Lin L, Li DF, Wang GK, Ren AJ, Qin YW, Yuan WJ, Jing Q. MicroRNAs are dynamically regulated in hypertrophic hearts, miR-199a is essential for the maintenance of cell size in cardiomyocytes. J Cell Physiol. 2010;225:437–443. doi: 10.1002/jcp.22217. [DOI] [PubMed] [Google Scholar]
- 601.Southworth R. Hexokinase-mitochondrial interaction in cardiac tissue: implications for cardiac glucose uptake, the 18FDG lumped constant and cardiac protection. J Bioenerg Biomembr. 2009;41:187–193. doi: 10.1007/s10863-009-9207-9. [DOI] [PubMed] [Google Scholar]
- 602.Southworth R, Davey KA, Warley A, Garlick PB. A reevaluation of the roles of hexokinase I and II in the heart. Am J Physiol Heart Circ Physiol. 2007;292:H378–H386. doi: 10.1152/ajpheart.00664.2006. [DOI] [PubMed] [Google Scholar]
- 603.Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA. 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Staal SP, Hartley JW. Thymic lymphoma induction by the AKT8 murine retrovirus. J Exp Med. 1988;167:1259–1264. doi: 10.1084/jem.167.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Stewart BE, Rice RH. Differentiation-associated expression of the proto-oncogene pim-1 in cultured human keratinocytes. J Invest Dermatol. 1995;105:699–703. doi: 10.1111/1523-1747.ep12324482. [DOI] [PubMed] [Google Scholar]
- 606.Stiles BL. PI-3-K and AKT: onto the mitochondria. Adv Drug Deliv Rev. 2009;61:1276–1282. doi: 10.1016/j.addr.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 607.Stirone C, Boroujerdi A, Duckles SP, Krause DN. Estrogen receptor activation of phosphoinositide-3 kinase, akt, nitric oxide signaling in cerebral blood vessels: rapid and long-term effects. Mol Pharmacol. 2005;67:105–113. doi: 10.1124/mol.104.004465. [DOI] [PubMed] [Google Scholar]
- 608.Stout BA, Bates ME, Liu LY, Farrington NN, Bertics PJ. IL-5 and granulocyte-macrophage colony-stimulating factor activate STAT3 and STAT5 and promote Pim-1 and cyclin D3 protein expression in human eosinophils. J Immunol. 2004;173:6409–6417. doi: 10.4049/jimmunol.173.10.6409. [DOI] [PubMed] [Google Scholar]
- 609.Sucharov C, Bristow MR, Port JD. miRNA expression in the failing human heart: functional correlates. J Mol Cell Cardiol. 2008;45:185–192. doi: 10.1016/j.yjmcc.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Sugden PH. Ras, Akt, and mechanotransduction in the cardiac myocyte. Circ Res. 2003;93:1179–1192. doi: 10.1161/01.RES.0000106132.04301.F5. [DOI] [PubMed] [Google Scholar]
- 611.Sugden PH, Clerk A. Akt like a woman: gender differences in susceptibility to cardiovascular disease. Circ Res. 2001;88:975–977. doi: 10.1161/hh1001.091864. [DOI] [PubMed] [Google Scholar]
- 612.Sun D, Nguyen N, DeGrado TR, Schwaiger M, Brosius FC., 3rd Ischemia induces translocation of the insulin-responsive glucose transporter GLUT4 to the plasma membrane of cardiac myocytes. Circulation. 1994;89:793–798. doi: 10.1161/01.cir.89.2.793. [DOI] [PubMed] [Google Scholar]
- 613.Sun H, Kerfant BG, Zhao D, Trivieri MG, Oudit GY, Penninger JM, Backx PH. Insulin-like growth factor-1 and PTEN deletion enhance cardiac L-type Ca2+ currents via increased PI3Kalpha/PKB signaling. Circ Res. 2006;98:1390–1397. doi: 10.1161/01.RES.0000223321.34482.8c. [DOI] [PubMed] [Google Scholar]
- 614.Sun L, Shukair S, Naik TJ, Moazed F, Ardehali H. Glucose phosphorylation and mitochondrial binding are required for the protective effects of hexokinases I and II. Mol Cell Biol. 2008;28:1007–1017. doi: 10.1128/MCB.00224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Sun YP, Wang WD, Zheng XC, Wang JJ, Ma SC, Xu YJ. Levels of serum brain natriuretic peptide and the correlation to heart function in children with Kawasaki disease. ] Zhongguo Dang Dai Er Ke Za Zhi. 12:169–171. [PubMed] [Google Scholar]
- 616.Sussman M. “AKT”. Aing lessons for stem cells: regulation of cardiac myocyte and progenitor cell proliferation. Trends Cardiovasc Med. 2007;17:235–240. doi: 10.1016/j.tcm.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Sussman MA. Mitochondrial integrity: preservation through Akt/Pim-1 kinase signaling in the cardiomyocyte. Exp Rev Cardiovasc Ther. 2009;7:929–938. doi: 10.1586/erc.09.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Sussman MA, Lim HW, Gude N, Taigen T, Olson EN, Robbins J, Colbert MC, Gualberto A, Wieczorek DF, Molkentin JD. Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science. 1998;281:1690–1693. doi: 10.1126/science.281.5383.1690. [DOI] [PubMed] [Google Scholar]
- 619.Takahashi A, Kureishi Y, Yang J, Luo Z, Guo K, Mukhopadhyay D, Ivashchenko Y, Branellec D, Walsh K. Myogenic Akt signaling regulates blood vessel recruitment during myofiber growth. Mol Cell Biol. 2002;22:4803–4814. doi: 10.1128/MCB.22.13.4803-4814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Takaishi H, Konishi H, Matsuzaki H, Ono Y, Shirai Y, Saito N, Kitamura T, Ogawa W, Kasuga M, Kikkawa U, Nishizuka Y. Regulation of nuclear translocation of forkhead transcription factor AFX by protein kinase B. Proc Natl Acad Sci USA. 1999;96:11836–11841. doi: 10.1073/pnas.96.21.11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Tang J, Wang J, Kong X, Yang J, Guo L, Zheng F, Zhang L, Huang Y, Wan Y. Vascular endothelial growth factor promotes cardiac stem cell migration via the PI3K/Akt pathway. Exp Cell Res. 2009;315:3521–3531. doi: 10.1016/j.yexcr.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 622.Taniyama Y, Ito M, Sato K, Kuester C, Veit K, Tremp G, Liao R, Colucci WS, Ivashchenko Y, Walsh K, Shiojima I. Akt3 overexpression in the heart results in progression from adaptive to maladaptive hypertrophy. J Mol Cell Cardiol. 2005;38:375–385. doi: 10.1016/j.yjmcc.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 623.Tarantino C, Paolella G, Cozzuto L, Minopoli G, Pastore L, Parisi S, Russo T. miRNA 34a, 100, and 137 modulate differentiation of mouse embryonic stem cells. FASEB J. 2010;24:3255–3263. doi: 10.1096/fj.09-152207. [DOI] [PubMed] [Google Scholar]
- 624.Tateishi K, Ashihara E, Honsho S, Takehara N, Nomura T, Takahashi T, Ueyama T, Yamagishi M, Yaku H, Matsubara H, Oh H. Human cardiac stem cells exhibit mesenchymal features and are maintained through Akt/GSK-3beta signaling. Biochem Biophys Res Commun. 2007;352:635–641. doi: 10.1016/j.bbrc.2006.11.096. [DOI] [PubMed] [Google Scholar]
- 625.Tatsuguchi M, Seok HY, Callis TE, Thomson JM, Chen JF, Newman M, Rojas M, Hammond SM, Wang DZ. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2007;42:1137–1141. doi: 10.1016/j.yjmcc.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, tuberin and hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 627.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, Gross C, Engelhardt S, Ertl G, Bauersachs J. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 628.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 629.Tian R, Abel ED. Responses of GLUT4-deficient hearts to ischemia underscore the importance of glycolysis. Circulation. 2001;103:2961–2966. doi: 10.1161/01.cir.103.24.2961. [DOI] [PubMed] [Google Scholar]
- 630.Timmers L, Henriques JP, de Kleijn DP, Devries JH, Kemperman H, Steendijk P, Verlaan CW, Kerver M, Piek JJ, Doevendans PA, Pasterkamp G, Hoefer IE. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–510. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 631.Timolati F, Ott D, Pentassuglia L, Giraud MN, Perriard JC, Suter TM, Zuppinger C. Neuregulin-1 beta attenuates doxorubicin-induced alterations of excitation-contraction coupling and reduces oxidative stress in adult rat cardiomyocytes. J Mol Cell Cardiol. 2006;41:845–854. doi: 10.1016/j.yjmcc.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 632.Tissier R, Waintraub X, Couvreur N, Gervais M, Bruneval P, Mandet C, Zini R, Enriquez B, Berdeaux A, Ghaleh B. Pharmacological postconditioning with the phytoestrogen genistein. J Mol Cell Cardiol. 2007;42:79–87. doi: 10.1016/j.yjmcc.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 633.Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P, Leri A. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 634.Torigoe Y, Takahashi N, Hara M, Yoshimatsu H, Saikawa T. Adrenomedullin improves cardiac expression of heat-shock protein 72 and tolerance against ischemia/reperfusion injury in insulin-resistant rats. Endocrinology. 2009;150:1450–1455. doi: 10.1210/en.2008-1052. [DOI] [PubMed] [Google Scholar]
- 635.Tramontano AF, Muniyappa R, Black AD, Blendea MC, Cohen I, Deng L, Sowers JR, Cutaia MV, El-Sherif N. Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-dependent pathway. Biochem Biophys Res Commun. 2003;308:990–994. doi: 10.1016/s0006-291x(03)01503-1. [DOI] [PubMed] [Google Scholar]
- 636.Tremblay ML, Giguere V. Phosphatases at the heart of FoxO metabolic control. Cell Metab. 2008;7:101–103. doi: 10.1016/j.cmet.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 637.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95:230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 638.Tsang S, Liu J, Wong TM. Testosterone and cardioprotection against myocardial ischemia. Cardiovasc Hematol Disord Drug Targets. 2007;7:119–125. doi: 10.2174/187152907780830888. [DOI] [PubMed] [Google Scholar]
- 639.Tseng A, Stabila J, McGonnigal B, Yano N, Yang MJ, Tseng YT, Davol PA, Lum LG, Padbury JF, Zhao TC. Effect of disruption of Akt-1 of lin-c-kit+ stem cells on myocardial performance in infarcted heart. Cardiovasc Res. 2010;87:704–712. doi: 10.1093/cvr/cvq110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Tsujita Y, Kato T, Sussman MA. Evaluation of left ventricular function in cardiomyopathic mice by tissue Doppler and color M-mode Doppler echocardiography. Echocardiography. 2005;22:245–253. doi: 10.1111/j.0742-2822.2005.04014.x. [DOI] [PubMed] [Google Scholar]
- 641.Tsujita Y, Muraski J, Shiraishi I, Kato T, Kajstura J, Anversa P, Sussman MA. Nuclear targeting of Akt antagonizes aspects of cardiomyocyte hypertrophy. Proc Natl Acad Sci USA. 2006;103:11946–11951. doi: 10.1073/pnas.0510138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Tzur G, Levy A, Meiri E, Barad O, Spector Y, Bentwich Z, Mizrahi L, Katzenellenbogen M, Ben-Shushan E, Reubinoff BE, Galun E. MicroRNA expression patterns and function in endodermal differentiation of human embryonic stem cells. PLoS One. 2008;3:e3726. doi: 10.1371/journal.pone.0003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Uchiyama T, Engelman RM, Maulik N, Das DK. Role of Akt signaling in mitochondrial survival pathway triggered by hypoxic preconditioning. Circulation. 2004;109:3042–3049. doi: 10.1161/01.CIR.0000130647.29030.90. [DOI] [PubMed] [Google Scholar]
- 644.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 645.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 646.Van den Brom CE, Huisman MC, Vlasblom R, Boontje NM, Duijst S, Lubberink M, Molthoff CF, Lammertsma AA, van der Velden J, Boer C, Ouwens DM, Diamant M. Altered myocardial substrate metabolism is associated with myocardial dysfunction in early diabetic cardiomyopathy in rats: studies using positron emission tomography. Cardiovasc Diabetol. 2009;8:39. doi: 10.1186/1475-2840-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Van der Velden JL, Schols AM, Willems J, Kelders MC, Langen RC. Glycogen synthase kinase 3 suppresses myogenic differentiation through negative regulation of NFATc3. J Biol Chem. 2008;283:358–366. doi: 10.1074/jbc.M707812200. [DOI] [PubMed] [Google Scholar]
- 648.Van Eickels M, Grohe C, Cleutjens JP, Janssen BJ, Wellens HJ, Doevendans PA. 17Beta-estradiol attenuates the development of pressure-overload hypertrophy. Circulation. 2001;104:1419–1423. doi: 10.1161/hc3601.095577. [DOI] [PubMed] [Google Scholar]
- 649.Van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 651.Vander Haar E, Lee S, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nature Cell Biol. 2007;9:316–U126. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 652.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nature Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 653.Varambally S, Cao Q, Mani RS, Shankar S, Wang X, Ateeq B, Laxman B, Cao X, Jing X, Ramnarayanan K, Brenner JC, Yu J, Kim JH, Han B, Tan P, Kumar-Sinha C, Lonigro RJ, Palanisamy N, Maher CA, Chinnaiyan AM. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322:1695–1699. doi: 10.1126/science.1165395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Von Lewinski D, Voss K, Hulsmann S, Kogler H, Pieske B. Insulin-like growth factor-1 exerts Ca2+-dependent positive inotropic effects in failing human myocardium. Circ Res. 2003;92:169–176. doi: 10.1161/01.res.0000051885.70159.12. [DOI] [PubMed] [Google Scholar]
- 655.Vuolteenaho O, Ruskoaho H. Gender matters: estrogen protects from cardiac hypertrophy. Trends Endocrinol Metab. 2003;14:52–54. doi: 10.1016/s1043-2760(03)00006-7. [DOI] [PubMed] [Google Scholar]
- 656.Walsh K. Akt signaling and growth of the heart. Circulation. 2006;113:2032–2034. doi: 10.1161/CIRCULATIONAHA.106.615138. [DOI] [PubMed] [Google Scholar]
- 657.Wang F, He Q, Sun Y, Dai X, Yang XP. Female adult mouse cardiomyocytes are protected against oxidative stress. Hypertension. 55:1172–1178. doi: 10.1161/HYPERTENSIONAHA.110.150839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Wang J, Xu R, Lin F, Zhang S, Zhang G, Hu S, Zheng Z. MicroRNA: novel regulators involved in the remodeling and reverse remodeling of the heart. Cardiology. 2009;113:81–88. doi: 10.1159/000172616. [DOI] [PubMed] [Google Scholar]
- 659.Wang P, Lloyd SG, Zeng H, Bonen A, Chatham JC. Impact of altered substrate utilization on cardiac function in isolated hearts from Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2005;288:H2102–H2110. doi: 10.1152/ajpheart.00935.2004. [DOI] [PubMed] [Google Scholar]
- 660.Wang XH, Qian RZ, Zhang W, Chen SF, Jin HM, Hu RM. MicroRNA-320 expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clin Exp Pharmacol Physiol. 2009;36:181–188. doi: 10.1111/j.1440-1681.2008.05057.x. [DOI] [PubMed] [Google Scholar]
- 661.Wang Z, Bhattacharya N, Weaver M, Petersen K, Meyer M, Gapter L, Magnuson NS. Pim-1: a serine/threonine kinase with a role in cell survival, proliferation, differentiation and tumorigenesis. J Vet Sci. 2001;2:167–179. [PubMed] [Google Scholar]
- 662.Watcharasit P, Thiantanawat A, Satayavivad J. GSK3 promotes arsenite-induced apoptosis via facilitation of mitochondria disruption. J Appl Toxicol. 2008;28:466–474. doi: 10.1002/jat.1296. [DOI] [PubMed] [Google Scholar]
- 663.Watson RT, Pessin JE. GLUT4 translocation: the last 200 nanometers. Cell Signal. 2007;19:2209–2217. doi: 10.1016/j.cellsig.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 664.Webster KA. Aktion in the nucleus. Circ Res. 2004;94:856–859. doi: 10.1161/01.RES.0000126699.49835.5D. [DOI] [PubMed] [Google Scholar]
- 665.Wei W, Jin J, Schlisio S, Harper JW, Kaelin WG., Jr The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell. 2005;8:25–33. doi: 10.1016/j.ccr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 666.Welsh GI, Miller CM, Loughlin AJ, Price NT, Proud CG. Regulation of eukaryotic initiation factor eIF2B: glycogen synthase kinase-3 phosphorylates a conserved serine which undergoes dephosphorylation in response to insulin. FEBS Lett. 1998;421:125–130. doi: 10.1016/s0014-5793(97)01548-2. [DOI] [PubMed] [Google Scholar]
- 667.Wright JJ, Kim J, Buchanan J, Boudina S, Sena S, Bakirtzi K, Ilkun O, Theobald HA, Cooksey RC, Kandror KV, Abel ED. Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding. Cardiovasc Res. 2009;82:351–360. doi: 10.1093/cvr/cvp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;127:5–19. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 669.Xie X, Cao F, Sheikh AY, Li Z, Connolly AJ, Pei X, Li RK, Robbins RC, Wu JC. Genetic modification of embryonic stem cells with VEGF enhances cell survival and improves cardiac function. Cloning Stem Cells. 2007;9:549–563. doi: 10.1089/clo.2007.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Xing W, Yan W, Fu F, Jin Y, Ji L, Liu W, Wang L, Lv A, Duan Y, Zhang J, Zhang H, Gao F. Insulin inhibits myocardial ischemia-induced apoptosis and alleviates chronic adverse changes in post-ischemic cardiac structure and function. Apoptosis. 2009;14:1050–1060. doi: 10.1007/s10495-009-0378-y. [DOI] [PubMed] [Google Scholar]
- 671.Yamaguchi H, Wang HG. The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change. Oncogene. 2001;20:7779–7786. doi: 10.1038/sj.onc.1204984. [DOI] [PubMed] [Google Scholar]
- 672.Yamashita K, Kajstura J, Discher DJ, Wasserlauf BJ, Bishopric NH, Anversa P, Webster KA. Reperfusion-activated Akt kinase prevents apoptosis in transgenic mouse hearts overexpressing insulin-like growth factor-1. Circ Res. 2001;88:609–614. doi: 10.1161/01.res.88.6.609. [DOI] [PubMed] [Google Scholar]
- 673.Yan B, Zemskova M, Holder S, Chin V, Kraft A, Koskinen PJ, Lilly M. The PIM-2 kinase phosphorylates BAD on serine 112 and reverses BAD-induced cell death. J Biol Chem. 2003;278:45358–45367. doi: 10.1074/jbc.M307933200. [DOI] [PubMed] [Google Scholar]
- 674.Yan HL, Xue G, Mei Q, Wang YZ, Ding FX, Liu MF, Lu MH, Tang Y, Yu HY, Sun SH. Repression of the miR-17–92 cluster by p53 has an important function in hypoxia-induced apoptosis. EMBO J. 2009;28:2719–2732. doi: 10.1038/emboj.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Yanagawa B, Nagaya N. Adrenomedullin: molecular mechanisms and its role in cardiac disease. Amino Acids. 2007;32:157–164. doi: 10.1007/s00726-005-0279-5. [DOI] [PubMed] [Google Scholar]
- 676.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 677.Yang H, Kong W, He L, Zhao JJ, O’Donnell JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, Cheng JQ. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- 678.Yang L, Sun M, Sun XM, Cheng GZ, Nicosia SV, Cheng JQ. Akt attenuation of the serine protease activity of HtrA2/Omi through phosphorylation of serine 212. J Biol Chem. 2007;282:10981–10987. doi: 10.1074/jbc.M700445200. [DOI] [PubMed] [Google Scholar]
- 679.Yang ZZ, Tschopp O, Di-Poi N, Bruder E, Baudry A, Dummler B, Wahli W, Hemmings BA. Dosage-dependent effects of Akt1/protein kinase Balpha (PKBalpha) and Akt3/PKBgamma on thymus, skin, cardiovascular and nervous system development in mice. Mol Cell Biol. 2005;25:10407–10418. doi: 10.1128/MCB.25.23.10407-10418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Yin C, Wang X, Kukreja RC. Endogenous microRNAs induced by heat-shock reduce myocardial infarction following ischemia-reperfusion in mice. FEBS Lett. 2008;582:4137–4142. doi: 10.1016/j.febslet.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Yin H, Chao L, Chao J. Kallikrein/kinin protects against myocardial apoptosis after ischemia/reperfusion via Akt-glycogen synthase kinase-3 and Akt-Bad. 14-3-3 signaling pathways. J Biol Chem. 2005;280:8022–8030. doi: 10.1074/jbc.M407179200. [DOI] [PubMed] [Google Scholar]
- 682.Yin H, Zhang J, Lin H, Qiao Y, Wang R, Lu H, Liang S. Effect of traditional Chinese medicine Shu-mai-tang on angiogenesis, arteriogenesis and cardiac function in rats with myocardial ischemia. Phytother Res. 2009;23:92–98. doi: 10.1002/ptr.2565. [DOI] [PubMed] [Google Scholar]
- 683.Yoeli-Lerner M, Chin YR, Hansen CK, Toker A. Akt/protein kinase b and glycogen synthase kinase-3beta signaling pathway regulates cell migration through the NFAT1 transcription factor. Mol Cancer Res. 2009;7:425–432. doi: 10.1158/1541-7786.MCR-08-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Yu J, Li M, Qu Z, Yan D, Li D, Ruan Q. SDF-1/CXCR4-mediated migration of transplanted bone marrow stromal cells toward areas of heart myocardial infarction through activation of PI3K/Akt. J Cardiovasc Pharmacol. 55:496–505. doi: 10.1097/FJC.0b013e3181d7a384. [DOI] [PubMed] [Google Scholar]
- 685.Yue TL, Nerurkar SS, Bao W, Jucker BM, Sarov-Blat L, Steplewski K, Ohlstein EH, Willette RN. In vivo activation of peroxisome proliferator-activated receptor-delta protects the heart from ischemia/reperfusion injury in Zucker fatty rats. J Pharmacol Exp Ther. 2008;325:466–474. doi: 10.1124/jpet.107.135327. [DOI] [PubMed] [Google Scholar]
- 686.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 687.Zhai P, Gao S, Holle E, Yu X, Yatani A, Wagner T, Sadoshima J. Glycogen synthase kinase-3alpha reduces cardiac growth and pressure overload-induced cardiac hypertrophy by inhibition of extracellular signal-regulated kinases. J Biol Chem. 2007;282:33181–33191. doi: 10.1074/jbc.M705133200. [DOI] [PubMed] [Google Scholar]
- 688.Zhang D, Mott JL, Chang SW, Stevens M, Mikolajczak P, Zassenhaus HP. Mitochondrial DNA mutations activate programmed cell survival in the mouse heart. Am J Physiol Heart Circ Physiol. 2005;288:H2476–H2483. doi: 10.1152/ajpheart.00670.2004. [DOI] [PubMed] [Google Scholar]
- 689.Zhang H, Li M, Han Y, Hong L, Gong T, Sun L, Zheng X. Down-regulation of miR-27a might reverse multidrug resistance of esophageal squamous cell carcinoma. Dig Dis Sci. 2010;55:2545–2551. doi: 10.1007/s10620-009-1051-6. [DOI] [PubMed] [Google Scholar]
- 690.Zhang HB, Stallock JP, Ng JC, Reinhard C, Neufeld TP. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev. 2000;14:2712–2724. doi: 10.1101/gad.835000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Zhang XJ, Xiong ZB, Tang AL, Ma H, Ma YD, Wu JG, Dong YG. Rosiglitazone-induced myocardial protection against ischaemia-reperfusion injury is mediated via a phosphatidylinositol 3-kinase/Akt-dependent pathway. Clin Exp Pharmacol Physiol. 2010;37:156–161. doi: 10.1111/j.1440-1681.2009.05232.x. [DOI] [PubMed] [Google Scholar]
- 693.Zhang Y, Gao XS, Saucedo LJ, Ru BG, Edgar BA, Pan DJ. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nature Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 694.Zhang Z, Deb A, Pachori A, He W, Guo J, Pratt R, Dzau VJ. Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. J Mol Cell Cardiol. 2009;46:370–377. doi: 10.1016/j.yjmcc.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Zheng Z, Liu Z. CD151 gene delivery activates PI3K/Akt pathway and promotes neovascularization after myocardial infarction in rats. Mol Med. 2006;12:214–220. doi: 10.2119/2006-00037.Zheng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Zheng ZZ, Liu ZX. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates CD151-induced endothelial cell proliferation and cell migration. Int J Biochem Cell Biol. 2007;39:340–348. doi: 10.1016/j.biocel.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 697.Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3:245–252. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 698.Zhu L, Lemoine S, Babatasi G, Lepage O, Massetti M, Gerard JL, Hanouz JL. Sevoflurane- and desflurane-induced human myocardial post-conditioning through Phosphatidylinositol-3-kinase/Akt signalling. Acta Anaesthesiol Scand. 2009;53:949–956. doi: 10.1111/j.1399-6576.2009.02009.x. [DOI] [PubMed] [Google Scholar]
- 699.Zhu M, Feng J, Lucchinetti E, Fischer G, Xu L, Pedrazzini T, Schaub MC, Zaugg M. Ischemic postconditioning protects remodeled myocardium via the PI3K-PKB/Akt reperfusion injury salvage kinase pathway. Cardiovasc Res. 2006;72:152–162. doi: 10.1016/j.cardiores.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 700.Zuurbier CJ, Keijzers PJ, Koeman A, Van Wezel HB, Hollmann MW. Anesthesia’s effects on plasma glucose and insulin and cardiac hexokinase at similar hemodynamics and without major surgical stress in fed rats. Anesth Analg. 2008;106:135–142. doi: 10.1213/01.ane.0000297299.91527.74. [DOI] [PubMed] [Google Scholar]