Abstract
Glycosylation is one of the most significant protein PTMs. The biological activities of proteins are dramatically changed by the glycans associated with them. Thus, structural analysis of the glycans of glycoproteins in complex biological or clinical samples is critical in correlation with the functions of glycans with diseases. Profiling of glycans by HPLC-MS is a commonly used technique in analyzing glycan structures and quantifying their relative abundance in different biological systems. Methods relied on MS require isolation of glycans from negligible salts and other contaminant ions since salts and ions may interfere with the glycans, resulting in poor glycan ionization. To accomplish those objectives, glycan isolation and clean-up methods including SPE, liquid-phase extraction, chromatography, and electrophoresis have been developed. Traditionally, glycans are isolated from proteins or peptides using a combination of hydrophobic and hydrophilic columns: proteins and peptides remain on hydrophobic absorbent while glycans, salts, and other hydrophilic reagents are collected as flowthrough. The glycans in the flowthrough are then purified through graphite-activated carbon column by hydrophilic interaction LC. Yet, the drawback in these affinity-based approaches is nonspecific binding. As a result, chemical methods by hydrazide or oxime have been developed for solid-phase isolation of glycans with high specificity and yield. Combined with high-resolution MS, specific glycan isolation techniques provide tremendous potentials as useful tools for glycomics analysis.
Keywords: Dynamic covalent chemistry, Hydrazide, Immobilization, MALDI-TOF MS, Solid-phase extraction
1 Introduction
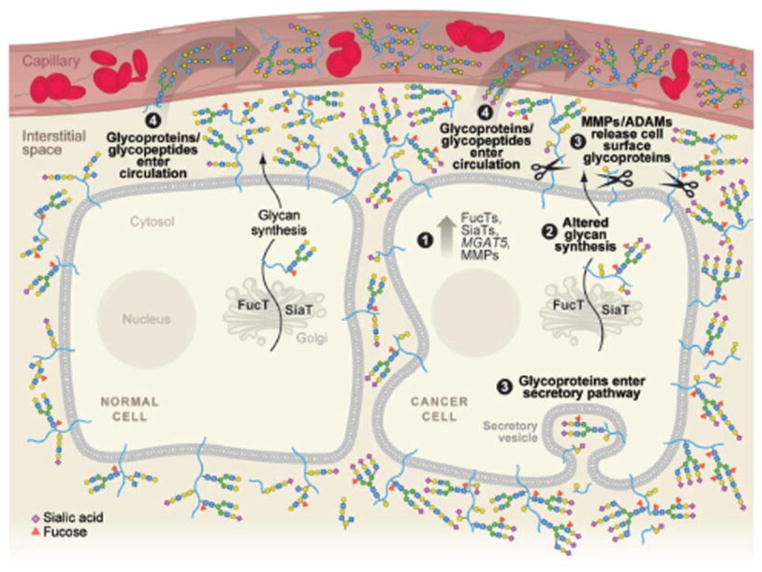
Glycomics has been a critical facet of postgenome science since glycans ubiquitously modify a great number of proteins (Fig. 1). Glycoproteins play key roles in many physiological events such as signal transduction, inflammation, tumorgenesis, cell development, and differentiation [1]. It has been well recognized that glycoprotein analysis is an important field of interest especially for diagnostics, prognostics, or therapeutic targets since the majority of human membrane and secreted proteins is glycosylated [2,3]. Numerous diseases have been attributed to upregulation or downregulation on one or several glycoproteins including coronary heart disease [4], congenital disorders of glycosylation [5], Tn syndrome [6], inflammatory bowel disease [7], prostate cancer [8], and ovarian cancer [9]. Additionally, glycoproteins modified with different glycans may exhibit different biological functions [10]. Alteration of glycans could dramatically change glycoprotein functions; thus, it is intrinsically related to the disease development [8]. For example, glycosylated proteins are released from malignant cells carrying disease-related carbohydrate epitopes into the interstitial space where glycoproteins further reach the circulation (Fig. 1). As a result, glycans are particularly interesting targets that offer a few major advantages over proteins, including biological design, amplification effect and stability [11].
Figure 1.
Schematic illustration of protein glycosylation in malignant cells. The glycosylated proteins are released from malignant cells carrying disease-related carbohydrate epitopes into the interstitial space, whether glycoproteins further reach the circulation. Reprint and permission from [11].
In general, glycan analysis starts with glycoprotein extraction from tissues, cells, or body fluids followed by glycan isolation, clean-up and/or separation, and detection. To study glycoproteins, glycans and their glycosylation sites, glycoproteins are enriched and their glycans are released by an enzyme or chemical reaction before digestion by trypsin [12]. Both formerly glycosylated peptides and released glycans are analyzed for identification of glycoproteins, glycosylation sites, and glycan structures by techniques such as ESI-MS or MALDI-MS. Currently, several strategies including chemical immobilization and affinity capture of glycans using lectin reagents have been developed to study glycoproteins [12–17]. These techniques have been successfully applied to quantitative glycoproteomics using MS in a variety of diseases [18–20]. However, isolation of glycans from complex mixtures with high specificity using chemical approaches remains to be developed. We reviewed current glycomics technologies for glycan purification, isolation, separation, and detection.
2 Glycan purification
Due to hydrophilic nature of glycans, analysis by MS becomes challenging when the glycans are analyzed in the mixture of hydrophobic proteins and peptides that are more efficiently ionized and suppress the ionization of glycans. Purification of glycans from complex mixtures of salts, detergents, proteins, and peptides prior to their detection by MS is critical because salts and detergents further interfere with the ionization of glycans by MS analysis.
Glycans for MS analysis are generally prepared in several steps. After glycoproteins are isolated, they are either directly analyzed, or digested to peptides. Then, the glycoproteins or glycopeptides are enzymatically treated to release N-linked glycans or chemically treated to obtain O-linked glycans. Third, the glycans are purified from the rest of the mixture consisting of peptides, other enzymes or chemical reagents used in the glycan releasing reaction. Methods to effectively separate or isolate glycans from the mixture are crucial for identification of glycans by MS.
One of the common techniques for glycan and peptide separation is to use hydrophilic interaction liquid chromatography (HILIC) [21–23]. Zwitterionic type of HILIC column demonstrates reasonable performance on separation of glycans, glycopeptides, and nonglycopeptides by recognition of isomeric glycans. The separation is mainly based on different combination of hydrophilicity and hydrophobicity of glycans and peptides [24]. However, without derivatization of glycans, the retention of glycans on HILIC absorbent is usually maintained low, so that some glycans can be lost in the flow-through and significantly decrease glycan recovery. Many researchers took advantage of graphitized carbon columns as an RP medium to purification and separation of unlabeled glycans for MS analysis [25–27]. We investigated the separation efficiency of glycan-peptide isolation using graphitized carbon column for mixture of glycans, peptides, and salts. Results clearly indicated that glycans elute in a lower concentration of ACN while peptides tend to elute at a higher concentration. However, in an intermediate ACN concentration, both glycans and peptides were eluted simultaneously [28]. These findings show that single dimensional (1D) chromatography is insufficient for glycan isolation.
Due to the fact that the unmodified glycans have little or no retention on hydrophobic absorbent, such as on C-8 and C-18, glycans in the protein/peptide mixture can be isolated using 2D chromatography or electrophoresis [29, 30]. For example, glycans were purified upon release from glycoprotein using 2D chromatography consisting of a C-8 column integrated with a graphitized carbon column [31]. Using the 2D procedure, peptides are retained on the C-8 column while glycans and other hydrophilic substances are collected in flow-through solution. Glycans are then enriched on a graphitized carbon column by hydrophobic interaction while salts and other hydrophilic molecules in solution are washed away in flow-through. Such sample clean-up method is straightforward and promising for development of a microfluidic platform in which hydrophobic resins are packed in the first channel and the graphitized resins are packed in the second channel. Cleanup can be achieved by passing sample through both channels in sequence, where the first channel is intersected with the second channel.
The potential concerns on these chromatographic methods are binding capacity between analytes and absorbent since glycans and nonglycan molecules can compete with each other by interaction with the absorbents. Thus, glycan purification performance is largely dependent on their differences on binding capacity. Although glycans are usually hydrophilic, their properties can vary with respect to their carbohydrate compositions and size of glycans. On the other hand, peptides have a wide range of hydrophobicity when different side chains exist [32, 33]. As it was observed, low molecular peptides were co-eluted with glycans, affecting the glycan identification by MS [34,35]. In addition, some glycans were even weakly bound on C8 or C18 absorbents, enabling the glycans to be lost, further affecting the overall yield of glycan recovery. Nevertheless, multidimensional microfluidic platform-based chromatographic technique could be a promising approach to replace traditional chromatographic separation in increasing the glycan recovery.
3 Dynamic covalent chemistry for glycan isolation
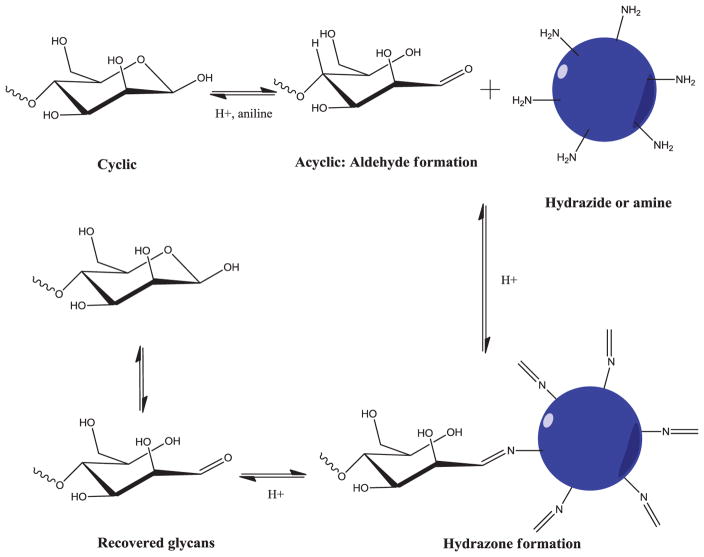
Dynamic covalent chemistry (DCC) is a chemical reaction that can be carried out in a reversible manner under certain conditions and equilibrium control [36]. Targeted molecules are conjugated and released via reversible bond formation, so that DCC has a great potential in fields of drug discovery and biology [37–40]. Glycans have single reducing end after they are released by either PNGase F or β-elimination. The reducing end co-exists in both a cyclic and an acyclic form, with the latter structure involving an aldehyde group that can conjugate with hydrazide groups [41]. At a neutral pH, the majority of the glycans are in the cyclic form that minimizes hydrazone formation. By addition of aniline, the acyclic structure dominates the glycan reducing end and tremendously increases glycan conjugation to the hydrazide group because aniline reacts to reducing ends and forms aniline Schiff base intermediates [38, 42]. Even without being reduced, hydra-zone bond is stable in weak base condition for its relatively low hydrolysis rate [43]. Further, decrease of buffer pH can significantly increase hydrazone hydrolysis and completely release glycans (Fig. 2). Thus, it allows capture of glycans to hydrazide resin in mild acid solution while peptides, detergent, and other reagents are removed using base buffer.
Figure 2.
Schematic diagram of glycan capture and release on solid-phase via dynamic covalent chemistry. Glycans are catalyzed by addition of aniline to formation of Schiff-based intermediate and further reacted to hydrazide or amine. The hydra-zone bond is stable at pH 7 or mild base condition, but can be easily hydrolyzed in acid condition e.g., 10% formic acid solution. Reprinted with permission from [28].
The DCC was applied for glycan isolation in complex sample mixture [28, 44]. In this method, glycans are released from denatured glycoproteins or trypsin digested glycopeptides. Then, the released glycans are conjugated to a solid support resin containing hydrazide groups via the glycan reducing ends. The glycan conjugated resin is further washed to remove nonspecific binding species followed by incubation of the glycan conjugated resin under acidic conditions (pH < 3.0) to release the glycans by hydrazone hydrolysis. Alternatively, glycans could be hydrolyzed from resins via competition reaction using high concentration of formaldehyde (e.g., 10× over glycan concentration) to minimize loss of sialic acid in acid solution [45] or stabilize sialic acid by amidation [46] and esterification [47]. The released glycans are then collected and analyzed by MS. The procedure is briefly illustrated in Fig. 3.
Figure 3.
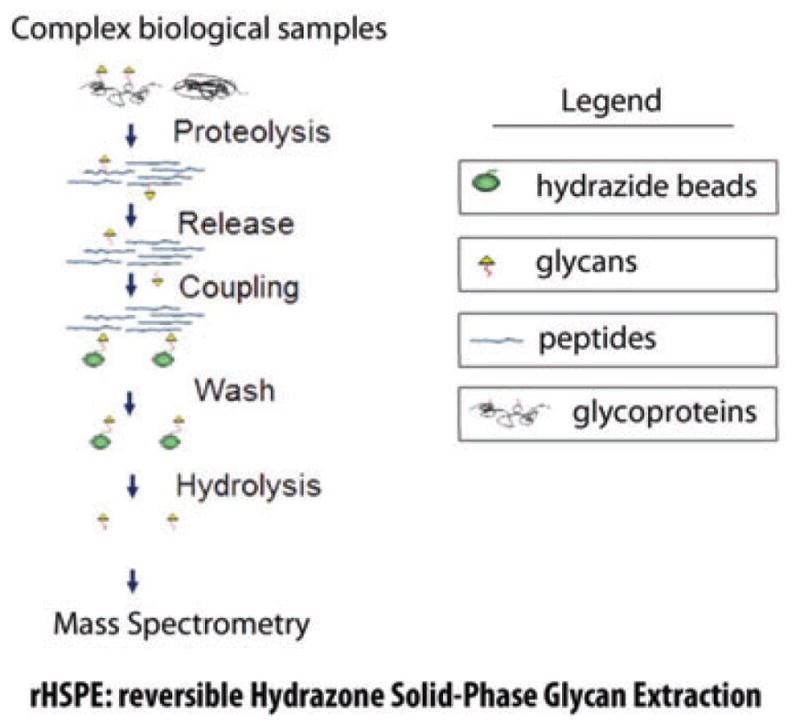
Schematic illustration of glycan capture procedure by reversible hydrazone solid-phase glycan extraction (rHSPE). Glycoproteins are first trypsin digested; glycans are then released by either PNGase F for N-linked glycans or β-elimination for O-linked glycans; glycans are conjugated to hydrazide beads; nonglycan molecules are removed by washing; conjugated glycans are finally hydrolyzed from solid phase either by acid hydrolysis or competition reaction by formaldehyde solution. Reprinted with permission from [28].
To develop the solid-phase glycan capture method, we used a set of standard glycans to determine conditions for glycan conjugation and hydrolysis. It has been concluded that conjugation has been performed in optimum conditions when pH ranges from 4 to 6 [48, 49]. In neutral pH, glycan conjugation is less than 10% by estimation of the intensity ratio between glycan and its conjugation products in MALDI-MS detection. Glycan–hydrazide conjugation is impressively increased up to approximately 90% at pH 5.0 in the presence of aniline. Without addition of aniline, less than 50% conjugation can be achieved at pH 5.0. The glycan–hydrazide conjugation is completely hydrolyzed in 10% formic acid. It should be noted that hydrazone is reversible, but its conjugation and dissociation rates are not identical under different pH and reagent conditions [43]. For example, conjugation of sulfonic acid hydrazide and benzaldehyde at pH 2.47 with the rate constant for hydrazone formation is approximately 0.76 × 10−4 s−1 and hydrolysis constant is 0.36 × 10−4 s−1. At pH 4.48, formation constant drops to 0.20 × 10−4 s−1 while hydrolysis constant drops further to 0.08 × 10−4 s−1. When the conjugation is conducted on solid phase, hydrolysis can thus be readily controlled by pH. The conjugated hydrazide glycan can be maintained under weak base buffer conditions for the removal of nonglycan material and then the hydrazone can be hydrolyzed under acid conditions for recovery of glycans. In addition, use of aniline can facilitate formation of aldehyde at the reducing end while minimizing ring structure formation with hydroxyl in C5 [38]. Comparatively, solid-phase conjugation has great advantages over solution based reaction, since buffer can be easily exchanged on solid support resins. The glycan loss due to hydrolysis can be minimized since quick pH change can be performed and much lower hydrolysis is expected at a higher pH condition. For instance, half-life of aliphatic aldehyde-based hydrazone conjugation is longer than 120 h at pH 7.4 while half-life of same compound drops to shorter than 2 h at pH 5.5 [50].
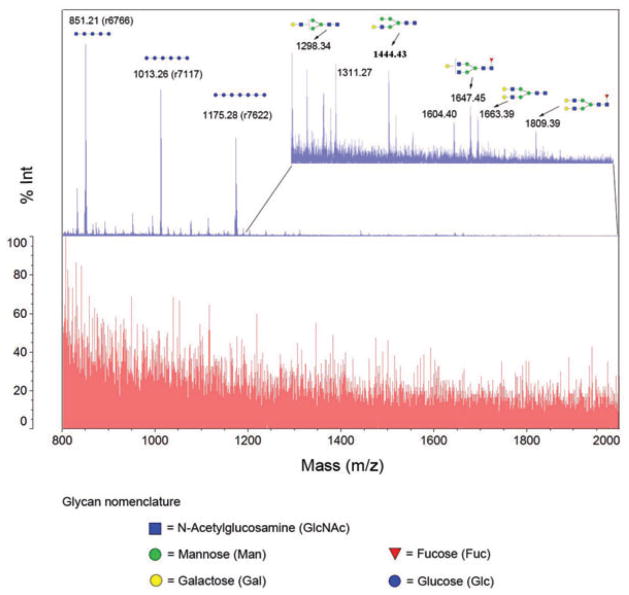
The reversible hydrazone solid-phase glycan extraction (rHSPE) was applied for isolation of glycans from human serum sample. We mixed 2 μL of serum with 3 μL of 10 mM standard glycans (DP4: maltotetraose; DP5: maltopentose; DP6: Maltohexanose; DP7: maltoheptaose). The mixture was denatured and digested by trypsin. After deglycosylation by PNGase F, the sample was used for glycan isolation by rHSPE. The results are shown in Fig. 4. Several conclusions were drawn by use of rHSPE. First, rHSPE is specific on glycan capture and all four spiked DPs were clearly observed after rHSPE capture. They were not detectable before glycan capture (Fig. 4). Second, rHSPE has the potential to extract glycans from complex biological samples. In this study, 17 N-linked glycans from serum were identified by MS after capture-release process using this method, as well as four standard glycans that were spiked to human serum. We expected that additional glycans were present in human serum samples, especially the complex glycans with terminal sialic acids. The derivatization of sialic acids by permethylation or esterification [46, 47] will facilitate the identification of sialylated glycans by MS. In addition, release of the glycans from hydrazide resins using less acidic conditions will preserve the sialylated glycans. Increased amount of serum samples can be also used to identify low abundant glycans.
Figure 4.
Glycan isolation from human serum with addition of standard glycans and peptides by hydrazide beads. The bottom spectrum was the sample mixture without glycan isolation. The top spectrum was isolated glycans from hydrazide beads after conjugation, washing, and hydrolysis. Reprinted with permission from [28].
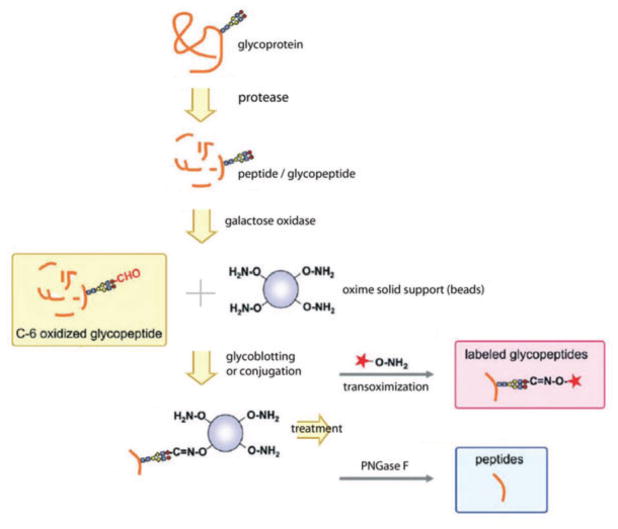
Glycopeptides can also be captured on a solid support through oxime group [51] as shown in Fig. 5. Glycopeptides are first treated with galactose oxidase to produce an aldehyde, which can conjugate to a solid support on its oxime. Other nonconjugated substances in complex biological samples can be readily washed away for isolation and purification of the glycopeptides. More importantly, captured glycopeptides can be either cleaved by PNGase F to collect deglycosylated peptides, or chemoselectively reacted with the tag by transoximization. Both peptides and glycans can be released by incubation in 4 N HCl [52]. If glycoproteins are captured via oxidization of glycans with terminal galactose, other types of glycan are not applicable. Sialylated glycans would have to be desialylated to be digested with the galactose oxidase enzyme. Moreover, periodate oxidation of sialylated glycans can be used to conjugate glycoproteins by aniline-catalyzed oxime ligation [53]. Similarly, we can study sialylated glycoproteins using solid-phase approach using hydrazide chemistry [54].
Figure 5.
Solid-phase glycoblotting and transoximization for glycomics and glycoproteomics analyses. Digested glycopeptides are oxidized on antennary galactose; C-6 oxidized glycopeptides are conjugated with oxime to immobilize glycopeptides; glycopeptides are labeled with tag by transoximization reaction, or glycopeptides are released from solid-phase by PNGase F treatment. Reprinted with permission from [51].
Recently, hydrazide beads were also used to react with glycans and further label immobilized glycans via a transoximization approach [55]. Glycan mixtures obtained from enzyme-digested glycoproteins are first captured by beads functionalized with oxime groups. A fluorescent tag is used to release labeled glycans and label the reducing end of glycan by transoximization chemistry. The transoximization produces multiple tag exchange functions that are useful for glycan purification by immobilization. This technology may provide a powerful platform for construction of glycan microarrays where naturally derived glycans are used as other contents.
4 Solid-phase glycan capture
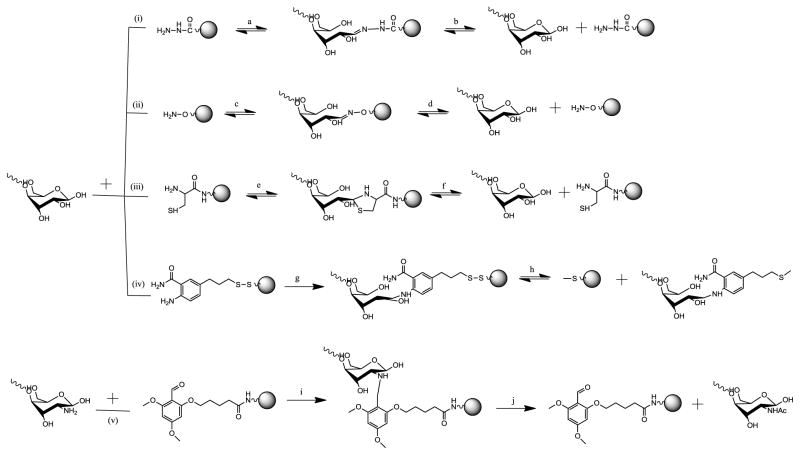
The key issue for enhanced glycan isolation by DCC is effective capturing on solid phase. Critical parameters for a solid-phase glycan capture are capture efficiency, covalent bond stability, and bond cleavability. The common glycans consist of several building blocks, including sialic acid, hexose (mannose, glucose, galactose etc), hexosamine (N-acetylglucosamine, N-acetylgalactosamine), deoxyhexose (fucose), pentose (xylose), and uronic acid (glucuronic acid and iduronic acid). All these monosaccharides have similar reducing ends. However, they have different functional groups such as carboxylic acid for sialic acid and uronic acid, amine for N-acetylhexosamine. The immobilization sites for glycans could be any of them. Method development for immobilization of glycan on solid phase via reducing end, amine or carboxylic acid is summarized in Fig. 6 and compared in Table 1.
Figure 6.
Methods for solid-phase capture and release of underivatized glycans. (i) Hydrazide chemistry for reducing end capture release. (A) pH 3–6 in methanol-acetic acid, microwave, 20 min. (B) 10% formic acid, or 200 mM formaldehyde at room temperature; (ii) Hydroxylamine chemistry for reducing end capture release. (C) DMF-water (1:1), pH 4.8, 40°C, 24 h. (D) 4N HCl hydrolysis; (iii) cysteinamide chemistry for reducing end capture release. (E) ACN-water (1:2), pH 4.0, room temperature, 48 h. (F) KCL in DI, 50°C, pH <6, (acetate and acetic acid); (iv) disulfide chemistry for reducing end. (G) DMSO-acetic acid (7:3), 50 mM sodium cyanoborohydride, 65°C, 3 h. (H) Disulfide bond can be cleaved by 16 mM DTT, 60°C, 1 h; (v) Chemoselective reductive alkylation chemistry for amine glycans. (I) Sodium cyanoborohydride in DMF-acetic acid (99:1). (J) Glycan is released in acetic anhydride-pyridine, TFA-water (19:1) [72]. Reprinted with permission from [57].
Table 1.
Summary of solid-phase methods for glycan isolation
| Linkage | Isolation method | Reference | Application | Pros and cons |
|---|---|---|---|---|
| Hydrazide | Formation of hydrazone between glycan and hydrazide; release of glycans by hydrolysis | Yang et al. [28] | N-glycan isolation from serum | Pros: conjugation and hydrolysis of glycans can be controlled by pH; fast capture and release Cons: reversible hydrazone bonds reduce glycan recovery |
| Miura et al. [103] | O-glycan enrichment from mucin | |||
| Lee et al. [104] | Carbohydrate microarray | |||
| Hydroxylamine | Formation covalent bonding between oxime and reducing end of glycans; release of glycan by hydrolysis | Thygesen et al. [30] | Oxime functionalized AuNP for protein–carbohydrate interactions | Pros: ease of formation under mild aqueous conditions; high nucleophilicity via the α-effect; good stability under a wide range of pH; versatility in the choice of supports Cons: difficulty due to reduced hydrolysis compared to hydrazone bond |
| Vila-Perello et al. [58] | Immobilization of oligosaccharide on specific protein-carbohydrate sensor surface | |||
| Dendane et al. [105] | Immobilization of oxime bond on to the inner wall of fused-silica capillary and on the surface of glass slides | |||
| Cysteinamide | Anchoring of cysteine-terminated resins through formation of a thiazolidine linkage | Guillaumie et al. [57,62] | Immobilized amine-bond of cysteinamide on solid-phase and capture glycan via thiazolidine linkage | Pros: quantitative coupling; thiazolidine stability over a wide range of conditions Cons: coupling in multiple steps |
| Disulfide | Formation of disulfide bond on solid-phase support; release of glycan through cleavage of disulfide bond | Guillaumie et al. [57,106,107] | (GalA)n functionalized sepharose supports; glycan reducing end conjugation with aminooxyacetamide | Pros: quantitative coupling of glycans; stable bond; glycan capture completion Cons: residue left on glycan reducing ends |
| Reductive alkylation | Formation of a chemoselective reductive alkylation of the 2-amino group; release of glycans by hydrolysis | Jensen et al. [72]; Rolborg et al. [108] | Unprotected D-glucosamine and glucosamine derivatives are attached to a trialkoxybenzyl linker on a solid support | Cons: quantitative coupling of glycans; good glycan capture yield; easy to release of glycans by hydrolysis at acid condition Pros: only suitable for glucosamine and its derivatives |
Common method of capturing glycans is through their reducing ends as described previously since released glycans by enzyme or chemical reaction have single reducing end. Also, cleavable bonds formed at reducing ends could be potentially used to recover intact glycans. In weak acidic conditions, reducing ends tend to be arranged in acyclic form [38], creating aldehyde group for conjugation. Aldehydes can also conjugate with functional groups such as hydrazide (i), hydroxylamine (ii), cysteinamide (iii), and amine (iv, v), formation of hydrazone, oxime, thiazolidine, and imine, respectively [56, 57] (Fig. 6).
Hydrazide chemistry has been successfully demonstrated for capture and release of glycans via their reducing ends [55]. Immobilization of glycan reducing ends via an oxime bond has been studied by several groups [52, 58–61]. The conditions for the formation of the oxime are dimethylformamide and 0.1 M aqueous sodium acetate [62]. Glycans are dissolved in the solvent and incubated with the resin for 24 h at 40°C. Without further reduction, the immobilized glycans can be cleaved off the resin by incubation under acid solution (Fig. 6 (ii) c and d) e.g., 4 N-hydrochloride [52]. The oxime reversible linkage has several advantages over other linkers. First, the conjugation can be readily implemented under rather mild aqueous conditions since the oxime is more nucleophilic than hydrazide due to its α-effect. Second, oxime linkage is quite stable in a wide range of pH, providing stability for glycan purification and other substance removal at minimum sample loss. This strategy has been employed for glycoblotting glycans from crude proteolytic digest mixture [55]. The captured glycans can also be used for enzyme-linked immunosorbent assay detection, in which lactose and N-acetylchitooligosaccharides are anchored on a solid support via oxime formation at reducing ends [63].
Cysteinamide can also anchor glycan reducing end via its aldehyde group [62]. Pectin fractions in a water-ACN mixture at pH 4.0 are immobilized onto cysteine-terminated solid resins by formation of a thiazolidine linkage (Fig. 6 (iii)). The thiazolidine linker is stable under neutral and weak base conditions. Addition of potassium and acetic acid could release intact glycans from solid resins, recovery of purified glycans from SPE. Studies have shown that the dissociation rate of thiazolidine linkage was highly dependent on salt concentration and pH of the solution [64, 65]. The thiazolidine-based solid phase has been applied for synthesis of hydrogels as corneal adhesives for healing wounds [65]. Chitosan has been similarly immobilized on nonporous glass beads through thiazolidine linker and verified using NMR and infrared resonance [66].
The major disadvantage of using hydrazone, oxime, or thiazolidine is the instability of these structures under certain conditions. As a result, a few glycans immobilized on solid supports can be inevitably lost during multiple cleaning processes. A permanent covalent conjugation or permanent covalent chemistry may provide an ideal scenario to improving glycan capture efficiency and recovery yield. An example is to develop a bifunctional reagent (Fig. 6 iv) that contains a terminal amine at one end and a terminal disulfide at the other end. The reducing ends can be conjugated to the amine that is converted to a stable bond by further reduction step, similar to glycan fluorescence labeling by aminobenzamide [49,67] or aminobenzoic acid [22,68]. The disulfide terminal will enable immobilization onto a solid support, such as gold via self-assembled monolayer [69–71]. Glycans are then enriched on the solid support and released by cleaving disulfide bond via reduction. However, this approach modifies glycan-reducing ends by adding fixed molecular weight, resulting in complicated data analysis.
Other type of glycans e.g., glucosamine subunit, can be captured by a similar approach in which the amine on the glucosamine conjugates to the aldehyde groups on the backbone amide linker (BAL) solid-phase support [72, 73]. BAL has been applied to side-chain anchoring for peptides and glycans [72, 74]. Glucosamine is first immobilized onto the BAL solid support by the formation of the Schiff base and its subsequent reduction (Fig. 6 v). At a later stage, the conjugated glucosamines can be released from the solid support by treatment with TFA containing acetic anhydride and pyridine. By this approach, the released glycan is modified with acetic group increased molecular weight by CH3CO− (43 Da).
Many glycans also contain sialic acid, especially in a variety of disease sample from serum [75,76], tissues [77–79], and body fluids [80–82]. As an alternative approach, several labs developed an approach to specifically analyze sialylated glycans instead of analyzing global glycans in specimen, such as mannose and focusylated glycans [83–85]. For instance, oxime reactive groups are anchored onto a solid support, and these groups can react with the oxidized sialic acid on sialylated glycans. Briefly, glycoproteins or glycopeptides are mixed with oxime beads at 37°C for 2 h after sialic acid is oxidized in 1 mM sodium periodate aqueous solution at 0°C for 15 min [54,83,85,86]. After removal of other substances, sialylated glycoproteins or glycopeptides can be remained on beads. Then, the sialylated glycopeptides are directly analyzed after hydrolysis of oxime linkage under acid condition; both formerly sialylated N-glycopeptides and their glycans can be studied by PNGase F treatment and/or oxime linkage hydrolysis.
5 Labeling glycans for structural and quantitative analyses
Isolated glycans at their native form or labeled at the reducing ends can be further analyzed by electrophoresis, chromatography, and MS. Many studies have demonstrated the separation of glycans using CE coupling with MS for qualitative and quantitation analysis [87–89]. More effectively, LC is applied for glycan profiling since it possesses high separation resolution and a variety of separation media are available. The normal phase LC–MS can identify details of underivatized glycans in a few hours with a sensitivity that is in the fentomole range [90–92]. On the other hand, RP-LC has been developed for profiling and characterizing underivatized or labeled glycans by fluorescence detection and structural determination by MS/MS [25,31,93,94]. In many instances, underivatized glycans cannot be reliably and robustly identified by either chromatography or MS. Therefore, labeling and/or modification of glycans are an effective means widely used to increase glycan stability and hydrophobicity. For instance, glycan permethylation significantly enhances stability, facilitates absolute linkage identification by MS/MS [95, 96], and stabilizes the sialic acid residues [97]. Glycans are often labeled with a variety of reagents [97] including 2-aminopyridine [98], 2-aminobenzoic acid and 2-aminobenzamide [49], 2-aminonaphthalene trisulfonic acid [99], and 1-aminopyrene-3,6,8-trisulfonic acid [100]. Glycans that have been labeled by different fluorescence reagents showed considerable differences in detection sensitivity [101]. Thus, their detection enhancement in MALDI-TOF MS was also reported to be attributed to the labeling [102]. Since detailed discussion of labeling and MS is beyond scope of this review, further information on those topics can be found in numerous literatures.
6 Conclusions
In this review, we have summarized several technologies developed for glycan purification. Chromatographic techniques are simple and straightforward means to partially or completely purify glycans by 1D or 2D approach. Integration of C-18 or C-8 with graphitized carbon has been traditionally used for glycan preparation. Purification efficiency and recovery of glycan by chromatography could further be improved by minimizing the nonspecific binding of glycans. As an alternative approach, SPE of glycans presents itself as a reliable technique for glycan preparation since glycans can be efficiently isolated from other substances by dynamic or permanent covalent chemistry. Serum glycan isolation has been demonstrated using reversible hydrazone SPE of glycans. Other reversible linkers such as oxime, thiazolidine, and disulfide can be similarly applied for glycan isolation. Permanent covalent chemistry can significantly increase glycan capture and recovery although structures of the released glycans are modified at their reducing ends.
Glycan isolation by a solid-phase support can be applied in microfluidics. For example, packing of hydrazide beads in microfluidic can immobilize glycopeptides, followed by glycopeptides or glycans separation in the second channel via RP-LC. On-bead glycan permethylation can be performed online in microfluidics, offering several advantages over in-solution solid-phase methods including automation, easy interfacing with MS, simplified sample cleanup, minimized sample loss, and versatile detections. We expect that these technologies incorporated with the state-of-art MS will make a tremendous progress in understanding the protein glycosylation on disease progressions and identification of glycan biomarkers for the goal of disease prognosis and diagnosis.
Acknowledgments
The work was support by National Institutes of Health, National Heart, Lung, and Blood Institute (P01HL107153 and N01-HV-00240), and National Cancer Institute (U01CA152813 and U24CA160036).
Abbreviations
- BAL
backbone amide linker
- DCC
dynamic covalent chemistry
- HILIC
hydrophilic interaction liquid chromatography
- rHSPE
reversible hydrazone solid-phase glycan extraction
Footnotes
The authors have declared no conflict of interest.
References
- 1.Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; NY: 2009. [PubMed] [Google Scholar]
- 2.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krueger KE, Srivastava S. Posttranslational protein modifications. Mol Cell Proteomics. 2006;5:1799–1810. doi: 10.1074/mcp.R600009-MCP200. [DOI] [PubMed] [Google Scholar]
- 4.Sönmez H, Öztürk ZG, Ulutin T, Domaniç N, et al. Carbohydrate-deficient transferrin and sialidase levels in coronary heart disease. Thromb Res. 2000;99:311–315. doi: 10.1016/s0049-3848(00)00262-0. [DOI] [PubMed] [Google Scholar]
- 5.Endo T, Manya H. O-Mannosylation in Mammalian Cells. Humana Press Inc; Totowa, NJ: 2006. [DOI] [PubMed] [Google Scholar]
- 6.Butler M, Quelhas D, Critchley AJ, Carchon H, et al. Detailed glycan analysis of serum glycoproteins of patients with congenital disorders of glycosylation indicates the specific defective glycan processing step and provides an insight into pathogenesis. Glycobiology. 2003;13:601–622. doi: 10.1093/glycob/cwg079. [DOI] [PubMed] [Google Scholar]
- 7.Ju T, Cummings RD. Protein glycosylation: chaperone mutation in Tn syndrome. Nature. 2005;437:1252. doi: 10.1038/4371252a. [DOI] [PubMed] [Google Scholar]
- 8.Kyselova Z, Mechref Y, Al Bataineh MM, Dobrolecki LE, et al. Alterations in the serum glycome due to metastatic prostate cancer. J Proteome Res. 2007;6:1822–1832. doi: 10.1021/pr060664t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leiserowitz G, Lebrilla C, Miyamoto S, An H, et al. Glycomics analysis of serum: a potential new biomarker for ovarian cancer? Int J Gynecol Cancer. 2008;18:470–475. doi: 10.1111/j.1525-1438.2007.01028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turnbull JE, Field RA. Emerging glycomics technologies. Nat Chem Biol. 2007;3:74–77. doi: 10.1038/nchembio0207-74. [DOI] [PubMed] [Google Scholar]
- 11.Drake PM, Cho W, Li B, Prakobphol A, et al. Sweetening the pot: adding glycosylation to the biomarker discovery equation. Clin Chem. 2010;56:223–236. doi: 10.1373/clinchem.2009.136333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Li X, Martin DB, Aebersold R. Identification and quantification of N-linked glycoproteins using hydrazide chemistry, stable isotope labeling and mass spectrometry. Nat Biotechnol. 2003;21:660–666. doi: 10.1038/nbt827. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Aebersold R. Isolation of glycoproteins and identification of their N-linked glycosylation sites. Methods Mol Biol. 2006;328:177–185. doi: 10.1385/1-59745-026-X:177. [DOI] [PubMed] [Google Scholar]
- 14.Kaji H, Saito H, Yamauchi Y, Shinkawa T, et al. Lectin affinity capture, isotope-coded tagging and mass spectrometry to identify N-linked glycoproteins. Nat Biotechnol. 2003;21:667–672. doi: 10.1038/nbt829. [DOI] [PubMed] [Google Scholar]
- 15.Ueda K, Takami S, Saichi N, Daigo Y, et al. Development of serum glycoproteomic profiling technique; simultaneous identification of glycosylation sites and site-specific quantification of glycan structure changes. Mol Cell Proteomics. 2010;9:1819–1828. doi: 10.1074/mcp.2010/000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sparbier K, Wenzel T, Kostrzewa M. Exploring the binding profiles of ConA, boronic acid and WGA by MALDI-TOF/TOF MS and magnetic particles. J Chromatogr B. 2006;840:29–36. doi: 10.1016/j.jchromb.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Wu Z, Zhang L, Lu H, et al. Highly specific enrichment of glycopeptides using boronic acid-functionalized mesoporous silica. Anal Chem. 2008;81:503–508. doi: 10.1021/ac801912t. [DOI] [PubMed] [Google Scholar]
- 18.Domon B, Aebersold R. Mass spectrometry and protein analysis. Science. 2006;312:212–217. doi: 10.1126/science.1124619. [DOI] [PubMed] [Google Scholar]
- 19.Liu T, Qian WJ, Gritsenko MA, Camp DG, II, et al. Human plasma N-glycoproteome analysis by immunoaffinity subtraction, hydrazide chemistry, and mass spectrometry. J Proteome Res. 2005;4:2070–2080. doi: 10.1021/pr0502065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stahl-Zeng J, Lange V, Ossola R, Eckhardt K, et al. High sensitivity detection of plasma proteins by multiple reaction monitoring of N-glycosites. Mol Cell Proteomics. 2007;6:1809–1817. doi: 10.1074/mcp.M700132-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.de Boer AR, Hokke CH, Deelder AM, Wuhrer M. Serum antibody screening by surface plasmon resonance using a natural glycan microarray. Glycoconjugate J. 2008;25:75–84. doi: 10.1007/s10719-007-9100-x. [DOI] [PubMed] [Google Scholar]
- 22.Ruhaak LR, Huhn C, Waterreus WJ, De Boer AR, et al. Hydrophilic interaction chromatography-based high-throughput sample preparation method for N-glycan analysis from total human plasma glycoproteins. Anal Chem. 2008;80:6119–6126. doi: 10.1021/ac800630x. [DOI] [PubMed] [Google Scholar]
- 23.Rudd PM, Guile GR, Harvey D, Opdenakker G, et al. Oligosaccharide sequencing technology. Nature. 1997;388:205–207. doi: 10.1038/40677. [DOI] [PubMed] [Google Scholar]
- 24.Takegawa Y, Deguchi K, Keira T, Ito H, et al. Separation of isomeric 2-aminopyridine derivatized N-glycans and N-glycopeptides of human serum immunoglobulin G by using a zwitterionic type of hydrophilic-interaction chromatography. J Chromatogr A. 2006;1113:177–181. doi: 10.1016/j.chroma.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Rudd PM, Dwek RA. Rapid, sensitive sequencing of oligosaccharides from glycoproteins. Curr Opin Biotech. 1997;8:488–497. doi: 10.1016/s0958-1669(97)80073-0. [DOI] [PubMed] [Google Scholar]
- 26.Packer NH, Lawson MA, Jardine DR, Redmond JW. A general approach to desalting oligosaccharides released from glycoproteins. Glycoconjugate J. 1998;15:737–747. doi: 10.1023/a:1006983125913. [DOI] [PubMed] [Google Scholar]
- 27.Larsen MR, Højrup P, Roepstorff P. Characterization of gel-separated glycoproteins using two-step proteolytic digestion combined with sequential microcolumns and mass spectrometry. Mol Cell Proteomics. 2005;4:107–119. doi: 10.1074/mcp.M400068-MCP200. [DOI] [PubMed] [Google Scholar]
- 28.Yang S, Zhang H. Glycan analysis by reversible reaction to hydrazide beads and mass spectrometry. Anal Chem. 2012;84:2232–2238. doi: 10.1021/ac202769k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bones J, Mittermayr S, O’Donoghue N, Guttman A, et al. Ultra performance liquid chromatographic profiling of serum N-glycans for fast and efficient identification of cancer associated alterations in glycosylation. Anal Chem. 2010;82:10208–10215. doi: 10.1021/ac102860w. [DOI] [PubMed] [Google Scholar]
- 30.Thomsson KA, Karlsson NG, Hansson GC. Liquid chromatography–electrospray mass spectrometry as a tool for the analysis of sulfated oligosaccharides from mucin glycoproteins. J Chromatogr A. 1999;854:131–139. doi: 10.1016/s0021-9673(99)00625-1. [DOI] [PubMed] [Google Scholar]
- 31.Aldredge D, An HJ, Tang N, Waddell K, et al. Annotation of a serum N-glycan library for rapid identification of structures. J Proteome Res. 2012;11:1958–1968. doi: 10.1021/pr2011439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monera OD, Sereda TJ, Zhou NE, Kay CM, et al. Relationship of sidechain hydrophobicity and α-helical propensity on the stability of the single-stranded amphipathic α-helix. J Pept Sci. 1995;1:319–329. doi: 10.1002/psc.310010507. [DOI] [PubMed] [Google Scholar]
- 33.de Planque MRR, Bonev BB, Demmers JAA, Greathouse DV, et al. Interfacial anchor properties of tryptophan residues in transmembrane peptides can dominate over hydrophobic matching effects in peptide-lipid interactions. Biochemistry. 2003;42:5341–5348. doi: 10.1021/bi027000r. [DOI] [PubMed] [Google Scholar]
- 34.Kotani Y, Matsuda S, Wen TC, Sakanaka M, et al. A hydrophilic peptide comprising 18 amino acid residues of the prosaposin sequence has neurotrophic activity in vitro and in vivo. J Neurochem. 1996;66:2197–2200. doi: 10.1046/j.1471-4159.1996.66052197.x. [DOI] [PubMed] [Google Scholar]
- 35.Louis JM, Dyda F, Nashed NT, Kimmel AR, et al. Hydrophilic peptides derived from the transframe region of Gag-Pol inhibit the HIV-1 protease. Biochemistry. 1998;37:2105–2110. doi: 10.1021/bi972059x. [DOI] [PubMed] [Google Scholar]
- 36.Rowan SJ, Cantrill SJ, Cousins GRL, Sanders JKM, et al. Dynamic covalent chemistry. Angew Chem Int Ed. 2002;41:898–952. doi: 10.1002/1521-3773(20020315)41:6<898::aid-anie898>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 37.Otto S, Furlan RLE, Sanders JKM. Dynamic combinatorial chemistry. Drug Discov Today. 2002;7:117–125. doi: 10.1016/s1359-6446(01)02086-4. [DOI] [PubMed] [Google Scholar]
- 38.Bhat VT, Caniard AM, Luksch T, Brenk R, et al. Nucleophilic catalysis of acylhydrazone equilibration for protein-directed dynamic covalent chemistry. Nat Chem. 2010;2:490–497. doi: 10.1038/nchem.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramström O, Lehn JM. Drug discovery by dynamic combinatorial libraries. Nat Rev Drug Discov. 2002;1:26–36. doi: 10.1038/nrd704. [DOI] [PubMed] [Google Scholar]
- 40.Erlanson DA, Wells JA, Braisted AC. Tethering: fragment-based drug discovery. Annu Rev Biophys Biomol Struct. 2004;33:199–223. doi: 10.1146/annurev.biophys.33.110502.140409. [DOI] [PubMed] [Google Scholar]
- 41.Zhi Z, Powell AK, Turnbull JE. Fabrication of carbohydrate microarrays on gold surfaces: direct attachment of nonderivatized oligosaccharides to hydrazide-modified self-assembled monolayers. Anal Chem. 2006;78:4786–4793. doi: 10.1021/ac060084f. [DOI] [PubMed] [Google Scholar]
- 42.Dirksen A, Dirksen S, Hackeng TM, Dawson PE. Nucleophilic catalysis of hydrazone formation and transimination: implications for dynamic covalent chemistry. J Am Chem Soc. 2006;128:15602–15603. doi: 10.1021/ja067189k. [DOI] [PubMed] [Google Scholar]
- 43.Levrand B, Fieber W, Lehn JM, Herrmann A. Controlled release of volatile aldehydes and ketones from dynamic mixtures generated by reversible hydrazone formation. Helv Chim ACTA. 2007;90:2281–2314. [Google Scholar]
- 44.Abe M, Shimaoka H, Fukushima M, Nishimura SI. A cross-linked polymer possessing a high density of hydrazide groups: high-throughput glycan purification and labeling for high-performance liquid chromatography analysis. Polym J. 2012;44:269–277. [Google Scholar]
- 45.Kalia J, Raines RT. Hydrolytic stability of hydrazones and oximes. Angew Chem Int Ed. 2008;47:7523–7526. doi: 10.1002/anie.200802651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sekiya S, Wada Y, Tanaka K. Derivatization for stabilizing sialic acids in MALDI-MS. Anal Chem. 2005;77:4962–4968. doi: 10.1021/ac050287o. [DOI] [PubMed] [Google Scholar]
- 47.Miura Y, Shinohara Y, Furukawa J, Nagahori N, et al. Rapid and simple solid-phase esterification of sialic acid residues for quantitative glycomics by mass spectrometry. Chem-Eur J. 2007;13:4797–4804. doi: 10.1002/chem.200601872. [DOI] [PubMed] [Google Scholar]
- 48.Rothenberg BE, Hayes BK, Toomre D, Manzi AE, et al. Biotinylated diaminopyridine: an approach to tagging oligosaccharides and exploring their biology. Proc Natl Acad Sci USA. 1993;90:11939–11943. doi: 10.1073/pnas.90.24.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bigge J, Patel T, Bruce J, Goulding P, et al. Nonselective and efficient fluorescent labeling of glycans using 2-amino benzamide and anthranilic acid. Anal Biochem. 1995;230:229–238. doi: 10.1006/abio.1995.1468. [DOI] [PubMed] [Google Scholar]
- 50.Kale AA, Torchilin VP. Design, synthesis, and characterization of pH-sensitive PEG-PE conjugates for stimuli-sensitive pharmaceutical nanocarriers: the effect of substitutes at the hydrazone linkage on the ph stability of PEG-PE conjugates. Bioconjugate Chem. 2007;18:363–370. doi: 10.1021/bc060228x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimaoka H, Kuramoto H, Furukawa J, Miura Y, et al. One-pot solid-phase glycoblotting and probing by transoximization for high-throughput glycomics and glycoproteomics. Chem-Eur J. 2007;13:1664–1673. doi: 10.1002/chem.200601613. [DOI] [PubMed] [Google Scholar]
- 52.Nishimura SI, Niikura K, Kurogochi M, Matsushita T, et al. High-throughput protein glycomics: combined use of chemoselective glycoblotting and MALDI-TOF/TOF mass spectrometry. Angew Chem Int Ed. 2005;44:91–96. doi: 10.1002/anie.200461685. [DOI] [PubMed] [Google Scholar]
- 53.Zeng Y, Ramya T, Dirksen A, Dawson PE, et al. High-efficiency labeling of sialylated glycoproteins on living cells. Nat Methods. 2009;6:207–209. doi: 10.1038/nmeth.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian Y, Esteva FJ, Song J, Zhang H. Altered expression of sialylated glycoproteins in breast cancer using hydrazide chemistry and mass spectrometry. Mol Cell Protoemics. 2012 doi: 10.1074/mcp.M111.011403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furukawa J, Shinohara Y, Kuramoto H, Miura Y, et al. Comprehensive approach to structural and functional glycomics based on chemoselective glycoblotting and sequential tag conversion. Anal Chem. 2008;80:1094–1101. doi: 10.1021/ac702124d. [DOI] [PubMed] [Google Scholar]
- 56.Zatsepin TS, Stetsenko DA, Arzumanov AA, Romanova EA, et al. Synthesis of peptide-oligonucleotide conjugates with single and multiple peptides attached to 2′-aldehydes through thiazolidine, oxime, and hydrazine linkages. Bioconjugate Chem. 2002;13:822–830. doi: 10.1021/bc020016+. [DOI] [PubMed] [Google Scholar]
- 57.Larsen K, Thygesen MB, Guillaumie F, Willats WGT, et al. Solid-phase chemical tools for glycobiology. Carbohyd Res. 2006;341:1209–1234. doi: 10.1016/j.carres.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 58.Vila-Perelló M, Gutiérrez Gallego R, Andreu D. A simple approach to well-defined sugar-coated surfaces for interaction studies. ChemBioChem. 2005;6:1831–1838. doi: 10.1002/cbic.200500125. [DOI] [PubMed] [Google Scholar]
- 59.Langenhan JM, Griffith BR, Thorson JS. Neoglycorandomization and chemoenzymatic glycorandomization: two complementary tools for natural product diversification. J Nat Prod. 2005;68:1696–1711. doi: 10.1021/np0502084. [DOI] [PubMed] [Google Scholar]
- 60.Thygesen MB, Sauer J, Jensen KJ. Chemoselective capture of glycans for analysis on gold nanoparticles: carbohydrate oxime tautomers provide functional recognition by proteins. Chem-Eur J. 2009;15:1649–1660. doi: 10.1002/chem.200801521. [DOI] [PubMed] [Google Scholar]
- 61.Cló E, Blixt O, Jensen KJ. Chemoselective reagents for covalent capture and display of glycans in microarrays. Eur J Org Chem. 2010;2010:540–554. [Google Scholar]
- 62.Guillaumie F, Thomas ORT, Jensen KJ. Immobilization of pectin fragments on solid supports: novel coupling by thiazolidine formation. Bioconjugate Chem. 2002;13:285–294. doi: 10.1021/bc0155364. [DOI] [PubMed] [Google Scholar]
- 63.Satoh A, Fukui E, Yoshino S, Shinoda M, et al. Comparison of methods of immobilization to enzyme-linked immunosorbent assay plates for the detection of sugar chains. Anal Biochem. 1999;275:231–235. doi: 10.1006/abio.1999.4329. [DOI] [PubMed] [Google Scholar]
- 64.Fife TH, Natarajan R, Shen C, Bembi R. Mechanism of thiazolidine hydrolysis. Ring opening and hydrolysis of 1, 3-thiazolidine derivatives of p-(dimethylamino) cinnamaldehyde. J Am Chem Soc. 1991;113:3071–3079. [Google Scholar]
- 65.Saiz C, Wipf P, Manta E, Mahler G. Reversible thiazolidine exchange: a new reaction suitable for dynamic combinatorial chemistry. Org Lett. 2009;11:3170–3173. doi: 10.1021/ol901104a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu X, Tokura S, Nishi N, Sakairi N. A novel method for immobilization of chitosan onto nonporous glass beads through a 1,3-thiazolidine linker. Polymer. 2003;44:1021–1026. [Google Scholar]
- 67.Royle L, Campbell MP, Radcliffe CM, White DM, et al. HPLC-based analysis of serum N-glycans on a 96-well plate platform with dedicated database software. Anal Biochem. 2008;376:1–12. doi: 10.1016/j.ab.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 68.Anumula K. Quantitative determination of monosaccharides in glycoproteins by high-performance liquid chromatography with highly sensitive fluorescence detection. Anal Biochem. 1994;220:275–283. doi: 10.1006/abio.1994.1338. [DOI] [PubMed] [Google Scholar]
- 69.Peelen D, Smith LM. Immobilization of amine-modified oligonucleotides on aldehyde-terminated alka-nethiol monolayers on gold. Langmuir. 2005;21:266–271. doi: 10.1021/la048166r. [DOI] [PubMed] [Google Scholar]
- 70.Quétard C, Bourgerie S, Normand-Sdiqui N, Mayer R, et al. Novel glycosynthons for glycoconjugate preparation: oligosaccharylpyroglutamylanilide derivatives. Bioconjugate Chem. 1998;9:268–276. doi: 10.1021/bc970122p. [DOI] [PubMed] [Google Scholar]
- 71.Castner DG, Hinds K, Grainger DW. X-ray photo-electron spectroscopy sulfur 2p study of organic thiol and disulfide binding interactions with gold surfaces. Lang-muir. 1996;12:5083–5086. [Google Scholar]
- 72.Jensen KJ, Alsina J, Songster MF, Vágner J, et al. Backbone amide linker (BAL) strategy for solid-phase synthesis of C-terminal-modified and cyclic peptides. J Am Chem Soc. 1998;120:5441–5452. [Google Scholar]
- 73.Boas U, Brask J, Jensen KJ. Backbone amide linker in solid-phase synthesis. Chem Rev. 2009;109:2092–2118. doi: 10.1021/cr068206r. [DOI] [PubMed] [Google Scholar]
- 74.Mende F, Seitz O. 9-fluorenylmethoxycarbonyl-based solid-phase synthesis of peptide α-thioesters. Angew Chem Int Ed. 2011;50:1232–1240. doi: 10.1002/anie.201005180. [DOI] [PubMed] [Google Scholar]
- 75.Zhao J, Simeone DM, Heidt D, Anderson MA, et al. Comparative serum glycoproteomics using lectin selected sialic acid glycoproteins with mass spectrometric analysis: application to pancreatic cancer serum. J Proteome Res. 2006;5:1792–1802. doi: 10.1021/pr060034r. [DOI] [PubMed] [Google Scholar]
- 76.Freeze HH, Aebi M. Altered glycan structures: the molecular basis of congenital disorders of glycosylation. Curr Opin Struc Biol. 2005;15:490–498. doi: 10.1016/j.sbi.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 77.Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446:1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 78.Martin LT, Marth JD, Varki A, Varki NM. Genetically altered mice with different sialyltransferase deficiencies show tissue-specific alterations in sialylation and sialic acid 9-O-acetylation. J Biol Chem. 2002;277:32930–32938. doi: 10.1074/jbc.M203362200. [DOI] [PubMed] [Google Scholar]
- 79.Dube DH, Bertozzi CR. Glycans in cancer and inflammation-potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 80.Suzuki Y, Ito T, Suzuki T, Holland RE, Jr, et al. Sialic acid species as a determinant of the host range of influenza A viruses. J Virol. 2000;74:11825–11831. doi: 10.1128/jvi.74.24.11825-11831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Varki A. Sialic acids in human health and disease. Trands Mol Med. 2008;14:351–360. doi: 10.1016/j.molmed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tajiri M, Yoshida S, Wada Y. Differential analysis of site-specific glycans on plasma and cellular fibronectins: application of a hydrophilic affinity method for glycopeptide enrichment. Glycobiology. 2005;15:1332–1340. doi: 10.1093/glycob/cwj019. [DOI] [PubMed] [Google Scholar]
- 83.Nilsson J, Rüetschi U, Halim A, Hesse C, et al. Enrichment of glycopeptides for glycan structure and attachment site identification. Nat Methods. 2009;6:809–811. doi: 10.1038/nmeth.1392. [DOI] [PubMed] [Google Scholar]
- 84.Kurogochi M, Matsushista T, Amano M, Furukawa J, et al. Sialic acid-focused quantitative mouse serum glycoproteomics by multiple reaction monitoring assay. Mol Cell Proteomics. 2010;9:2354–2368. doi: 10.1074/mcp.M110.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kurogochi M, Amano M, Fumoto M, Takimoto A, et al. Reverse glycoblotting allows rapid-enrichment glycoproteomics of biopharmaceuticals and disease-related biomarkers. Angew Chem Int Ed. 2007;46:8808–8813. doi: 10.1002/anie.200702919. [DOI] [PubMed] [Google Scholar]
- 86.Almaraz RT, Tian Y, Bhattarcharya R, Tan E, et al. Metabolic flux increases glycoprotein sialylation: implications for cell adhesion and cancer metastasis. Mol Cell Proteomics. 2012;11 doi: 10.1074/mcp.M112.017558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang Y, Mechref Y, Novotny MV. Microscale nonreductive release of O-linked glycans for subsequent analysis through MALDI mass spectrometry and capillary electrophoresis. Anal Chem. 2001;73:6063–6069. doi: 10.1021/ac015534c. [DOI] [PubMed] [Google Scholar]
- 88.Gennaro LA, Delaney J, Vouros P, Harvey DJ, et al. Capillary electrophoresis/electrospray ion trap mass spectrometry for the analysis of negatively charged derivatized and underivatized glycans. Rapid Commun Mass Spectrom. 2002;16:192–200. doi: 10.1002/rcm.564. [DOI] [PubMed] [Google Scholar]
- 89.Hernández-Borges J, Neusüß C, Cifuentes A, Pelzing M. On-line capillary electrophoresis-mass spectrometry for the analysis of biomolecules. Electrophoresis. 2004;25:2257–2281. doi: 10.1002/elps.200405954. [DOI] [PubMed] [Google Scholar]
- 90.Wuhrer M, Koeleman CAM, Deelder AM, Hokke CH. Normal-phase nanoscale liquid chromatography-mass spectrometry of underivatized oligosaccharides at low-femtomole sensitivity. Anal Chem. 2004;76:833–838. doi: 10.1021/ac034936c. [DOI] [PubMed] [Google Scholar]
- 91.Küster B, Wheeler SF, Hunter AP, Dwek RA, et al. Sequencing of N-linked oligosaccharides directly from protein gels: in-gel deglycosylation followed by matrix-assisted laser desorption/ionization mass spectrometry and normal-phase high-performance liquid chromatography. Anal Biochem. 1997;250:82–101. doi: 10.1006/abio.1997.2199. [DOI] [PubMed] [Google Scholar]
- 92.Naka R, Kamoda S, Ishizuka A, Kinoshita M, et al. Analysis of total N-glycans in cell membrane fractions of cancer cells using a combination of serotonin affinity chromatography and normal phase chromatography. J Proteome Res. 2006;5:88–97. doi: 10.1021/pr0502976. [DOI] [PubMed] [Google Scholar]
- 93.Chen X, Flynn GC. Analysis of N-glycans from recombinant immunoglobulin G by on-line reversed-phase high-performance liquid chromatography/mass spectrometry. Anal Biochem. 2007;370:147–161. doi: 10.1016/j.ab.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 94.Costello CE, Contado-Miller JM, Cipollo JF. A glycomics platform for the analysis of permethylated oligosaccharide alditols. J Am Chem Soc. 2007;18:1799–1812. doi: 10.1016/j.jasms.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kang P, Mechref Y, Novotny MV. High-throughput solid-phase permethylation of glycans prior to mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:721–734. doi: 10.1002/rcm.3395. [DOI] [PubMed] [Google Scholar]
- 96.Harvey DJ. Matrix-assisted laser desorption/ionization mass spectrometry of carbohydrates. Mass Spec Reviews. 1999;18:349–450. doi: 10.1002/(SICI)1098-2787(1999)18:6<349::AID-MAS1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 97.Ruhaak L, Zauner G, Huhn C, Bruggink C, et al. Glycan labeling strategies and their use in identification and quantification. Anal Bioanal Chem. 2010;397:3457–3481. doi: 10.1007/s00216-010-3532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ikenaka T, Matsushima Y. A highly sensitive method for analyses of sugar moieties of glycoproteins by fluorescence labeling. J Biochem. 1981;90:407–414. doi: 10.1093/oxfordjournals.jbchem.a133487. [DOI] [PubMed] [Google Scholar]
- 99.Chiesa C, O’Neill RA. Capillary zone electrophoresis of oligosaccharides derivatized with various aminonaphthalene sulfonic acids. Electrophoresis. 1994;15:1132–1140. doi: 10.1002/elps.11501501171. [DOI] [PubMed] [Google Scholar]
- 100.Evangelista RA, Liu MS, Chen FTA. Characterization of 9-aminopyrene-1,4,6-trisulfonate derivatized sugars by capillary electrophoresis with laser-induced fluorescence detection. Anal Chem. 1995;67:2239–2245. doi: 10.1006/abio.1995.1474. [DOI] [PubMed] [Google Scholar]
- 101.Rudd PM, Colominas C, Royle L, Murphy N, et al. A high-performance liquid chromatography based strategy for rapid, sensitive sequencing of N-linked oligosaccharide modifications to proteins in sodium dodecyl sulphate polyacrylamide electrophoresis gel bands. Proteomics. 2001;1:285–294. doi: 10.1002/1615-9861(200102)1:2<285::AID-PROT285>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 102.Morelle W, Slomianny MC, Diemer H, Schaeffer C, et al. Structural characterization of 2-aminobenzamide-derivatized oligosaccharides using a matrix-assisted laser desorption/ionization two-stage time-of-flight tandem mass spectrometer. Rapid Commun Mass Spectrom. 2005;19:2075–2084. doi: 10.1002/rcm.2033. [DOI] [PubMed] [Google Scholar]
- 103.Miura Y, Kato K, Takegawa Y, Kurogochi M, et al. Glycoblotting-assisted O-glycomics: ammonium carbamate allows for highly efficient O-glycan release from glycoproteins. Anal Chem. 2010;82:10021–10029. doi: 10.1021/ac101599p. [DOI] [PubMed] [Google Scholar]
- 104.Lee M, Shin I. Facile preparation of carbohydrate microarrays by site-specific, covalent immobilization of un-modified carbohydrates on hydrazide-coated glass slides. Org Lett. 2005;7:4269–4272. doi: 10.1021/ol051753z. [DOI] [PubMed] [Google Scholar]
- 105.Dendane N, Hoang A, Guillard L, Defrancq E, et al. Efficient surface patterning of oligonucleotides inside a glass capillary through oxime bond formation. Bioconjugate Chem. 2007;18:671–676. doi: 10.1021/bc060254v. [DOI] [PubMed] [Google Scholar]
- 106.Guillaumie F, Sterling JD, Jensen KJ, Thomas ORT, et al. Solid-supported enzymatic synthesis of pectic oligogalacturonides and their analysis by MALDI-TOF mass spectrometry. Carbohyd Res. 2003;338:1951–1960. doi: 10.1016/s0008-6215(03)00321-5. [DOI] [PubMed] [Google Scholar]
- 107.Guillaumie F, Justesen SFL, Mutenda KE, Roepstorff P, et al. Fractionation, solid-phase immobilization and chemical degradation of long pectin oligogalacturonides. Initial steps towards sequencing of oligosaccharides. Carbohyd Res. 2006;341:118–129. doi: 10.1016/j.carres.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 108.Tolborg JF, Petersen L, Jensen KJ, Mayer C, et al. Solid-phase oligosaccharide and glycopeptide synthesis using glycosynthases. J Org Chem. 2002;67:4143–4149. doi: 10.1021/jo0163445. [DOI] [PubMed] [Google Scholar]