Summary
Duchenne Muscular Dystrophy (DMD) is an X-linked lethal muscle wasting disease characterized by muscle fiber degeneration and necrosis. The progressive pathology of DMD can be explained by an insufficient regenerative response resulting in fibrosis and adipose tissue formation. BMPs are known to inhibit myogenic differentiation and in a previous study we found increased expression of the BMP family member BMP4 in DMD myoblasts. The aim of the current study was therefore to investigate whether inhibition of BMP signaling could be beneficial for myoblast differentiation and muscle regeneration processes in a DMD context. All tested BMP inhibitors, Noggin, dorsomorphin and LDN-193189, were able to accelerate and enhance myogenic differentiation. However, dorsomorphin repressed both BMP and TGFβ signaling and was found to be toxic to primary myoblast cell cultures. In contrast, Noggin was found to be a potent and selective BMP inhibitor and was therefore tested in vivo in a DMD mouse model. Local adenoviral-mediated overexpression of Noggin in muscle resulted in increased expression of the myogenic regulatory genes Myog and Myod1 and improved muscle histology. In conclusion, our results suggest that repression of BMP signaling may constitute an attractive adjunctive therapy for DMD patients.
Keywords: DMD, BMP signaling, myogenic differentiation, BMP antagonists
Introduction
Duchenne Muscular Dystrophy (DMD) is a lethal X-linked muscle wasting disorder caused by large deletions, insertions or point mutations in the DMD gene, which encodes the dystrophin protein. DMD muscle pathology has a progressive nature. The absence of functional dystrophin protein induces muscle fiber degeneration and necrosis. Subsequent local inflammation triggers fibrosis and fatty tissue infiltration, which results in replacement of muscle fibers with fibrotic and fatty tissue and loss of muscle function (reviewed in (Blake et al., 2002)). Although no treatment exists to date that can reverse the progressive muscle pathology of DMD, substantial effort and progress has been made in the development of novel therapies for DMD, which can roughly be divided into two groups; therapies aiming for restoration of dystrophin expression and therapies aiming for improvement of the overall condition of the muscle by repressing the molecular pathways that aggravate DMD pathology. The complexity of molecular pathways involved in the progressive pathophysiology of the disease makes it difficult to identify all the molecular players involved in DMD pathology, but several key players have been identified by expression profiling (Chen et al., 2000; Haslett et al., 2002; Pescatori et al., 2007; Sterrenburg et al., 2006). Importantly, signaling cascades that are known to be pro-inflammatory and pro-fibrotic, such as the nuclear Factor-κB (NF-κB) and Transforming Growth Factor-β1 (TGFβ1) pathways, were reported to be increased in DMD patients and in the mdx mouse model for DMD (Acharyya et al., 2007; Bernasconi et al., 1995; Chen et al., 2005; Cohn et al., 2007). In addition, TGFβ1 and the related family member myostatin have been described to act as direct negative regulators of muscle mass and muscle regeneration by repressing proliferation and differentiation of muscle stem cells (also known as satellite cells) and may therefore play a role in the further impairment of muscle regeneration in DMD. Several studies showed that blocking the myostatin- and TGFβ-induced signaling cascades improved the dystrophic phenotype and muscle function of mdx mice by counteracting fibrosis and/or stimulating muscle regeneration (Bogdanovich et al., 2002; Cohn et al., 2007; Grounds and Torrisi, 2004; Haidet et al., 2008). The results of these studies provide insight in the molecular mechanism of DMD pathology and hold promise that specific pathways can be targeted in the future to improve DMD. However, the complete spectrum of molecular players involved in pathological processes such as fibrosis, inflammation and regeneration and their spatiotemporal interplay during the progression of the disease remains to be elucidated.
BMPs are secreted proteins that form a large subfamily within the TGFβ superfamily and which fulfill essential roles during embryonic development and in adult life. The specificity of downstream signaling cascades depends on the specific interaction of BMP proteins with different type I and type II receptor kinases, which subsequently activate intracellular Smad1/5/8 proteins as well as other protein kinases such as p38 MAP kinase (Miyazono et al., 2010). By genome wide expression profiling, we previously identified BMPs as potential novel players in DMD pathology. In muscles of mdx mice the expression of several BMP signaling components was found to be increased (Turk et al., 2005). In addition, BMP4 levels were found to be consistently elevated in myoblast cultures derived from DMD patients compared to myoblasts isolated from healthy individuals, and finally the BMP antagonist gremlin 2 was found to be downregulated in DMD muscle (Pescatori et al., 2007; Sterrenburg et al., 2006). These findings suggest that increased BMP signaling may be directly involved in DMD pathology.
Although the exact role and potential impact of deregulated BMP signaling on DMD pathology is not known, several studies show that BMPs have a profound repressive effect on myogenic differentiation. In myoblast cell culture both BMP2 and BMP4 repress myogenic differentiation and stimulate differentiation towards the osteoblast lineage (Dahlqvist et al., 2003; Katagiri et al., 1997; Yamamoto et al., 1997). During embryonic muscle differentiation inhibition of local BMP signaling by secretion of BMP antagonists such as Noggin and Gremlin is crucial for proper differentiation of muscle progenitors cells (Linker et al., 2003; Reshef et al., 1998; Tzahor et al., 2003). These natural BMP inhibitors can bind to BMP dimers, thereby preventing them from binding to and activating of their receptors. Noggin loss-of-function results in perinatal death in mice and a range of developmental defects, including severe reduction of skeletal muscle size, presumably as a consequent of defective terminal muscle differentiation (Tylzanowski et al., 2006). In contrast, to date no function of BMP signaling has been reported during muscle regeneration in adult muscle. Interestingly, however, one study reported high levels of BMP4 in a subset of skeletal muscle stem cells (the so-called side-population) in fetal muscle, which presumably is important for proliferation of satellite cells (Frank et al., 2006). Two recent studies report that BMP signaling is active in quiescent satellite cells in adult muscle and that BMP signaling is activated upon muscle damage (Clever et al., 2010; Fukada et al., 2007). In addition, loss of function of Id1 and Id3, two downstream targets of BMP signaling cascades, results in a proliferation defect of the satellite cells after muscle damage in mice (Clever et al., 2010). This suggests that BMP signaling plays a yet unidentified role in adult muscle regeneration and that elevated BMP signaling in dystrophic DMD muscle may interfere with the muscle regeneration process in DMD. We hypothesize that the use of BMP antagonists may be useful to inhibit BMP signaling in DMD muscle and may ameliorate the progressive DMD pathology. The objective of this study was therefore to determine the effect of different BMP inhibitors on myogenic differentiation in vitro and the effect of selective BMP repression in a DMD mouse model.
Results
Endogenous BMP signaling is repressed during myoblast differentiation
To determine BMP activity during myoblast differentiation, we induced myogenic differentiation of C2C12 mouse myoblasts and subsequently determined pSmad1/5/8 levels at different time points. After the switch to low serum containing differentiation medium, C2C12 myoblasts fused and differentiated into multinucleated myotubes (Fig. 1A). As expected, the level of the myogenic transcription factor Myog increased from day 1 onwards (Fig. 1B). Interestingly, Bmp4 expression and pSmad1/5/8 levels peaked two days after initiation of myogenic differentiation and at this stage myogenin was already present (Fig. 1B, C). The subsequent decrease of pSmad1/5/8 levels after two days correlated with a decrease in endogenous Bmp4 expression (Fig. 1B, C). This was consistent with CAGE/SAGE expression analysis of undifferentiated versus differentiated C2C12 myoblasts, were Bmp4 was found to be the only BMP ligand whose expression decreased upon myogenic differentiation (M. Hestand, unpublished). Together these results show that BMP signaling is actively repressed upon myogenic differentiation.
Fig. 1. Endogenous BMP signaling during myogenic differentiation.
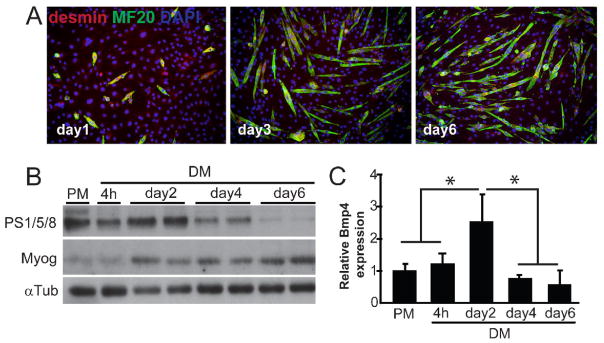
(A) Myogenic differentiation of C2C12 cells. Immunofluorescent staining of myogenic marker desmin in red and differentiation marker Myosin Heavy Chain (MyHC) in green. After 3 days of differentiation in low serum medium, formation of myotubes was observed. (B) Western blot analysis of pSmad1/5/8 and Myog levels on C2C12 protein lysates at different time points of differentiation. αTubulin is shown as a loading control in all samples. (C) qRT-PCR analysis of Bmp4 expression on C2C12 cDNA at different time points of differentiation. Fold expression relative to PM is shown and error bars indicate s.d. of triplicate experiments. *P<0.05. PM, proliferation medium; DM, differentiation medium.
Inhibition of BMP signaling by three BMP inhibitors
Several BMP antagonists have been used to inhibit BMP signaling in vitro and in vivo. To determine which BMP inhibitors can specifically inhibit BMP signaling in C2C12 myoblasts, we subsequently performed a luciferase reporter assay using three established BMP inhibitors: Noggin, dorsomorphin and LDN-193189 (LDN). Noggin can bind to specific BMPs, thereby inhibiting their binding to BMP receptors and blocking activation of downstream signaling (Li et al., 2008). The compounds dorsomorphin and the structurally related LDN are small molecule kinase inhibitors that bind and inhibit the kinase domain of BMP type I receptors (Cuny et al., 2008; Yu et al., 2008a,b). Luciferase reporter assays employing a BMP-responsive reporter construct (BRE-luc) and a TGFβ/Smad3-responsive reporter construct (CAGA-luc) were used to measure the potency and specificity of the inhibitors. Dorsomorphin repressed both basal and BMP4-induced BRE-luc activity (2-fold and 5-fold respectively), but only at a relatively high concentration of 4μM (Fig. 2A,B). In addition, dorsomorphin inhibited CAGA-luc activity at a comparable dose (Fig. 2C), showing that this compound cannot solely be regarded as a BMP antagonist. LDN repressed both basal and BMP4-induced BRE-activity at a lower dose of 120nM (Fig. 2A, B), whereas CAGA-luc activity was only inhibited at a ten-fold higher dose (Fig. 2C), suggesting that specific BMP inhibition with this compound can be achieved at lower concentrations. Noggin also repressed both basal and BMP4-induced BRE-activity (5-fold and 40-fold respectively; Fig. 2A, B), but had no effect on CAGA-luc activity at low or high dose (Fig. 2C). Together these results suggest that Noggin is the most selective BMP inhibitor of the three compounds tested and can be used to specifically inhibit BMP signaling.
Fig. 2. Effect of BMP inhibitors on BMP and TGFβ signaling.
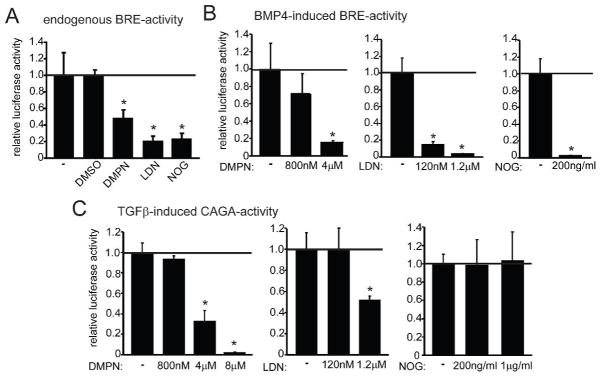
(A) Luciferase reporter assay with the BRE-luc reporter showing the activity of endogenous BMP signaling in transfected C2C12 cells treated with or without BMP antagonists Noggin (NOG; 200ng/ml), LDN-193189 (LDN; 120nM) and dorsomorphin (DMPN; 4μM).(B) Luciferase reporter assay with BRE-luc showing the activity of BMP4-induced signaling in transfected C2C12 cells treated with or without different concentrations of BMP antagonists (C) Luciferase reporter assay with the CAGA-luc reporter showing the activity of TGFβ-induced signaling in transfected C2C12 cells treated with or without different concentrations of BMP antagonists. Error bars indicate s.d. of triplicate samples and the luciferase activity relative to the control is shown in each graph; *P<0.01.
Inhibition of BMP signaling potentiates myoblast differentiation
We next determined the effect of Noggin, dorsomorphin and LDN on myogenic differentiation of C2C12 myoblasts. C2C12 cells were switched to differentiation medium with or without BMP antagonists and after three days of differentiation the cells were stained for desmin and myosin heavy chain (MyHC) to determine the differentiation index (percentage of MyHC+ cells) and fusion index (average number of nuclei/MyHC+ cell). All three BMP inhibitors were able to increase the differentiation and fusion index in C2C12 myoblasts compared to the control, showing their ability to enhance myogenic differentiation (Fig. 3A,B). In addition, BMP4-induced inhibition of myogenic differentiation was antagonized by Noggin and LDN (Fig. S1A). Next, we determined the effect of the BMP inhibitors on the levels of pSmad1/5/8 and Myog at different time points of differentiation. All three inhibitors decreased pSmad1/5/8 levels after 24h and 48h treatment (Fig. 3C). After 24 hours of differentiation, the protein level of Myog was significantly increased after treatment with the BMP inhibitors compared to the control, whereas the difference was less apparent after two days, suggesting that treatment resulted in precocious myogenic differentiation (Fig. 3C). The apparent acceleration of myogenic differentiation was confirmed in Noggin-treated cells by immunofluorescent staining of Myog, where the difference in percentage Myog+ nuclei compared to control cells was more apparent at the first three days of differentiation compared to day 4 and 6 (Fig. 3D and Fig. S1B). Next, we determined the effect of the BMP inhibitors on the myogenic differentiation of human primary myoblast cultures obtained from muscle biopsies of healthy volunteers (Aartsma-Rus et al., 2002). Consistent with the results obtained in C2C12 cells, treatment with LDN and Noggin enhanced differentiation in primary myoblast cultures (Fig. 4). Surprisingly, dorsomorphin was found to be toxic in primary myoblast cell cultures and the cells demonstrated a reduced capacity to differentiate and fuse (Fig. 4). Together these results suggest that Noggin and LDN, but not dorsomorphin, are suitable compounds to enhance myogenic differentiation.
Fig. 3. Effect of BMP inhibitors on myogenic differentiation.
(A) Immunofluorescent staining of desmin and MyHC showing the effect of Noggin (NOG; 200ng/ml), LDN-193189 (LDN; 120nM) and dorsomorphin (DMPN; 4 μM) on myogenic differentiation of C2C12 cells. Experiment was performed in duplo and representative pictures are shown. (B) Quantification of immunofluorescent staining in (A) showing the differentiation index and fusion index of C2C12 cells treated with or without the different BMP antagonists. Error bars indicate the s.d. of 10 areas measured in duplo. *P<0.01 (C) Western blot analysis of C2C12 protein lysates showing the pSmad1/5/8 levels and Myogenin levels after treatment with the different compounds after 1 day and 2 days of differentiation. αTubulin is shown as a loading control in all samples. (D) Immunofluorescent staining of Myog (in green) and desmin (myogenic cells; in red) of C2C12 cells differentiated for 3 days and 6 days with or without Noggin (200ng/ml). Top row shows merged pictures with DAPI (in blue) staining the total nuclei in the field. Representative pictures are shown from experiments performed in duplo. The graph on the right shows the quantification of the percentage of Myog+ nuclei compared to the total amount of nuclei. Error bars indicate the s.d. of 10 areas measured in duplo. *P<0.01
Fig. 4. Effect of BMP inhibitors on myogenic differentiation of primary human myoblasts.
Immunofluorescent staining of human primary myoblasts differentiated for 7 days with our without Noggin (NOG; 200ng/ml), LDN-193189 (LDN; 120nM) and dorsomorphin (DMPN; 4 μM). Staining shows desmin (myogenic cells; in red) and MyHC (differentiated myotubes; in green). Experiment was performed in duplo and representative pictures are shown. Error bars indicate s.d. of 10 measured areas in duplo; *P<0.01
overexpression of BMP antagonist Noggin ameliorates muscle dystrophy in mdx utrn+/− mice
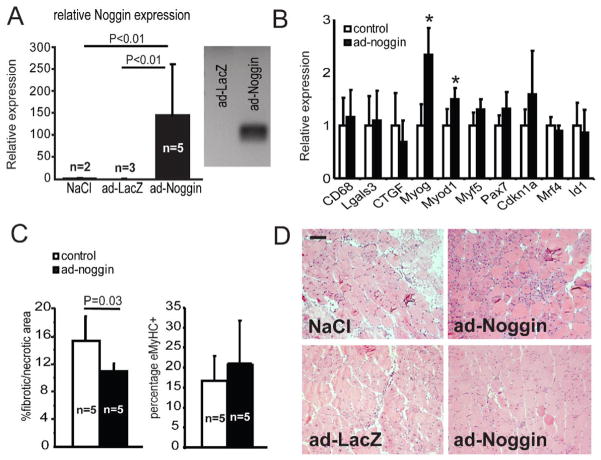
To test the hypothesis that specific inhibition of BMP signaling may alleviate the dystrophic phenotype, we used Noggin to asses the effect of BMP inhibition in vivo in mdx utrn+/− mice. In mdx utrn+/− and mdx utrn−/− mice the loss-of-function of the dystrophin-related protein utrophin results in a more severe dystrophic muscle phenotype than seen in the mdx mice (Deconinck et al., 1997; Zhou et al., 2008). We hypothesized that the effect of Noggin overexpression on the dystrophic phenotype would be more evident in mdx utrn+/− mice compared to mdx mice, since the dystrophic phenotype is more prominent in these mice ((Zhou et al., 2008) and M. Putten, unpublished). For the in vivo overexpression, we used a Noggin adenoviral vector that was previously used in another study to repress BMP activity in vivo (Glaser et al., 2003). Five mice at the age of four weeks were injected intramuscular in the gastrocnemius muscle with the human Noggin cDNA containing adenovirus. As a control we injected the contralateral gastrocnemius muscle of each mouse with either LacZ adenoviral vector (n=3) or saline solution (n=2). After four weeks we isolated the muscles and we performed X-gal staining on sections to verify LacZ activity in the control injected gastrocnemius muscle. High LacZ activity was seen in regenerating fibers (Fig. S2A and data not shown), although there was high variability between different injected muscles (data not shown). PCR and qRT-PCR analysis of the ad-Noggin-injected muscles showed that human Noggin was detectable in all injected muscles, although the expression levels of Noggin in the different injected muscles showed a high level of variation (Fig. 5A and Fig. S2B). Isolation of the amplified product and subsequent sequence analysis verified the presence of human Noggin (data not shown).
Fig. 5. Adenoviral-mediated overexpression of Noggin in dystrophic muscle.
(A) The left panel shows qRT-PCR analysis of control (n=5) and ad-Noggin (n=5) gastrocnemius cDNA with primers specific for human Noggin. The right panel shows a representative gel with RT-PCR product amplified with human Noggin specific primers. The amplified products of all ad-Noggin samples were isolated, sequenced and identified as human Noggin. (B) qRT-PCR analysis of myogenic, inflammation and fibrosis marker gene expression in gastrocnemius samples of control (n=5) and ad-Noggin (n=5). Error bars represents s.d. *P<0.01 (C) Quantitative analysis of fibrotic/necrotic areas as determined with H&E staining and the percentage of eMyHC+ fibers of control (n=5) and ad-Noggin (n=5) muscle sections. Error bars represents s.d. (D) Representative pictures of H&E stainings of control muscle (NaCl and LacZ) and contralateral ad-Noggin injected muscle. Scale bar = 100 μm
We determined the expression of several genes involved in the process of regeneration, fibrosis and inflammation to determine the effect of Noggin overexpression on dystrophic muscle pathology. Myog and Myod1 are involved in myogenesis and have been described as being negatively regulated by BMP signaling in myoblast cell culture (Dahlqvist et al., 2003; Vinals and Ventura, 2004). During adult muscle regeneration these genes are activated in the process of satellite cell proliferation and differentiation (Creuzet et al., 1998; Dahlqvist et al., 2003; Jin et al., 2000). qRT-PCR analysis showed that the expression of Myog and Myod1 was significantly higher in the ad-Noggin-injected muscles compared to control samples (on average 2-fold and 1.3-fold; Fig. 5B and Fig. S2B). The muscles with the highest expression of Noggin also showed a decrease in the expression of fibrosis-related genes CTGF and Col1a1 and of the BMP-target gene Id1, although on average the differences between control and ad-Noggin-injected muscles were not significant (Fig. 5B and Fig. S2B). The expression of genes involved in inflammation (CD68 and Lgals3) showed no significant difference compared to the control groups (Fig. 5B). To determine the effect on muscle histology, we performed H&E staining, collagen staining and embryonic myosin heavy chain (eMyHC) staining on muscle sections. Histological analysis of control and ad-Noggin injected muscles showed that the average percentage of fibrosis/necrotic areas determined with H&E staining was decreased by 20% in the ad-Noggin-treated group (Fig. 5C, D). Furthermore, the number of eMyHC+ fibers increased slightly in ad-Noggin-injected samples, although this trend was not significant (Fig. 5C and data not shown). No significant differences were found in the amount of collagen staining, fiber size or the percentage of fibers containing centrally located nuclei (data not shown).
In summary, we found that overexpression of Noggin induced the expression of myogenic marker genes in mdx utrn+/− muscles and alleviated the dystrophic phenotype.
Discussion
In this study we showed that different BMP antagonists can efficiently accelerate and enhance myogenic differentiation in vitro and provide the first evidence that BMP antagonists may be beneficial in counteracting the dystrophic pathology of DMD. BMPs are established inhibitors of myogenesis; during embryogenesis repression of BMPs is necessary for proper initiation of myogenic differentiation of myoblasts. In this study we show that BMP signaling is downregulated during myogenic differentiation as evidenced by the decrease of pSmad1/5/8 levels and correlated reduction in endogenous Bmp4 expression. Inhibition of endogenous BMP signaling as well as BMP4-induced signaling by treatment with BMP antagonists Noggin, dorsomorphin or LDN, accelerated and enhanced myogenic differentiation in C2C12 cells. The mechanism by which these BMP antagonists repress BMP signaling is different. Noggin is a secreted protein that selectively sequesters BMPs, including BMP4, and therefore inhibits both Smad-dependent BMP signaling and Smad-independent BMP signaling. In contrast, the small molecule compounds dorsomorphin and its structural derivative LDN bind to the kinase domains of the BMP type I receptors. Both Noggin and LDN were able to induce myogenic differentiation in human primary myoblast cultures, showing that these BMP antagonists have a comparable effect on human myoblasts. Dorsomorphin, however, was found to repress myogenic differentiation of primary myoblasts, which is presumably a result of non-specific effects of the compound. Several other studies showed that dorsomorphin indeed also targets other kinases such as AMPK (Yu et al., 2008b) and VEGF type-2 receptor (Flk1/KDR) (Hao et al., 2010). In addition, we show in this study that dorsomorphin has a repressive effect on Smad3-dependent TGFβ-activity. The reported inductive effect of dorsomorphin on myoblast apoptosis (Niesler et al., 2007) could explain the effects observed in primary myoblast cultures in our study, suggesting this compound is not suitable to enhance myogenic differentiation in vivo. LDN inhibits TGFβ activity and also VEGF type-2 receptor at higher doses (this study and Hao et al., 2010) but other aspecific effects have not been described yet and we observed no toxicity in vitro, suggesting a low dose of this compound can be used to specifically inhibit BMP signaling and enhance myogenic differentiation. In a previous study, our group showed that DMD patient myoblasts exhibited elevated BMP4 expression and in addition are more sensitive for BMP signaling. Although we tried to determine the effect of the different antagonists on myogenic differentiation of three different DMD patient myoblast cultures, we were not able to effectively induce myogenic differentiation of these cells. This is presumably because these cell cultures had a low percentage of myoblasts as a result of high passage number. Nonetheless there was a tendency that the cells treated with Noggin and LDN had an increased differentiation index (data not shown).
Based on our in vitro results we decided to overexpress Noggin using an adenoviral vector for selective in vivo inhibition of BMP signaling. Our results show that Noggin can induce Myog and Myod1 expression in vivo in dystrophic muscle of mdx utrn+/− mice. In addition, significant improvement of muscle histology was seen, although no significant difference in average was found in the number of eMyHC+ fibers and collagen levels between control and ad-Noggin samples. In a pilot experiment we already observed improved muscle histology after two weeks of the ad-Noggin injection (n=2, data not shown). The observed variance in response after ad-Noggin injection is most likely the result of the considerable variance in Noggin expression when using the adenoviral vector. This is consistent with our observation that the most pronounced effect on gene expression was detected in muscle with the highest expression of Noggin. Moreover, it was previously shown that the full length protein is not very stable in vivo and overexpression of a modified Noggin protein, which lacks a heparin binding site was shown to have an increased half-life and to be more stable in vivo (Glaser et al., 2003). Further studies are therefore needed to investigate the effect of BMP inhibition in DMD context using a more stable and efficient method of expression. Nonetheless, our results suggest that local inhibition of BMP signaling effects the expression of genes involved in the muscle regeneration process resulting in improvement of the dystrophic phenotype.
BMP antagonists, such as recombinant Noggin, dorsomorphin and its structural derivative LDN, have been used to effectively antagonize BMP signaling in vivo (Cuny et al., 2008; Glaser et al., 2003; Lories et al., 2005; Wang et al., 2009; Yu et al., 2008a). This suggests that in the future BMP antagonists may be of therapeutic use to treat diseases where BMP signaling is aberrantly activated, such as Fibrodysplasia Ossificans Progressiva and, in our case, DMD (Sterrenburg et al., 2006; Yu et al., 2008a). However several important issues have to be addressed before BMP antagonists can be considered as a therapeutic option. First, it will be important to determine the potential role of BMP signaling in normal and dystrophic muscle. It has been described that BMP signaling is active in quiescent satellite cells and is downregulated upon activation of this cell population after muscle damage (Fukada et al., 2007). Recently, a novel role of BMP signaling in the regulation of satellite cell proliferation has been suggested (Clever et al., 2010), but the molecular mechanism and the potential role in dystrophic pathology is currently not known. Furthermore, overexpression of Noggin inhibits the activity of several members of the BMP family (Balemans and Van Hul, 2002) and therefore it is not exactly known which ligands and signaling components may contribute to the pathology of DMD. Second, the effect of long term treatment with BMP inhibitors is not known and may have serious side-effects. For example, it has been described that ectopic expression of Noggin in the mouse skeleton reduces bone density and increases the occurrence of spontaneous fractures (Devlin et al., 2003). Finally, additional signaling cascades, such as induced by TGFβ and myostatin, also likely play an important role in DMD pathology and it will therefore be important to determine the additive or synergistic effect when targeting multiple pathways. Intriguingly, it is known that overexpression of follistatin or a soluble form of the type 2 receptor kinase Acvr2B, both proteins that inhibit the activity of BMPs, myostatin and activin, result in an excessive increase in muscle mass that cannot solely be explained by loss of function of myostatin (Gilson et al., 2009; Lee, 2007), showing that other ligands are involved in the regulation of muscle mass. Future experiments will therefore be focused on further dissecting the role the different signaling pathways in DMD pathology.
Materials & methods
Cell culture and transfections
The mouse myoblast cell line C2C12 was maintained in DMEM supplemented with 10% FBS, Pen/strep and Glutamax (Gibco). Primary human myoblasts were maintained in F-10 HAM medium supplemented with 20% FBS and pen/strep and grown at 37 °C at 5% CO2. For the differentiation assay, both the C2C12 cells and primary human myoblasts were cultured in differentiation medium after cells reached 80%–90% confluency, which is composed of DMEM (Gibco) supplemented with 2% FBS. C2C12 cells were grown at 37°C in a humidified incubator 10% CO2. Transient transfections and reporter assays were performed in triplicates as previously described with BRE-luc and CAGA12-luc reporter constructs (Dennler et al., 1998; Korchynskyi and ten Dijke, 2002). In all reporter assays, a β-galactosidase expression plasmid was co-transfected and served as a control to correct for transfection efficiency. The experiments were performed in triplicates. For BRE-luc and CAGA-Luc reporter assays, the luciferase activity was analyzed 8 hours after BMP4 (10ng/ml) TGF-β3 (5 ng/ml) addition with our without the different concentrations of the BMP antagonists. Recombinant human BMP4 was a gift from Yongping Cai and Anne-Marie Cleton-Jansen, Department of Pathology, Leiden University Medical Center, Leiden, The Netherlands. Dorsomorphin was purchased from Biomol (BML-275). Noggin protein used in cell culture was a gift from Regeneron, USA.
Mice
Male mdx/utrn+/− mice were obtained through crossing of mdx and utrn−/−. At 4 weeks of age, mice were anesthetized with isoflurane and injected intramuscular in the gastrocnemius and triceps muscles with ad-Noggin (1×109 pfu/injection), ad-lacZ (1×109 pfu/injection) or saline. The adeno containing Noggin cDNA was obtained from Regeneron, the adeno containing the LacZ cDNA was in the same backbone (serotype 5). Four weeks after injection muscles were isolated and analyzed. The animal experiments were carried out with approval of the Animal Experimental Commission of the Leiden University Medical Center, Leiden and according to the Dutch Government guidelines.
Histology
Muscle tissue for histological analysis and RNA isolation was snap frozen in liquid nitrogen-cooled 2-methylbutane (Sigma Aldrich, The Netherlands) and sectioned at 8 μm with a Shandon cryotome (Thermo Fisher Scientific Co., Pittsburgh, PA, USA). Sections were fixed using ice cold acetone for 5 minutes and stained with hematoxylin and eosin (Sigma Aldrich, The Netherlands). For the X-gal staining, the sections were fixed for 4 minutes using cold fixative solution (0.2% glutaraldehyde, 1.5% paraformaldehyde, 2mM MgCl2, 5mM EGTA and 100mM Sodium-phosphate-buffer pH 7.3) and washed twice in PBS. Sections are stained overnight in the dark at room temperature with staining solution (5mM K3Fe(CN)6, 5mM K4Fe(CN)6, 1mg/ml X-gal, 2mM MgCl2 and 100mM Sodium-phosphate-buffer pH 7.3). Then the sections were washed with PBS, dehydrated and embedded in Pertex (Histolab, Göteborg). Pictures were taken using a Leica microscope and analyzed using ImageJ software as described (van Putten et al., in press).
Immunofluorescence and Western blotting
Antibody used for immunoblotting were as following: actin (Sigma), Myogenin (F5D; BD Pharmingen), Smad phosphorylation was detected using PS1 antibody recognizing phosphorylated SMAD1/5/8 (Persson et al., 1998). Western blotting was performed as previously described. Antibodies used for immunofluorescence were as following: Desmin, Myosin heavy chain (MF20; Developmental Studies Hybridoma Bank), embryonic Myosin (eMyHC; F1.652, DSHB). Immunofluorescencent staining and western blotting protocol was performed as previously described (Aartsma-Rus et al., 2003).
quantitative Real-Time PCR (qRT-PCR)
Total RNA was isolated from muscle tissue using the RNAII RNA isolation kit (Machery Nagel) according to the manufacturers protocol. Subsequently, cDNA was reverse transcribed from 1ug total RNA using the Revert Aid protocol (Fermentas) with random hexamer primers. Quantitative Real-Time PCR analysis was performed using the Roche 480 lightcycler and the relative expression level of the genes of interest was determined in triplicate for each sample using the 2−ΔΔCT method using Genex Analysis software (Biorad). Values were normalized to Gapdh expression. Measurements were performed in triplo and statistical analysis between groups was performed using a two-tailed Students t-test. The sequences of the different primers used in this study can be provided upon request.
Supplementary Material
Acknowledgments
We thank Dr. Aris Economides and Dr. Paschalis Sideras for providing us with with the Noggin protein and the Noggin adenovirus. This work was financially supported by the Dutch Organization for Scientific Research (Zon-MW) (VICI Grant NWO 918.66.606), the Dutch Ministery for Economic Affairs (IOP Genomics grant IGE7001), the Dutch Parent Project and the Centre for Biomedical Genetics.
References
- Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PM, Carathers M, Li ZW, Beg AA, Ghosh S, Sahenk Z, et al. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aartsma-Rus A, Bremmer-Bout M, Janson AA, den Dunnen JT, van Ommen GJ, van Deutekom JC. Targeted exon skipping as a potential gene correction therapy for Duchenne muscular dystrophy. Neuromuscul Disord. 2002;12(Suppl 1):S71–S77. doi: 10.1016/s0960-8966(02)00086-x. [DOI] [PubMed] [Google Scholar]
- Aartsma-Rus A, Janson AA, Kaman WE, Bremmer-Bout M, den Dunnen JT, Baas F, van Ommen GJ, van Deutekom JC. Therapeutic antisense-induced exon skipping in cultured muscle cells from six different DMD patients. Hum Mol Genet. 2003;12:907–914. doi: 10.1093/hmg/ddg100. [DOI] [PubMed] [Google Scholar]
- Balemans W, Van Hul HW. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R. Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine. J Clin Invest. 1995;96:1137–1144. doi: 10.1172/JCI118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–421. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- Chen YW, Nagaraju K, Bakay M, McIntyre O, Rawat R, Shi R, Hoffman EP. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology. 2005;65:826–834. doi: 10.1212/01.wnl.0000173836.09176.c4. [DOI] [PubMed] [Google Scholar]
- Chen YW, Zhao P, Borup R, Hoffman EP. Expression profiling in the muscular dystrophies: identification of novel aspects of molecular pathophysiology. J Cell Biol. 2000;151:1321–1336. doi: 10.1083/jcb.151.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clever JL, Sakai Y, Wang RA, Schneider D. Inefficient Skeletal Muscle Repair in Inhibitor of Differentiation (Id) Knockout Mice Suggests a Crucial Role for BMP Signaling During Adult Muscle Regeneration. Am J Physiol Cell Physiol. 2010 doi: 10.1152/ajpcell.00388.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn RD, van EC, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, et al. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13:204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creuzet S, Lescaudron L, Li Z, Fontaine-Perus J. MyoD, myogenin, and desmin-nls-lacZ transgene emphasize the distinct patterns of satellite cell activation in growth and regeneration. Exp Cell Res. 1998;243:241–253. doi: 10.1006/excr.1998.4100. [DOI] [PubMed] [Google Scholar]
- Cuny GD, Yu PB, Laha JK, Xing X, Liu JF, Lai CS, Deng DY, Sachidanandan C, Bloch KD, Peterson RT. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett. 2008;18:4388–4392. doi: 10.1016/j.bmcl.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist C, Blokzijl A, Chapman G, Falk A, Dannaeus K, Ibanez CF, Lendahl U. Functional Notch signaling is required for BMP4-induced inhibition of myogenic differentiation. Development. 2003;130:6089–6099. doi: 10.1242/dev.00834. [DOI] [PubMed] [Google Scholar]
- Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, Watt DJ, Dickson JG, Tinsley JM, Davies KE. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell. 1997;90:717–727. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin RD, Du Z, Pereira RC, Kimble RB, Economides AN, Jorgetti V, Canalis E. Skeletal overexpression of noggin results in osteopenia and reduced bone formation. Endocrinology. 2003;144:1972–1978. doi: 10.1210/en.2002-220918. [DOI] [PubMed] [Google Scholar]
- Frank NY, Kho AT, Schatton T, Murphy GF, Molloy MJ, Zhan Q, Ramoni MF, Frank MH, Kohane IS, Gussoni E. Regulation of myogenic progenitor proliferation in human fetal skeletal muscle by BMP4 and its antagonist Gremlin. J Cell Biol. 2006;175:99–110. doi: 10.1083/jcb.200511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada S, Uezumi A, Ikemoto M, Masuda S, Segawa M, Tanimura N, Yamamoto H, Miyagoe-Suzuki Y, Takeda S. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells. 2007;25:2448–2459. doi: 10.1634/stemcells.2007-0019. [DOI] [PubMed] [Google Scholar]
- Gilson H, Schakman O, Kalista S, Lause P, Tsuchida K, Thissen JP. Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am J Physiol Endocrinol Metab. 2009;297:E157–E164. doi: 10.1152/ajpendo.00193.2009. [DOI] [PubMed] [Google Scholar]
- Glaser DL, Economides AN, Wang L, Liu X, Kimble RD, Fandl JP, Wilson JM, Stahl N, Kaplan FS, Shore EM. In vivo somatic cell gene transfer of an engineered Noggin mutein prevents BMP4-induced heterotopic ossification. J Bone Joint Surg Am. 2003;85-A:2332–2342. doi: 10.2106/00004623-200312000-00010. [DOI] [PubMed] [Google Scholar]
- Grounds MD, Torrisi J. Anti-TNFalpha (Remicade) therapy protects dystrophic skeletal muscle from necrosis. FASEB J. 2004;18:676–682. doi: 10.1096/fj.03-1024com. [DOI] [PubMed] [Google Scholar]
- Haidet AM, Rizo L, Handy C, Umapathi P, Eagle A, Shilling C, Boue D, Martin PT, Sahenk Z, Mendell JR, et al. Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc Natl Acad Sci U S A. 2008;105:4318–4322. doi: 10.1073/pnas.0709144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, Hopkins CR, Lindsley CW, Hong CC. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010;5:245–253. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett JN, Sanoudou D, Kho AT, Bennett RR, Greenberg SA, Kohane IS, Beggs AH, Kunkel LM. Gene expression comparison of biopsies from Duchenne muscular dystrophy (DMD) and normal skeletal muscle. Proc Natl Acad Sci U S A. 2002;99:15000–15005. doi: 10.1073/pnas.192571199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Murakami N, Saito Y, Goto Y, Koishi K, Nonaka I. Expression of MyoD and myogenin in dystrophic mice, mdx and dy, during regeneration. Acta Neuropathol. 2000;99:619–627. doi: 10.1007/s004010051172. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Akiyama S, Namiki M, Komaki M, Yamaguchi A, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 inhibits terminal differentiation of myogenic cells by suppressing the transcriptional activity of MyoD and myogenin. Exp Cell Res. 1997;230:342–351. doi: 10.1006/excr.1996.3432. [DOI] [PubMed] [Google Scholar]
- Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem. 2002;277:4883–4891. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- Lee SJ. Quadrupling muscle mass in mice by targeting TGF-beta signaling pathways. PLoS ONE. 2007;2:e789. doi: 10.1371/journal.pone.0000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZB, Kollias HD, Wagner KR. Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem. 2008;283:19371–19378. doi: 10.1074/jbc.M802585200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker C, Lesbros C, Stark MR, Marcelle C. Intrinsic signals regulate the initial steps of myogenesis in vertebrates. Development. 2003;130:4797–4807. doi: 10.1242/dev.00688. [DOI] [PubMed] [Google Scholar]
- Lories RJ, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 2005;115:1571–1579. doi: 10.1172/JCI23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- Niesler CU, Myburgh KH, Moore F. The changing AMPK expression profile in differentiating mouse skeletal muscle myoblast cells helps confer increasing resistance to apoptosis. Exp Physiol. 2007;92:207–217. doi: 10.1113/expphysiol.2006.034736. [DOI] [PubMed] [Google Scholar]
- Persson U, Izumi H, Souchelnytskyi S, Itoh S, Grimsby S, Engstrom U, Heldin CH, Funa K, ten Dijke P. The L45 loop in type I receptors for TGF-beta family members is a critical determinant in specifying Smad isoform activation. FEBS Lett. 1998;434:83–87. doi: 10.1016/s0014-5793(98)00954-5. [DOI] [PubMed] [Google Scholar]
- Pescatori M, Broccolini A, Minetti C, Bertini E, Bruno C, D’amico A, Bernardini C, Mirabella M, Silvestri G, Giglio V, et al. Gene expression profiling in the early phases of DMD: a constant molecular signature characterizes DMD muscle from early postnatal life throughout disease progression. FASEB J. 2007;21:1210–1226. doi: 10.1096/fj.06-7285com. [DOI] [PubMed] [Google Scholar]
- van Putten M, de Winter CL, van Roon-Mom W, van Ommen GJ, ‘t Hoen PAC, Aartsma-Rus A. A three months mild functional test regime does not affect disease parameters in young mdx mice. Neuromuscul Disord. 2010 doi: 10.1016/j.nmd.2010.02.004. in press. [DOI] [PubMed] [Google Scholar]
- Reshef R, Maroto M, Lassar AB. Regulation of dorsal somitic cell fates: BMPs and Noggin control the timing and pattern of myogenic regulator expression. Genes Dev. 1998;12:290–303. doi: 10.1101/gad.12.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterrenburg E, van der Wees CG, White SJ, Turk R, de Menezes RX, van Ommen GJ, den Dunnen JT, ‘t Hoen PA. Gene expression profiling highlights defective myogenesis in DMD patients and a possible role for bone morphogenetic protein 4. Neurobiol Dis. 2006;23:228–236. doi: 10.1016/j.nbd.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Turk R, Sterrenburg E, de Meijer EJ, van Ommen GJ, den Dunnen JT, ‘t Hoen PA. Muscle regeneration in dystrophin-deficient mdx mice studied by gene expression profiling. BMC Genomics. 2005;6:98. doi: 10.1186/1471-2164-6-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylzanowski P, Mebis L, Luyten FP. The Noggin null mouse phenotype is strain dependent and haploinsufficiency leads to skeletal defects. Dev Dyn. 2006;235:1599–1607. doi: 10.1002/dvdy.20782. [DOI] [PubMed] [Google Scholar]
- Tzahor E, Kempf H, Mootoosamy RC, Poon AC, Abzhanov A, Tabin CJ, Dietrich S, Lassar AB. Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev. 2003;17:3087–3099. doi: 10.1101/gad.1154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinals F, Ventura F. Myogenin protein stability is decreased by BMP-2 through a mechanism implicating Id1. J Biol Chem. 2004;279:45766–45772. doi: 10.1074/jbc.M408059200. [DOI] [PubMed] [Google Scholar]
- Wang L, Harrington L, Trebicka E, Shi HN, Kagan JC, Hong CC, Lin HY, Babitt JL, Cherayil BJ. Selective modulation of TLR4-activated inflammatory responses by altered iron homeostasis in mice. J Clin Invest. 2009;119:3322–3328. doi: 10.1172/JCI39939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Akiyama S, Katagiri T, Namiki M, Kurokawa T, Suda T. Smad1 and smad5 act downstream of intracellular signalings of BMP-2 that inhibits myogenic differentiation and induces osteoblast differentiation in C2C12 myoblasts. Biochem Biophys Res Commun. 1997;238:574–580. doi: 10.1006/bbrc.1997.7325. [DOI] [PubMed] [Google Scholar]
- Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, et al. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med. 2008a;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008b;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Rafael-Fortney JA, Huang P, Zhao XS, Cheng G, Zhou X, Kaminski HJ, Liu L, Ransohoff RM. Haploinsufficiency of utrophin gene worsens skeletal muscle inflammation and fibrosis in mdx mice. J Neurol Sci. 2008;264:106–111. doi: 10.1016/j.jns.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.