Abstract
Pericytes are a heterogeneous group of extensively branched cells located in microvessels where they make focal contacts with endothelium. Pericytes stabilize blood vessels, regulate vascular tone, synthesize matrix, participate in repair and serve as progenitor cells, among other functions. Recent work has highlighted the role of pericytes and pericyte-like cells in fibrosis, where chronic injury triggers pericyte proliferation and differentiation into collagen-secretory, contractile myofibroblasts with migration away from vessels, causing microvascular rarefaction. In this review I summarize the developmental origins of kidney pericytes and perivascular fibroblasts, discuss pericyte to myofibroblast transition in type I diabetic nephropathy and describe the regulation of pericyte differentiation into myofibroblasts as a therapeutic target for treatment of diabetic nephropathy.
Keywords: Pericyte, myofibroblast, fibrosis, interstitium, diabetic nephropathy
THE RENAL INTERSTITIUM IN DIABETIC NEPHROPATHY
Renal cortical interstitial expansion is generally accepted as the best histologic correlate of renal functional decline in glomerular diseases generally and type I diabetic nephropathy in particular.1-3 While this interstitial expansion has historically been attributed to glomerulosclerosis with secondary ischemic or proteinuric damage to the remainder of the nephron, glomerular histologic changes do not correlate as well with renal function at least in type II diabetes.4 The earliest interstitial lesions in type I diabetic nephropathy are characterized by increased interstitial cell number but not collagen content. The identity of these cells in human biopsy specimens is most likely the myofibroblast, a contractile, migratory cell that secrete matrix proteins that forms interstitial scar tissue. Myofibroblasts are abundant in the interstitium of human kidneys in diabetic nephropathy, and the expression of myofibroblast markers also closely correlates with progressive diabetic nephropathy.5
Recent evidence implicates peritubular capillary loss as a central mediator of chronic tubular hypoxia causing progression of chronic kidney disease.6 Interstitial expansion and fibrosis causes capillary dropout, suggesting that interstitial fibrosis, rather than representing a passive marker of glomerulosclerosis, may actively promote tubular hypoxia in adjacent tubules, leading to nephron loss and kidney functional decline independent of glomerular disease. Because fibrosis is the final common pathway of multiple separate kidney diseases it represents a logical treatment target. Given the reversibility of interstitial lesions in principle,7 targeting interstitial expansion represents a viable therapeutic strategy for type I diabetic nephropathy. Designing such strategies requires full understanding of where myofibroblasts originate, and the signaling pathways that regulate their proliferation and differentiation. This review will summarize data implicating the pericyte as a myofibroblast precursor cell and will highlight several signaling pathways that regulate pericyte to myofibroblast transition that may as therapeutic targets for type I diabetic nephropathy.
PERICYTE MORPHOLOGY, MARKERS AND FUNCTION
Pericytes are contractile, branched cells located in microvessels such as capillaries and postcapillary venules (For recent review see 8). They are completely or partially embedded within the microvascular basement membrane, and are closely apposed to endothelial cells with which they make focal contacts (Figure 1).9 Historically, pericytes were first described by Rouget when he termed them adventitial cells.10 In 1923 Zimmerman distinguished three forms of these cells that he termed pericytes, the name that endures today, the precapillary, midcapillary and postcapillary pericytes.11 Kidney pericytes were identified by Courtnoy and Boyles9 in 1983 using electron microscopy, and more recently by others.12 Kidney pericytes have features of both the fibroblast and the smooth muscle cell and are partially sheathed with matrix that represents a duplication of the capillary basement membrane which is often found to be incomplete between pericyte and endothelial cell, enabling close apposition and interdigitation between both cell types (Figure 1A). Pericyte-endothelial cell contacts, which include gap junctions, adhesion plaques and peg-socket junctions, are sites of active signaling between pericytes and endothelial cells.8, 13 Many kidney pericytes span from the peritubular capillary to the tubule with processes abutting both endothelium and the tubular basement membrane.12, 14
Figure 1. Kidney Pericyte Morphology.
A. Electron micrograph of pericyte processes with pale cytoplasm (#) encircling endothelial cells in tumor stroma (*). Note that these pericyte processes lie underneath the capillary basement membrane. Image courtesy of Brian Eyden, PhD, reprinted with permission.24B. Fluorescence image of adult mouse kidney interstitium from a FoxD1-GFPCre; R26tdTomato bigenic mouse. Note the finely branched processes emanating from the pericyte cell bodies, extending around tubular basement membrane. C. An interstitial kidney pericyte encircling a peritubular capillary, with the endothelial cell nucleus visible (*).
Because pericytes are long, branched cells that make occasional endothelial cell contacts, distinguishing kidney pericytes from kidney fibroblasts, which are also branched interstitial cells, is challenging, and definitive markers have not been described to separate these two stromal cell types. Indeed, some renal pathologists argue that kidney ‘pericytes’ are in fact simply kidney fibroblasts, that introducing the term pericyte to refer to these interstitial cells is unnecessary.15 While it is true that molecular markers separating kidney pericytes and fibroblasts have not been discovered to date, and that in most or perhaps all cases, the cells that we and others have referred to as pericytes are the same cells that others describe as fibroblasts,14, 16 several lines of evidence indicate that kidney pericytes are independent of fibroblasts. From a histologic perspective, some kidney intersitial cells fulfill nearly all of the histologic criteria for pericytes (apposition to endothelial cells with sites of close contact underneath capillary basement membrane).9 From a functional perspective pericytes support vasculature and in kidney have been extensively characterized as cells closely apposed to vasa recta capillaries, where they regulate medullary bloodflow.17, 18 Pericytes also have progenitor cell functions, something not typically ascribed to fibroblasts19 and the importance of pericytes as progenitors of scar-forming stroma is now being recognized in other organs.20 For these reasons I will term these cells pericytes, recognizing that the full diversity of kidney stromal cells is still being defined and therefore remains a controversial and unsettled issue.
The expression of pericyte marker proteins is an important adjunct to the morphologic identification of these cells, however it is critical to appreciate that there is no universally-accepted molecular definition of the pericyte across organs. α-smooth muscle actin (αSMA) is a well characterized marker of pericytes in some organs and is expressed by pre- and post-capillary pericytes, however mid-capillary pericytes do not express αSMA.21 Kidney pericytes, being of Zimmerman's mid-capillary variety, are αSMA-negative.22 Kidney pericyte markers include platelet-derived growth factor receptor-β (PDGFRβ),23 but this may also be expressed by fibroblasts. Kidney pericytes are also CD31- and CD45- and exhibit variable expression of other proteins including NG2 and CD73.
Pericyte functions are broad, and include regulation of renal bloodflow through microvessel contractility, urinary concentration and sodium absorption and these effects influence systemic blood pressure. Pericytes also stabilize endothelium, in part through secretion of TGF-β which inhibits endothelial cell division. They also secrete matrix proteins such as fibronectin and laminin.24 A large body of literature suggests that pericytes have progenitor cell functions, though much of the data is indirect. Pericytes are osteogenic, and may contribute to vascular calcification. More recently, pericytes in muscle have been shown to directly contribute to muscle regeneration after injury. Very recent studies have shown that pericytes do give rise to multipotent mesenchymal stem cells.19
INTERSTITIAL MYOFIBROBLASTS IN FIBROTIC DISEASE AND THEIR ORIGIN
Myofibroblasts are reactive cells present under conditions of injury or pathology such as cancer. They are characterized by cellular processes giving the cells a stellate appearance (Figure 2). These cells secrete matrix and have abundant rough endoplasmic reticulum and collagen secretion granules.24 The best marker of the myofibroblast is alpha-smooth muscle actin (α-SMA) which forms bundles of myofilaments called stress fibers. These stress fibers are critical in connecting the myofibroblast to extracellular matrix, and when the cell contracts this exerts mechanical forces on matrix causing reorganization during wound healing. Other myofibroblast markers include plasma membrane fibronectin and vimentin. In kidney, Collagen-1α1 expression defines the myofibroblast, consistent with the matrix-secretory function of these cells.22
Figure 2. Myofibroblast Morphology.
Electron micrograph of a myofibroblast, with stellate appearance. This cell has abundant myofilaments in cytoplasm (*) as well as dense fibronectin along the cell surface (arrows). Image courtesy of Brian Eyden, PhD, reprinted with permission.24
The cellular origin of the myofibroblast has been topic of investigation for decades, and evidence implicates several different cellular sources including expansion of local fibroblast pools, smooth muscle cell myofibroblast progenitors, endothelium and epithelium. Indirect evidence has also implicated the pericyte as a myofibroblast progenitor, but no lineage tracing study had provided definitive proof.22, 25, 26 We have performed such genetic lineage analysis and defined the kidney pericyte as the primary myofibroblast progenitor in fibrotic kidney disease. We first genetically traced epithelial cells in fibrosis, in order to determine whether they might serve as myofibroblast progenitors through the process of Epithelial to Mesenchymal Transition (EMT), however we found no evidence that EMT contributes to the interstitial myofibroblast pool, at least using the rigorous definition, which assumes direct interconversion of an epithelial cell to a myofibroblast progenitor27. Next we utilized an inducible CreERt2 driven by the FoxD1 to genetically tag interstitial pericytes. FoxD1 is expressed in stroma surrounding cap mesenchyme during nephrogenesis, and FoxD1+ cells do not have epithelial potential, as cap mesenchyme does, but rather they are fated to differentiate into kidney pericytes and perivascular fibroblasts. We genetically labeled 20% of all the interstitial pericytes/perivascular fibroblasts with a single dose of Tamoxifen during development. After fibrosis in adult kidney, this cohort of pulse-genetically-labeled pericytes expanded 15-fold, all acquired αSMA expression and represented 20% of the total myofibroblast pool, providing unequivocal results implicating the interstitial pericyte/perivascular fibroblast as the myofibroblast progenitor (Figure 3).28, 29 These studies did not suggest the existence of alternative myofibroblast progenitor pools in these fibrosis models, although this is a difficult point to prove and lineage tracing does implicate endothelial cells as an alternative source of myofibroblasts in kidney fibrosis.30 Clearly more lineage analysis is required to confirm that pericytes are the predominant source of kidney myofibroblasts and to quantitate the degree to which other cell types (endothelium or fibroblast, for example) contribute to the myofibroblast pool.
Figure 3. Pericyte to Myofibroblast Transition.
A. Genetically labeled pericytes with fine process identified in fluorescence in a FoxD1-GFPCre; R26tdTomato bigenic mouse kidney. B. Ten days after unilateral ureteral obstruction, there is an expansion of labeled cells in interstitium, reflecting proliferation and differentiation of pericytes into myofibroblasts during chronic injury.
Recent work from the Yanagita lab has provided additional insight into the developmental origins of both FoxD1-derived pericytes and adult kidney myofibroblasts. Using a myelin protein zero-Cre (P0-Cre) driver, Asada and colleagues lineage labeled a cohort of extrarenal cells in the neural crest, and show that these cells migrate into mouse kidney at e13.5, where they surround cap mesenchyme, a portion of them are FoxD1+ and the cells later acquire expression of PDGFRβ and CD73 and reside in kidney interstitium.16 In adult, they go on to show that these cells differentiate into αSMA+ myofibroblasts under conditions of chronic disease (and lose ability to express erythropoietin). Importantly, they show that 94% of myofibroblasts derive from Po-Cre-labeled precursurs. Since much of the labeled cells also express FoxD1 once they migrate into kidney, the findings are consistent with the model that pericytes/fibroblasts (Asada and colleagues term the PO-labeled cells fibroblasts) are the primary myofibroblast precursor. While these lineage tracing studies have not been performed in diabetic nephropathy models, fibrosis is the final common pathway of chronic kidney diseases and the biology is likely to be similar, though it will be important in the future to confirm these findings in relevant diabetic nephropathy models.
Several questions concerning kidney stromal cell heterogeneity remain. Are all PDGFRβ cells in kidney pericytes or only a fraction, with the balance being fibroblasts? Do a subset of these cells serve as myofibroblast progenitors or do they all share this potential? The question of pericyte heterogeneity is one that has been examined in the past and is being reassessed currently. Brigid Hogan's group has examined this question in lung fibrosis. As with our results in kidney, they found no evidence that lung myofibroblasts derive from Type II epithelial alveolar cells through EMT, using two different epithelial CreERt2 drivers (Surfactant protein C-CreERt2 and Scgb1a1-CreERt2). They did observe proliferation of NG2+ pericyte-like cells in lung fibrosis, however these cells did not acquire high-level αSMA expression.31 Whether lung myofibroblasts differ in αSMA expression, or if there is a separate NG2- pericyte-like myofibroblast progenitor in lung remains to be clarified. These studies point to heterogeneity among lung stromal populations, and highlight the important need to better understand stromal and pericyte cell subtypes and their lineage relationships in fibrotic disease.
PATHWAYS REGULATING PERICYTE DIFFERENTIATION
Multiple pathways regulate pericyte-myofibroblast transition. I will highlight four pathways, Hh-Gli, TGFβ, PDGF and CTGF, focusing on recent developments (Figure 4). There are other important pathways that regulate pericyte differentiation, such as endothelin and the renin-angiotensin-aldosterone pathway but these are outside the scope of the current review.
Figure 4. Myofibroblast Origins and Signaling.
Cartoon depicting the developmental origins of pericytes and fibroblasts in kidney from a FoxD1+ progenitor in mesenchyme during development. In the adult, pericytes can be activated by various growth factors to differentiate into myofibroblasts. Whether adult pericytes (or a subset) have the potential to differentiate into other cell types besides myofibroblasts, as do some pericytes in other vascular beds, remains an open question in kidney.
HEDGEHOG-GLI SIGNALING
The Hedgehog (Hh) family of morphogens regulates a diverse range of developmental processes in the mammalian embryo, including ventralization of the neural tube, patterning and growth of limbs and face, the formation of organs such as lung and gut, development of hair follicles and decisions of left-right asymmetry.32, 33 In kidney development, Sonic hedgehog (Shh) is expressed in collecting duct epithelia and regulates adjacent mesenchymal cell proliferation and differentiation, and either germline Shh deletion or deletion of Shh from collecting duct leads to severe renal developmental abnormalities including renal aplasia or hypoplasia.34-36 Hh ligands are secreted, lipid-modified proteins that can act at short or long distances by binding to the membrane receptor Patched1 (Ptch1) on target cells, thereby releasing tonic inhibition by Ptch1 on the transmembrane protein Smoothened (Smo). Derepressed Smo translocates to the primiary cilium, inhibiting production of the truncated repressor forms of the Gli2 and Gli3 transcription factors and promoting preservation of their full-length activator forms which induce transcription of hedgehog target genes, including Gli1 and Ptch1 – both of which serve as readouts of Hh pathway activation (Figure 5A).37 Hh signaling has multiple, context-dependent downstream effects such as controlling expression of patterning genes like Pax2 and Sall1 or regulating cell cycle by activating Cyclin D1 and N-Myc.36
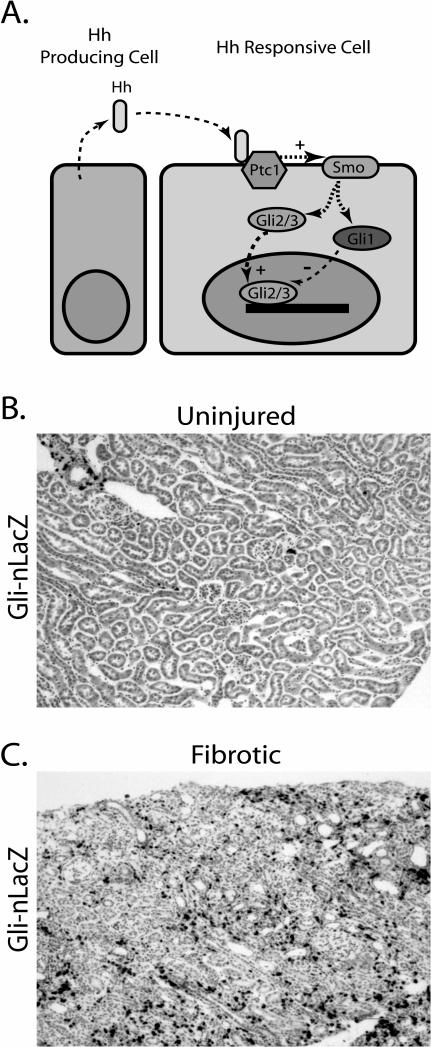
Figure 5. Hedgehog-Gli1 Signaling in Fibrosis.
A. Hh ligand is produced by one cell and acts in a paracrine fashion by binding to Ptch1 on a different cell. This binding releases tonic inhibition of Smo by Ptch1. Smo subsequently activates Gli1-3 effectors, and Gli2/3 translocates to the nucleus where it activates target gene transcription. B. Kidney cortex from a Gli1-nLacZ reporter mouse at ten weeks, stained with Xgal. C. After unilateral ureteral obstruction there is a dramatic upregulation of Gli1-nLacZ expression in kidney interstitium, reflecting activation of the Hh-Gli pathway in kidney pericytes (Fabian and Humphreys, unpublished).
Emerging evidence implicates the Hh pathway in pericyte-myofibroblast transition. In cancer and solid organ injury models, epithelial-derived Hh ligands can be reactivated in pathologic states to transmit signals to surrounding mesenchymal cells. In carcinogenesis, for example, Hh ligands from the epithelial tumor act on adjacent stroma to promote a favorable tumor microenvirment.38-40 In murine bladder injury, epithelial Shh induces Wnt expression in surrounding stromal cells, which in turn stimulates stromal and epithelial proliferation in a paracrine signaling loop.41 Hh pathway reactivation has also been implicated in organ fibrosis. Both chronic cholestasis and nonalcoholic steatohepatitis are characterized by increased Hh signaling during fibrosis,42, 43 and Hh signaling promotes activation of hepatic stellate cells to the myofibroblastic phenotype.44 In lung fibrosis, Shh is upregulated in airway epithelial cells, and Ptch1 expression is increased in the pulmonary interstitium.45 We have shown that kidney pericytes respond to Hh ligands during injury (Figure 5B,C, Fabian and Humphreys, unpublished observations). Collectively, these results suggest that mesenchymal cells may be targets of Hh signaling in adult disease, just as they are in development.
Recently, proof of principle for targeting myofibroblasts in pathologic states was provided by Olive and colleagues in a mouse pancreatic cancer model.40 This tumor is characterized by a dense stromal cell/myofibroblast matrix, with deficient vasculature. These authors showed that tumor-derived Hh ligand drove myofibroblast proliferation in this model. Inhibiting Hh-Gli signaling with a specific inhibitor (IPI-926)46 decreased tumor stroma and enhanced vasculature – allowing more efficient delivery of chemotherapy. Whether such a strategy might alleviate interstitial fibrosis in diabetic nephropathy is of course an unexplored question, but the concept of targeting myofibroblasts appears to be a promising one and deserves further attention.
TRANSFORMING GROWTH FACTOR-β
The pro-fibrotic cytokine TGFβ is a central mediator of renal fibrosis and abundant data implicate it as a critical mediator of diabetic nephropathy.47, 48 TGFβ is markedly increased in all diabetic nephropathy animal models as well as human biopsies of diabetic nephropathy,49, 50 Mice that overexpress TGFβ51 or are given exogenous TGFβ52 develop renal fibrosis. Antibodies against either TGFβ53 or its receptor54 reduce fibrotic disease including in animal models of diabetic nephropathy,55 as does genetic deletion of the TGFβ effector Smad3.56, 57
Despite intense investigation, the mechanisms by which TGFβ induces interstitial fibrosis are not clearly understood and even the renal cell types that respond to TGFβ are debated. In epithelial cells, TGFβ exposure induces expression of mesenchymal proteins and downregulates E-cadherin, so for many years it was believed that TGFβ induced EMT, whereby epithelial cells transdifferentiate into interstitial myofibroblasts and contribute to matrix secretion.58, 59 However, our results suggest that EMT does not occur in vivo in fibrotic renal disease28 although it occurs readily in vitro. Others have recently come to the same conclusion.60-62
These findings suggest that injured epithelia act in a paracrine fashion to activate pericyte proliferation and differentiation into myofibroblasts. Proximal tubular epithelium is perhaps the best characterized source of TGFβ in diabetic nephropathy.63, 64 Indeed, cell cycle arrest is an important up-stream contributor to the production of TGFβ.65 Canonical TGFβ signaling operates through the type I TGFβ receptor, activin linked kinase 5 (ALK5) heterodimerized with TGFβ receptor type II. ALK5 subsequently activates downstream Smad signaling, which triggers pericyte proliferation and myofibroblast differentiation.66 In addition, TGFβ is a direct inducer of fgf-2 in renal fibroblasts/pericytes 67, and fgf-2 is one of the most potent stimulators of renal fibroblast proliferation known.68 Studies in vitro have shown that the pro-proliferative effects of TGFβ are almost entirely mediated through autocrine fgf-2 production.69
PLATELET-DERIVED GROWTH FACTOR
The PDGF system consists of four ligand isoforms (PDGF-A, -B, -C and –D) and two receptors (PDGFR-α and –β). Known roles for PDGF signaling includes wound healing, fibrosis, atherosclerosis and cancer.70 The PDGF system is upregulated in multiple models of kidney fibrosis, including unilateral ureteral obstruction, Thy 1.1 glomerulonephritis, lupus and ischemia-reperfusion injury. Lassila et al. has shown that PDGF-B is strongly induced in streptozotocin-induced diabetes in ApoE knockout mice, and that inhibition of PDGFR signaling with imatinib reduced albuminuria and interstitial fibrosis.71 More recently, Chen et al. extended these observations, showing that in kidney fibrosis all four PDGF isoforms are induced broadly throughout kidney, with PDGFR-α and –β expression expressed exclusively in pericytes and myofibroblasts. Inhibition of PDGF signaling, either by imatinib, or neutralizing PDGFR antibodies, attenuated macrophage infiltration and fibrosis.72 These studies provide encouragement that the pericyte PDGF system represents a viable therapeutic target in diabetic nephropathy.
CONNECTIVE TISSUE GROWTH FACTOR
CTGF, also called CCN2, is a member of the CCN family of matricellular proteins, and is a potent inducer of extracellular matrix expression and has complex effects on angiogenesis. CTGF binds integrins including integrin β173 and heparan sulfate proteoglycans in a cell-specific manner, to promote adhesion as well as fibrogenesis. Mice deficient in integrin β1 exhibit abnormal pericyte migration, do not form proper contacts to endothelium and have defects in microvascular stability.74 Its expression is induced by TGFβ, but CTGF also enhances TGF-β-mediated signaling. In addition, CTGF can be induced independent of TGFβ by advanced-glycation end-products (AGE), important by-products of the hyperglycemic environment. CTGF is linked to pericytes because they can be induced to express CTGF by injury, and pericyte-derived CTGF subsequently inhibits angiogenesis by binding VEGF and by promoting fibrosis through modulation of the activity of other growth factors important in fibrosis (reviewed in 75).
Much evidence links increased CTGF expression to diabetic nephropathy, as both plasma and urine levels of CTGF correlate with both albuminuria and renal progression in diabetic nephropathy.76, 77 Furhtermore, growing evidence indicates that blockade of CTGF ameliorates fibrosis, and that this effect is through inhibition of myofibroblast formation. Blockade of CTGF by neutralizing antibody or siRNA reduced aSMA expression and fibrosis in a lung injury model,78 and CTGF conditional deletion inhibited skin fibrosis also with reduced myofibroblast numbers.79 A phase I trial investigating the safety of an anti-CTGF antibody for treatment of diabetic nephropathy was recently completed, and a phase II trial is currently underway.80
TRANSLATION TO HUMAN DIABETIC NEPHROPATHY
Since diabetes is a disease characterized by clinical silence for many years, during which time fibrosis develops, it is clear that translation to the clinic will require better identification of patients at risk of progressing. This will include biomarkers, as described elsewhere in this issue, and imaging modalities, to diagnose and track diabetic nephropathy earlier and quantitatively. While anti-CTGF therapies are already in human trials to treat diabetic nephropathy, a number of targeted therapies are being tested in humans for other indications but could be useful in targeting pericytes during diabetic nephropathy. Imatinib inhibits both PDGFRβ and c-abl and is widely used to treat gastrointestinal stromal tumors, but preclinical evidence indicates it has potential efficacy in slowing progression of diabetic nephropathy.71 A variety of hedgehog inhibitors are in clinical trials at present, where their activity in treating cancers such as basal cell carcinoma or medulloblastoma is being assessed. Whether such agents might be effective in slowing the progression of diabetic nephropathy is an open question.
CONCLUSIONS
New therapeutic approaches to treat diabetic nephropathy are urgently needed. The pericyte has recently gained attention as an important cell in development of kidney fibrosis, and inhibiting the differentiation of pericytes into scar-forming myofibroblasts holds promise as a new strategy to prevent diabetic nephropathy progression.
Acknowledgements
I thank Brian Eyden, PhD, for electron micrographs, and the Humphreys lab for spirited discussions. This work was supported by National Institutes of Health grant DK088923 and a grant from the Harvard Stem Cell Institute.
References
- 1.Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968;2:363–366. doi: 10.1016/s0140-6736(68)90589-8. [DOI] [PubMed] [Google Scholar]
- 2.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20:1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 3.Katz A, Caramori ML, Sisson-Ross S, et al. An increase in the cell component of the cortical interstitium antedates interstitial fibrosis in type 1 diabetic patients. Kidney Int. 2002;61:2058–2066. doi: 10.1046/j.1523-1755.2002.00370.x. [DOI] [PubMed] [Google Scholar]
- 4.Fioretto P, Mauer M, Brocco E, et al. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia. 1996;39:1569–1576. doi: 10.1007/s001250050616. [DOI] [PubMed] [Google Scholar]
- 5.Essawy M, Soylemezoglu O, Muchaneta-Kubara EC, et al. Myofibroblasts and the progression of diabetic nephropathy. Nephrol Dial Transplant. 1997;12:43–50. doi: 10.1093/ndt/12.1.43. [DOI] [PubMed] [Google Scholar]
- 6.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens. 2004;13:1–7. doi: 10.1097/00041552-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Fioretto P, Steffes MW, Sutherland DE, et al. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339:69–75. doi: 10.1056/NEJM199807093390202. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Flores L, Gutierrez R, Madrid JF, et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 9.Courtnoy P, Boyles J. Fibronectin in the microvasculature: Localization in the pericyte-endothelial interstitium. J Ultrastruct Res. 1983;83:258–273. doi: 10.1016/s0022-5320(83)90133-8. [DOI] [PubMed] [Google Scholar]
- 10.Rouget C. Memoire sur le developpement, la structure et les proprietes physiologiques des capillaries sanguins et lymphatiques. Arch Physiol Norm Pathol. 1873;5:603–663. [Google Scholar]
- 11.Zimmerman K. Der feinere bau der blutcapillaren. Z Anat Entwicklungsgeschichte. 1923;68:29–36. [Google Scholar]
- 12.Takahashi-Iwanaga H. The three-dimensional cytoarchitecture of the interstitial tissue in the rat kidney. Cell Tissue Res. 1991;264:269–281. doi: 10.1007/BF00313964. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto K. Pericyte-endothelial gap junctions in developing rat cerebral capillaries: a fine structural study. Anat Rec. 1995;242:562–565. doi: 10.1002/ar.1092420412. [DOI] [PubMed] [Google Scholar]
- 14.Kaissling B, Hegyi I, Loffing J, et al. Morphology of interstitial cells in the healthy kidney. Anat Embryol (Berl) 1996;193:303–318. doi: 10.1007/BF00186688. [DOI] [PubMed] [Google Scholar]
- 15.Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest. 2011;121:468–474. doi: 10.1172/JCI44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asada N, Takase M, Nakamura J, et al. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest. 2011;121:3981–3990. doi: 10.1172/JCI57301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park F, Mattson DL, Roberts LA, et al. Evidence for the presence of smooth muscle alpha-actin within pericytes of the renal medulla. Am J Physiol. 1997;273:R1742–1748. doi: 10.1152/ajpregu.1997.273.5.R1742. [DOI] [PubMed] [Google Scholar]
- 18.Pallone TL, Silldorff EP. Pericyte regulation of renal medullary blood flow. Exp Nephrol. 2001;9:165–170. doi: 10.1159/000052608. [DOI] [PubMed] [Google Scholar]
- 19.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Goritz C, Dias DO, Tomilin N, et al. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 21.Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol. 1991;113:147–154. doi: 10.1083/jcb.113.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin SL, Kisseleva T, Brenner DA, et al. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundberg C, Ljungstrom M, Lindmark G, et al. Microvascular pericytes express platelet-derived growth factor-beta receptors in human healing wounds and colorectal adenocarcinoma. Am J Pathol. 1993;143:1377–1388. [PMC free article] [PubMed] [Google Scholar]
- 24.Eyden B. The myofibroblast: a study of normal, reactive and neoplastic tissues, with an emphasis on ultrastructure. part 2 - tumours and tumour-like lesions. J Submicrosc Cytol Pathol. 2005;37:231–296. [PubMed] [Google Scholar]
- 25.Gabbiani G. The cellular derivation and the life span of the myofibroblast. Pathol Res Pract. 1996;192:708–711. doi: 10.1016/S0344-0338(96)80092-6. [DOI] [PubMed] [Google Scholar]
- 26.Rajkumar VS, Howell K, Csiszar K, et al. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res Ther. 2005;7:R1113–1123. doi: 10.1186/ar1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. The American journal of pathology. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grgic I, Duffield JS, Humphreys BD. The origin of interstitial myofibroblasts in chronic kidney disease. Pediatr Nephrol. 2011 doi: 10.1007/s00467-011-1772-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeisberg EM, Potenta SE, Sugimoto H, et al. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rock JR, Barkauskas CE, Cronce MJ, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 33.Mao J, Kim BM, Rajurkar M, et al. Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract. Development. 2010;137:1721–1729. doi: 10.1242/dev.044586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002;129:5301–5312. doi: 10.1242/dev.129.22.5301. [DOI] [PubMed] [Google Scholar]
- 35.Cain JE, Rosenblum ND. Control of mammalian kidney development by the Hedgehog signaling pathway. Pediatr Nephrol. 2010 doi: 10.1007/s00467-010-1704-x. [DOI] [PubMed] [Google Scholar]
- 36.Hu MC, Mo R, Bhella S, et al. GLI3-dependent transcriptional repression of Gli1, Gli2 and kidney patterning genes disrupts renal morphogenesis. Development. 2006;133:569–578. doi: 10.1242/dev.02220. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki H, Nishizaki Y, Hui C, et al. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999;126:3915–3924. doi: 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- 38.Tian H, Callahan CA, DuPree KJ, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci U S A. 2009;106:4254–4259. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yauch RL, Gould SE, Scales SJ, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 40.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin K, Lee J, Guo N, et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472:110–114. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Omenetti A, Porrello A, Jung Y, et al. Hedgehog signaling regulates epithelial mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118:3331–3342. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Syn WK, Choi SS, Liaskou E, et al. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology. 2011;53:106–115. doi: 10.1002/hep.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi SS, Omenetti A, Witek RP, et al. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1093–1106. doi: 10.1152/ajpgi.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart GA, Hoyne GF, Ahmad SA, et al. Expression of the developmental Sonic hedgehog (Shh) signalling pathway is up-regulated in chronic lung fibrosis and the Shh receptor patched 1 is present in circulating T lymphocytes. J Pathol. 2003;199:488–495. doi: 10.1002/path.1295. [DOI] [PubMed] [Google Scholar]
- 46.Tremblay MR, Lescarbeau A, Grogan MJ, et al. Discovery of a potent and orally active hedgehog pathway antagonist (IPI-926). J Med Chem. 2009;52:4400–4418. doi: 10.1021/jm900305z. [DOI] [PubMed] [Google Scholar]
- 47.Ziyadeh FN, Sharma K. Overview: combating diabetic nephropathy. J Am Soc Nephrol. 2003;14:1355–1357. doi: 10.1097/01.asn.0000065608.37756.58. [DOI] [PubMed] [Google Scholar]
- 48.Zhu Y, Usui HK, Sharma K. Regulation of transforming growth factor beta in diabetic nephropathy: implications for treatment. Seminars in nephrology. 2007;27:153–160. doi: 10.1016/j.semnephrol.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto T, Nakamura T, Noble NA, et al. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakharova OV, Taal MW, Brenner BM. Pathogenesis of diabetic nephropathy: focus on transforming growth factor-beta and connective tissue growth factor. Current opinion in nephrology and hypertension. 2001;10:727–738. doi: 10.1097/00041552-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Kopp JB, Factor VM, Mozes M, et al. Transgenic mice with increased plasma levels of TGF-beta 1 develop progressive renal disease. Laboratory investigation; a journal of technical methods and pathology. 1996;74:991–1003. [PubMed] [Google Scholar]
- 52.Ledbetter S, Kurtzberg L, Doyle S, et al. Renal fibrosis in mice treated with human recombinant transforming growth factor-beta2. Kidney international. 2000;58:2367–2376. doi: 10.1046/j.1523-1755.2000.00420.x. [DOI] [PubMed] [Google Scholar]
- 53.Border WA, Okuda S, Languino LR, et al. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor β1. Nature. 1990;346:371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- 54.Kasuga H, Ito Y, Sakamoto S, et al. Effects of anti-TGF-beta type II receptor antibody on experimental glomerulonephritis. Kidney international. 2001;60:1745–1755. doi: 10.1046/j.1523-1755.2001.00990.x. [DOI] [PubMed] [Google Scholar]
- 55.Zimmerman BJ, Holt JW, Paulson JC, et al. Molecular determinants of lipid mediator-induced leukocyte adherence and emigration in rat mesenteric venules. Am J Physiol. 1994;266:H847–H853. doi: 10.1152/ajpheart.1994.266.3.H847. [DOI] [PubMed] [Google Scholar]
- 56.Sato M, Muragaki Y, Saika S, et al. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. The Journal of clinical investigation. 2003;112:1486–1494. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Inazaki K, Kanamaru Y, Kojima Y, et al. Smad3 deficiency attenuates renal fibrosis, inflammation,and apoptosis after unilateral ureteral obstruction. Kidney international. 2004;66:597–604. doi: 10.1111/j.1523-1755.2004.00779.x. [DOI] [PubMed] [Google Scholar]
- 58.Burns WC, Twigg SM, Forbes JM, et al. Connective tissue growth factor plays an important role in advanced glycation end product-induced tubular epithelial-to-mesenchymal transition: implications for diabetic renal disease. J Am Soc Nephrol. 2006;17:2484–2494. doi: 10.1681/ASN.2006050525. [DOI] [PubMed] [Google Scholar]
- 59.Hills CE, Squires PE. TGF-beta1-induced epithelial-to-mesenchymal transition and therapeutic intervention in diabetic nephropathy. American journal of nephrology. 2010;31:68–74. doi: 10.1159/000256659. [DOI] [PubMed] [Google Scholar]
- 60.Bielesz B, Sirin Y, Si H, et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. The Journal of clinical investigation. 2010;120:4040–4054. doi: 10.1172/JCI43025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Endo T, Okuda T, Nakamura J, et al. Exploring the Origin of the Cells Responsible for Regeneration and Fibrosis in the Kidneys. J Am Soc Nephrol. 2010;21:36A. (Abstract) [Google Scholar]
- 62.Koesters R, Kaissling B, Lehir M, et al. Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. The American journal of pathology. 2010;177:632–643. doi: 10.2353/ajpath.2010.091012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fraser D, Brunskill N, Ito T, et al. Long-term exposure of proximal tubular epithelial cells to glucose induces transforming growth factor-beta 1 synthesis via an autocrine PDGF loop. The American journal of pathology. 2003;163:2565–2574. doi: 10.1016/s0002-9440(10)63611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang M, Fraser D, Phillips A. ERK, p38, and Smad signaling pathways differentially regulate transforming growth factor-beta1 autoinduction in proximal tubular epithelial cells. The American journal of pathology. 2006;169:1282–1293. doi: 10.2353/ajpath.2006.050921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang L, Besschetnova TY, Brooks CR, et al. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–543. 531, 143. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Geest RJ, Klaassen I, Vogels IM, et al. Differential TGF-{beta} signaling in retinal vascular cells: a role in diabetic retinopathy? Invest Ophthalmol Vis Sci. 2010;51:1857–1865. doi: 10.1167/iovs.09-4181. [DOI] [PubMed] [Google Scholar]
- 67.Strutz F, Zeisberg M, Renziehausen A, et al. TGF-beta 1 induces proliferation in human renal fibroblasts via induction of basic fibroblast growth factor (FGF-2). Kidney international. 2001;59:579–592. doi: 10.1046/j.1523-1755.2001.059002579.x. [DOI] [PubMed] [Google Scholar]
- 68.Strutz F, Zeisberg M, Hemmerlein B, et al. Basic fibroblast growth factor expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney international. 2000;57:1521–1538. doi: 10.1046/j.1523-1755.2000.00997.x. [DOI] [PubMed] [Google Scholar]
- 69.Vasko R, Koziolek M, Ikehata M, et al. Role of basic fibroblast growth factor (FGF-2) in diabetic nephropathy and mechanisms of its induction by hyperglycemia in human renal fibroblasts. American journal of physiology. 2009;296:F1452–1463. doi: 10.1152/ajprenal.90352.2008. [DOI] [PubMed] [Google Scholar]
- 70.Floege J, Eitner F, Alpers CE. A new look at platelet-derived growth factor in renal disease. J Am Soc Nephrol. 2008;19:12–23. doi: 10.1681/ASN.2007050532. [DOI] [PubMed] [Google Scholar]
- 71.Lassila M, Jandeleit-Dahm K, Seah KK, et al. Imatinib attenuates diabetic nephropathy in apolipoprotein E-knockout mice. J Am Soc Nephrol. 2005;16:363–373. doi: 10.1681/ASN.2004050392. [DOI] [PubMed] [Google Scholar]
- 72.Chen YT, Chang FC, Wu CF, et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011;80:1170–1181. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]
- 73.Chen Y, Abraham DJ, Shi-Wen X, et al. CCN2 (connective tissue growth factor) promotes fibroblast adhesion to fibronectin. Mol Biol Cell. 2004;15:5635–5646. doi: 10.1091/mbc.E04-06-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abraham S, Kogata N, Fassler R, et al. Integrin beta1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. Circ Res. 2008;102:562–570. doi: 10.1161/CIRCRESAHA.107.167908. [DOI] [PubMed] [Google Scholar]
- 75.van Nieuwenhoven FA, Jensen LJ, Flyvbjerg A, et al. Imbalance of growth factor signalling in diabetic kidney disease: is connective tissue growth factor (CTGF, CCN2) the perfect intervention point? Nephrol Dial Transplant. 2005;20:6–10. doi: 10.1093/ndt/gfh570. [DOI] [PubMed] [Google Scholar]
- 76.Nguyen TQ, Tarnow L, Andersen S, et al. Urinary connective tissue growth factor excretion correlates with clinical markers of renal disease in a large population of type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2006;29:83–88. doi: 10.2337/diacare.29.1.83. [DOI] [PubMed] [Google Scholar]
- 77.Nguyen TQ, Tarnow L, Jorsal A, et al. Plasma connective tissue growth factor is an independent predictor of end-stage renal disease and mortality in type 1 diabetic nephropathy. Diabetes Care. 2008;31:1177–1182. doi: 10.2337/dc07-2469. [DOI] [PubMed] [Google Scholar]
- 78.Ponticos M, Holmes AM, Shi-wen X, et al. Pivotal role of connective tissue growth factor in lung fibrosis: MAPK-dependent transcriptional activation of type I collagen. Arthritis Rheum. 2009;60:2142–2155. doi: 10.1002/art.24620. [DOI] [PubMed] [Google Scholar]
- 79.Liu S, Shi-wen X, Abraham DJ, et al. CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthritis Rheum. 2011;63:239–246. doi: 10.1002/art.30074. [DOI] [PubMed] [Google Scholar]
- 80.Adler SG, Schwartz S, Williams ME, et al. Phase 1 study of anti-CTGF monoclonal antibody in patients with diabetes and microalbuminuria. Clin J Am Soc Nephrol. 2010;5:1420–1428. doi: 10.2215/CJN.09321209. [DOI] [PMC free article] [PubMed] [Google Scholar]