Abstract
Background
A prospective cohort study was conducted to characterize the temporal sequence of microbial and inflammatory events immediately preceding Escherichia coli recurrent urinary tract infection (rUTI).
Methods
Women with acute cystitis and a history of UTI within the previous year self-collected periurethral and urine samples daily and recorded measurements of urine leukocyte esterase, symptoms, and sexual intercourse daily for 3 months. rUTI strains were characterized by pulsed-field gel electrophoresis and genomic virulence profiling. Urinary cytokine levels were measured.
Results
There were 38 E. coli rUTIs in 29 of 104 women. The prevalence of periurethral rUTI strain carriage increased from 46% to 90% during the 14 days immediately preceding rUTI, with similar increases in same-strain bacteriuria (from 7% to 69%), leukocyte esterase (from 31% to 64%), and symptoms (from 3% to 43%), most notably 2–3 days before rUTI (P < .05 for all comparisons). Intercourse with periurethral carriage of the rUTI strain also increased before rUTI (P = .008). Recurrent UTIs preceded by bacteriuria, pyuria, and symptoms were caused by strains less likely to have P fimbriae than other rUTI strains (P = .002).
Conclusions
Among women with frequent rUTIs, the prevalences of periurethral rUTI strain carriage, bacteriuria, pyuria, and intercourse dramatically increase over the days preceding rUTI. A better understanding of the pathogenesis of rUTI will lead to better prevention strategies.
Acute uncomplicated urinary tract infections (UTIs) affect millions of women each year and carry an annual cost to society of >$1.6 billion [1]. At least one-quarter of women with UTIs will experience recurrent UTIs (rUTIs) with repeated clinical evaluation, antibiotic use, and morbidity [2]. Despite the high incidence and significant clinical and economic impact of rUTI on society, our understanding of the pathogenesis of rUTI is incomplete.
Uncomplicated rUTI has traditionally been thought to result from repeated ascending infection of the bladder by intestinal bacteria (most commonly Escherichia coli) that colonize the vaginal vestibule and distal urethra in large numbers [3–10]. Bacterial strains that cause repeat infections may reside in the intestine, vagina, or periurethra between episodes of infection [11–14]. Newer evidence from mouse models suggests that E. coli also lie dormant within a bladder mucosal reservoir and later emerge to cause reinfection [15–18]. To better understand the pathogenesis of rUTI, however, a more detailed description of the temporal sequence of microbial and inflammatory events immediately preceding the onset of rUTI is needed. We therefore prospectively studied microbial characteristics, periurethral uropathogen carriage, bacteriuria, the urinary inflammatory response, symptoms, and sexual intercourse just before E. coli rUTI in women.
METHODS
Study subjects
From January 2003 through December 2006, premenopausal women aged 18–49 years with a self-reported history of at least 1 UTI during the past year and a current diagnosis of acute cystitis (dysuria, frequency, or urgency with a concentration of a uropathogen in urine ≥1 × 102 colony-forming units [CFUs]/mL) were recruited at the student health center of the University of Washington. Exclusion criteria included known anatomic or functional abnormalities of the urinary tract, chronic illness requiring medical supervision, pregnancy or planned pregnancy during the next 3 months, and symptoms or signs of acute pyelonephritis.
Study design
The study design was approved by the Human Subjects Review Committee at the University of Washington, and all subjects provided written informed consent. During the enrollment UTI evaluation, subjects provided a medical history and underwent genitourinary examination. Periurethral, midstream urine, and rectal specimens were collected before treatment of their enrollment UTI. Subjects were then taught proper techniques for daily home collection and storage of periurethral and urine specimens and for performance of daily dipstick measurements of urine leukocyte esterase (Multistix; Bayer) and were instructed to record all episodes of sexual intercourse, urinary symptoms (dysuria, frequency, or urgency), and the leukocyte esterase reading (0–3+) in a daily diary. Subjects were followed for 3 months and were asked to return to the clinic monthly and whenever they felt that they had a rUTI. Investigators were available by telephone on weekends. Follow-up clinic visits were similar to the enrollment visit.
E. coli rUTI was defined as the presentation of a subject to the clinic for medical evaluation of symptoms of acute cystitis (dysuria, frequency, or urgency) with a concentration of E. coli in urine ≥1 × 102 CFUs/mL, regardless of the enrollment UTI urine isolate [19]. If E. coli was not isolated, the organism present in the urine at the greatest quantity was considered to be the causal uropathogen. The term 14-day window refers to the 14 days before and the day of rUTI. The term outside the 14-day window refers to all follow-up days >14 days before or after any UTI among all women in the analysis cohort. Women who fulfilled all of the criteria for UTI (symptoms, bacteriuria, and pyuria) but did not attend the clinic were classified as having what we have termed preclinical UTI.
Specimen collection and processing
Periurethral specimens were collected using a sterile, cotton-tipped swab and placed in a BBL Port-A-Cul specimen collection and transportation tube (Becton Dickinson) containing a reduced transport medium. Clean-catch midstream urine specimens were collected in a sterile container at the clinic or in a sterile BD Vacutainer urine tube (Becton Dickinson) containing a lyophilized preservative including boric acid and sodium formate at home. Rectal specimens were collected monthly at the clinic using a sterile, rayon-tipped swab and placed in Amies medium (BBL CultureSwab Plus; Becton Dickinson). All clinical specimens were refrigerated and transported to the laboratory within 24 h. Home specimens were stored at room temperature and were brought to the clinic by the subject or were transported to the laboratory by courier within 72 h.
Specimens were inoculated onto blood agar, MacConkey agar, and colistin–nalidixic acid plates. Plates were incubated at 37°C for 48 h, and organisms were further identified using standard methods [20]. All potential uropathogens were identified from periurethral cultures. All uropathogens present in midstream urine at ≥1 × 102 CFUs/mL in clinical specimens and at ≥1 × 103 CFUs/mL in home specimens were identified and quantified. E. coli were characterized as β-hemolytic or nonhemolytic. Periurethral and urine isolates were stored at 4°C for 14 days. When E. coli rUTI occurred, all stored peri-urethral and urinary E. coli isolates from the preceding 14 days with the same hemolysis pattern as the subject’s rUTI urine isolate were stocked at −70°C. From rectal specimen cultures, 1 colony of each morphotype of gram-negative rod and a sweep of gram-positive isolates were stocked at −70°C.
Pulsed-field gel electrophoresis (PFGE) was used to characterize the causative E. coli rUTI strain isolated from urine on the day subjects presented for medical evaluation of rUTI. PFGE was also used to identify the first appearance of the rUTI strain in the periurethra and urine within the 14-day window. All subsequent E. coli isolates from the periurethra and urine, respectively, recovered before the rUTI and having the same hemolysis pattern as the rUTI strain were assumed to also be the rUTI strain. PFGE was also performed on enrollment urine E. coli isolates and on 1 colony of each morphotype (or at least 3 colonies) of E. coli from the rectal specimen collected during the most recent clinic visit previous to the rUTI visit. PFGE methods have been published elsewhere [21]. All E. coli strains causing enrollment UTIs and rUTIs were tested for genetic determinants of selected virulence factors known to be associated with UTI (P fimbriae, hemolysin, cytotoxic necrotizing factor, iron-regulated gene A homologue adhesin, Dr-binding adhesin, and S fimbriae) by an established multiplex polymerase chain reaction–based assay [22, 23].
Urine (500 μL) from midstream urine specimens collected on the day of rUTI and on the 3 preceding days were stored at −70°C for later testing for the cytokines interleukin 6 (IL-6), interleukin 8 (IL-8), CXCL-1, CXCL-5, CXCL-6, and intercellular adhesion molecule 1 (ICAM-1). These cytokines all have been associated with the early stages of mucosal inflammation [24–26]. Before testing, specimens were centrifuged at 13,250 g for 2 min, and the supernatants were removed for analysis. The supernatant was subjected to a sandwich enzyme-linked immunosorbent assay technique (Duoset; R&D Systems).
Effect of storage on culture and cytokine results
Subjects submitted 77%, 92%, and 97% of home-collected urine specimens to the research clinic within 1, 2, and 3 days, respectively, of collection. Experiments were performed to assess the quality of the home-collected urine and periurethral specimens. First, E. coli was isolated by culture from urine specimens collected during routine clinic visits at a rate (9%) similar to those for home-collected specimens that arrived in the laboratory after 1 (8%), 2 (8%), and 3 (6%) days of home storage at room temperature. Second, the quantity of E. coli isolated from paired urine specimens collected at home and at the clinic on the same day revealed 88% agreement in log CFUs per milliliter. Third, in separate pilot experiments in which aliquots of urine were cultured on the day of collection and after 1, 2, and 3 days of storage in boric acid and sodium formate preservative at room temperature, there was no trend toward an increasing or decreasing quantity of E. coli with storage. However, similar experiments with periurethral specimens showed that semiquantitative counts of E. coli increased with each day of storage in the transport medium. Thus, analyses of periurethral culture results used qualitative rather than semiquantitative results. Levels of urinary cytokines were not significantly altered when freshly collected samples were compared with those stored in preservative for up to 7 days at room temperature or 4°C.
Statistical analysis
The analysis cohort consisted of women who met the enrollment criteria and had at least 1 day of follow-up. The unit of analysis was rUTI, because analyses of the data using study subject as the unit of analysis revealed no patient-specific biases. Analyses focused on the 14-day windows before E. coli rUTIs. Variables were analyzed by subject-day within the 14-day windows. Comparison days included all subject-days outside the 14-day windows among women who had E. coli rUTI and among women who did not experience rUTI. Subject-days from women with rUTIs caused by bacteria other than E. coli were not included in the comparison group.
The McNemar test was used to evaluate the change in point prevalence of selected variables in the 14-day windows. The sign test was used to compare median urine cytokine values on day −3 and the day of rUTI. The Fisher exact test was used for the comparison of virulence profiles.
RESULTS
Description of cohort
Of 114 women initially enrolled in the study, 8 had a negative enrollment urine culture, and 2 had no follow-up. Thus, there were 104 women in the analysis cohort (table 1). Eighty-five percent of enrollment UTIs were caused by E. coli. Median follow-up was 90 days (range, 3–106 days), and 80% of women were followed up for >60 days.
Table 1.
Selected Characteristics of Study Subjects (n =104)
| Characteristic | Value |
|---|---|
| Age, median (range), years | 22 (18–41) |
| Race | |
| White | 77 (74) |
| Asian | 17 (16) |
| Other | 10 (10) |
| Hispanic | 5 (5) |
| At least some college | 101 (97) |
| Never married | 82 (79) |
| Never pregnant | 98 (94) |
| ≥3 lifetime UTIs | 91 (88)a |
| No. of UTIs in past year, median (range) | 3 (2–9)a |
| Months since most recent UTI before enrollment, median (range) | 2 (<1 to 11) |
| Antibiotic at enrollment | |
| Trimethoprim-sulfamethoxazole | 83 (80) |
| Nitrofurantoin | 18 (17) |
| Ciprofloxacin or other | 3 (3) |
| Sexually active | 104 (100) |
| Birth control methodsb | |
| Hormonal | 63 (61) |
| Condom | 50 (48) |
| Other | 6 (6) |
NOTE. Data are no. (%) of subjects, unless otherwise indicated. UTI, urinary tract infection.
Including the UTI at enrollment.
Percentages total >100% because some women used multiple birth control methods.
Thirty-six of 104 women had a total of 49 rUTIs. E. coli caused 38 rUTIs (78%) among 29 women. Seven women, including 1 who also had an E. coli rUTI, had 10 rUTIs caused by bacteria other than E. coli, and 1 woman was treated for rUTI at another facility, from which culture results were unavailable. By PFGE, 25 E. coli rUTIs (67%) were caused by a strain that had been documented to cause the enrollment UTI or a previous rUTI in that subject during the study.
Temporal sequence of events preceding E. coli rUTI
There were 489 subject-days included within the 14-day windows preceding the 38 E. coli rUTIs. Periurethral specimens, urine specimens, diary-reported symptom data, and diary-reported sexual intercourse data were available for 93%–99% of subject-days within the 14-day windows, and leukocyte esterase measurements were available for 87%. There were 5289 subject-days outside the 14-day windows for comparison, including 953 subject-days among women with E. coli rUTI and 4336 subject-days among women who did not experience rUTI. Culture and diary reported data were available for 92%–96% of comparison days.
The prevalence of any growth of periurethral carriage of the rUTI strain was 46% fourteen days before E. coli rUTI, after which it increased steadily to 90% one day before rUTI (P < .001 for day −14 vs day −1) (figure 1). In comparison, peri-urethral carriage of E. coli (any strain) was present on 55% of subject-days outside the 14-day windows. The rUTI strain was cultured from the feces before 29 (78%) of 37 E. coli rUTIs.
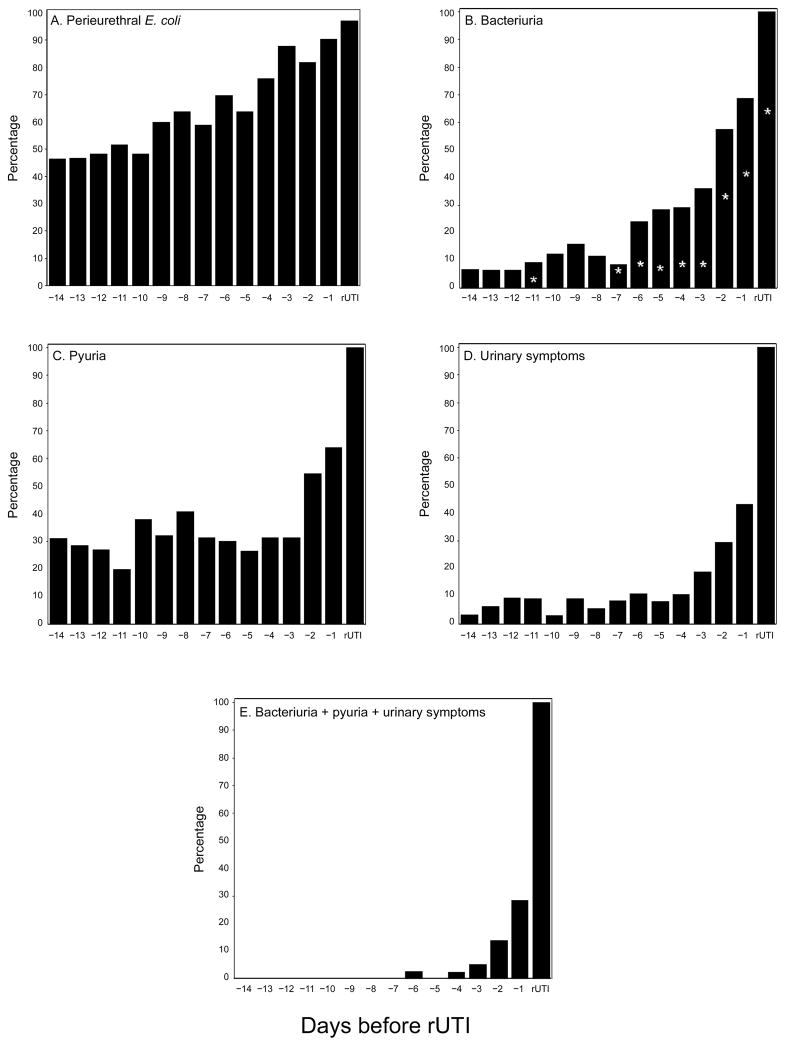
Figure 1.
Point prevalence of the recurrent urinary tract infection (rUTI) strain during the 14 days before Escherichia coli rUTI (n = 38) for any growth in the periurethra (P < .001 for day −14 vs day −1) (A); ≥1 × 103 colony-forming units (CFUs)/mL in urine (P < .001 for day −14 vs day −1; asterisks denote ≥1 × 105 CFUs/mL) (B); trace levels of urine leukocyte esterase or more (P = .04 for day −14 vs day −1) (C); dysuria, frequency, or urgency (P < .001 for day −14 vs day −1) (D); and the combination of bacteriuria, pyuria, and urinary symptoms (preclinical UTI; P = .004 for day −14 vs day −1) (E). Data are the percentage of women with the indicated characteristic on the days before E. coli rUTI. P values were determined by the McNemar test.
The prevalence of bacteriuria with a concentration of the rUTI strain ≥1 × 103 CFUs/mL was 7% fourteen days before E. coli rUTI, after which it began a steady rise during the week before rUTI and then increased dramatically over 2–3 days to 69% one day before rUTI (P < .001 for day −14 vs day −1) (figure 1). The prevalence of bacteriuria with a concentration of the rUTI strain ≥1 × 105 CFUs/mL also increased dramatically over 2–3 days to 43% one day before rUTI (P < .001) (figure 1). In comparison, bacteriuria with E. coli (any strain) at a concentration ≥1 × 103 CFUs/mL and ≥1 × 105 CFUs/mL was present on 7.4% and 0.9%, respectively, of subject-days outside the 14-day windows.
The prevalence of any detectable urine leukocyte esterase was 31% fourteen days before E. coli rUTI but increased dramatically over 2–3 days to 64% one day before rUTI (P = .04 for day −14 vs day −1) (figure 1). In comparison, urine leukocyte esterase was present on 21% of subject-days outside the 14-day windows. The prevalence of reported symptoms of dysuria, frequency, or urgency was 3% fourteen days before E. coli rUTI and remained low until dramatically increasing over 2–3 days to 43% one day before rUTI (P < .001 for day −14 vs day −1) (figure 1). Symptoms were reported on only 4% of subject-days outside the 14-day windows.
Eleven women had the combination of E. coli bacteriuria with a concentration of the rUTI strain ≥1 × 103 CFUs/mL, pyuria, and urinary symptoms before presentation to the clinic (preclinical UTI) within the 14-day window, including 17 subject-days within 2–3 days before rUTI in 10 women (P = .004 for day −14 vs day −1) (figure 1). These occurrences were not temporally related to closure of the clinic on weekends, and there was no clear trend toward increasing or decreasing symptom severity on these days compared with rUTI days. Strains of E. coli that caused rUTI preceded by preclinical UTI were significantly less likely to have P fimbriae than were strains that caused rUTI without prior preclinical UTI (0/11 vs 14/26; P = .002), and there was a strong trend in the same direction for all of the other virulence determinants tested for (table 2). There were only 8 subject-days outside the 14-day windows on which a woman had E. coli bacteriuria, pyuria, and symptoms.
Table 2.
Proportion of Recurrent Urinary Tract Infection (rUTI) Escherichia coli Strains with Selected Virulence Determinants, by the Presence or Absence of Preclinical UTI before the rUTI
| Virulence determinant | Preclinical UTI | Pa | |
|---|---|---|---|
| No | Yes | ||
| P fimbriae | 14/26 (54) | 0/11 (0) | .002 |
| Hemolysin | 15/26 (58) | 3/11 (27) | .15 |
| Cytotoxic necrotizing factor | 14/26 (54) | 3/11 (27) | .17 |
| IrgA homologue adhesin | 6/26 (23) | 1/11 (9) | .65 |
| Afimbrial/Dr-binding adhesin | 7/24 (29) | 1/11 (9) | .39 |
| S fimbriae | 5/26 (19) | 1/11 (9) | .65 |
NOTE. Data are the proportion (%) of strains with the indicated virulence determinant. Preclinical UTI was defined as the occurrence of the combination of E. coli bacteriuria with ≥1 × 103 colony-forming units of the rUTI strain per milliliter, pyuria, and urinary symptoms on days before a subject presented to the research clinic for medical evaluation of her rUTI. IrgA, iron-regulated gene A.
Fisher exact test.
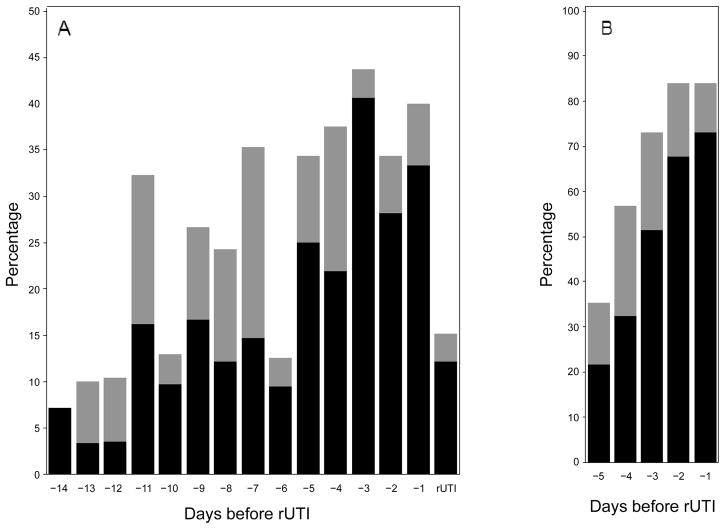
The prevalence of concurrent sexual intercourse and peri-urethral carriage of the rUTI strain was 7% fourteen days before E. coli rUTI and increased before rUTI (P = .008 for day −14 vs day −1), with a peak of 41% three days before rUTI (figure 2). In contrast, the combination of sexual intercourse and periurethral carriage of E. coli (any strain) occurred on only 14% of subject-days outside the 14-day windows. The cumulative prevalence of concurrent sexual intercourse and peri-urethral carriage of the rUTI strain within 5 days of rUTI was 73% (figure 2).
Figure 2.
A, Point prevalence of sexual intercourse either with (black) or without (gray) periurethral carriage of the recurrent urinary tract infection (rUTI) strain during the 14 days before Escherichia coli rUTI (P = .008 for intercourse with periurethral carriage for day −14 vs day −1). B, Cumulative prevalence of sexual intercourse either with (black) or without (gray) periurethral carriage of the rUTI strain during the 5 days before E. coli rUTI. Data are the percentage of women with the indicated characteristic on the days before E. coli rUTI. P values were determined by the McNemar test.
Median urine concentrations of IL-6 and IL-8 three days before the 18 E. coli rUTIs for which data were available from each of the preceding 3 days were 1.7 and 52 pg/mL, respectively. Urinary cytokine levels did not increase in a stepwise manner but rose dramatically on the day of rUTI to 9.3 and 519 pg/mL, respectively (P = .008 for IL-6 and P = .03 for IL-8 for day −3 vs day of rUTI) (figure 3). Outside the 14-day windows, median urinary concentrations of IL-6 and IL-8 were 1.7 and 32 pg/mL, respectively.
Figure 3.
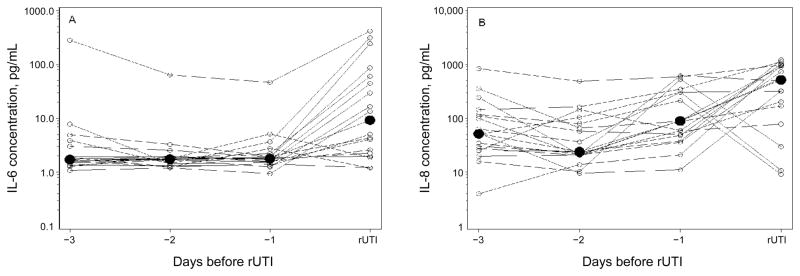
Concentrations of interleukin 6 (IL-6) (A) and interleukin 8 (IL-8) (B) in the urine during the 3 days before Escherichia coli recurrent urinary tract infection (rUTI) (n = 18). Individual (white circles) and median (black circles) concentrations are shown. P = .008 and P = .03 for changes in median concentrations from day −3 to the day of rUTI for IL-6 and IL-8, respectively. P values were determined by the sign test.
DISCUSSION
This is the first prospective study of the microbial, inflammatory, clinical, and behavioral events occurring immediately before E. coli rUTI in women. Although it has been widely accepted that periurethral carriage of the causative uropathogen generally precedes rUTI [3–10], we demonstrated that prolonged periurethral E. coli carriage often preceded a dramatic increase in the prevalence of bacteriuria, pyuria, and symptoms during the 2–3-day period before women presented with rUTI. These temporal trends were statistically significant, and the prevalences of these clinical and microbiological parameters were much higher during the latter days within the 14-day windows compared with subject-days outside the 14-day windows, suggesting that our findings accurately depict the sequence of events immediately preceding E. coli rUTI. rUTIs were diagnosed when women presented to the clinic with typical symptoms and a laboratory diagnosis was confirmed. However, analysis of self-collected laboratory and diary data indicated that 11 women developed symptoms, pyuria, and bacteriuria several days before their presentation to the clinic, a condition we have termed preclinical UTI. The study was not designed to query reasons for delayed presentation, but, given that no barriers to clinic presentation were identified, our findings may in part reflect a variable threshold for symptom tolerance. Interestingly, E. coli rUTI strains associated with pre-clinical UTI exhibited fewer virulence determinants than did those that caused a sudden onset of rUTI, and the pathogenic role played by preclinical UTI bears further investigation.
It remains uncertain what initiates this chain of events before rUTI. Sexual intercourse has been strongly linked in time and frequency to UTI [27–31], but previous studies have not examined the periurethral flora at the time of intercourse. In the present study, concurrent sexual intercourse and periurethral carriage with the rUTI strain preceded 73% of E. coli rUTIs within 5 days and peaked 3 days before presentation for medical evaluation. Sexual intercourse likely plays an important role in facilitating both periurethral colonization with E. coli and movement of the rUTI strain from the periurethra into the bladder [31–33]. However, even in this universally sexually active population, sexual intercourse was not universal before E. coli rUTI (nor did it always lead to rUTI), and thus further research is warranted to better define “triggers” of UTIs other than intercourse.
In a limited number of studies, urinary concentrations of IL-6 and IL-8 have been shown to be elevated at the time of UTI and have correlated with clinical symptoms and signs of inflammation [34–39]. However, these cytokines have not previously been measured in a systematic fashion on the days immediately preceding UTI. Median IL-6 and IL-8 concentrations concurrent with rUTI in the present study were similar to those previously found for cystitis [38–39]. However, urinary concentrations of IL-6 and IL-8 did not increase along with the presence of symptoms and pyuria before E. coli rUTI. It is possible that the assays used were insensitive to small or local changes sufficient to give rise to symptoms or that other cytokines are responsible for initiating the sequence of early events before rUTI. However, urinary concentrations of CXCL-1, CXCL-5, CXCL-6, and ICAM-1 also did not appear to correlate with the onset of symptoms and pyuria before rUTI (data not shown).
In accordance with the findings of previous studies [11–13], most E. coli rUTIs (67%) were repeat infections with the same strain, suggesting a persistent reservoir for infection. E. coli that cause rUTI have been found to reside in the fecal flora [12, 14, 40], as was found for 78% of E. coli rUTIs in this study, and periurethral carriage of intestinal E. coli before bacteriuria has been interpreted to support the ascending pathway to bladder infection. On the other hand, recent studies in mice have shown that E. coli may invade and replicate within bladder epithelial cells and reemerge later from quiescent reservoirs to cause infection [15–18]. In addition, there is new evidence that this pathway may occur in women with cystitis, in whom evidence of intracellular bacterial communities has been found in exfoliated cells in urine [41], and bacteria have been found in bladder epithelium between UTIs [42, 43]. Of note, we did observe the appearance of the rUTI strain in urine without prior periurethral carriage before 2 E. coli rUTIs (data not shown), a pattern potentially consistent with a bladder source of reinfection. However, our study design lacked the sensitivity to determine the role played by a bladder reservoir in the temporal development of these 2 or any of the 38 E. coli rUTIs observed in our study.
The strengths of this study include the prospective follow-up of a large cohort of women and their excellent compliance in the daily collection of data and specimens over a prolonged period, thus providing data on days affected and unaffected by rUTI. The capture of extensive data, including urinary cytokine levels, in the days leading up to rUTI and the use of PFGE and virulence profiling to molecularly characterize the rUTI strains allowed for detailed characterization of the sequence of microbial and inflammatory events preceding E. coli rUTI. The main limitation was the inability, based on costs, to quantify E. coli present at <1 × 103 CFUs/mL in self-collected midstream urine specimens and to routinely characterize every E. coli isolate within the 14-day window by PFGE. Because study subjects included women with a history of rUTI, the findings of this study may not necessarily reflect the events preceding sporadic UTI.
In conclusion, among women with relatively frequent rUTIs, the development of E. coli rUTI begins 2–3 days before many women present for medical evaluation. Sexual intercourse in the setting of periurethral colonization appears to be the most important trigger event for rUTI among young women. The existence of a bladder reservoir and its interplay with the intestinal reservoir as potential sources of periurethral colonization and bacteriuria must be defined further. Finally, future prevention and treatment strategies should target the events preceding rUTI. For example, efforts to eradicate periurethral carriage by means of a topical microbicide, vaginal probiotic, targeted antibiotics, or vaccine or attempts to reduce the formation of other latent uropathogen reservoirs may reduce the risk of rUTI. Because the mechanism of rUTI may vary from woman to woman, informed approaches to prevention and treatment specific to individual patients may ultimately be necessary.
Acknowledgments
Financial support: National Institutes of Health (Specialized Centers of Research grant P50 AR 049475 and National Institute of Diabetes and Digestive and Kidney Diseases grant P01 DK 53369).
We thank Elaine Jong and D. C. Dugdale, medical directors, and the staff at the University of Washington Hall Health Center for helping with subject enrollment; Carol Winter and Ellen Cassen for subject enrollment and Natalie DeShaw for assistance with patient care and data collection at Hall Health Center; Marsha Cox, Cheryl Wobbe, and Sheila Manuguid at the University of Washington UTI Research Laboratory for laboratory assistance; and Jim Johnson at the University of Minnesota and David Rosen and Tom Hannan at Washington University for their helpful comments on the manuscript.
Footnotes
Potential conflicts of interest: none reported.
Presented in part: 46th Annual Meeting of the Infectious Diseases Society of America/48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 25–28 October 2008 (abstract L-607); 44th Annual Meeting of the Infectious Diseases Society of America, Toronto, 12–15 October 2006 (oral abstract 1158).
References
- 1.Foxman B, Barlow R, D’arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10:509–15. doi: 10.1016/s1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 2.Foxman B. Recurring urinary tract infection: incidence and risk factors. Am J Public Health. 1990;80:331–3. doi: 10.2105/ajph.80.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stamey TA, Timothy M, Millar M, Mihara G. Recurrent urinary infections in adult women: the role of introital enterobacteria. Calif Med. 1971;115:1–19. [PMC free article] [PubMed] [Google Scholar]
- 4.Marsh FP, Murray M, Panchamia P. The relationship between bacterial cultures of the vaginal introitus and urinary infection. Br J Urol. 1972;44:368–75. doi: 10.1111/j.1464-410x.1972.tb10093.x. [DOI] [PubMed] [Google Scholar]
- 5.Stamey T. The role of introital enterobacteria in recurrent urinary infections. J Urol. 1973;109:467–72. doi: 10.1016/s0022-5347(17)60454-3. [DOI] [PubMed] [Google Scholar]
- 6.Stamey T, Sexton C. The role of vaginal colonization with enterobacteriaceae in recurrent urinary infections. J Urol. 1975;113:214–7. doi: 10.1016/s0022-5347(17)59447-1. [DOI] [PubMed] [Google Scholar]
- 7.Kunin CM, Polyak F, Postel E. Periurethral bacterial flora in women: prolonged intermittent colonization with Escherichia coli. JAMA. 1980;243:134–9. [PubMed] [Google Scholar]
- 8.Cooper J, Brumfitt W, Hamilton-Miller JMT. The role of periurethral colonization in the aetiology of recurrent urinary infection in women. Br J Obstet Gynaecol. 1980;87:1145–51. doi: 10.1111/j.1471-0528.1980.tb04488.x. [DOI] [PubMed] [Google Scholar]
- 9.Pfau A, Sacks T. The bacterial flora of the vaginal vestibule, urethra, and vagina in premenopausal women with recurrent urinary tract infections. J Urol. 1981;126:630–4. doi: 10.1016/s0022-5347(17)54661-3. [DOI] [PubMed] [Google Scholar]
- 10.Brumfitt W, Gargan RA, Hamilton-Miller JMT. Periurethral entero-bacterial carriage preceding urinary infection. Lancet. 1987;1:824–6. doi: 10.1016/s0140-6736(87)91606-0. [DOI] [PubMed] [Google Scholar]
- 11.Brauner A, Jacobson SH, Kuhn I. Urinary Escherichia coli causing recurrent infections: a prospective follow-up of biochemical phenotypes. Clin Nephrol. 1992;38:318–23. [PubMed] [Google Scholar]
- 12.Russo TA, Stapleton A, Wenderoth S, Hooton TM, Stamm WE. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J Infect Dis. 1995;172:440–5. doi: 10.1093/infdis/172.2.440. [DOI] [PubMed] [Google Scholar]
- 13.Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;22:91–9. doi: 10.1093/clinids/22.1.91. [DOI] [PubMed] [Google Scholar]
- 14.Schlager TA, Hendley JO, Bell AL, Whittam TS. Clonal diversity of Escherichia coli colonizing stools and urinary tracts of young girls. Infect Immun. 2002;70:1225–9. doi: 10.1128/IAI.70.3.1225-1229.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulvey MA, Lopez-Boado YS, Wilson CL, et al. Induction and evasion of host defenses by type-1 piliated uropathogenic Escherichia coli. Science. 1998;282:1494–7. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 16.Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. PNAS. 2000;97:8829–35. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69:4572–9. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–7. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 19.Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. Diagnosis of coliform infection in acutely dysuric women. New Engl J Med. 1982;307:463–8. doi: 10.1056/NEJM198208193070802. [DOI] [PubMed] [Google Scholar]
- 20.Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of clinical microbiology. 9. Washington, DC: American Society for Microbiology Press; 2007. [Google Scholar]
- 21.Renter DG, Sargeant JM, Oberst RD, Samadpour M. Diversity, frequency, and persistence of Escherichia coli 0157 strains from range cattle environments. Appl Environ Microbiol. 2003;69:542–7. doi: 10.1128/AEM.69.1.542-547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson JR. Microbial virulence determinants and the pathogenesis of urinary tract infection. Infect Dis Clin North Am. 2003;17:261–78. doi: 10.1016/s0891-5520(03)00027-8. [DOI] [PubMed] [Google Scholar]
- 23.Johnson JR, Stell AL. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J Infect Dis. 2000;181:261–72. doi: 10.1086/315217. [DOI] [PubMed] [Google Scholar]
- 24.Svanborg C, Godaly G, Hedlund M. Cytokine responses during mucosal infections: role in disease pathogenesis and host defense. Curr Opin Microbiol. 1999;2:99–105. doi: 10.1016/s1369-5274(99)80017-4. [DOI] [PubMed] [Google Scholar]
- 25.Otto G, Burdick M, Streiter R, Godaly G. Chemokine response to febrile urinary tract infection. Kidney Int. 2005;68:62–70. doi: 10.1111/j.1523-1755.2005.00381.x. [DOI] [PubMed] [Google Scholar]
- 26.Van Damme J, Wuyts A, Froyen G, et al. Granulocyte chemotactic protein-2 and related CXC chemokines: from gene regulation to receptor usage. J Leukoc Biol. 1997;62:563–9. doi: 10.1002/jlb.62.5.563. [DOI] [PubMed] [Google Scholar]
- 27.Remis RS, Gurwith MJ, Gurwith D, Hargrett-Bean NT, Layde PM. Risk factors for urinary tract infection. Am J Epidemiol. 1987;126:685–94. doi: 10.1093/oxfordjournals.aje.a114708. [DOI] [PubMed] [Google Scholar]
- 28.Nicolle LE, Harding GKM, Preksaitis J, Ronald AR. The association of urinary tract infection with sexual intercourse. J Infect Dis. 1982;146:579–83. doi: 10.1093/infdis/146.5.579. [DOI] [PubMed] [Google Scholar]
- 29.Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. New Engl J Med. 1996;335:468–74. doi: 10.1056/NEJM199608153350703. [DOI] [PubMed] [Google Scholar]
- 30.Scholes D, Hooton TM, Roberts PL, Stapleton AE, Gupta K, Stamm WE. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177–82. doi: 10.1086/315827. [DOI] [PubMed] [Google Scholar]
- 31.Hooton TM, Hillier S, Johnson C, Roberts PL, Stamm WE. Escherichia coli bacteriuria and contraceptive method. JAMA. 1991;265:64–9. [PubMed] [Google Scholar]
- 32.Bran JL, Levison ME, Kaye D. Entrance of bacteria into the female urinary bladder. New Engl J Med. 1972;286:626–9. doi: 10.1056/NEJM197203232861203. [DOI] [PubMed] [Google Scholar]
- 33.Buckley RM, McGuckin M, MacGregor RR. Urine bacterial counts after sexual intercourse. New Engl J Med. 1978;298:321–4. doi: 10.1056/NEJM197802092980607. [DOI] [PubMed] [Google Scholar]
- 34.Benson M, Jodal U, Andreasson A, Karlsson A, Rydberg J, Svanborg C. Interleukin-6 response to urinary tract infection in childhood. Pediatr Infect Dis J. 1994;13:612–6. doi: 10.1097/00006454-199407000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Hedges S, Stenqvist K, Lidin-Janson G, Martinell J, Sandberg T, Svanborg C. Comparison of urine and serum concentrations of interleukin-6 in women with acute pyelonephritis or asymptomatic bacteriuria. J Infect Dis. 1992;166:653–6. doi: 10.1093/infdis/166.3.653. [DOI] [PubMed] [Google Scholar]
- 36.Otto G, Braconier J, Andreasson A, Svanborg C. Interleukin-6 and disease severity in patients with bacteremic and nonbacteremic febrile urinary tract infection. J Infect Dis. 1999;179:172–9. doi: 10.1086/314534. [DOI] [PubMed] [Google Scholar]
- 37.Ko YC, Mukaida N, Ishiyama S, et al. Elevated interleukin-8 levels in the urine of patients with urinary tract infections. Infect Immun. 1993;61:1307–14. doi: 10.1128/iai.61.4.1307-1314.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davidoff R, Yamaguchi R, Leach GE, Park E, Lad PM. Multiple urinary cytokine levels of bacterial cystitis. J Urol. 1997;157:1980–5. [PubMed] [Google Scholar]
- 39.Jacobson SH, Hylander B, Wretlind B, Brauner A. Interleukin-6 and interleukin-8 in serum and urine in patients with acute pyelonephritis in relation to bacterial-virulence-associated traits and renal function. Nephron. 1994;67:172–9. doi: 10.1159/000187923. [DOI] [PubMed] [Google Scholar]
- 40.Moreno E, Andreu A, Perez T, Sabate M, Johnson JR, Prats G. Relationship between Escherichia coli strains causing urinary tract infection in women and the dominant faecal flora of the same hosts. Epidemiol Infect. 2006;134:1015–23. doi: 10.1017/S0950268806005917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Medicine. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elliott TSJ, Slack RCB, Bisho MC. Scanning electron microscopy of human bladder mucosa in acute and chronic urinary tract infection. Br J Urol. 1984;56:38–43. doi: 10.1111/j.1464-410x.1984.tb07160.x. [DOI] [PubMed] [Google Scholar]
- 43.Elliott TSJ, Reed L, Slack RCB, Bishop MC. Bacteriology and ultra-structure of the bladder in patients with urinary tract infection. J Infect. 1985;11:191–9. doi: 10.1016/s0163-4453(85)92997-4. [DOI] [PubMed] [Google Scholar]