Summary
A number of autoimmune diseases, including multiple sclerosis, are mediated by self-reactive T cells that have escaped the deletional mechanisms of central tolerance. Usually, these T cells are kept at bay through peripheral tolerance mechanisms including regulation through coinhibitory receptors and suppression by regulatory T cells (Tregs). However, if these mechanisms fail, self-reactive T cells are activated and autoimmune responses ensue. This review outlines how the coinhibitory receptors CTLA-4, PD-1, Tim-3 and TIGIT act at different checkpoints to inhibit autoreactive T cells and suppress the development of CNS autoimmunity. Loss of each of these receptors predisposes to autoimmunity, indicating a non-redundant role in maintaining peripheral tolerance. At the same time, their functional patterns seem to overlap to a large degree. We therefore propose that only the concerted action of a combination of inhibitory receptors is able to maintain peripheral tolerance and prevent autoimmunity.
Keywords: Costimulation, CTLA-4, TIGIT, PD-1, Tim-3
Introduction
In order to combat the plethora of ever evolving pathogens, the adaptive immune system has developed T and B cells with an almost unlimited repertoire of receptors capable of recognizing and eliminating all imaginable kinds of foreign antigens (Ags). However, as this repertoire of receptors is generated in a random process, it also contains receptors specific for self-antigens. Self-reactive immune cells are controlled through mechanisms of central and peripheral tolerance. However, when autoreactive cells escape these mechanisms, they pose a serious threat as they can induce tissue inflammation and autoimmunity. Depending on the expression profile of their Ag, self-reactive cells may induce organ-specific autoimmune diseases, such as type I diabetes and Multiple Sclerosis (MS), or systemic autoimmune diseases like systemic lupus erythematosus (SLE). In essence, due to the natural presence of self-reactive immune cells in the repertoire, everyone has the potential to develop autoimmunity, but certain environmental and genetic factors increase susceptibility or can trigger an autoimmune attack. The most important genetic risk factor is the MHC genotype. Since MHC molecules select the (autoreactive) T cell repertoire and determine the ability of T cells to respond to a specific Ag, this association also highlights the central role of self-reactive T cells in the pathogenesis of many autoimmune diseases. This review outlines how inhibitory receptors keep these autoreactive T cells in check, focusing on their role in preventing T cell-mediated autoimmunity in the central nervous system. We will first introduce MS and how T cells contribute to the disease and will then discuss how the inhibitory receptors CTLA-4, TIGIT, PD-1, and Tim-3 act as negative regulators in MS pathogenesis.
Multiple Sclerosis and experimental autoimmune encephalomyelitis
MS is a chronic autoimmune disease of the central nervous system (CNS) in which the myelin sheath is under autoimmune attack causing inflammation and demyelination of the white matter, which leads to progressive paralysis. The lesions in brain, spinal cord and optic nerve are characterized by demyelination, infiltration of immune cells and activation of phagocytes at the site of the lesion. It is not well understood what causes the disease, but it is widely accepted that autoreactive T cells play an important role in inducing tissue inflammation in MS. A current hypothesis proposes that autoreactive T cells are activated by chance in the peripheral immune compartment and traffic to the CNS, where they get re-activated by self-antigen to induce inflammation and demyelination. This process is also recapitulated in experimental autoimmune encephalomyelitis (EAE), the animal model for MS.
MS is a clinically and pathologically heterogeneous disease. According to the disease course it can be subdivided into primary progressive MS, relapsing-remitting MS which often evolves into the secondary progressive form, and fulminant MS (1). The histopathological pattern of CNS lesions varies among patients and during different stages of the disease. In acute and relapsing-remitting MS, lymphocytic infiltrates form perivascular cuffs that consist of T cells, activated macrophages and microglia. In these lesions, emerging classical focal plaques are characterized by demyelination, axonal injury and axonal loss in the white matter of the CNS. Some patients display complement deposition in the lesions suggesting the involvement of B cells and antibodies (Abs) in disease pathogenesis. Moreover, in some patients with very severe and rapidly progressive disease, lymphoid follicle-like structures that contain B cells, plasma cells, T cells and dendritic cells have been observed in the leptomeninges (2).
The animal model EAE has been used extensively to study the cellular and molecular mechanisms that lead to MS. Like MS, EAE is an autoimmune disease characterized by autopathogenic T cells that specifically home to the CNS. Since the CNS is an immunologically privileged site protected by the blood brain barrier, it usually does not contain significant numbers of lymphocytes. However, once activated, T cells can cross the blood brain barrier and induce pathology (3, 4). Myelin-reactive T cells get re-activated in the CNS, produce pro-inflammatory cytokines, and promote infiltration and activation of other cell types including T cells, macrophages and resident microglia. This results in inflammation, demyelination and oligodendrocyte death, which subsequently lead to loss of axonal function and clinical disease.
Myelin is formed by oligodendrocytes and wrapped around the axons of neurons. It serves as electric insulation to facilitate rapid propagation of electric impulses. Myelin contains different proteins, namely myelin basic protein (MBP), proteolipid protein (PLP), myelin-associated glycoprotein (MAG) and Connexin32. In the CNS, myelin additionally contains myelin oligodendrocyte glycoprotein (MOG). In general, EAE can be induced by immunization with MBP, PLP and MOG. However, clinical disease and lesion distribution differ depending on the genetic background and the antigen used for immunization. Thus, SJL mice immunized with the encephalitogenic peptide PLP139-151 develop EAE with a relapsing-remitting disease course. The animals suffer from acute paralytic attacks, which are followed by periods of remission. The lesions in this mouse strain are predominantly located in the spinal cord (5, 6). On the other hand, immunization of C57BL/6 mice with MOG35-55 peptide results in a chronic or chronic progressive form of EAE: the affected mice develop ascending paralysis with lesions in brain, spinal cord and optic nerve (7).
In addition to disease induction by active immunization, EAE can also be induced by passive transfer of myelin-specific T cells. Myelin-specific T cells can either be obtained from previously immunized mice or from mice that transgenically express myelin-reactive TCRs. We have previously generated TCR transgenic mice (2D2), that recognize the MOG35-55 peptide in the context of the MHC class II molecule I-Ab (8). 2D2 TCR transgenic mice and T cells derived from these mice have proven to be a valuable tool in studying the mechanisms of EAE pathogenesis and development of CNS autoimmunity.
T helper cell subsets and their role in autoimmunity
Costimulation and T cell activation
Productive T cell activation, expansion and differentiation requires two independent signals: 1) T cell receptor (TCR) ligation through interaction with the antigenic peptide/MHC complex and 2) the interaction of costimulatory receptors on T cells with costimulatory ligands expressed on antigen presenting cells (APCs). Depending on the molecules and pathways engaged, costimulation can deliver either positive or negative signals and thus determines the outcome of TCR engagement. Positive signals are required for T cell proliferation, differentiation and survival. The absence of costimulation or a preferential engagement of negative costimulatory molecules results in a failure to induce an immune response or induces a state of tolerance (9–11). The interactions between a number of ligands expressed on APCs and their receptors expressed on T cells are capable of providing a costimulatory signal. However, CD28 and CTLA-4 represent the most prominent costimulatory molecules as they constitute the best-studied costimulatory pathway. CD28 is constitutively expressed on the surface of T cells and interacts with two ligands (CD80 and CD86) on the surface of APCs to induce a positive costimulatory signal into T cells. CTLA-4 binds to the same set of ligands but with much higher affinity (12). In contrast to CD28, CTLA-4 is not expressed in naïve or resting T cells but is upregulated on the cell surface following T cell activation and induces a negative signal into T cells (13–16). Costimulatory ligands that deliver a positive signal, such as CD28, are essential for optimal T cell proliferation and effector function as well as for preventing programmed cell death (17–19). Conversely, engagement of coinhibitory receptors such as CTLA-4 inhibits T cell proliferation and activation and induces a state of T cell unresponsiveness, anergy and tolerance (20). In addition to regulating effector T cell responses directly, negative costimulatory molecules like CTLA-4 can also indirectly regulate T cell responses by modulating the function of regulatory T cells (Tregs), which also control T cell activation and induce self-tolerance (21).
The strict control of T cell responses through costimulatory molecules is of utmost importance to a functioning immune system, as dysregulation of T cell responses results in pathology. Failure to keep T cell responses in check causes excessive immune activation and autoimmunity as exemplified in CTLA-4 knock out (KO) mice, which succumb to multi-organ autoimmune disease (13, 14). This is also reflected in the genetic association of a number of mutations in costimulatory molecules with predisposition to autoimmune diseases (22). In this review we will focus on the role of inhibitory receptors and pathways in controlling autoreactive T cell responses that lead to CNS autoimmunity, namely CTLA-4, PD-1, Tim3 and the novel coinhibitory receptor TIGIT.
Effector T cells
Autoimmune tissue inflammation is induced when self-reactive T helper cells are activated and acquire an appropriate pro-inflammatory effector phenotype. In addition to the direct damage caused by the resulting pro-inflammatory milieu, T helper cells also provide help to cytotoxic T cells (CTL) and autoreactive B cells. The subsequent expansion and maturation of self-reactive CTLs and B cells leads to tissue destruction and the production of isotype switched autoantibodies, which further contribute to tissue inflammation and damage. The ability of autoreactive T cells to induce autoimmunity and tissue inflammation is not only dictated by their specificity for self-antigen but, more importantly, by their effector functions. Upon interaction with the self- or a cross-reactive antigen, T helper cells activate, expand, and differentiate into various effector T cell subsets. Depending on the cytokines they produce, these T cell subsets have very different properties. T helper cells include the well-defined Th1 and Th2 effector subsets, as well as the more recently described Th17 and Th9 subsets. In addition, T helper cells can acquire regulatory phenotypes and differentiate into Treg or Tr1 cells (23).
Th1 cells are generated from naïve T helper cells by TCR engagement and STAT1 signaling, induced by interferons (IFN). Phosphorylated STAT1 induces expression of the Th1 specific transcription factor T-bet, which then drives Th1 differentiation by transactivating the Th1 signature cytokine IFN-γ and the specific subunit of the receptor for interleukin (IL)-12, IL-12Rβ2. Thus, the cell becomes responsive to IL-12, which is produced by activated APCs, and subsequent IL-12 signaling through STAT4 further stabilizes the Th1 phenotype. For decades, Th1 cells were thought to be the main inducers of organ-specific autoimmune tissue inflammation, whereas Th2 cells and their signature cytokine IL-4 were thought to be rather protective in autoimmunity. A number of observations supported the role of IFN-γ and Th1 cells in the induction of MS and EAE: IFN-γ is present in the CNS lesions of mice and MS patients (24, 25); Th1 cells can attract and activate destructive macrophages in the CNS lesions (26); myelin-specific Th1-clones can adoptively transfer EAE (26, 27); T-bet deficient mice are highly resistant to EAE (28); and IFN-γ treatment exacerbates disease in MS patients (29). However, this hypothesis was challenged when it was shown that animals lacking key Th1-associated factors including the signature molecules IFN-γ, IFN-γR, STAT-1, and IL-12p35 are actually more susceptible to multiple autoimmune diseases including EAE (28, 30, 31), experimental autoimmune uveitis (EAU) (32), and collagen-induced arthritis (CIA) (33).
The discovery that the cytokine IL-23, which is important for the generation of IL-17-producing Th17 cells, is crucial for the development of autoimmunity (31, 34), shifted the focus towards Th17 cells. Th17 cells are particularly suited for the promotion of autoimmune tissue inflammation due to production of their signature cytokine IL-17, which induces the secretion of multiple pro-inflammatory cytokines and chemokines from responding parenchymal cells (35). Accordingly, elevated levels of IL-17 are detected in several autoimmune diseases including MS (36), rheumatoid arthritis (37) and psoriasis (38). Furthermore, IL-17 neutralizing Abs ameliorate EAE (39) and IL-17 deficient animals develop attenuated CIA and EAE (40, 41). These studies demonstrated the importance of Th17 cells in EAE and other autoimmune diseases, and prompted a re-evaluation of the role of other effector T cells in the induction of autoimmune tissue inflammation previously thought to be driven by Th1 cells. Differentiation of Th17 cells is driven by a combination of the cytokines TGF-β and IL-6 (42, 43), or TGF-β plus IL-21 (44), and depends on STAT3 signaling (45). IL-1β can synergize with IL-6 to induce murine Th17 cell differentiation, and, together with TGF-β, IL-6, and IL-21, IL-1β has also been described as a critical differentiation factor for human Th17 cells (46). IL-6 and TGF-β signals ultimately lead to the expression of the Th17 specific transcription factor RORγt, which transactivates many Th17-associated factors including the signature cytokines IL-17A and IL-17F (47). Differentiation of naïve T cells into Th17 cells does not require IL-23. However, IL-23 is essential for terminal differentiation of Th17 cells (48), stabilization of their effector phenotype (49, 50), and possibly for long-term survival/expansion of pathogenic Th17 cells in EAE (51).
In the context of EAE, we have demonstrated that upon adoptive transfer into WT recipient mice MOG-specific Th1 and Th17 cells can both induce autoimmune tissue inflammation independently of each other albeit with different pathological phenotypes (50). Indeed, it has become clear that both cell types play a role in autoimmune pathogenesis and need to be controlled to prevent development of autoimmunity and tissue inflammation. Whether Th1 or Th17 cells are dominant may vary not only between different stages of the disease but also between patients (52) and thus may offer an explanation for the heterogeneity seen in many autoimmune diseases, and especially in MS.
Regulatory T cells
Central tolerance eliminates most self-reactive T cells through negative selection in the thymus during development. However, some autoreactive T cells escape negative selection and can, even in healthy individuals, be detected in the peripheral immune compartment (53). The fact that these autoreactive cells do not generally induce autoimmune disease can be attributed to mechanisms of peripheral tolerance including anergy, clonal deletion and suppression by Tregs, which act to keep the self-reactive T cell repertoire in check.
Tregs were initially identified as CD4+CD45RBlow or CD4+CD25+ T cells that are able to control autoimmunity in mice. When T cells depleted of these populations were transferred into T cell-deficient hosts, the recipients spontaneously developed autoimmune disease in multiple organs. Co-transfer of Tregs could prevent disease onset, suggesting that this T cell subset normally suppresses self-reactive T cell responses (54, 55). A clear definition of Tregs came about with the identification of Foxp3 as a master transcription factor for Tregs. Forced expression of Foxp3 results in the acquisition of a Treg phenotype and suppressor function (56–58). The fundamental importance of Foxp3+Tregs in the maintenance of immune tolerance was underscored by the observation that Scurfy mice and human IPEX (immunodysregulation polyendocrinopathy enteropathy X-linked) syndrome patients have genetic mutations in the Foxp3 gene and consequently develop multi-organ autoimmune disease (59, 60).
We now know that Tregs constitute approximately 10% of the peripheral CD4+ T cell pool and that the majority of Tregs arise naturally in the thymus (nTreg). However, naïve CD4+ T cells in the periphery can also acquire Foxp3 expression and convert into Tregs (iTreg) (61). In vitro the conversion into Foxp3+ Tregs is induced by the cytokine TGF-β and Treg differentiation can be facilitated by IL-2 and retinoic acid (62–64). In vivo approximately 10% of CD4+CD25− T cells convert into Foxp3 expressing Tregs six weeks after transfer into congenic hosts (65) and continuous low dose administration of antigen without inflammatory stimuli also induces the conversion of CD4+CD25− T cells into Foxp3+ Tregs in vivo (66). Furthermore, antigen presentation by immature DCs or the cytokine milieu generated by tolerogenic DCs might also promote conversion of Foxp3− T cells into Foxp3+ Tregs (67).
Like Foxp3− T cells, Tregs are activated through recognition of their cognate Ag in the context of MHC. However, once activated, Tregs are able to suppress responder T cells irrespective of their Ag specificity (68). Several partially overlapping and redundant mechanisms of Treg-mediated suppression have been described including secretion of soluble mediators (cytokines), contact-dependent suppression, as well as alteration of APC function (69). Tregs may secrete the immunosuppressive cytokines IL-10, TGF-β and IL-35, all of which can directly inhibit the function of responder T cells and APCs (70–72). In addition, Tregs can suppress immune responses through cell-cell-contact-dependent mechanisms. These include direct suppression of effector T cells through granzyme A- and perforin-dependent cytolysis (73), generation of pericellular adenosine catalyzed by CD39 and CD73 on Tregs (74, 75) as well as suppression through membrane bound TGF-β (76). Tregs also express high levels of coinhibitory receptors and can outcompete effector T cells for positive signals (77). Furthermore, Tregs are capable of contact-dependent killing of APCs (78), conditioning of DCs to downmodulate costimulatory ligands, and express indoleamine 2,3-dioxygenase (IDO) (79), and trans-endocytosis of costimulatory ligands. Thus, Tregs can indirectly suppress immune responses by altering the APC compartment.
Since their discovery, numerous genome-wide transcriptional profiles have been generated on Tregs. These data allowed for the definition of a common molecular Treg cell signature consisting of a set of over- and under-expressed genes that distinguishes Tregs from conventional T cells (80). Interestingly, although Foxp3 is a key regulator of Tregs and was confirmed as the master transcription factor, not all aspects of the lineage are regulated by this transcription factor (81, 82). These findings also highlighted that Tregs represent a very heterogeneous population. A series of recent reports has subdivided Tregs into functional subphenotypes specialized for the suppression of different Th subsets. T-bet, the master transcription factor essential for differentiation and function of Th1 cells, was shown to also be expressed by a subset of Foxp3+ Tregs. Intriguingly, T-bet+ Tregs turned out to be essential for the efficient control of Th1 effectors in that T-bet-deficient Foxp3+ Tregs failed to control Th1-mediated immune responses (83). Similarly, IRF4, a transcription factor involved in inducing IL-4, is required for Treg-mediated control of Th2 responses (84), loss of STAT3 in Tregs results in selective dysregulation of Th17 responses (85), and Bcl-6-expressing Tregs are essential for controlling T follicular helper cells and germinal center responses (86, 87). Like different effector T cell lineages, different Treg subsets can also be distinguished by their expression of different sets of chemokine receptors that allow them to home to the relevant tissues (88). This emerging diversity in Treg populations adds another layer of complexity to immune responses. Not only can effector T cell responses go out of bound and cause autoimmunity but failure to activate and recruit the right type of Treg to control a specific type of effector T cells can also promote development of autoimmunity.
CTLA-4
Genetic linkage of CTLA-4 to MS susceptibility
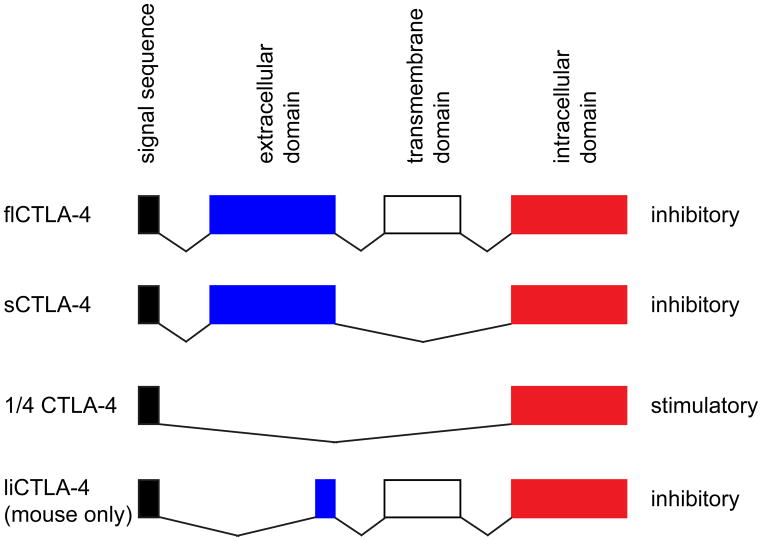
CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, CD152) is the most prominent example of an inhibitory molecule and is upregulated on the cell surface following T cell activation (15). Loss of CTLA-4 induces early lethality and multi-organ autoimmunity, suggesting that CTLA-4 may be a key molecule for the regulation of autoimmune disease (13, 14). Indeed, a number of studies have demonstrated a genetic linkage of polymorphisms in the CTLA-4 gene with susceptibility to MS. The first single nucleotide polymorphism (SNP) associated with MS susceptibility is located at position +49 (G>A) in exon 1 of the CTLA-4 gene (11, 89). Several follow-up studies have yielded mixed results. While some studies clearly confirmed the initially reported linkage, the genetic association did not hold true in when other populations were examined (90–92). Interestingly, some studies that could not confirm the linkage of the A allele at position +49 with susceptibility to MS, found an association with either clinical disability or with the progressive form of the disease but not with relapsing-remitting MS (93–95). In addition to differences in patient cohorts, differences in sample size and study design can strongly influence these results. This is particularly important considering that later studies revealed that several other SNPs in the CTLA-4 gene are also linked to MS and have to be controlled for in the cohorts analyzed (96). Moreover, further work has revealed that the genetic linkage of a number of autoimmune diseases to CTLA-4 is not due to polymorphisms in the coding sequence of CTLA-4 but due to differential generation of splice variants of CTLA-4 (22, 97). In addition to the full-length CTLA-4 (flCTLA-4), three other splice variants of CTLA-4 exist (Figure 1). A soluble form of CTLA-4 (sCTLA-4) lacking the transmembrane region is created by splicing out exon 3 (98). Like flCTLA-4, sCTLA-4 is an inhibitory molecule and seems to play an important role in Treg function (99, 100). 1/4 CTLA-4 lacks both exons 2 and 3, which code for the ligand binding and transmembrane domains. Genetic over-expression of 1/4CTLA-4 suggests that, in contrast to all other splice variants, this isoform of CTLA-4 is not inhibitory but promotes T cell activation and development of autoimmunity (101). Lastly, ligand independent CTLA-4 (liCTLA-4) lacks most of the Ig domain and thereby the domain that interacts with B7 ligands (22, 97). In mice this isoform appears to have a strong inhibitory function, however, it is not expressed in humans (22, 102). Interestingly, the splice variants of CTLA-4 associated with susceptibility to autoimmune diseases differ in mice and humans. In mouse, autoimmunity is associated with the generation of ligand liCTLA-4 (97), whereas in humans autoimmunity is associated with two SNPs (CT60 and +49G>A) where in both cases the protective A allele favors the generation of sCTLA-4 over the full-length protein (97). Intriguingly, recent studies indicate that plasma sCTLA-4 levels are decreased in MS patients (103). However, how this might regulate generation or effector functions of autoreactive T cells is not well understood.
Figure 1. Splice variants of CTLA-4.
Full length CTLA-4 (flCTLA-4) is composed of four exons representing the signal sequence, extracellular domain, transmembrane domain, and intracellular domain. Splice variants of the gene are generated by alternative splicing of these exons resulting in the formation of soluble CTLA-4 (sCTLA-4), which lacks the transmembrane domain, 1/4 CTLA-4 which lacks exons 2 and 3, and ligand-independent CTLA-4 (liCTLA-4) which lacks exon 2. In general, CTLA-4 acts as an inhibitory molecule. However, the 1/4 CTLA-4 isoform is stimulatory.
Functional role of CTLA-4 and its ligands in CNS autoimmunity
Activation of T cells may be important at several stages of EAE pathogenesis, including stimulation and clonal expansion within peripheral lymphoid tissues, entry and reactivation in the CNS, and mediating tissue destruction within the CNS parenchyma (104). Expression and ligation of costimulatory molecules can modulate T cell activation at each of these steps and determine disease outcome and a number of studies have addressed the role of CTLA-4 or its ligands in EAE.
Peripheral tolerance can be potently induced by intravenous administration of antigen-coupled ethylene carbodiimide-fixed splenocytes (105, 106). However, blocking CTLA-4 at the time of challenge completely reverses this tolerance (107), indicating that peripheral tolerance and thereby the lack of T cell activation is dependent on CTLA-4. Similarly, PLP139-151-specific CD4+ T cells primed in SJL mice in vivo show enhanced proliferation and a marked increase in pro-inflammatory cytokine secretion when restimulated in the presence of a CTLA-4 blocking Ab in vitro. Furthermore, blocking of CTLA-4 during in vitro activation cultures strongly enhances the ability of PLP-specific T cells to transfer disease (108). CTLA-4 blockade exacerbates EAE both in adoptive transfer as well as active immunization models of EAE, resulting in higher maximal disease scores and more inflammatory foci in the CNS (108–110). In a relapsing-remitting model of EAE treatment of mice during remission leads to an increase in relapse incidence and severity (108). Similarly, treatment of MBP immunized animals with a CTLA-4 blocking Ab also results in enhanced disease concomitant with elevated production of the pro-inflammatory cytokines IFN-γ and TNFα as well as an increase in mortality (109). More detailed studies on the consequences of CTLA-4 blockade in vivo revealed that anti-CTLA-4 Ab not only augments T cell responses to the inducing autoantigen but also accelerates epitope spreading and enhances T cell reactivity to relapse associated epitopes (108). CTLA-4 therefore plays a key role in limiting CNS autoimmunity at several stages of the disease, including initial activation, expansion of autoreactive T cells and de novo priming of T cells during relapse.
The fact that a number of mutations in CTLA-4 have been linked to MS and that costimulatory pathway blockade using anti-CTLA-4 Ab exacerbates EAE emphasizes the importance of this pathway for the control of CNS autoimmunity. Furthermore, costimulatory pathway blockade using CTLA-4 immunoglobulin (CTLA-4-Ig), which binds to both CD80 and CD86, can serve as a therapeutic agent for autoimmune diseases in humans and is now approved for treatment of rheumatoid arthritis patients under the name Belatacept (111, 112). This underscores the importance of the CD28/CTLA-4 – CD80/CD86 pathway in regulating the induction of autoimmune diseases. CTLA-4-Ig is able to block the activation and expansion of pathogenic T cells in vitro (109) and is also effective in blocking autoimmune disease of the CNS, as in vivo administration prevents the development of EAE (113, 114). Since CTLA-4-Ig binds to both CD80 and CD86 and completely blocks T cell costimulation through CD28, other studies have aimed at deciphering whether the two ligands have differential roles in regulating autoimmune responses. Interestingly, studies with Abs directed against either of the two ligands suggest that CD80 and CD86 can differentially regulate autoimmune responses. Treatment with anti-CD80 Abs results in reduced disease severity, prevents epitope spreading and blocks clinical relapses (115–117). In contrast, anti-CD86 increases disease severity (115, 116). This correlates with the observation that CD80 but not CD86 is upregulated in spleen and CNS upon disease induction (118). Furthermore, CD80 expression is enhanced in MS patients with rapidly progressing disease as well as during relapses (119, 120). We studied the relative importance of CD80 versus CD86 costimulation in the induction of EAE using B7-deficient mice and found that mice lacking either CD80 or CD86 are as susceptible to disease induction as wild type animals (121). However, double knockout mice are resistant to EAE induction by active immunization and show impaired T cell expansion and markedly reduced inflammatory infiltrates in the CNS (122). Further studies revealed that B7 costimulation not only plays a key role in initial T cell priming but also is essential during the effector phases of the disease, where it promotes T cell survival and sustained inflammation (122). Strikingly, MBP-specific CD4+ T cells from MS patients do not require B7 costimulation and are less responsive to CTLA-4 blockade, indicating a dysregulation in the requirement for costimulation in these individuals (123, 124).
CTLA-4 in regulatory T cells
The first indication that CTLA-4 might have effector T cell extrinsic functions came from a series of experiments using chimeric mice that harbor a mixture of wild type and CTLA-4-deficient cells. These chimeric mice fail to develop the fatal lymphoproliferative disease that is observed in CTLA-4-deficient mice implying that CTLA-4 not only has effector T cell intrinsic but also extrinsic functions in regulating immune responses (125, 126). CTLA-4 is indeed constitutively expressed on Tregs and CTLA4 is a target gene of Foxp3, suggesting that it is involved in the suppressive function of Tregs (127, 128). Initial studies on the role of CTLA-4 in Treg suppression using anti-CTLA-4 were controversial. The issue was clarified by a study using mice that specifically lack CTLA-4 in Tregs but have intact CTLA-4 expression in effector T cells (21). These conditional CTLA-4 KO mice display fatal lymphoproliferative disease and multiorgan autoimmunity, similar to what is observed in global CTLA-4 KO mice. However, the conditional KO mice display a later onset and prolonged survival indicating that CTLA-4 is important in both regulatory and effector T cells.
The role of CTLA-4 in Tregs was explored in great detail and it is now clear that CTLA-4 can contribute to Treg mediated suppression through a number of mechanisms. The high levels of CTLA-4 expressed on Tregs can compete with effector T cells for CD80/CD86 signals (77). Furthermore, CTLA-4 is instrumental in reducing the level of costimulatory B7 molecules on APCs as the observed reduction of CD80 and CD86 on APCs conditioned by Tregs is abrogated in the presence of CTLA-4 blocking Ab or when CTLA-4 KO Tregs are used (21, 129, 130). The regulation appears to be contact-dependent and specific for B7 molecules, as expression of MHC class II or CD40 are not affected by Tregs (131, 132). Recently, CTLA-4 expressing Tregs were shown to even be capable of physically removing B7 ligands from APCs (133). In this process termed trans-endocytosis, ligand engagement results in the transfer of B7 molecules from the APC to a CTLA-4 containing vesicle in the T cell. In addition to regulating the costimulatory potential of APCs, CTLA-4 can condition APCs by back-signaling and inducing the production of indoleamine 2,3-dioxygenase (IDO) (79, 134). IDO catabolizes tryptophan, which is required for T cell proliferation (135). The localized tryptophan depletion resulting from IDO induction therefore inhibits T cell responses by limiting T cell proliferation.
A number of studies confirmed that CTLA-4 contributes to Treg suppression in wild type cells. However, under certain circumstances CTLA-4 KO Tregs are functional and can compensate for the lack of CTLA-4 using alternative suppressive mechanisms (21, 136–140). The role of CTLA-4 in Tregs has also been explored comparing CTLA-4-deficient Tregs with wild type Tregs that expressed the same TCR (140, 141). In a diabetes model CTLA-4 expression on antigen-specific Tregs is crucial for inhibiting autoimmune islet destruction and disease. In contrast, in an EAE model TCR transgenic CTLA-4 KO Tregs are functional and able to completely suppress disease (141). In conclusion, it is clear that CTLA-4 plays an important role in Treg function. However, depending on the tissue site, the type of T cell priming and the nature of disease, other suppressive mechanisms might be able to compensate for CTLA-4. Nevertheless, the fulminant autoimmune phenotype observed in conditional CTLA-4 KO mice suggests that CTLA-4 is a central component for Treg suppression.
TIGIT
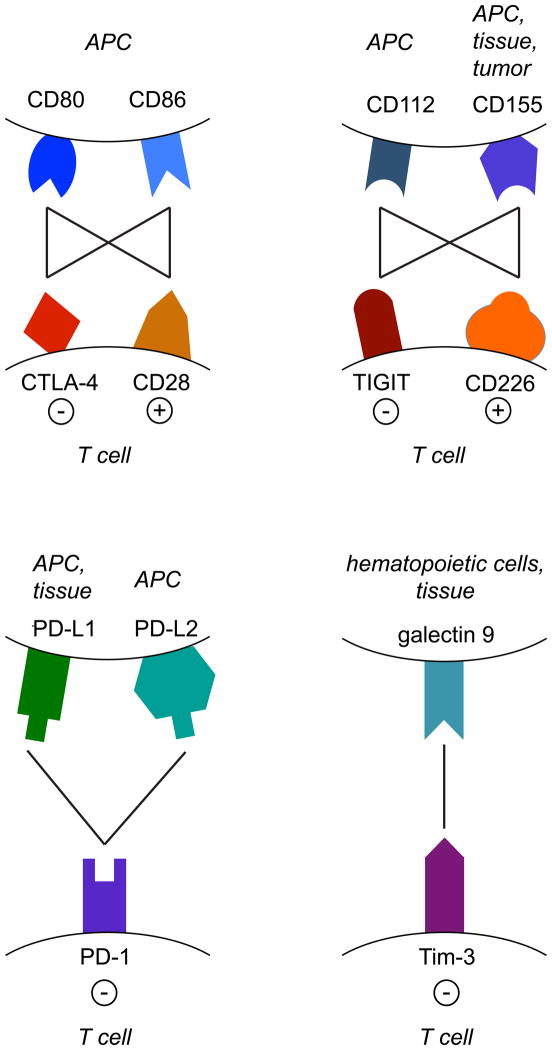
TIGIT (T cell immunoglobulin and ITIM domain; also called Vsig9, Vstm3 or WUCAM) is a CD28 family protein composed of an extracellular IgV domain, a transmembrane domain and a cytoplasmic tail containing two ITIM motifs (142–146). Expression has been reported on NK cells and T cells, particularly in memory T cells, follicular T helper cells and Tregs (142, 144, 145), and it is transiently induced upon T cell activation (147). Like CTLA-4, TIGIT binds two ligands CD155 (PVR) and CD112 (PVRL2, nectin-2), which are expressed on APCs (Figure 2) (142–144). In addition, CD155 is also expressed on a variety of non-hematopoietic cell types and was found to be upregulated on a number of tumors (148, 149). Both ligands are shared with CD226 (DNAM-1), which acts as a costimulatory molecule in T cells (150). The costimulary pathway formed by CD226/TIGIT and their ligands CD155/CD112 therefore forms a network that resembles the CD28/CTLA-4 – CD80/CD86 pathway, with CD226 and CD28 delivering a positive signal and TIGIT and CTLA-4 being inhibitory. Similar to the CD28/CTLA-4 costimulatory pathway, recent genome wide association scans have now also linked the CD226/TIGIT pathway to multiple autoimmune diseases in humans including type 1 diabetes and multiple sclerosis (151–154). However, in this pathway the association is with a SNP in the positive regulator CD226 (Gly307Ser), not with TIGIT.
Figure 2. Receptor-ligand networks of co-inhibitory receptors.
The CTLA-4/CD28-CD80/CD86 and TIGIT/CD226-CD112/CD155 networks both consist of an inhibitory (CTLA-4 and TIGIT) and a stimulatory (CD28 and CD226) receptor on T cells and two ligands expressed on APCs. In addition to APCs, CD155 is also expressed on tissues and tumor cells. PD-1 has two receptors: PD-L1, which is expressed on APCs and on a variety of tissues, and PD-L2, which is found on APCs. Tim-3 binds to galectin 9 expressed on hematopoietic cells and non-hematopoietic tissues. Lines indicate protein-protein interactions; − represents inhibition, + represents activation.
Initial data suggested that TIGIT does not have any T cell intrinsic function but instead acts on DCs and modulates their function by binding to the ligand CD155 (142). TIGIT interaction with CD155 was shown to inhibit IL-12p40 production and instead induce IL-10 from treated DCs. Consistent with these data, in vivo administration of TIGIT-Fc reduces the levels of IL-12p35 and p40, increases IL-10 expression and generates tolerogenic DCs that suppress T cell proliferation and IFN-γ production from responding T cells (142). However, more recent studies revealed that TIGIT directly inhibits cytotoxicity in NK cells (143). In this context, the inhibitory activity of TIGIT is completely dependent on the tyrosine at position 231 in the ITIM motif (143). We and others were able to show that TIGIT also has intrinsic effects on T cells and can inhibit T cell proliferation directly in the absence of APCs (144, 147, 155). Specifically, TIGIT seems to block productive T cell activation by targeting molecules that are upstream in the T cell activation pathway, such as components of the TCR complex. At the same time TIGIT promotes T cell maintenance and survival by driving expression of cytokine receptors and anti-apoptotic molecules (147).
Experiments using TIGIT-deficient mice confirmed that TIGIT also acts as an inhibitory molecule in vivo. Although TIGIT KO mice do not develop spontaneous autoimmunity, they display augmented T cell expansion upon immunization as well as increased levels of the pro-inflammatory cytokines IFN-γ and IL-17, whereas IL-10 levels are drastically reduced in these mice (147). Direct evidence for an inhibitory role of TIGIT in CNS autoimmunity came from a series of EAE experiments. In line with what was observed for other inhibitory receptors, blocking of TIGIT strongly increases EAE severity (144). Furthermore, TIGIT-deficient mice are more susceptible to EAE, while mice transgenically expressing TIGIT on T and B cells are protected from disease (144, 147). Although TIGIT-deficiency per se does not result in the induction of spontaneous autoimmunity, TIGIT KO mice do develop spontaneous EAE when crossed with MOG35-55-specific TCR transgenic 2D2 mice (156). TIGIT−/− x 2D2 mice start developing disease at 4 weeks of age and display atypical signs of neurologic dysfunction that are reminiscent of the atypical disease observed in Th17-driven EAE (147, 157). TIGIT might therefore play a role in regulating the threshold of T cell activation and maintaining peripheral tolerance.
In summary, even though at this point only a limited number of studies have investigated properties and functions of TIGIT, it shows many parallels with other inhibitory receptors, especially CTLA-4 (Table 1). Both molecules form part of a costimulatory network consisting of a positive and a negative regulator on T cells and two ligands expressed on APCs (Figure 2). Like CTLA-4, TIGIT binds its ligands with higher affinity than CD226 (142, 143). Both CTAL-4 and TIGIT have intrinsic inhibitory functions as blocking Abs exacerbate EAE (108–110, 144). Furthermore, like CTLA-4-Ig, soluble TIGIT can suppress autoimmune diseases when administered in vivo (113, 114, 144). Finally, both CTLA-4 and TIGIT are expressed on Tregs (128, 142, 144). However, whether TIGIT also plays a functional role on this cell subset remains to be determined.
Table 1.
Function of coinhibitory receptors in CNS autoimmunity.
| T cell intrinsic effect | Function in Tregs | KO phenotype | blocking Ab | recombinant protein | |
|---|---|---|---|---|---|
| CTLA-4 | inhibits proliferation inhibits pro- inflammatory cytokines |
reduction of B7 ligands on APCs IDO induction in APCs |
spontaneous autoimmunity | exacerbates EAE accelerates epitope spreading |
prevents EAE |
| TIGIT | inhibits proliferation inhibits pro- inflammatory cytokines |
spontaneous autoimmunity on susceptible background higher susceptibility to EAE |
exacerbates EAE | prevents EAE | |
| PD-1 | inhibits proliferation inhibits pro- inflammatory cytokines |
promotes Treg differentiation & stability augments suppression |
spontaneous autoimmunity higher susceptibility to EAE |
||
| Tim-3 | induces cell death inhibits IFNγ secretion |
exacerbates EAE | induces hyperproliferation of Th1 cells Tim-3 Tg mice are resistant to EAE |
The inhibitory receptors CTLA-4, TIGIT, PD-1, and Tim-3 share many properties. All four limit the secretion of the pro-inflammatory cytokine IFNγ from T cells and inhibit expansion of pro-inflammatory T cells, either by inhibiting proliferation or by inducing cell death. Loss of the inhibitory effect of these receptors, through blocking Abs or by genetic deficiency, exacerbates EAE and can result in spontaneous autoimmunity. In addition, CTLA-4 and PD-1 further dampen T cell responses by promoting the suppressive function of regulatory T cells.
PD-1
PD-1 and its ligands promote tolerance
PD-1 (Programmed Death 1) is a member of the immunoglobulin (Ig) superfamily with an N-terminal IgV-like extracellular domain. Its cytoplasmic domain contains both an immunoreceptor tyrosine-based inhibitory motif (ITIM) and an immunoreceptor tyrosine-based switch motif (ITSM), which is essential for PD-1 function (158, 159). PD-1 can be expressed on T and B cells, natural killer T cells, monocytes, and dendritic cells (DCs) upon activation, as well as on immature thymocytes during thymic development (160). PD-1 is induced by TCR signaling (161) and its expression is maintained in settings of persistent antigenic stimulation, such as chronic viral infection, cancer or autoimmunity. Specifically, PD-1 is highly expressed on non-functional, exhausted T cells associated with some of these conditions (162–165). PD-1 can bind to two ligands, PD-L1 and PD-L2, which differ in their expression pattern (Figure 2). PD-L1 is expressed on a wide range of hematopoietic as well as non-hematopoietic cells including T and B cells, DCs, macrophages, bone marrow-derived mast cells, mesenchymal stem cells, astrocytes and neurons (166) and can be upregulated on other tissue cells upon activation. PD-L2 is inducibly expressed on DCs, macrophages, bone marrow-derived mast cells and a subset of B1 (but not B2) B cells (167). The inflammatory milieu can regulate PD-L1 and PD-L2 expression and cytokines are potent inducers of the ligands. PD-L1 and PD-L2 also differ in their affinities for PD-1, with PD-L2 being the higher affinity binder. In contrast to PD-L2, PD-L1 also binds to CD80 (168).
The first indication that PD-1 might play a role in preventing autoimmunity came from PD-1 KO mice, which spontaneously develop a lupus like disease characterized by autoantibodies and a mild glomerulonephritis or cardiomyopathy, depending on the genetic background (169). PD-1 deficiency further accelerates the development of autoimmune disease if crossed onto susceptible backgrounds. In NOD mice, diabetes onset is usually observed at around 17 weeks of age in females. In the absence of PD-1, the age of onset is as early as 5 weeks and disease incidence increases from 30% to 100% (170). These findings suggested a key role for PD-1 in self-tolerance and further work has unraveled some of the mechanisms of PD-1 – PD-L1/PD-L2 control of autoimmune responses. PD-1 and its ligands regulate both central and peripheral tolerance in multiple ways. In the thymus, PD-L1 is broadly expressed on the thymic cortex and on thymocytes, while PD-L2 expression is restricted to the thymic medulla (171). PD-1 is expressed on CD4− CD8− (double negative) thymocytes, where it alters the signaling threshold and thereby limits positive selection (172, 173). However, its role in negative selection is controversial (174, 175). Peripheral tolerance mechanisms are necessary to keep autoreactive T cells that have escaped negative selection in check. The PD-1 pathway contributes to this process by inhibiting the expansion of self-reactive T cells, thereby limiting the initial activation phase. PD-L1 and PD-L2 are expressed on immature DCs, allowing them to control peripheral T cell activation. Loss of PD-1 on T cells compromises peripheral tolerance as tolerance induced by immature DCs is no longer effective (176). Furthermore PD-1 – PD-L1 interactions also influence reactivation, expansion and effector function of autoreactive T cells in tissue (177). Interestingly, studies using bone marrow chimeras indicate that PD-L1 on non-hematopoietic cells largely contributes to tissue tolerance (178). Similarly, loss or blockade of the PD-1 – PD-L1 interaction results in rapid, exacerbated diabetes in the NOD strain (170, 179). Furthermore, the PD-1 pathway seems to limit peripheral T cell responses by maintaining T cell anergy (180). Collectively, these data demonstrate that the PD-1 pathway inhibits autoimmunity by regulating both central and peripheral tolerance.
In the CNS, PD-1 and its ligands are expressed on a variety of cell types. PD-1 is constitutively expressed in retinal neurons, while expression of PD-L1 and PD-L2 is induced by inflammation (181). All three receptors are found on cellular infiltrates within the meninges during active EAE, suggesting a role for the PD-1 pathway in CNS autoimmunity (171). PD-L1 is also expressed on vascular endothelial cells and astrocytes. Furthermore, IFN-γ induces expression of PD-L1 on microglia and IL-12 promotes expression on CD11b+ APCs during EAE (182, 183). Initial indication that the PD-1 pathway plays a role in EAE came from studies using neutralizing Abs. Blocking of PD-1 or PD-L2 during disease induction results in augmented expansion of MOG-specific T cells, earlier onset and increased inflammatory infiltrates and disease severity (184). However, later studies indicated that the relative contribution of the two ligands strongly depends on the genetic background (185). Further studies using KO mice suggest that PD-1 and PD-L1 but not PD-L2 are predominantly responsible for suppression of EAE (186, 187). PD-1 and PD-L1-deficient T cells secrete increased amounts of IFN-γ and IL-17 upon in vitro restimulation and the respective KO mice develop more severe EAE. In contrast, EAE in PD-L2 KO mice is comparable to wild type controls (186). Interestingly, for the control of EAE, PD-L1 expression is not only important on T cells and APCs but also on host tissue (187).
In humans, PD-1 polymorphisms have been associated with a number of autoimmune diseases, including MS (188). One SNP (G7146A) is located in an intronic sequence that represents a Runx1 binding site and may alter mRNA stability or expression levels (189). In addition, the SNP is associated with reduced PD-1-mediated suppression of IFN-γ secretion by T cells and a progressive disease course in MS patients (190). Interestingly, IFN-β, the primary immunomodulatory treatment for MS, enhances PD-L1 expression on APCs in vitro as well as in vivo following IFN-β therapy (191). These data suggest that IFN-β may be acting through modulating the PD-1 pathway. Due to its increasingly recognized role in inhibiting autoimmunity, the PD-1 pathway has become a new target for the development of novel immunomodulatory therapies. In a first approach, genetically modified DCs that present MOG peptide in the context of MHC class II and overexpress PD-L1 were used to tolerize mice before EAE induction (192). Administration can reduce T cell expansion, CNS infiltrates and clinical scores. Importantly, DCs presenting MOG in the context of PD-L1 overexpression are able to dampen disease even when administered therapeutically. Another approach using a recombinant adenovirus to express PD-L1 also showed promising results in a lupus-like model of autoimmunity (193).
Role of the PD-1 pathway in regulatory T cells
PD-1 and PD-L1 are highly expressed on Tregs and may play an important role in their function (194). In vitro, PD-L1 promotes the conversion of conventional T cells into iTregs, enhances and promotes maintenance of Foxp3 expression, and augments suppressive function of iTregs (195). Furthermore, in vivo tissue expression of PD-L1 may promote conversion of conventional T cells and differentiated Th1 cells into iTregs (196, 197). Neurons, which express PD-L1, also promote the conversion of encephalitogenic T cells into Tregs capable of inhibiting EAE (198). Surprisingly, the role of the PD-1 pathway in Treg induction was supported by EAE studies aimed at determining the effect of pertussis toxin (PT) in disease induction (199). PT was originally thought to increase the permeability of the blood brain barrier and thereby promote disease induction in EAE (200, 201). Later studies revealed that PT administration decreases the frequency and function of Tregs (202, 203). In PD-1 deficient mice EAE induction does not require PT as these mice already harbor reduced frequencies of Tregs. In contrast, PT is essential for disease induction in wild type mice (199). PD-1 KO mice also show two- to three-fold reduced Treg frequencies upon immunization, and PD-1 KO T cells display a marked defect in Treg conversion in vitro. In vivo, PT treatment also downregulates PD-1 expression on Tregs without altering other phenotypic characteristics (199). It is therefore likely that PT also reduces Treg stability and suppressive function. In summary, there is clear evidence that the PD-1 pathway promotes Treg differentiation and function and thereby contributes to limiting CNS inflammation.
Tim-3
Tim-3 (T cell immunoglobulin and mucin domain-3) was first identified as a molecule specifically expressed on CD4+ Th1 (and Tc1) but not Th2 cells (204). More recently, Tim-3 has also been found on Th17 cells although at lower levels than on Th1 cells (205–207). In addition to full length Tim-3, a soluble splice variant that lacks the mucin and transmembrane domain has been described (208); however, the biological function of this isoform remains unclear. Galectin-9, a β-galactose binding protein, is the ligand for Tim-3 (Figure 2) (209). Galectin-9 triggering of Tim-3 on T cells induces calcium flux, cell aggregation, and cell death in vitro and administration of galectin-9 in vivo causes selective loss of IFN-γ producing T cells (209). Together these data indicate that Tim-3 serves as an inhibitory molecule that controls pro-inflammatory Th1, and possibly Th17, responses, thereby preventing uncontrolled inflammation and immunopathology.
Tim-3/Tim-3 ligand interactions negatively regulate EAE
Th1 and Th17 cells, both of which express Tim-3, can induce EAE (157). As Tim-3 functions as an inhibitory receptor on T cells, it would be predicted to play an important role in regulating this disease. Indeed, Tim-3-expressing CD4+ and CD8+ T cells are predominantly found in the CNS of mice with EAE and accumulate as disease progresses (204). Treatment of mice immunized for the development of EAE with an anti-Tim-3 Ab leads to hyperacute disease characterized by higher numbers and activation of CD11b+ cells in demyelinating lesions, an increase in inflammatory foci in the CNS, and increased mortality relative to mice treated with control immunoglobulin (204). Similarly, blockade of Tim-3 signaling with a Tim-3-Ig fusion protein results in hyper-proliferation of Th1 cells, increased release of the Th1 cytokines IFN-γ and IL-2 (208), and knock-down of galectin-9 in mice immunized for EAE exacerbates disease (209). Conversely, in vivo administration of galectin-9 results in selective loss of IFN-γ-producing cells and attenuates Th1 responses (209).
Tim-3 in human disease
In human T cells, Tim-3 is also expressed on Th1 but not Th2 cells and is found at low levels on Th17 cells (206). Examination of Tim-3 in MS has shown that CD4+ T cell clones from the cerebrospinal fluid (CSF) of MS patients express lower levels of Tim-3 than clones from the CSF of control subjects and that decreased Tim-3 expression is accompanied by significantly higher levels of IFN-γ production (210). Interestingly, further Th1 polarization in vitro significantly augments IFN-γ secretion but not Tim-3 expression among CSF clones from MS patients relative to those from control subjects. In keeping with the negative regulatory function of Tim-3 discovered in mouse T cells, blockade of Tim-3 on T cells from normal control subjects enhances IFN-γ secretion (211). Surprisingly, this is not the case in T cells from untreated MS patients, suggesting a T cell intrinsic dysregulation of both Tim-3 expression and function in T cells from MS patients. Indeed, dysregulation of Tim-3 expression in T cells is also observed in other autoimmune diseases. In T cells from Psoriasis patients both Th1 and Th17 cells exhibit defects in the ability to upregulate Tim-3 expression upon stimulation relative to cells from patients with atopic dermatitis and healthy donors (212). On the other hand, in patients with Rheumatoid arthritis (RA), high expression of Tim-3 on T cells correlates with both lower disease activity and lower levels of TNF-α (213). Together with the data from MS patients, these observations indicate an association between dysregulation of Tim-3 expression and function in T cells with human autoimmune disease. Decreased expression of Tim-3 in autoimmune conditions results in reduced inhibition of pro-inflammatory Th1 and Th17 cells and allows these autopathogenic T cells to go unchecked. In line with this, treatment of MS patients with either IFN-β or glatiramer acetate results in both restoration of Tim-3 expression and function in T cells from MS patients (211). Similarly, increased Tim-3 expression on T cells has been noted in RA patients that enter remission after treatment (213). Together these results underscore the importance of Tim-3 in negatively regulating pathogenic T cell responses in human autoimmunity.
In addition to autoimmune diseases, Tim-3 has recently gained attention in other settings of persistent antigenic T cell stimulation, namely chronic viral infection (214–216) and cancer (162, 217–219). In these settings Tim-3 marks exhausted T cells, that are characterized by failure to proliferate and exert effector function upon antigen encounter (reviewed in (220)). In contrast to autoimmunity, in these diseases the chronic activation results in sustained expression of inhibitory receptors and functional T cell inactivation. PD-1 was the first coinhibitory receptor to be linked with an exhausted T cell phenotype and blockade of the PD-1 pathway partially reverses the dysfunctional state and restores T cell effector function (165). However, PD-1 – PD-L1 blockade does not completely reverse T cell exhaustion, indicating that additional mediators are involved. Indeed, recent studies demonstrated that in certain models Tim-3 is co-expressed on a large fraction of PD-1+ exhausted T cells (162, 216, 221). Intriguingly, co-expression of PD-1 and Tim-3 is associated with more severe T cell exhaustion, while PD-1+Tim-3− T cells still retain some effector function. In these models, blockade of the PD-1 pathway only partially restores T cell function but combined blockade of the Tim-3 and PD-1 pathways synergistically improves T cell responses (162, 216). Thus, T cell exhaustion appears to be a stepwise process, in which the accumulation of several inhibitory receptors is required for full inactivation of effector T cells. In autoimmunity, the situation is reversed as failure to express inhibitory molecules such as Tim-3 allows immune responses to go unabated. Therefore the induction of T cell exhaustion, while detrimental in chronic viral infections and cancer, would be beneficial in autoimmunity. The association of Tim-3 with T cell exhaustion together with the observations that both Tim-3 expression and function are restored in T cells from MS and RA patients that have undergone treatment raises the possibility that the drugs used to treat these diseases may function by creating an environment that is permissive for Tim-3 expression and the induction of exhaustion in autoreactive T cells.
Concluding remarks
Self-reactive T cell responses are kept in check via peripheral tolerance mechanisms including expression of coinhibitory receptors and suppression through Tregs. However, failure or dysfunction of these inhibitory mechanisms can result in autoimmunity as autoreactive T cells are constantly exposed to and activated by their cognate antigen. Interestingly, the combination of inhibitory receptors that keeps autoimmunity at bay is also found in other settings of constant antigen exposure, namely chronic infections and cancer. However, in these situations expression of the inhibitory receptors results in a state of T cell unresponsiveness and exhaustion. Each individual receptor alone might not necessarily have a strong effect on T cells or might only result in a partial inhibition of T cell function. However, the stepwise accumulation of several inhibitory receptors characterizes a state of deep exhaustion, marked by a profound loss of effector T cell function. Thus, early exhaustion is defined by a partial loss of effector function and is marked by the expression of PD-1. As exhaustion progresses, other inhibitory molecules such as LAG-3 and Tim-3 are co-expressed, resulting in complete loss of T cell function. CTLA-4 is expressed on exhausted cells. However, whether CTLA-4 actively contributes to exhaustion seems to depend on the pathogenic challenge at hand (165, 222, 223). Based on these findings, it appears that the appropriate combination of inhibitory receptors is key to inducing the correct state of T cell unresponsiveness in the peripheral immune compartment. Likewise, maintaining peripheral tolerance might depend on the combination of inhibitory receptors expressed on autoreactive T cells. If the balance of these receptors is disturbed, the correct state of tolerance can no longer be maintained and autoimmune responses are elicited. Given the similarities between the different receptors regarding function, expression pattern and autoimmune diseases they are associated with (Table 1), it is likely that coinhibitory receptors might be co-regulated. Cytokines or specific transcription factors may promote their concerted expression and, as the inhibitory receptors accumulate, a state of tolerance is achieved. The challenge might therefore lie in determining the correct combination of inhibitory receptors necessary to prevent development of autoimmunity and defining the factors that induce their expression, yet keeping immune responses to viral infections and tumors unaffected.
References
- 1.Lassmann H. Pathology of multiple sclerosis. In: Compston A, Ebers G, Lassmann H, McDonald I, Matthews B, Wekerle H, editors. McAlpine’s Multiple Sclerosis. 3. Hong Kong: Churchill Livingstone; 1998. pp. 323–358. [Google Scholar]
- 2.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 4.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 5.Sobel RA, Greer JM, Kuchroo VK. Minireview: Autoimmune Responses to Myelin Proteolipid Protein. Neurochemical Research. 1994;19:915–921. doi: 10.1007/BF00968701. [DOI] [PubMed] [Google Scholar]
- 6.Berger T, Weerth S, Kojima K, Linington C, Wekerle H, Lassmann H. Experimental autoimmune encephalomyelitis: the antigen specificity of T lymphocytes determines the topography of lesions in the central and peripheral nervous system. Lab Invest. 1997;76:355–364. [PubMed] [Google Scholar]
- 7.Mendel I, Kerlero de Rosbo N, Ben-Nun A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur J Immunol. 1995;25:1951–1959. doi: 10.1002/eji.1830250723. [DOI] [PubMed] [Google Scholar]
- 8.Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin Oligodendrocyte Glycoprotein-specific T cell Receptor Transgenic mice Develop Spontaneous Autoimmune Optic Neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 10.Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 11.Boussiotis VA, Freeman GJ, Gray G, Gribben J, Nadler LM. B7 but not intercellular adhesion molecule-1 costimulation prevents the induction of human alloantigen-specific tolerance. J Exp Med. 1993;178:1753–1763. doi: 10.1084/jem.178.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linsley P, Brady W, Urnes M, Grosmaire L, Damle N, Ledbetter J. CTLA-4 is a second receptor for the B cell activation antigen B7. Journal of Experimental Medicine. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 15.Freeman GJ, et al. Uncovering of functional alternative CTLA4 counter-receptor in B7-deficient mice. Science. 1993;262:907–909. doi: 10.1126/science.7694362. [DOI] [PubMed] [Google Scholar]
- 16.Alegre ML, et al. Regulation of surface and intracellular expression of CTLA4 on mouse T cells. J Immunol. 1996;157:4762–4770. [PubMed] [Google Scholar]
- 17.Cerdan C, et al. Prolonged IL-2 receptor alpha/CD25 expression after T cell activation via the adhesion molecules CD2 and CD28. Demonstration of combined transcriptional and post-transcriptional regulation. J Immunol. 1992;149:2255–2261. [PubMed] [Google Scholar]
- 18.de Boer M, Kasran A, Kwekkeboom J, Walter H, Vandenberghe P, Ceuppens JL. Ligation of B7 with CD28/CTLA-4 on T cells results in CD40 ligand expression, interleukin-4 secretion and efficient help for antibody production by B cells. Eur J Immunol. 1993;23:3120–3125. doi: 10.1002/eji.1830231212. [DOI] [PubMed] [Google Scholar]
- 19.Boise LH, et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. [PubMed] [Google Scholar]
- 20.Perez V, Vanparijis L, Biuckians A, Zheng X, Strom T, Abbas A. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 21.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 22.Vijayakrishnan L, et al. An autoimmune disease-associated CTLA-4 splice variant lacking the B7 binding domain signals negatively in T cells. Immunity. 2004;20:563–575. doi: 10.1016/s1074-7613(04)00110-4. [DOI] [PubMed] [Google Scholar]
- 23.Jäger A, Kuchroo VK. Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand J Immunol. 2010;72:173–184. doi: 10.1111/j.1365-3083.2010.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ando DG, Clayton J, Kono D, Urban JL, Sercarz EE. Encephalitogenic T cells in the B10. PL model of experimental allergic encephalomyelitis (EAE) are of the Th-1 lymphokine subtype. Cell Immunol. 1989;124:132–143. doi: 10.1016/0008-8749(89)90117-2. [DOI] [PubMed] [Google Scholar]
- 25.Traugott U, Lebon P. Multiple sclerosis: involvement of interferons in lesion pathogenesis. Ann Neurol. 1988;24:243–251. doi: 10.1002/ana.410240211. [DOI] [PubMed] [Google Scholar]
- 26.Kuchroo VK, Martin CA, Greer JM, Ju ST, Sobel RA, Dorf ME. Cytokines and adhesion molecules contribute to the ability of myelin proteolipid protein-specific T cell clones to mediate experimental allergic encephalomyelitis. J Immunol. 1993;151:4371–4382. [PubMed] [Google Scholar]
- 27.Baron J, Madri J, Ruddle N, Hashim G, Janeway C., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987;37:1097–1102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- 30.Ferber IA, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 31.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 32.Jones LS, et al. IFN-gamma-deficient mice develop experimental autoimmune uveitis in the context of a deviant effector response. J Immunol. 1997;158:5997–6005. [PubMed] [Google Scholar]
- 33.Matthys P, Vermeire K, Mitera T, Heremans H, Huang S, Billiau A. Anti-IL-12 antibody prevents the development and progression of collagen-induced arthritis in IFN-gamma receptor-deficient mice. Eur J Immunol. 1998;28:2143–2151. doi: 10.1002/(SICI)1521-4141(199807)28:07<2143::AID-IMMU2143>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 34.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matusevicius D, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 37.Aarvak T, Chabaud M, Miossec P, Natvig JB. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J Immunol. 1999;162:1246–1251. [PubMed] [Google Scholar]
- 38.Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645–649. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 39.Hofstetter HH, et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Ishigame H, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 42.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 43.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang XO, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 46.Yang L, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou L, Littman DR. Transcriptional regulatory networks in Th17 cell differentiation. Curr Opin Immunol. 2009;21:146–152. doi: 10.1016/j.coi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng Y, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 49.Awasthi A, et al. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jäger A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X, et al. Crucial role of interleukin-7 in T helper type 17 survival and expansion in autoimmune disease. Nat Med. 2010;16:191–197. doi: 10.1038/nm.2077. [DOI] [PubMed] [Google Scholar]
- 52.Axtell RC, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gallegos AM, Bevan MJ. Central tolerance: good but imperfect. Immunol Rev. 2006;209:290–296. doi: 10.1111/j.0105-2896.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 54.Powrie F, Mason D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22low subset. J Exp Med. 1990;172:1701–1708. doi: 10.1084/jem.172.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 56.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 57.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 58.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 59.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 60.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 61.Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev. 2011;241:260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen W, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 64.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 65.Liang S, Alard P, Zhao Y, Parnell S, Clark SL, Kosiewicz MM. Conversion of CD4+ CD25− cells into CD4+ CD25+ regulatory T cells in vivo requires B7 costimulation, but not the thymus. J Exp Med. 2005;201:127–137. doi: 10.1084/jem.20041201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi T, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 69.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 70.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J Exp Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Collison LW, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 73.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 74.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 75.Deaglio S, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bour-Jordan H, Bluestone JA. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev. 2009;229:41–66. doi: 10.1111/j.1600-065X.2009.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925–3932. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grohmann U, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 80.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10:689–695. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 81.Sugimoto N, et al. Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol. 2006;18:1197–1209. doi: 10.1093/intimm/dxl060. [DOI] [PubMed] [Google Scholar]
- 82.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 83.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chaudhry A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Linterman MA, et al. Foxp3(+) follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chung Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ligers A, Xu C, Saarinen S, Hillert J, Olerup O. The CTLA-4 gene is associated with multiple sclerosis. J Neuroimmunol. 1999;97:182–190. doi: 10.1016/s0165-5728(99)00072-7. [DOI] [PubMed] [Google Scholar]
- 90.Kantarci OH, et al. CTLA4 is associated with susceptibility to multiple sclerosis. J Neuroimmunol. 2003;134:133–141. doi: 10.1016/s0165-5728(02)00395-8. [DOI] [PubMed] [Google Scholar]
- 91.Suppiah V, et al. The CTLA4 +49 A/G*G-CT60*G haplotype is associated with susceptibility to multiple sclerosis in Flanders. J Neuroimmunol. 2005;164:148–153. doi: 10.1016/j.jneuroim.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 92.Roxburgh RH, et al. No evidence of a significant role for CTLA-4 in multiple sclerosis. J Neuroimmunol. 2006;171:193–197. doi: 10.1016/j.jneuroim.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 93.Fukazawa T, et al. CTLA-4 gene polymorphism may modulate disease in Japanese multiple sclerosis patients. J Neurol Sci. 1999;171:49–55. doi: 10.1016/s0022-510x(99)00251-8. [DOI] [PubMed] [Google Scholar]
- 94.Maurer M, Ponath A, Kruse N, Rieckmann P. CTLA4 exon 1 dimorphism is associated with primary progressive multiple sclerosis. J Neuroimmunol. 2002;131:213–215. doi: 10.1016/s0165-5728(02)00275-8. [DOI] [PubMed] [Google Scholar]
- 95.Bilinska M, et al. Progression of multiple sclerosis is associated with exon 1 CTLA-4 gene polymorphism. Acta Neurol Scand. 2004;110:67–71. doi: 10.1111/j.1600-0404.2004.00271.x. [DOI] [PubMed] [Google Scholar]
- 96.Karabon L, et al. The CTLA-4 gene polymorphisms are associated with CTLA-4 protein expression levels in multiple sclerosis patients and with susceptibility to disease. Immunology. 2009;128:e787–796. doi: 10.1111/j.1365-2567.2009.03083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ueda H, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 98.Magistrelli G, et al. A soluble form of CTLA-4 generated by alternative splicing is expressed by nonstimulated human T cells. Eur J Immunol. 1999;29:3596–3602. doi: 10.1002/(SICI)1521-4141(199911)29:11<3596::AID-IMMU3596>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 99.Oaks MK, Hallett KM, Penwell RT, Stauber EC, Warren SJ, Tector AJ. A native soluble form of CTLA-4. Cell Immunol. 2000;201:144–153. doi: 10.1006/cimm.2000.1649. [DOI] [PubMed] [Google Scholar]
- 100.Gerold KD, Zheng P, Rainbow DB, Zernecke A, Wicker LS, Kissler S. The soluble CTLA-4 splice variant protects from type 1 diabetes and potentiates regulatory T-cell function. Diabetes. 2011;60:1955–1963. doi: 10.2337/db11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu SM, et al. Overexpression of the Ctla-4 isoform lacking exons 2 and 3 causes autoimmunity. J Immunol. 2011;188:155–162. doi: 10.4049/jimmunol.1102042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Araki M, et al. Genetic evidence that the differential expression of the ligand-independent isoform of CTLA-4 is the molecular basis of the Idd5. 1 type 1 diabetes region in nonobese diabetic mice. J Immunol. 2009;183:5146–5157. doi: 10.4049/jimmunol.0802610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang H, et al. Plasma sCD28, sCTLA-4 levels in neuromyelitis optica and multiple sclerosis during relapse. J Neuroimmunol. 2012;243:52–55. doi: 10.1016/j.jneuroim.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 104.Hickey W. T lymphocyte entry and antigen recognition in the central nervous system. In: Ader R, Felton D, Cohen N, editors. Psycoimmunoloty. Academic Press; New York: 1991. pp. 149–176. [Google Scholar]
- 105.Miller SD, Wetzig RP, Claman HN. The induction of cell-mediated immunity and tolerance with protein antigens coupled to syngeneic lymphoid cells. J Exp Med. 1979;149:758–773. doi: 10.1084/jem.149.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eagar TN, Karandikar NJ, Bluestone JA, Miller SD. The role of CTLA-4 in induction and maintenance of peripheral T cell tolerance. Eur J Immunol. 2002;32:972–981. doi: 10.1002/1521-4141(200204)32:4<972::AID-IMMU972>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 108.Karandikar NJ, Eagar TN, Vanderlugt CL, Bluestone JA, Miller SD. CTLA-4 downregulates epitope spreading and mediates remission in relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 2000;109:173–180. doi: 10.1016/s0165-5728(00)00322-2. [DOI] [PubMed] [Google Scholar]
- 109.Perrin PJ, Maldonado JH, Davis TA, June CH, Racke MK. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J Immunol. 1996;157:1333–1336. [PubMed] [Google Scholar]
- 110.Hurwitz AA, Sullivan TJ, Krummel MF, Sobel RA, Allison JP. Specific blockade of CTLA-4/B7 interactions results in exacerbated clinical and histologic disease in an actively-induced model of experimental allergic encephalomyelitis. J Neuroimmunol. 1997;73:57–62. doi: 10.1016/s0165-5728(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 111.Westhovens R, et al. Safety and efficacy of the selective costimulation modulator abatacept in patients with rheumatoid arthritis receiving background methotrexate: a 5-year extended phase IIB study. J Rheumatol. 2009;36:736–742. doi: 10.3899/jrheum.080813. [DOI] [PubMed] [Google Scholar]
- 112.Kremer JM, et al. Treatment of rheumatoid arthritis with the selective costimulation modulator abatacept: twelve-month results of a phase iib, double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52:2263–2271. doi: 10.1002/art.21201. [DOI] [PubMed] [Google Scholar]
- 113.Cross A, Girard T, Giacoletto K, Evans R, Karr R. CTLA4-Ig treatment prevents murine experimental autoimmune encephalomyelitis. J Neuroimmunol. 1994;54:P01.01 (abstract). [Google Scholar]
- 114.Khoury S, et al. CD28-B7 costimulatory blockade by CTLA4Ig prevents actively induced experimental autoimmune encephalomyelitis and inhibits Th1 but spares Th2 cytokines in the central nervous system. Journal of Immunology. 1995;155:4521–4524. [PubMed] [Google Scholar]
- 115.Kuchroo VK, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 116.Racke MK, et al. Distinct roles for B7-1 (CD-80) and B7-2 (CD-86) in the initiation of experimental allergic encephalomyelitis. J Clin Invest. 1995;96:2195–2203. doi: 10.1172/JCI118274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miller SD, et al. Blockade of CD28/B7-1 interaction prevents epitope spreading and clinical relapses of murine EAE. Immunity. 1995;3:739–745. doi: 10.1016/1074-7613(95)90063-2. [DOI] [PubMed] [Google Scholar]
- 118.Karandikar NJ, Vanderlugt CL, Eagar T, Tan L, Bluestone JA, Miller SD. Tissue-specific up-regulation of B7-1 expression and function during the course of murine relapsing experimental autoimmune encephalomyelitis. J Immunol. 1998;161:192–199. [PubMed] [Google Scholar]
- 119.Mena E, Rohowsky-Kochan C. Expression of costimulatory molecules on peripheral blood mononuclear cells in multiple sclerosis. Acta Neurol Scand. 1999;100:92–96. doi: 10.1111/j.1600-0404.1999.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 120.Genc K, Dona DL, Reder AT. Increased CD80(+) B cells in active multiple sclerosis and reversal by interferon beta-1b therapy. J Clin Invest. 1997;99:2664–2671. doi: 10.1172/JCI119455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chang TC, Jabs C, Sobel RA, Kuchroo VK, Sharpe AH. Studies in B7-deficient mice reveal a critical role for B7 costimulation in both the initiation and effector phases of. Journal of Experimental Medicine. 1999;190:733–740. doi: 10.1084/jem.190.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang TT, Jabs C, Sobel RA, Kuchroo VK, Sharpe AH. Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis. J Exp Med. 1999;190:733–740. doi: 10.1084/jem.190.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Scholz C, Patton KT, Anderson DE, Freeman GJ, Hafler DA. Expansion of autoreactive T cells in multiple sclerosis is independent of exogenous B7 costimulation. J Immunol. 1998;160:1532–1538. [PubMed] [Google Scholar]
- 124.Oliveira EM, et al. CTLA-4 dysregulation in the activation of myelin basic protein reactive T cells may distinguish patients with multiple sclerosis from healthy controls. J Autoimmun. 2003;20:71–81. doi: 10.1016/s0896-8411(02)00106-3. [DOI] [PubMed] [Google Scholar]
- 125.Bachmann MF, Kohler G, Ecabert B, Mak TW, Kopf M. Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J Immunol. 1999;163:1128–1131. [PubMed] [Google Scholar]
- 126.Tivol EA, Gorski J. Re-establishing peripheral tolerance in the absence of CTLA-4: complementation by wild-type T cells points to an indirect role for CTLA-4. J Immunol. 2002;169:1852–1858. doi: 10.4049/jimmunol.169.4.1852. [DOI] [PubMed] [Google Scholar]
- 127.Wu Y, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 128.Gavin MA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 129.Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006;118:240–249. doi: 10.1111/j.1365-2567.2006.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schildknecht A, et al. FoxP3+ regulatory T cells essentially contribute to peripheral CD8+ T-cell tolerance induced by steady-state dendritic cells. Proc Natl Acad Sci U S A. 2010;107:199–203. doi: 10.1073/pnas.0910620107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 133.Qureshi OS, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fallarino F, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 135.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kataoka H, et al. CD25(+)CD4(+) regulatory T cells exert in vitro suppressive activity independent of CTLA-4. Int Immunol. 2005;17:421–427. doi: 10.1093/intimm/dxh221. [DOI] [PubMed] [Google Scholar]
- 137.Read S, et al. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ise W, et al. CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms. Nat Immunol. 2010;11:129–135. doi: 10.1038/ni.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jain N, Nguyen H, Chambers C, Kang J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc Natl Acad Sci U S A. 2010;107:1524–1528. doi: 10.1073/pnas.0910341107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schmidt EM, et al. Ctla-4 controls regulatory T cell peripheral homeostasis and is required for suppression of pancreatic islet autoimmunity. J Immunol. 2009;182:274–282. doi: 10.4049/jimmunol.182.1.274. [DOI] [PubMed] [Google Scholar]
- 141.Verhagen J, et al. Enhanced selection of FoxP3+ T-regulatory cells protects CTLA-4-deficient mice from CNS autoimmune disease. Proc Natl Acad Sci U S A. 2009;106:3306–3311. doi: 10.1073/pnas.0803186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yu X, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 143.Stanietsky N, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2009;106:17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Levin SD, et al. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur J Immunol. 2010;41:902–915. doi: 10.1002/eji.201041136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Boles KS, et al. A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC. Eur J Immunol. 2009;39:695–703. doi: 10.1002/eji.200839116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stengel KF, et al. Structure of TIGIT immunoreceptor bound to poliovirus receptor reveals a cell-cell adhesion and signaling mechanism that requires cis-trans receptor clustering. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1120606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Joller N, et al. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186:1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 149.Casado JG, et al. Expression of adhesion molecules and ligands for activating and costimulatory receptors involved in cell-mediated cytotoxicity in a large panel of human melanoma cell lines. Cancer Immunol Immunother. 2009;58:1517–1526. doi: 10.1007/s00262-009-0682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bottino C, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198:557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hafler JP, et al. CD226 Gly307Ser association with multiple autoimmune diseases. Genes Immun. 2009;10:5–10. doi: 10.1038/gene.2008.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Maiti AK, et al. Non-synonymous variant (Gly307Ser) in CD226 is associated with susceptibility to multiple autoimmune diseases. Rheumatology (Oxford) 2010;49:1239–1244. doi: 10.1093/rheumatology/kep470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Du Y, et al. Association of the CD226 single nucleotide polymorphism with systemic lupus erythematosus in the Chinese Han population. Tissue Antigens. 2010;77:65–67. doi: 10.1111/j.1399-0039.2010.01568.x. [DOI] [PubMed] [Google Scholar]
- 154.Suzuki T, et al. Non-synonymous variant (Gly307Ser) in CD226 is associated with susceptibility in Japanese rheumatoid arthritis patients. Mod Rheumatol. 2012 doi: 10.1007/s10165-012-0609-x. [DOI] [PubMed] [Google Scholar]
- 155.Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 Axis Regulates Human T Cell Function. J Immunol. 2012 doi: 10.4049/jimmunol.1103627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang X, et al. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity. 2004;20:337–347. doi: 10.1016/s1074-7613(04)00051-2. [DOI] [PubMed] [Google Scholar]
- 159.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nishimura H, et al. Developmentally regulated expression of the PD-1 protein on the surface of double-negative (CD4−CD8−) thymocytes. Int Immunol. 1996;8:773–780. doi: 10.1093/intimm/8.5.773. [DOI] [PubMed] [Google Scholar]
- 161.Agata Y, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 162.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 165.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 166.Yamazaki T, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 167.Zhong X, Tumang JR, Gao W, Bai C, Rothstein TL. PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and phosphatidylcholine binding. Eur J Immunol. 2007;37:2405–2410. doi: 10.1002/eji.200737461. [DOI] [PubMed] [Google Scholar]
- 168.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7–1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 170.Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci U S A. 2005;102:11823–11828. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liang SC, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 172.Nishimura H, Honjo T, Minato N. Facilitation of beta selection and modification of positive selection in the thymus of PD-1-deficient mice. J Exp Med. 2000;191:891–898. doi: 10.1084/jem.191.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Keir ME, Latchman YE, Freeman GJ, Sharpe AH. Programmed death-1 (PD-1):PD-ligand 1 interactions inhibit TCR-mediated positive selection of thymocytes. J Immunol. 2005;175:7372–7379. doi: 10.4049/jimmunol.175.11.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Blank C, Brown I, Marks R, Nishimura H, Honjo T, Gajewski TF. Absence of programmed death receptor 1 alters thymic development and enhances generation of CD4/CD8 double-negative TCR-transgenic T cells. J Immunol. 2003;171:4574–4581. doi: 10.4049/jimmunol.171.9.4574. [DOI] [PubMed] [Google Scholar]
- 175.Thangavelu G, Parkman JC, Ewen CL, Uwiera RR, Baldwin TA, Anderson CC. Programmed death-1 is required for systemic self-tolerance in newly generated T cells during the establishment of immune homeostasis. J Autoimmun. 2011;36:301–312. doi: 10.1016/j.jaut.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 176.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 177.Brown JA, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 178.Keir ME, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ansari MJ, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fife BT, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chen L, et al. Constitutive neuronal expression of the immune regulator, programmed death 1 (PD-1), identified during experimental autoimmune uveitis. Ocul Immunol Inflamm. 2009;17:47–55. doi: 10.1080/09273940802491884. [DOI] [PubMed] [Google Scholar]
- 182.Cheng X, Zhao Z, Ventura E, Gran B, Shindler KS, Rostami A. The PD-1/PD-L pathway is up-regulated during IL-12-induced suppression of EAE mediated by IFN-gamma. J Neuroimmunol. 2007;185:75–86. doi: 10.1016/j.jneuroim.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Magnus T, et al. Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: implications for immune responses and autoimmunity in the CNS. J Neurosci. 2005;25:2537–2546. doi: 10.1523/JNEUROSCI.4794-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Salama AD, et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med. 2003;198:71–78. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhu B, et al. Differential role of programmed death-ligand 1 [corrected] and programmed death-ligand 2 [corrected] in regulating the susceptibility and chronic progression of experimental autoimmune encephalomyelitis. J Immunol. 2006;176:3480–3489. doi: 10.4049/jimmunol.176.6.3480. [DOI] [PubMed] [Google Scholar]
- 186.Carter LL, et al. PD-1/PD-L1, but not PD-1/PD-L2, interactions regulate the severity of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2007;182:124–134. doi: 10.1016/j.jneuroim.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 187.Latchman YE, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 189.Prokunina L, et al. A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans. Nat Genet. 2002;32:666–669. doi: 10.1038/ng1020. [DOI] [PubMed] [Google Scholar]
- 190.Kroner A, et al. A PD-1 polymorphism is associated with disease progression in multiple sclerosis. Ann Neurol. 2005;58:50–57. doi: 10.1002/ana.20514. [DOI] [PubMed] [Google Scholar]
- 191.Schreiner B, et al. Interferon-beta enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J Neuroimmunol. 2004;155:172–182. doi: 10.1016/j.jneuroim.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 192.Hirata S, Senju S, Matsuyoshi H, Fukuma D, Uemura Y, Nishimura Y. Prevention of experimental autoimmune encephalomyelitis by transfer of embryonic stem cell-derived dendritic cells expressing myelin oligodendrocyte glycoprotein peptide along with TRAIL or programmed death-1 ligand. J Immunol. 2005;174:1888–1897. doi: 10.4049/jimmunol.174.4.1888. [DOI] [PubMed] [Google Scholar]
- 193.Ding H, et al. Delivering PD-1 inhibitory signal concomitant with blocking ICOS co-stimulation suppresses lupus-like syndrome in autoimmune BXSB mice. Clin Immunol. 2006;118:258–267. doi: 10.1016/j.clim.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 194.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25 high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 195.Francisco LM, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Krupnick AS, et al. Murine vascular endothelium activates and induces the generation of allogeneic CD4+25+Foxp3+ regulatory T cells. J Immunol. 2005;175:6265–6270. doi: 10.4049/jimmunol.175.10.6265. [DOI] [PubMed] [Google Scholar]
- 197.Amarnath S, et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3:111ra120. doi: 10.1126/scitranslmed.3003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med. 2006;12:518–525. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- 199.Wang C, Li Y, Proctor TM, Vandenbark AA, Offner H. Down-modulation of programmed death 1 alters regulatory T cells and promotes experimental autoimmune encephalomyelitis. J Neurosci Res. 2010;88:7–15. doi: 10.1002/jnr.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Linthicum DS, Munoz JJ, Blaskett A. Acute experimental autoimmune encephalomyelitis in mice. I. Adjuvant action of Bordetella pertussis is due to vasoactive amine sensitization and increased vascular permeability of the central nervous system. Cell Immunol. 1982;73:299–310. doi: 10.1016/0008-8749(82)90457-9. [DOI] [PubMed] [Google Scholar]
- 201.Bruckener KE, el Baya A, Galla HJ, Schmidt MA. Permeabilization in a cerebral endothelial barrier model by pertussis toxin involves the PKC effector pathway and is abolished by elevated levels of cAMP. J Cell Sci. 2003;116:1837–1846. doi: 10.1242/jcs.00378. [DOI] [PubMed] [Google Scholar]
- 202.Chen X, Winkler-Pickett RT, Carbonetti NH, Ortaldo JR, Oppenheim JJ, Howard OM. Pertussis toxin as an adjuvant suppresses the number and function of CD4+CD25+ T regulatory cells. Eur J Immunol. 2006;36:671–680. doi: 10.1002/eji.200535353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cassan C, et al. Pertussis toxin reduces the number of splenic Foxp3+ regulatory T cells. J Immunol. 2006;177:1552–1560. doi: 10.4049/jimmunol.177.3.1552. [DOI] [PubMed] [Google Scholar]
- 204.Monney L, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 205.Chen Y, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hastings WD, et al. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol. 2009;39:2492–2501. doi: 10.1002/eji.200939274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nakae S, Iwakura Y, Suto H, Galli SJ. Phenotypic differences between Th1 and Th17 cells and negative regulation of Th1 cell differentiation by IL-17. J Leukoc Biol. 2007;81:1258–1268. doi: 10.1189/jlb.1006610. [DOI] [PubMed] [Google Scholar]
- 208.Sabatos CA, et al. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4:1102–1110. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 209.Zhu C, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 210.Koguchi K, Anderson DE, Yang L, O’Connor KC, Kuchroo VK, Hafler DA. Dysregulated T cell expression of TIM3 in multiple sclerosis. J Exp Med. 2006;203:1413–1418. doi: 10.1084/jem.20060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yang L, Anderson DE, Kuchroo J, Hafler DA. Lack of TIM-3 immunoregulation in multiple sclerosis. J Immunol. 2008;180:4409–4414. doi: 10.4049/jimmunol.180.7.4409. [DOI] [PubMed] [Google Scholar]
- 212.Kanai Y, Satoh T, Igawa K, Yokozeki H. Impaired Expression of Tim-3 on Th17 and Th1 Cells in Psoriasis. Acta Derm Venereol. 2012 doi: 10.2340/00015555-1285. [DOI] [PubMed] [Google Scholar]
- 213.Liu Y, et al. Increased Tim-3 expression on peripheral lymphocytes from patients with rheumatoid arthritis negatively correlates with disease activity. Clin Immunol. 2010;137:288–295. doi: 10.1016/j.clim.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 214.Golden-Mason L, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jones RB, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jin HT, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fourcade J, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhou Q, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 220.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 221.Takamura S, et al. Premature terminal exhaustion of Friend virus-specific effector CD8+ T cells by rapid induction of multiple inhibitory receptors. J Immunol. 2010;184:4696–4707. doi: 10.4049/jimmunol.0903478. [DOI] [PubMed] [Google Scholar]
- 222.Kaufmann DE, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 223.Schurich A, et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology. 2011;53:1494–1503. doi: 10.1002/hep.24249. [DOI] [PubMed] [Google Scholar]