Abstract
Experiences of adversity in the early years of life alter the developing brain. However, evidence documenting this relationship often focuses on severe stressors and relies on peripheral measures of neurobiological functioning during infancy. The present study employed functional magnetic resonance imaging (fMRI) during natural sleep to examine associations between a more moderate environmental stressor (non-physical interparental conflict), and 6–12 month-old infants’ neural processing of emotional tone of voice. The primary question was whether interparental conflict experienced by infants is associated with neural responses to emotional tone of voice, particularly very angry speech. Results indicated that maternal report of higher interparental conflict was associated with infants’ heightened neural responses to very angry versus neutral speech across several brain regions implicated in emotion and stress reactivity and regulation (including rostral anterior cingulate cortex, caudate, thalamus and hypothalamus) – suggesting that even moderate environmental stress may be associated with brain functioning during infancy.
Keywords: Psychological Stress, Neuroimaging, Emotional Development, Infant Development
Prominent ideas about how the environment shapes development rest on an understanding that brain plasticity during the first years of life confers vulnerability for key neural systems involved in stress and emotion related functioning. The consequences of early life stress are increasingly understood via impact on these systems (Sánchez, Ladd, & Plotsky, 2001). Existing work with infants and young children has leveraged peripheral indicators of neuroendocrine functioning (e.g., cortisol; Loman & Gunnar, 2010), and direct measures of brain electrical activity (EEG; Nelson & McCleery, 2008) to increase understanding of how early adversity impacts neurobehavioral development.
The high spatial resolution of functional magnetic resonance imaging (fMRI), commonly used with older children and adults, has facilitated precise identification of neural networks linking early adversity with subsequent socioemotional functioning, with a focus on brain regions tied to initiation and regulation of the hypothalamic-pituitary-adrenal axis (HPA) stress response, including limbic (Tottenham et al., 2011) and medial prefrontal regions (Treadway et al., 2009). However, fMRI research in older children and adults fails to distinguish effects of early stress from subsequent recovery or development of psychopathology. Moreover, a predominant focus on severe stressors, such as institutional rearing or maltreatment (Hart & Rubia, 2012), leaves a gap in the empirical literature regarding effects of more moderate early adversity.
Non-physical interparental conflict is a more moderate source of early adversity that nevertheless appears to be associated with alterations in stress hormones, behavioral symptoms, and socioemotional problems during childhood (Cummings & Davies, 2010; Davies, Sturge-Apple, Cicchetti, & Cummings, 2007). Although more sparse, research with infants indicates that interparental conflict is associated with differences in physiological and behavioral indices of emotional reactivity and regulation as early as 6-months-of-age (Crockenberg, Leerkes, & Lekka, 2007; Moore, 2010). Interparental conflict may have an impact on early emotional development due to decreases in sensitive caregiving (Krishnakumar & Buehler, 2000), as well as direct exposure to aggressive interactions between caregivers (Crockenberg et al., 2007). Basic research suggests that 5-month-old infants discriminate between different emotional states, with expressions of anger eliciting greater attention and arousal than happy or neutral (Balaban, 1995; Grossmann, Oberecker, Koch, & Friederici, 2010; Grossmann, Striano, & Friederici, 2005). Moore (2009) showed that infants who witnessed vocal anger toward their mother demonstrated altered parasympathetic nervous system responses to an immediately subsequent stressful interaction with their mother. Specifically, they showed greater withdrawal of vagal tone and less recovery, indicative of greater physiological reactivity after this brief exposure to anger (Moore, 2009).
Early exposure to interparental conflict may also increase risk for later emotional and psychological problems. Interparental conflict is associated with lower baseline vagal tone (Porter et al., 2003) and greater withdrawal of vagal tone during a stressful interaction and recovery period in 6-month-olds (Moore, 2010), indicative of lower parasympathetic tone and greater stress reactivity. Variation in vagal reactivity acts as a moderator of risk for school-aged children exposed to conflict (El-Sheikh et al., 2009; El-Sheikh & Whitson, 2006). Despite the implication that some aspects of nervous system functioning may be shaped by family conflict during infancy, and subsequently increase risk for school-aged children, the ties between early exposure and subsequent vulnerability remain poorly understood. The autonomic and behavioral measures utilized to date represent outputs from multiple neural networks. Candidate neural networks linking early adversity and subsequent risk for psychopathology have not yet been identified.
Recent work demonstrates the feasibility of conducting fMRI research with infants during natural sleep (Redcay, Kennedy, & Courchesne, 2007), allowing for examination of specific neural regions and networks during the first years of life. This work also draws attention to the sensitivity of infants to environmental stimuli during sleep by documenting distinct patterns of neural activation depending on properties of speech (Dehaene-Lambertz, Dehaene, & Hertz-Pannier, 2002; Redcay, Haist, & Courchesne, 2008) and emotional tone (Blasi et al., 2011). The present study builds upon these methodological advances to characterize infants’ neural responses to emotional stimuli in the context of varying levels of interparental conflict.
Method
Participants
Families were recruited through flyers posted at local public sector human services agencies and advertisements on Craigslist. Twenty-four infants (8F/16M) aged 6–12-months (M=8.33, SD=1.90) completed the auditory fMRI paradigm during natural sleep; twenty infants had usable fMRI data (see below). Infants had no known neurological disorders, and lived with both biological parents. Exclusionary criteria included referrals or investigations by a public child protective services agency. Interparental conflict was assessed during screening to obtain sufficient range in the sample, using the Problem Solving Communication subscale from the Marital Satisfaction Inventory-Revised (Snyder, 1997), and selecting families based on established norms for distressed versus non-distressed couples.
Interparental Conflict Measures
Mothers rated interparental non-physical conflict levels since the birth of the child on the Psychological Aggression scale of the Conflicts Tactics Scale-Revised (Straus, Hamby, Boney-McCoy, & Sugarman, 1996) and the O’Leary-Porter Scale (Porter & O’Leary, 1980; see Supplementary Materials). The measures were highly reliable (α=.936 and .823, respectively) and correlated (r(22)=0.744, p< .001) allowing for creation of an average, composite score of maternal report of interparental conflict (see Supplementary Materials).
Auditory Stimuli
Auditory stimuli consisted of previously validated nonsense sentences spoken in very angry, mildly angry, happy and neutral tones of voice by a male adult (Pell, Paulmann, Dara, Alasseri, & Kotz, 2009). Nonsense sentences possessed phonological and grammatical properties of English, but content words were replaced by semantically meaningless sound strings (see Supplementary Materials).
fMRI Data Acquisition
Infants came in at their regular bedtime for scanning (see Supplementary Materials). Neuroimaging data were collected on a Siemens Allegra 3.0T scanner with a phased array coil. Consistent with previous neuroimaging research using auditory stimuli with sleeping toddlers (Redcay et al., 2007) the paradigm consisted of 20sec blocks separated by 15sec rest periods. Blocks for each emotion condition (very angry, mildly angry, happy and neutral) were presented four times per run in a semi-counterbalanced design based on a Williams’ Latin Square. T2-weighted echo-planar functional scans (9min 28sec; 284 whole brain volumes) were acquired during presentation of the paradigm (see Supplementary Materials). Prospective acquisition correction (PACE) was applied to adjust slice position and orientation, as well as to re-grid residual volume-to-volume motion in real-time during data acquisition for the purpose of reducing motion-induced effects (Thesen, Heid, Mueller, & Schad, 2000)
fMRI Data Analysis
Neuroimaging data were converted to Neuroimaging Informatics Technology Initiative (NIfTI) data format using MRIConvert (http://lcni.uoregon.edu/~jolinda/MRIConvert/). Brain images were extracted using BET from FSL (Beckmann et al., 2006; S. Smith, Bannister, Beckmann, & Brady, 2001; S. M. Smith, 2002) and BSE from BrainSuite09 (Sandor & Leahy, 1997; Shattuck, Sandor-Leahy, Schaper, Rottenberg, & Leahy, 2001). All other preprocessing steps were accomplished using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/), including realignment, registration, normalization, and smoothing with a 6mm FWHM kernel. Images were normalized to a standard template for the 8–11 month age range from the MRI Study of Normal Brain Development (Fonov et al., 2011; Fonov, Evans, Mckinstry, Almli, & Collins, 2009). Images with severe motion artifacts (greater than 2mm of motion and/or evidencing visual signs of motion artifacts) were removed from runs resulting in less than 2mm of motion per run (maximum=1.07mm). Each run retained at least 3 (out of a total of 4) blocks of each condition.
At the individual subject level, fixed-effects contrasts were computed to examine neural activation during presentation of each condition (very angry, mildly angry, happy, or neutral) versus rest, as well as the specific contrast of very angry versus neutral. Motion parameters in 6 directions were included as regressors of no interest. Functional runs for which the contrast of all auditory conditions to rest did not evidence auditory cortex activation at a relaxed threshold, p<.05 uncorrected, were excluded from analyses because it was not possible to ascertain whether basic sensory processing of stimuli occurred. Four of the 24 infants did not have at least one functional run for which clear auditory activation was detected and were thus excluded from further analyses. Resulting contrast images were entered into whole brain random-effects group analyses. Results were reported that exceeded a threshold of p<.05, FWE corrected for multiple comparisons across the whole brain (specifically p<.05 and 75 contiguous voxels, determined by NeuroElf’s AlphaSim (http://neuroelf.net/)). Regions of activation were identified based on anatomical landmarks, although infant template and MNI template coordinates are provided for reference.
Results
Effect of interparental conflict on processing very angry tone of voice
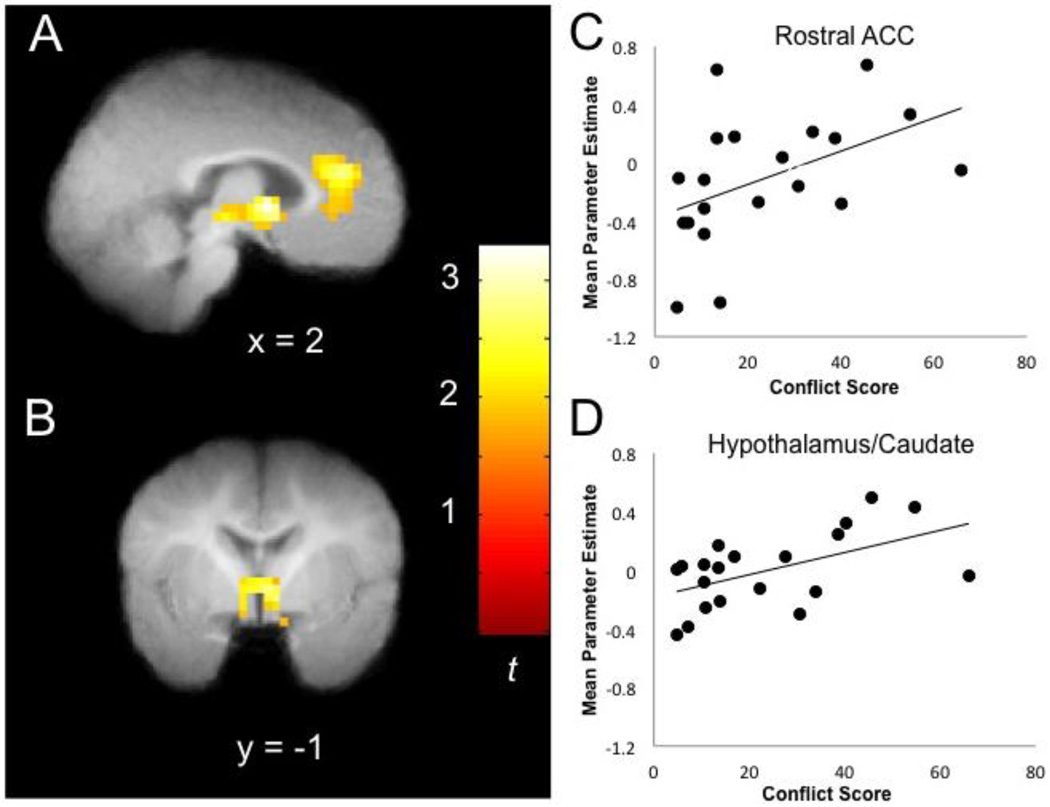
The primary research question focused on the extent to which the composite interparental conflict score was associated with infants’ neural responses to very angry auditory stimuli, relative to neutral auditory stimuli. A whole brain regression with interparental conflict score as the independent variable and neural activity during very angry relative to neutral tone of voice as the dependent variable revealed a significant cluster in rostral anterior cingulate cortex (ACC) as well as a subcortical cluster encompassing parts of the caudate, thalamus and hypothalamus (Table 1). Specifically, higher levels of interparental conflict were associated with greater activation in these regions during presentation of very angry compared to neutral speech (Figure 1, Panels A-B). In order to depict this association graphically, mean parameter estimates of activity (averaged across all voxels in each cluster) were extracted for each participant from both the rostral ACC and subcortical cluster during the very angry versus neutral contrast using MarsBaR region of interest toolbox for SPM (http://marsbar.sourceforge.net/). These mean parameter estimates for each participant in each cluster were then plotted as a function of conflict score. The graphs are not an additional statistical analysis, but illustrate the positive association between conflict and activation of these regions to very angry versus neutral speech that was demonstrated statistically with the fMRI analyses (Figure 1, Panels C-D). Results remained consistent when controlling for variation in infant age. These results were specific to the very angry versus neutral contrast. Exploratory analysis of the association between conflict and neural processing of happy speech are presented in Supplementary Materials.
Table 1.
Increased activation associated with higher interparental conflict for very angry > neutral
| Infant Atlas | MNI Atlas | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regression | Region | x | y | z | x | y | z | k | t | |
| Positive correlation between conflict and very angry > neutral | Anterior Cingulate | 3 | 29 | 13 | 4 | 36 | 17 | 88 | 2.72 | |
| Thalamus | R | 3 | −1 | 1 | 4 | −1 | 1 | 94 | 2.80 | |
| Thalamus | L | −6 | −4 | −2 | −7 | −5 | −3 | 2.29 | ||
| Caudate | L | −6 | 5 | 7 | −7 | 6 | 9 | 2.81 | ||
| Hypothalamus | L | −6 | −1 | −5 | −7 | −1 | −6 | 2.08 | ||
|
Activation to emotion stimuli after regressin gout interparental conflict | ||||||||||
| Infant Atlas | MNI Atlas | |||||||||
| Contrast | Region | x | y | z | x | y | z | k | t | |
| Conjunction All>Rest | Auditory Cortex | R | 42 | −13 | 1 | 51 | −16 | 1 | 214 | 4.95 |
| **Auditory Cortex | L | −42 | −16 | 7 | −51 | −20 | 9 | 50 | 3.22 | |
| Very Angry>Neutral | **Temporal pole | L | −45 | −1 | −8 | −55 | −1 | −10 | 53 | 2.96 |
| Mildly Angry>Neutral | No suprathreshold clusters | |||||||||
| Happy>Neutral | Lingual gyms | 0 | −55 | 5 | 0 | −68 | 6 | 473 | 2.96 | |
| Fusiform | L | −18 | −58 | −11 | −22 | −72 | −14 | 2.31 | ||
| Parahippocampal Gyrus | L | −15 | −19 | −17 | −18 | −23 | −22 | 2.14 | ||
| Putamen | L | −21 | 5 | 7 | −26 | 6 | 9 | 189 | 2.55 | |
| Mid-Cingulate | −9 | −1 | 28 | −11 | −1 | 36 | 2.00 | |||
| SMA | −3 | −1 | 49 | −4 | −1 | 63 | 2.00 | |||
| Superior Frontal Gyrus | L | −18 | 35 | 22 | −22 | 43 | 28 | 107 | 2.35 | |
| Middle Frontal Gyrus | L | −33 | 20 | 28 | −40 | 25 | 36 | 1.98 | ||
Note. Activations FWE corrected (p < .05, 75 voxels).
Subthreshold clusters reported (p<.05, uncorrected, 50 voxels). Coordinates without voxel numbers indicate submaxima within preceding cluster. k refers to the number of voxels within each cluster, t refers to the t statistic of the corresponding coordinates (local maxima or submaxima); SMA=Supplementary Motor Area.
Figure 1.
Note. Results are p<.05 FWE corrected and displayed on group mean structural image. Panels A and B show greater activity in the rostral ACC (panel A; infant atlas coordinates [3, 29, 13]; MNI [4, 36, 17]) and a subcortical cluster including hypothalamus, caudate, and thalamus (panel B; infant atlas coordinates [3, −1, 1]; MNI [4, −1, 1]) associated with higher conflict score for the very angry > neutral nonsense speech contrast. Panels C and D re-illustrate the association between conflict score and parameter estimates of activity in the rostral ACC (panel C) and the subcortical cluster including hypothalamus, caudate, and thalamus (panel D) for very angry > neutral nonsense speech.
Effect of different emotional tones of voice
Because conflict was associated with neural responses to very angry speech, brain activation during presentation of each emotional tone of voice was examined after covarying out individual differences in conflict (Table 1). Direct comparison of very angry relative to neutral did not reveal any clusters surviving FWE correction, although a cluster in the left temporal pole was just below this extent threshold. Comparison of happy relative to neutral revealed significant areas of activation in the left dorsolateral prefrontal cortex, putamen, and medial temporal and occipital cortices.
Discussion
While unusually adverse experiences such as institutional rearing or maltreatment are known to impact development of key neural networks, the present study suggests potential effects of a more moderate environmental stressor, non-physical interparental conflict. By taking advantage of recent methodological advances that allow for investigation of neural functioning during infancy with the higher spatial resolution afforded by fMRI (Blasi et al., 2011; Dehaene-Lambertz et al., 2010) this work provides novel evidence of associations between interparental conflict and patterns of infant brain functioning elicited by processing emotional speech during natural sleep.
Higher levels of interparental conflict were associated with greater activation to very angry tone of voice in the rostral ACC and subcortical structures including the hypothalamus. While we cannot be certain about the meaning of the activation patterns in these brain regions, many studies indicate their involvement in emotion and stress processing and regulation (Kober et al., 2008). The rostral ACC is implicated in emotion processing and regulation in typical populations (Kober et al., 2008) and its functioning is frequently altered in stress-related disorders (Fonzo et al., 2010; Kim et al., 2008). Research also demonstrates associations between early adversity and decreased volume of the ACC for adults with (Treadway et al., 2009) and without symptoms of psychopathology (Cohen et al., 2006), although the developmental pathway through which these structural differences emerge remains unknown.
The hypothalamus initiates activity of the HPA-axis. Activity of the HPA-axis in response to psychosocial stress is controlled by limbic brain structures involved in emotion processing and memory including the amygdala, hippocampus and ACC (Pruessner et al., 2010; Ulrich-Lai & Herman, 2009). The hypothalamus is thus viewed as a key link between emotional input, neuroendocrine functioning and stress reactivity (Kober et al., 2008). Extensive research has focused on alterations in the functioning of the HPA-axis (as indexed by the hormone cortisol) as a result of early life stress including more normative stressors, such as interparental conflict (Davies et al., 2007), and more extreme events such as neglect and abuse (Bruce, Fisher, Pears, & Levine, 2009). Specific patterns of HPA-axis functioning have also repeatedly been associated with mood disorders in adolescence and adulthood (Lopez-Duran, Kovacs, & George, 2009; Parker, Schatzberg, & Lyons, 2003).
The evidence reviewed above converges with extensive research using animal models to indicate the ACC and hypothalamus as part of neural networks that link early psychosocial adversity to subsequent difficulties with regulation of emotions and stress (Loman & Gunnar, 2010). However, this study is the first to document an association between an environmental stressor and the functioning of these specific brain regions during infancy. These regions were identified based on a whole brain regression as opposed to a priori specification as regions of interest. This allows for a more independent test of whether the findings in this study converge with existing knowledge about the role of these brain regions based on animal models and research with older children and adults (Hart & Rubia, 2012).
This study also provides novel evidence regarding infants’ neural processing of happy and angry emotional speech during sleep, regardless of the level of interparental conflict. The findings are broadly in line with a recent fMRI study indicating differentiation of sad versus neutral vocalizations in sleeping 3–7-month-olds (Blasi et al., 2011), although this study did not find differences between happy and neutral stimuli. We may have been better able to observe the latter pattern due to differences in the age ranges sampled and the stimuli (nonsense speech versus emotional vocalizations).
Limitations of the present study include the lack of observational assessment of interparental conflict and of a high intensity positive affect condition (e.g. very happy) to test whether the effects are specific to anger versus high intensity emotion. Additionally, recruitment through Craigslist and human services agencies may have skewed the sample towards being of lower socioeconomic-status. We also were unable to monitor and control for sleep state, which is an important issue to be addressed in future work (see Supplementary Materials). Future research will also benefit from longitudinal investigations and inclusion of behavioral measures to assess whether changes in neural functioning mediate between exposure to environmental stress and socioemotional development.
Despite these limitations, the present findings indicate that during a period when infants are particularly vulnerable due to complete dependence on caregivers and high levels of neural plasticity, moderate sources of environmental stress may be related to neural functioning in areas central to emotion and stress related processes. Moreover, far from being oblivious to parents’ conflict, infants’ processing of stressor relevant stimuli, such as angry tone of voice, may occur even during sleep.
Supplementary Material
Acknowledgments
Support was provided by: Center for Drug Abuse Prevention in the Child Welfare System (1-P30-DA023920), Ruth L. Kirschstein National Research Service Award (F31-10667639), and the Lewis Center for NeuroImaging (LCNI) at the University of Oregon. Special thanks are due to Scott Watrous at LCNI, Kyndal Yada at the Oregon Social Learning Center, and Weili Lin and Kathy Wilber at the BRIC, UNC School of Medicine.
References and Notes
- Balaban MT. Affective Influences on Startle in Five-Month-Old Infants: Reactions to Facial Expressions of Emotion. Child Development. 1995;66(1):28. doi: 10.1111/j.1467-8624.1995.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Woolrich MW, Behrens TEJ, David E, Devlin JT, Smith SM. Applying FSL to the FIAC Data: Model-Based and Model-Free Analysis of Voice and Sentence Repetition Priming. Human Brain Mapping. 2006;27(5):380–391. doi: 10.1002/hbm.20246. Applying. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi A, Mercure E, Lloyd-Fox S, Thomson A, Brammer M, Sauter D, Deeley Q, et al. Early Specialization for Voice and Emotion Processing in the Infant Brain. Current Biology. 2011;21(14):1–5. doi: 10.1016/j.cub.2011.06.009. Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher Pa, Pears KC, Levine S. Morning cortisol Levels in preschool-aged foster children: differential effects of maltreatment type. Developmental Psychobiology. 2009;51(1):14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Ra, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological Psychiatry. 2006;59(10):975–82. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Crockenberg SC, Leerkes EM, Lekka SK. Pathways from marital aggression to infant emotion regulation : The development of withdrawal in infancy. Infant Behavior & Development. 2007;30:97–113. doi: 10.1016/j.infbeh.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Cummings EM, Davies PT. Marital Conflict and Children: An Emotional Security Perspective. New York: The Guilford Press; 2010. p. 320. [Google Scholar]
- Davies PT, Sturge-Apple ML, Cicchetti D, Cummings EM. The Role of Child Adrenocortical Functioning in Pathways Between Interparental Conflict and Child Maladjustment. Developmental Psychology. 2007;43(4):918–930. doi: 10.1037/0012-1649.43.4.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional Neuroimaging of Speech Perception in Infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Montavont A, Jobert A, Allirol L, Dubois J, Hertz-Pannier L, Dehaene S. Language or music, mother or Mozart? Structural and environmental influences on infants’ language networks. Brain and language. 2010;114(2):53–65. doi: 10.1016/j.bandl.2009.09.003. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Kouros CD, Erath S, Cummings EM, Keller P, Staton L. Marital conflict and children’s externalizing behavior: interactions between parasympathetic and sympathetic nervous system activity. Monographs of the Society for Research in Child Development. 2009;74(1):1–79. doi: 10.1111/j.1540-5834.2009.00501.x. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Whitson S. Longitudinal relations between marital conflict and child adjustment: Vagal regulation as a protective factor. Journal of Family Psychology. 2006;20(1) doi: 10.1037/0893-3200.20.1.30. 101037/0893-320020130. Retrieved from http://psycnet.apa.org/journals/fam/20/1/30/ [DOI] [PubMed] [Google Scholar]
- Fonov VS, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54(1):313–327. doi: 10.1016/j.neuroimage.2010.07.033. Elsevier Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov VS, Evans AC, Mckinstry RC, Almli CR, Collins DL. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage. 2009;47(Supplement 1):S102. [Google Scholar]
- Fonzo Ga, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biological Psychiatry. 2010;68(5):433–41. doi: 10.1016/j.biopsych.2010.04.028. Elsevier Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, Oberecker R, Koch SP, Friederici AD. The developmental origins of voice processing in the human brain. Neuron. 2010;65(6):852–858. doi: 10.1016/j.neuron.2010.03.001. Elsevier Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, Striano T, Friederici AD. Infants’ electric brain responses to emotional prosody. Neuroreport. 2005;16(16):1825–1828. doi: 10.1097/01.wnr.0000185964.34336.b1. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16237335. [DOI] [PubMed] [Google Scholar]
- Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Frontiers in Human Neuroscience. 2012;6:1–24. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Chey J, Chung A, Bae S, Khang H, Ham B, Yoon SJ, et al. Diminished rostral anterior cingulate activity in response to threat-related events in posttraumatic stress disorder. Journal of psychiatric research. 2008;42(4):268–77. doi: 10.1016/j.jpsychires.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. NeuroImage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnakumar A, Buehler C. Interparental Conflict and Parenting Behaviors: A Meta-Analytic Review. Family Relations. 2000;49(1):25–44. [Google Scholar]
- Loman MM, Gunnar MR. Early experience and the development of stress reactivity and regulation in children. Neuroscience and Biobehavioral Reviews. 2010;34(6):867–876. doi: 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: a meta-analysis. Psychoneuroendocrinology. 2009;34(9):1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GA. Infants’ and mothers' vagal reactivity in response to anger. Journal of Child Psychology and Psychiatry. 2009;50(11):1392–1400. doi: 10.1111/j.1469-7610.2009.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GA. Parent conflict predicts infants’ vagal regulation in social interaction. Development and Psychopathology. 2010;22(1):23–33. doi: 10.1017/S095457940999023X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson Ca, McCleery JP. Use of event-related potentials in the study of typical and atypical development. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(11):1252–61. doi: 10.1097/CHI.0b013e318185a6d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Hormones and Behavior. 2003;43(1):60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Pell MD, Paulmann S, Dara C, Alasseri A, Kotz Sa. Factors in the recognition of vocally expressed emotions: A comparison of four languages. Journal of Phonetics. 2009;37(4):417–435. Elsevier. [Google Scholar]
- Porter B, O’Leary KD. Marital discord and childhood behavior problems. Journal of Abnormal Child Psychology. 1980;8(3):287–295. doi: 10.1007/BF00916376. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7410730. [DOI] [PubMed] [Google Scholar]
- Porter C, Wouden-Miller M, Silva S, Porter A. Marital harmony and conflict: Links to infants’ emotional regulation and cardiac vagal tone. Infancy. 2003;4(2):297–307. Retrieved from http://www.tandfonline.com/doi/abs/10.1207/S15327078IN0402_09. [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, Dagher A, et al. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations--2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35(1):179–91. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Redcay E, Haist F, Courchesne E. Functional neuroimaging of speech perception during a pivotal period in language acquisition. Developmental Science. 2008;11(2):237–252. doi: 10.1111/j.1467-7687.2008.00674.x. [DOI] [PubMed] [Google Scholar]
- Redcay E, Kennedy DP, Courchesne E. fMRI during natural sleep as a method to study brain function during early childhood. NeuroImage. 2007;38:696–707. doi: 10.1016/j.neuroimage.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Sandor S, Leahy R. Surface-based labeling of cortical anatomy using a deformable atlas. IEEE transactions on medical imaging. 1997;16(1):41–54. doi: 10.1109/42.552054. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. NeuroImage. 2001;13(5):856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- Smith S, Bannister P, Beckmann C, Brady M. FSL: New tools for functional and structural brain image analysis. NeuroImage. 2001;(13) 2001. Retrieved from http://c3s2i.free.fr/cv/fsl.pdf.
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder DK. Marital Satisfaction Inventory—Revised. CA: Western Psychological Services; 1997. [Google Scholar]
- Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB. The Revised Conflict Tactics Scales (CTS2) Journal of Family Issues. 1996;17(3):283–316. [Google Scholar]
- Sánchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Development and Psychopathology. 2001;13:419–449. doi: 10.1017/s0954579401003029. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11523842. [DOI] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magnetic Resonance in Medicine. 2000;44(3):457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10975899. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Developmental Science. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Grant MM, Ding Z, Hollon SD, Gore JC, Shelton RC. Early adverse events, HPA activity and rostral anterior cingulate volume in MDD. PloS one. 2009;4(3):e4887. doi: 10.1371/journal.pone.0004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai Y, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.