Abstract
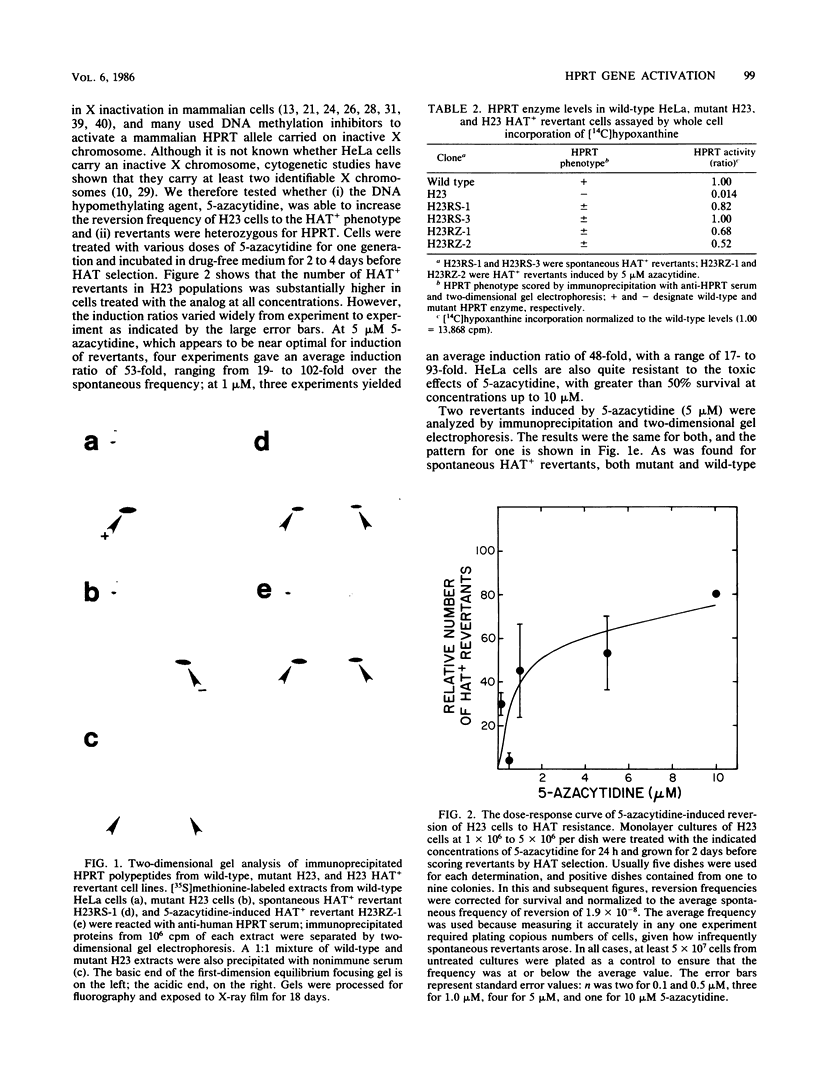
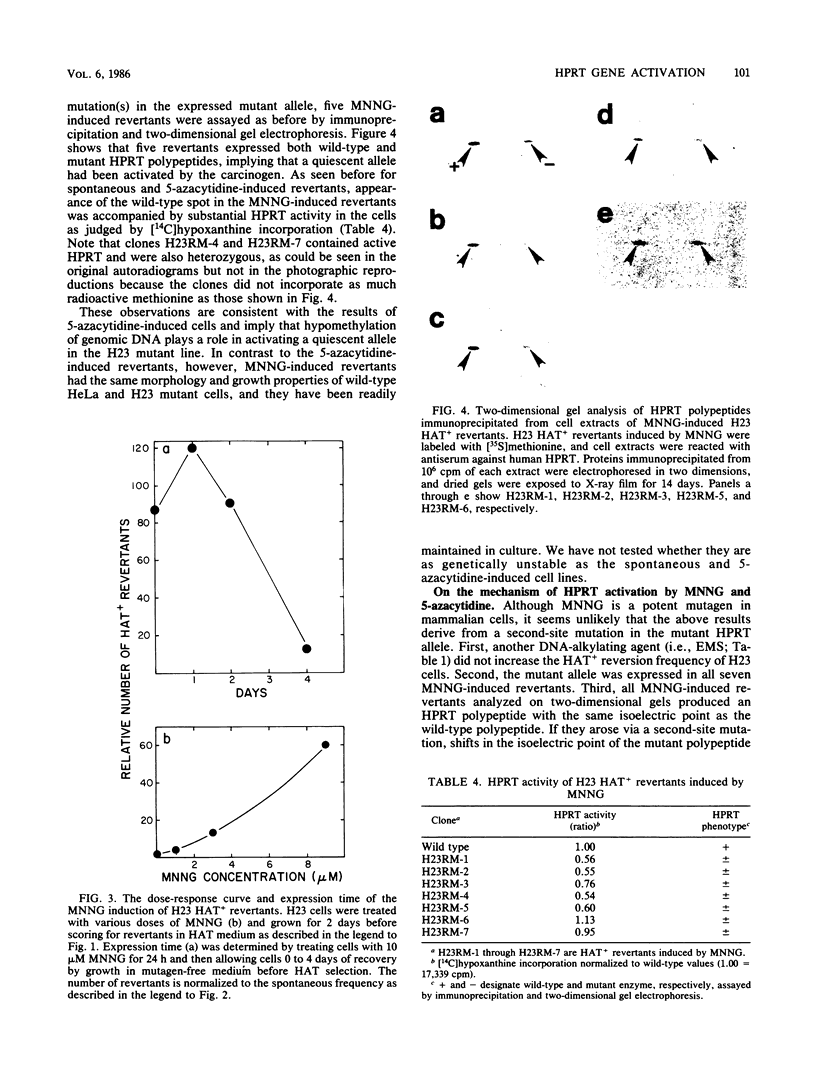
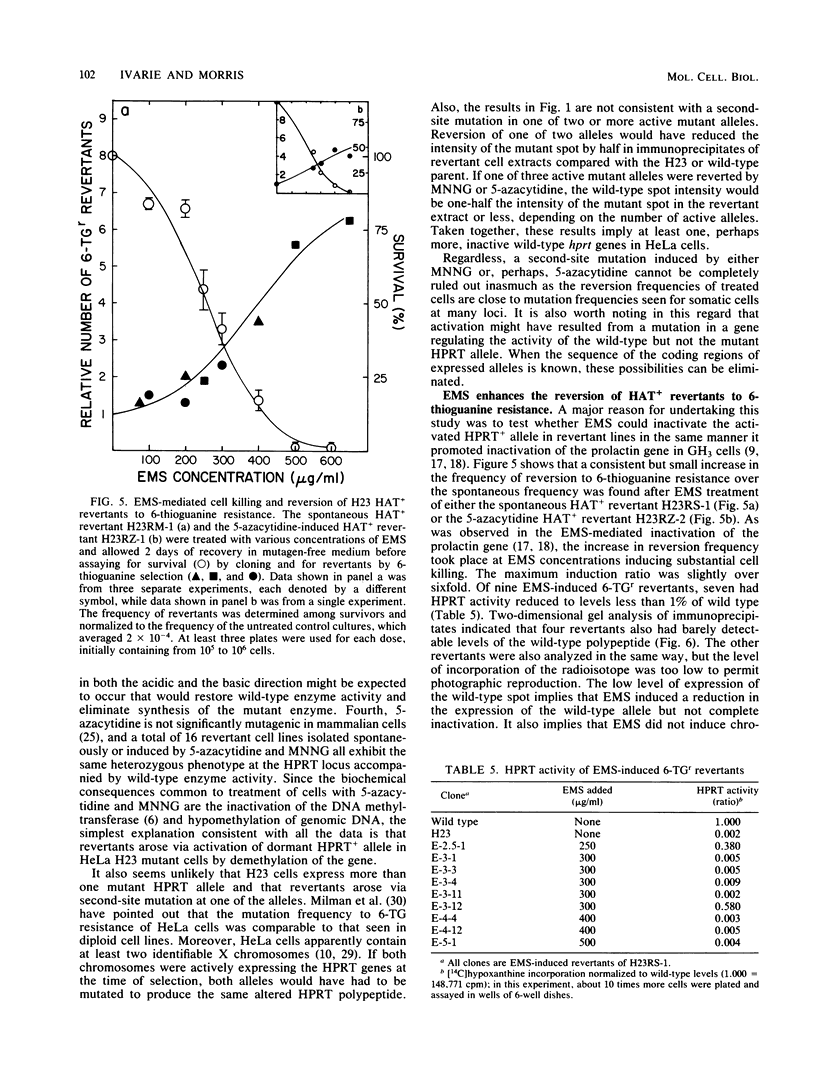
HeLA H23 cells are a mutant female human tumor cell line harboring defective hypoxanthine phosphoribosyltransferase (HPRT; IMP-pyrophosphate phosphoribosyltransferase, EC 2.4.2.8) as a result of a mutation that alters the isoelectric point of the enzyme (G. Milman, E. Lee, G. S. Changas, J. R. McLaughlin, and J. George, Jr., Proc. Natl. Acad. Sci. USA 73:4589-4592, 1976). As shown by Milman et al. and confirmed by us here, rare HAT+ revertants arise spontaneously at 1.9 X 10(-8) frequency and express both mutant and wild-type polypeptides. Thus, the H23 mutant also carries a silent wild-type HPRT allele that is activated in revertants. To test whether the silent allele was activated via hypomethylation of genomic DNA, H23 cells were treated with inhibitors of DNA methylation, and revertants were scored by HAT or azaserine selection. At an optimal dose of 5 microM 5-azacytidine, the reversion frequency was increased about 50-fold when assayed by HAT selection and over 1,000-fold when assayed by azaserine selection. HAT+ and azaserine revertants were heterozygous for HPRT, expressing both wild-type and mutant HPRT polypeptides. Like spontaneous revertants, they contained active HPRT enzyme and were genetically unstable, reverting at about 10(-4) frequency. Similar results were found after treatment with N-methyl-N'-nitro-N-nitrosoguanidine, a DNA-alkylating agent and potent inhibitor of mammalian DNA methylation. By contrast, the DNA-ethylating agent, ethyl methanesulfonate (EMS), did not increase the HAT+ reversion frequency; it did, however, increase the frequency by which H23 revertants heterozygous for HPRT reverted to 6-thioguanine resistance. Of nine EMS revertants, seven lacked HPRT activity and had a substantially reduced expression of the wild-type polypeptide. These observations support the hypothesis that DNA methylation plays an important role in human X-chromosome inactivation and that EMS can inactivate gene expression by promoting enzymatic methylation of genomic DNA as found previously for the prolactin gene in GH3 rat pituitary tumor cells (R. D. Ivarie and J. A. Morris, Proc. Natl. Acad. Sci. USA 79:2967-2970, 1982; R. D. Ivarie, J. A. Morris, and J. A. Martial, Mol. Cell. Biol. 2:179-189, 1982).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bestor T. H., Ingram V. M. Two DNA methyltransferases from murine erythroleukemia cells: purification, sequence specificity, and mode of interaction with DNA. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5559–5563. doi: 10.1073/pnas.80.18.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T. L., Drahovsky D. Hypomethylation of DNA in Raji cells after treatment with N-methyl-N-nitrosourea. Carcinogenesis. 1981;2(1):39–42. doi: 10.1093/carcin/2.1.39. [DOI] [PubMed] [Google Scholar]
- Boehm T. L., Drahovsky D. Inhibition of enzymatic DNA methylation by N-methyl-N-nitro-N-nitrosoguanidine in human Raji lymphoblast-like cells. Int J Biochem. 1981;13(12):1225–1232. doi: 10.1016/0020-711x(81)90068-9. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Kruh G. D. The HPRT locus. Cell. 1979 Jan;16(1):1–9. doi: 10.1016/0092-8674(79)90182-x. [DOI] [PubMed] [Google Scholar]
- Cox R. DNA methylase inhibition in vitro by N-methyl-N'-nitro-N-nitrosoguanidine. Cancer Res. 1980 Jan;40(1):61–63. [PubMed] [Google Scholar]
- Creusot F., Acs G., Christman J. K. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2'-deoxycytidine. J Biol Chem. 1982 Feb 25;257(4):2041–2048. [PubMed] [Google Scholar]
- Darlington G. J., Papaconstantinou J., Sammons D. W., Brown P. C., Wong E. Y., Esterman A. L., Kang J. Generation and chracterization of variants of mouse hepatoma cells with defects in hepato-specific gene expression. I. Albumin synthesis variants. Somatic Cell Genet. 1982 Jul;8(4):451–464. doi: 10.1007/BF01538707. [DOI] [PubMed] [Google Scholar]
- Drahovsky D., Wacker A. Inactivation of mammalian DNA methylase activities by n-methyl-n'-nitro-n-nitrosoguanidine. Eur J Cancer. 1975 Jul;11(7):517–519. doi: 10.1016/0014-2964(75)90154-1. [DOI] [PubMed] [Google Scholar]
- Farrance I. K., Ivarie R. Ethylation of poly(dC-dG).poly(dC-dG) by ethyl methanesulfonate stimulates the activity of mammalian DNA methyltransferase in vitro. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1045–1049. doi: 10.1073/pnas.82.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke U., Hammond D. S., Schneider J. A. The band patterns of twelve D 98-AH-2 marker chromosomes and their use for identification of intraspecific cell hybrids. Chromosoma. 1973;41(1):111–121. doi: 10.1007/BF00284079. [DOI] [PubMed] [Google Scholar]
- Fuscoe J. C., O'Neill J. P., Machanoff R., Hsie A. W. Quantification and analysis of reverse mutations at the hgprt locus in Chinese hamster ovary cells. Mutat Res. 1982 Sep;96(1):15–30. doi: 10.1016/0027-5107(82)90013-6. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Roufa D. J., Beaudet A. L., Caskey C. T. 8-Azaguanine resistance in mammalian cells. I. Hypoxanthine-guanine phosphoribosyltransferase. Genetics. 1972 Oct;72(2):239–252. doi: 10.1093/genetics/72.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J. A. 5-azacytidine-induced re-expression of alleles on the inactive X chromosome in a hybrid mouse cell line. Exp Cell Res. 1982 Sep;141(1):99–105. doi: 10.1016/0014-4827(82)90072-6. [DOI] [PubMed] [Google Scholar]
- Gruenbaum Y., Cedar H., Razin A. Substrate and sequence specificity of a eukaryotic DNA methylase. Nature. 1982 Feb 18;295(5850):620–622. doi: 10.1038/295620a0. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Baxter J. D., Morris J. A. Interaction of thyroid and glucocorticoid hormones in rat pituitary tumor cells. Specificity and diversity of the responses analyzed by two-dimensional gel electrophoresis. J Biol Chem. 1981 May 10;256(9):4520–4528. [PubMed] [Google Scholar]
- Ivarie R. D., Morris J. A. Induction of prolactin-deficient variants of GH3 rat pituitary tumor cells by ethyl methanesulfonate: reversion by 5-azacytidine, a DNA methylation inhibitor. Proc Natl Acad Sci U S A. 1982 May;79(9):2967–2970. doi: 10.1073/pnas.79.9.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarie R. D., Morris J. A., Martial J. A. Prolactin-deficient variants of GH3 rat pituitary tumor cells: linked expression of prolactin and another hormonally responsive protein in GH3 cells. Mol Cell Biol. 1982 Feb;2(2):179–189. doi: 10.1128/mcb.2.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarie R. D., O'Farrell P. H. The glucocorticoid domain: steroid-mediated changes in the rate of synthesis of rat hepatoma proteins. Cell. 1978 Jan;13(1):41–55. doi: 10.1016/0092-8674(78)90136-8. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Schacter B. S., O'Farrell P. H. The level of expression of the rat growth hormone gene in liver tumor cells is at least eight orders of magnitude less than that in anterior pituitary cells. Mol Cell Biol. 1983 Aug;3(8):1460–1467. doi: 10.1128/mcb.3.8.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M., Mohandas T., Shapiro L. J. Cell cycle-specific reactivation of an inactive X-chromosome locus by 5-azadeoxycytidine. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1215–1219. doi: 10.1073/pnas.79.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan B., DeMars R. Localized Derepression on the Human Inactive X Chromosone in Mouse-Human Cell Hybrids. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1510–1514. doi: 10.1073/pnas.72.4.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan M. B., Gowans B. J., Lieberman M. W. Methylation of deoxycytidine incorporated by excision-repair synthesis of DNA. Cell. 1982 Sep;30(2):509–516. doi: 10.1016/0092-8674(82)90248-3. [DOI] [PubMed] [Google Scholar]
- Kratzer P. G., Chapman V. M., Lambert H., Evans R. E., Liskay R. M. Differences in the DNA of the inactive X chromosomes of fetal and extraembryonic tissues of mice. Cell. 1983 May;33(1):37–42. doi: 10.1016/0092-8674(83)90332-x. [DOI] [PubMed] [Google Scholar]
- Landolph J. R., Jones P. A. Mutagenicity of 5-azacytidine and related nucleosides in C3H/10T 1/2 clone 8 and V79 cells. Cancer Res. 1982 Mar;42(3):817–823. [PubMed] [Google Scholar]
- Lester S. C., Korn N. J., DeMars R. Derepression of genes on the human inactive X chromosome: evidence for differences in locus-specific rates of derepression and rates of transfer of active and inactive genes after DNA-mediated transformation. Somatic Cell Genet. 1982 Mar;8(2):265–284. doi: 10.1007/BF01538681. [DOI] [PubMed] [Google Scholar]
- Lieberman M. W., Beach L. R., Palmiter R. D. Ultraviolet radiation-induced metallothionein-I gene activation is associated with extensive DNA demethylation. Cell. 1983 Nov;35(1):207–214. doi: 10.1016/0092-8674(83)90223-4. [DOI] [PubMed] [Google Scholar]
- Liskay R. M., Evans R. J. Inactive X chromosome DNA does not function in DNA-mediated cell transformation for the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4895–4898. doi: 10.1073/pnas.77.8.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller O. J., Miller D. A., Allderdice P. W., Dev V. G., Grewal M. S. Quinacrine fluorescent karyotypes of human diploid and heteroploid cell lines. Cytogenetics. 1971;10(5):338–346. doi: 10.1159/000130152. [DOI] [PubMed] [Google Scholar]
- Milman G., Lee E., Ghangas G. S., McLaughlin J. R., George M., Jr Analysis of HeLa cell hypoxanthine phosphoribosyltransferase mutants and revertants by two-dimensional polyacrylamide gel electrophoresis: evidence for silent gene activation. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4589–4593. doi: 10.1073/pnas.73.12.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Reactivation of an inactive human X chromosome: evidence for X inactivation by DNA methylation. Science. 1981 Jan 23;211(4480):393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- Pfohl-Leszkowicz A., Fuchs R. P., Dirheimer G. In vitro enzymatic methylation of DNA substituted by N-2-aminofluorene. FEBS Lett. 1984 Dec 3;178(1):59–63. doi: 10.1016/0014-5793(84)81240-5. [DOI] [PubMed] [Google Scholar]
- Pfohl-Leszkowicz A., Galiegue-Zouitina S., Bailleul B., Loucheux-Lefebvre M. H., Dirheimer G. Enzymatic methylation of DNA and poly(dG-dC) X poly(dG-dC) modified by 4-acetoxyaminoquinoline-1-oxide, the ultimate carcinogen of 4-nitroquinoline-1-oxide. FEBS Lett. 1983 Oct 31;163(1):85–88. doi: 10.1016/0014-5793(83)81169-7. [DOI] [PubMed] [Google Scholar]
- Ruchirawat M., Becker F. F., Lapeyre J. N. Interaction of DNA methyltransferase with aminofluorene and N-acetylaminofluorene modified poly(dC-dG). Nucleic Acids Res. 1984 Apr 11;12(7):3357–3372. doi: 10.1093/nar/12.7.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas C. E., Pfohl-Leszkowicz A., Lang M. C., Dirheimer G. Effect of modification by N-acetoxy-N-2-acetylaminofluorene on the level of DNA methylation. Nature. 1979 Mar 1;278(5699):71–72. doi: 10.1038/278071a0. [DOI] [PubMed] [Google Scholar]
- Sharp J. D., Capecchi N. E., Capecchi M. R. Altered enzymes in drug-resistant variants of mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3145–3149. doi: 10.1073/pnas.70.11.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steglich C., Grens A., Scheffler I. E. Chinese hamster cells deficient in ornithine decarboxylase activity: reversion by gene amplification and by azacytidine treatment. Somat Cell Mol Genet. 1985 Jan;11(1):11–23. doi: 10.1007/BF01534730. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Baker R. M. Isolation of mutants of cultured mammalian cells. Methods Cell Biol. 1973;6:209–281. doi: 10.1016/s0091-679x(08)60052-7. [DOI] [PubMed] [Google Scholar]
- Venolia L., Gartler S. M. Comparison of transformation efficiency of human active and inactive X-chromosomal DNA. Nature. 1983 Mar 3;302(5903):82–83. doi: 10.1038/302082a0. [DOI] [PubMed] [Google Scholar]
- Venolia L., Gartler S. M., Wassman E. R., Yen P., Mohandas T., Shapiro L. J. Transformation with DNA from 5-azacytidine-reactivated X chromosomes. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2352–2354. doi: 10.1073/pnas.79.7.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V. L., Jones P. A. Inhibition of DNA methylation by chemical carcinogens in vitro. Cell. 1983 Jan;32(1):239–246. doi: 10.1016/0092-8674(83)90514-7. [DOI] [PubMed] [Google Scholar]






