Abstract
Epilepsy is one of the most frequent neurological diseases. In focal medically refractory epilepsies, successful surgical treatment largely depends on the identification of epileptogenic zone. High-frequency oscillations (HFOs) between 80 and 500 Hz, which can be recorded with EEG, may be novel markers of the epileptogenic zone. This review discusses the clinical importance of HFOs as markers of epileptogenicity and their application in different types of epilepsies. HFOs are clearly linked to the seizure onset zone, and the surgical removal of regions generating them correlates with a seizure free post-surgical outcome. Moreover, HFOs reflect the seizure-generating capability of the underlying tissue, since they are more frequent after the reduction of antiepileptic drugs. They can be successfully used in pediatric epilepsies such as epileptic spasms and help to understand the generation of this specific type of seizures. While mostly recorded on intracranial EEGs, new studies suggest that identification of HFOs on scalp EEG or magnetoencephalography (MEG) is possible as well. Thus not only patients with refractory epilepsies and invasive recordings but all patients might profit from the analysis of HFOs. Despite these promising results, the analysis of HFOs is not a routine clinical procedure; most results are derived from relatively small cohorts of patients and many aspects are not yet fully understood. Thus the review concludes that even if HFOs are promising biomarkers of epileptic tissue, there are still uncertainties about mechanisms of generation, methods of analysis, and clinical applicability. Large multicenter prospective studies are needed prior to widespread clinical application.
Keywords: Epilepsy, Ripple, Fast ripple, EEG, Seizure, Infantile spasms
1. Introduction
Epilepsy is one of the most frequent neurological diseases with an overall incidence between 0.5 and 1% (Hauser et al., 1993; Holden et al., 2005). The primary therapy for epilepsy is antiepileptic drugs and there have been many new developments in this field over the recent years. Nevertheless about 30% of all epilepsies remain refractory to medication (Kwan and Brodie, 2000) and patients continue to have disabling seizures even after being treated with three or more different drugs. In medically refractory focal epilepsies the most promising treatment is the surgical removal of the epileptogenic zone (EZ), which is defined as “the minimum amount of cortex that must be resected (inactivated or completely disconnected) to produce seizure freedom” (Rose-now and Lüders, 2001). Patients however can only profit from this type of treatment if seizures are generated over a well-localized area and if this area can be removed safely. It is a major challenge in treating epilepsies and providing patients’ prognosis that mechanisms of seizure generation are not yet fully understood. Epileptologists therefore not only continuously look for better treatment but also try to find biomarkers of epileptogenicity, which allow to delineate brain regions that cause seizures or to identify patients in whom treatment options will fail.
In the last 10 years EEG activity above 70 Hz, the frequency that had been considered clinically significant came into the focus of research. These so-called high frequency oscillations (HFO) might be EEG biomarkers for epileptogenicity and this review will focus on their potential value as markers in clinical epilepsy.
In the presurgical evaluation of patients, epileptologists try to identify the seizure onset zone (SOZ) (Rosenow and Lüders, 2001). The SOZ is defined as the area from which clinical seizures start at the time of the presurgical evaluation. Electroencephalographic demonstration of the SOZ offers the most logical means to approximate the location of the EZ and it is hoped that in most of the cases both zones largely overlap. The EZ cannot be directly measured but is a theoretical construct which encompasses all areas potentially able to generate seizures. The only way to know whether the entire EZ has been removed is by looking at the post-surgical outcome. Patients will be seizure free in case of a complete removal of the EZ. Most epilepsy centers use a battery of presurgical diagnostic tests to evaluate their patients. The most basic are scalp EEGs and long-term EEG monitoring, seizure semiology observation, and anatomical MRI. Additional techniques include functional imaging such as SPECT or PET, source localization using magnetoencephalography (MEG) or high-resolution EEG and neuropsychological testing. Despite these efforts, results of the non-invasive investigations are often inconclusive and in these cases invasive EEG recordings are required. These recordings, which can be conducted by means of subdural electrodes and/or depth electrodes, are the most direct way to record electrical activity from brain tissue. Their obvious advantage over scalp EEG is their ability to demonstrate an early electrical signal preceding clinical seizure onset and devoid of artifact. The major limitation concerns invasiveness and the limited spatial sampling – one cannot be always certain that the earliest activity truly comes from the area where it was recorded. Also, the first ictal electrical change may manifest differently depending on the cortical regions from where it arises, on the underlying lesion, on the types of recording electrode, and even on age. In addition, the intracranial EEG signals at seizure onset can be ill-defined or widespread. For these reasons, and although the classical low-voltage fast discharge that slows, grows, and converts into rhythmic spiking is common, there are still no established electrophysiological criteria to identify unequivocally the SOZ and the definition of the surgical target often depends on additional information independent of intracranial EEG recordings.
This statement has been qualified, however, by recent studies proposing HFOs (80–500 Hz) recorded not only at seizure onset but also between seizures (the interictal period), as a putative new marker of the “area to be removed”. An interictal EEG marker for epileptogenic areas would also provide the additional advantage that epileptologists would not be dependent on waiting for spontaneous seizures to occur during the intracranial EEG investigation. The interpretation of invasive ictal studies requires the recording of several stereotyped clinical seizures to allow for a secure identification of the SOZ. Thus in some patients, even after withdrawal of medication, EEG recording over several days up to weeks is necessary to acquire enough ictal information. If interictal HFOs provide additional information on the localization of epileptogenic areas, the required recording time and accompanying discomfort and cost could be reduced.
1.1. Early microelectrode studies
For almost 50 years the clinical requirement of intracranial EEGs has provided a unique opportunity to also carry out chronic, extraoperative basic research studies on epilepsy using depth electrodes specially adapted with microelectrode arrays (Babb et al., 1973; Crandall et al., 1963). Since mesial temporal lobe epilepsy (MTLE) is the most common type of medically refractory epilepsy treated at surgical epilepsy centers (Engel, 2001), a large number of microelectrode studies have focused on neuronal mechanisms underlying epileptogenic properties of human mesial temporal lobe (MTL) structures. Some of them studied HFOs.
The first patient recordings of spontaneous interictal HFOs between 80 and 500 Hz were carried out using microelectrode arrays consisting of very fine flexible microwires that extended beyond the tip of clinical depth electrodes (Fried et al., 1999). In the studies by Bragin and co-workers at UCLA (Bragin et al., 1999a,b), microelectrodes positioned in hippocampus and entorhinal cortex (EC) of patients with MTLE captured HFOs bilaterally during episodes of non-rapid eye movement (non-REM) sleep that contained spectral frequencies between 80 and 200 Hz. These interictal HFOs strongly resembled the ripple oscillations found in the CA1 section of non-primate hippocampus, which reflect fast inhibitory postsynaptic potentials of synchronously discharging interneurons (Buzsaki et al., 1992; Ylinen et al., 1995). In addition to these “ripples”, another type of HFOs, labeled “fast ripples” because they had spectral frequencies in the range from 200 to 500 Hz, was found chiefly in hippocampus and EC ipsilateral to seizure onset. Whereas ripples were associated with rhythmic firing of presumed interneurons, fast ripples were believed to reflect abnormal synchronous burst firing of principal neurons in areas of seizure onset (Bragin et al., 1999b). These latter data were consistent with studies on ripples and fast ripples in the intrahippocampal kainic acid rat model of human MTLE (Bragin et al., 2000, 2002a).
Subsequent microelectrode studies involving larger samples of patients with MTLE provided additional evidence that rates of fast ripples, but not ripples, were significantly higher in MTL structures ipsilateral than contralateral to seizure onset (Staba et al., 2002, 2004). In relation to states of vigilance, rates of ripples were highest during non-REM sleep and lowest during REM sleep, similarly to the state-related changes in hippocampal ripples in normal rats (Ylinen et al., 1995), while fast ripples were highest during non-REM sleep, yet rates during REM sleep remained elevated and the same as rates during waking (Staba et al., 2004). Voltage-depth analysis in EC revealed spontaneous fast ripples localized to cell lamina corresponding with sites of evoked population spikes, while most ripples did not reverse in polarity across cell layers, suggesting that larger networks support the generation of ripples than fast ripples (Bragin et al., 2002b). While the single neuron correlates of fast ripples have not been studied in patients, one study found a large proportion of EC neurons discharged during the troughs of ripples with the maximum firing of putative pyramidal cells preceding the firing of putative interneuron by ~0.5 ms (Le Van Quyen et al., 2008). This latter pattern of EC interneuron firing in patients was similar to the firing of some interneurons during ripples in rats (Klausberger et al., 2003).
In spite of significant contributions of human microelectrode studies, our understanding of the mechanisms supporting the generation of HFOs and their function in epilepsy is incomplete. Results from chronic models of epilepsy, e.g. intrahippocampal kainic acid or pilocarpine are of special interest. Particularly important was the finding of fast ripples and ripples in the dentate gyrus of kianic acid-treated epileptic rats (Bragin et al., 2004). Since there is very little evidence that ripples occur in normal dentate gyrus, these data indicate both types of HFOs in this structure are pathologic; therefore, it must be considered that pathologic ripples exist in hippocampal and extra-hippocampal structures, e.g. CA3, CA1, and EC. Indeed, this latter possibility is consistent with a human microelectrode study that found a significantly greater number of ripples and fast ripples inside than outside the MTL SOZ (Worrell et al., 2008). It appears that spectral frequency alone cannot determine the pathological nature of HFOs (Engel et al., 2009), in as much as mixed events can also be recorded using recordings from micro- and macroelectrodes in humans (Blanco et al., 2010), therefore suggesting that epileptic HFOs might overlap with physiological HFOs in the ripple and fast ripple bands. At present the only characteristic feature that separates pathologic from normal HFOs derives from animal data that show pathologic HFOs reflect abnormal spontaneous burst of population spikes (Bragin et al., 2007, 2011). Clearly, additional studies are needed in patients with epilepsy, particularly in neocortex where the different types of HFOs and mechanisms underlying their generation are not known. These latter studies will likely require hybrid microelectrode–macroelectrode designs (Van Gompel et al., 2008) or high density microelectrode arrays that can be used to study the spatial and temporal properties of neocortical HFOs (Schevon et al., 2009), possibly identifying the neuronal firing patterns associated with normal and pathologic HFOs.
2. Clinical aspects of HFOs in epilepsy
2.1. HFOs as markers of epileptogenicity
Even if an increased number of microelectrode recordings have been performed over the last years, HFOs gained a broader interest in the clinical field when the first recordings were performed with electrodes in a standard clinical setting. Reasons for this broadened interest are most likely of practical nature. While most surgical epilepsy centers routinely use intracranial recordings, either chronic or intraoperatively, microelectrode recordings require special amplification and analysis techniques and are mainly used in strongly research oriented environments. Therefore with the option to use marcoelectrodes recordings many more centers can analyze HFOs, without having to change their equipment. Beside the costs, some centers are cautious about microelectrode recordings as it has been discussed whether the use of many small microwires instead of one macroelectrode may induce additional neuronal damage during the implantation. So far however these concerns were not confirmed (Howard et al., 1997; Ulbert et al., 2001).
Jirsch and co-workers (Jirsch et al., 2006) first described HFOs recorded with clinical EEG electrodes (electrodes manufactured at the Montreal Neurological Hospital and Institute, having a surface of 0.8 mm2) and showed a focal increase of HFOs during seizures in the SOZ. They also demonstrated that HFOs were not limited to MTL structures (the only ones so far explored with microelectrodes) but extended to neocortical areas. It rapidly became apparent that even with larger commercially available electrodes (5–7 mm2) the recording of ictal and interictal HFOs seems possible (Fig. 1; Akiyama et al., 2005; Blanco et al., 2010). It is however important to realize that no proof exists that HFOs recorded with microelectrodes are the same events as those recorded with macroelectrodes except for the fact that both events show a similarly close relation to the epileptic tissue. A typical HFO as seen with macroelectrodes is shown in Fig. 2.
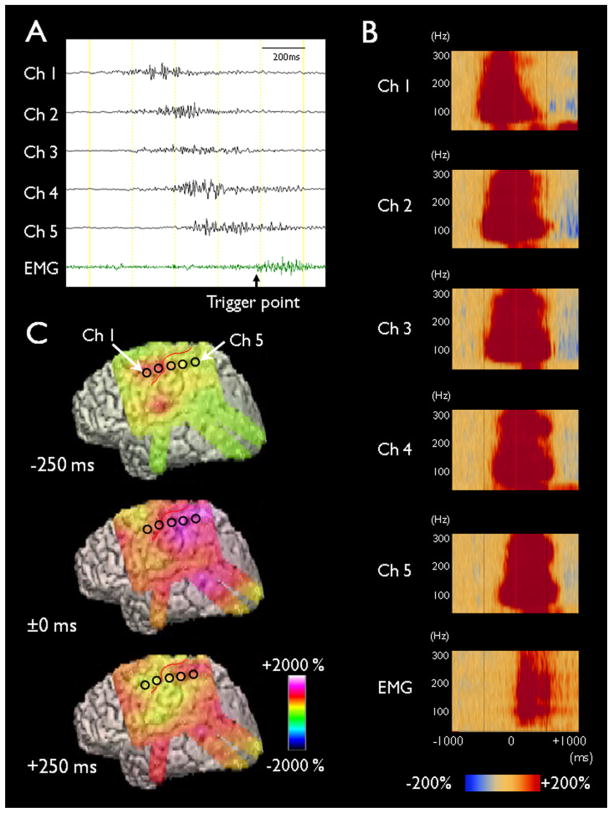
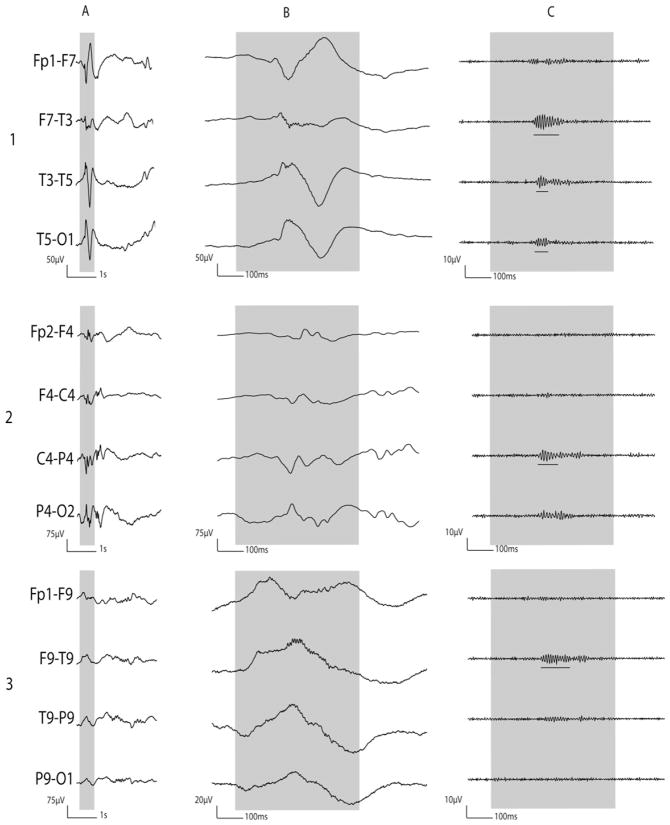
Fig. 1.
Ictal discharges associated with epileptic spasms. (A) Ictal ECoG traces are shown with a low-frequency filter of 53 Hz and a high-frequency filter of 300 Hz. Ictal augmentation of HFOs occurred at channel 1 and quickly involved the surrounding channels. The end of HFOs augmentation occurred at channel 1 and sequentially involved the surrounding channels; this observation was referred to as the “ictal doughnut phenomenon”. The trigger point for time-frequency analysis was placed at the EMG onset detected at right deltoid muscles. (B) Time-frequency plots derived from 62 spasms are shown. Augmentation of HFOs preceded the EMG onset (denoted as ±0 ms). (C) The amplitudes of HFOs associated with spasms are shown.
Source: Figure adapted from Nariai et al. (2011) with permission from John Wiley & Sons.
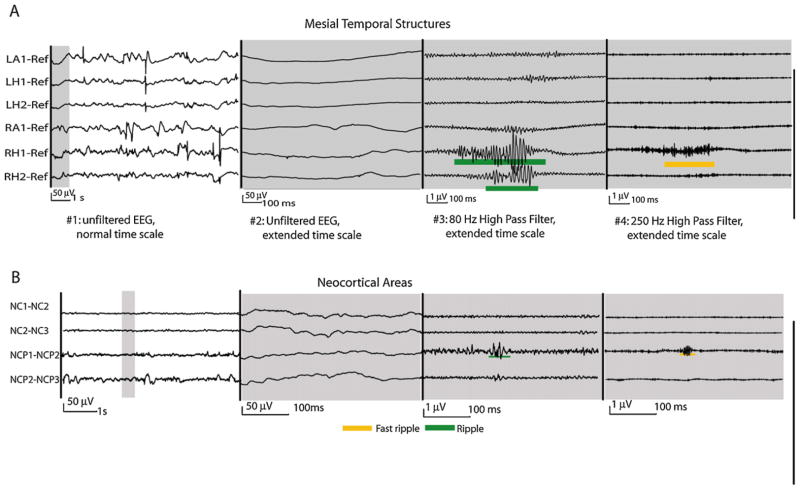
Fig. 2.
HFO recorded with macroelectrodes. This figure demonstrates ripples and fast ripples in the mesial temporal (A) and neocortical structures (B). HFOs are visualized on a different time scale and with different filter settings than the usual clinical EEG. In the two EEG segments on the left the amplitude scale is 50 times increase compared to those on the left to demonstrate the very small scale HFOs. HFOs in the mesial temporal structures are larger, of higher amplitude and more frequent than those in neocortical areas. Both EEGs derived from patients with non-lesional epilepsies.
Source: Figure adapted from Jacobs et al. (2009c) with permission from John Wiley & Sons.
The large number of studies with clinical electrodes allows evaluating HFOs as markers of epileptogenicity. The first step toward this goal was the proof that HFOs occur reliably in brain tissue generating seizures, thus that they have validity as markers. The link to the SOZ had already been established by microelectrode studies and was confirmed with clinical electrodes (Crepon et al., 2010; Jacobs et al., 2008). Moreover it was shown that HFOs are actually specific to areas of seizure generation and not to the substrate of pathological tissue in lesional epilepsy (Jacobs et al., 2009a). Macroelectrode studies usually covered a larger cortical surface than microelectrode studies. Thus it was also observed that in most patients HFOs were not limited to the SOZ but extended beyond. This observation raises the question whether these additional areas represent parts of the EZ or in contrast point toward HFOs being less specific for epileptogenic tissue. A study which correlated the strength of response (after discharge threshold) to cortical electrical stimulation with the occurrence of HFOs showed that areas outside the SOZ which generated HFOs also were more likely to show an epileptic response to stimulation (Jacobs et al., 2010a). This was interpreted as an indication that areas with HFOs outside the SOZ might be epileptogenic. The most convincing evidence however derives from the correlation between the surgical removal of HFO generating tissue and a good post-surgical outcome (Table 1). Ochi et al. (2007) showed a correlation between the removal of areas with ictal HFOs increase and a good post-surgical outcome. Later three independent studies with different recording methods and patient populations showed a correlation between the amount of removed HFO generating tissue and the post-surgical seizure outcome (Akiyama et al., 2011; Jacobs et al., 2010b; Wu et al., 2010). Wu and co-workers actually presented a case in which a two stage surgery was performed. After the first surgery HFO generating tissue remained in one local area and seizures continued. The HFO generating tissue was removed in the second surgery resulting in seizure freedom (Wu et al., 2010). This combined evidence points toward a strong association between HFOs and epileptogenicity.
Table 1.
Overview of studies correlating the post-surgical outcome with the removal of HFOs.
| Study | Patients | No. | Method | Surgery | HFO | Correlationa |
|---|---|---|---|---|---|---|
| Ochi et al. (2007) | Children | 9 | Subdural | Focal resection-Multilobectomy | Ictal R | R |
| Jacobs et al. (2010b) | Adults | 20 | Depth | Focal resection | Interictal R and FR | R and FR |
| Wu et al. (2010) | Children | 24 | ECOG intraoperative | Focal resection-Hemisperectomy | Interictal FR | FR |
| Akiyama et al. (2011) | Children | 28 | Subdural | Focal resection-Lobectomy | Interictal FR | FR |
| Nariai et al. (2011) | Children | 11 | Subdural | Focal resection-Lobectomy | Ictal R–epileptic spasms | R |
| Freiburg Epilepsy Centerb | Children and Adults | 22 | Subdural | Focal resection | Interictal R and FR | R and FR |
R = Ripples; FR = fast ripples.
Correlation between areas which generate events and the post-surgical outcome in regard to seizures.
Unpublished data of the first author.
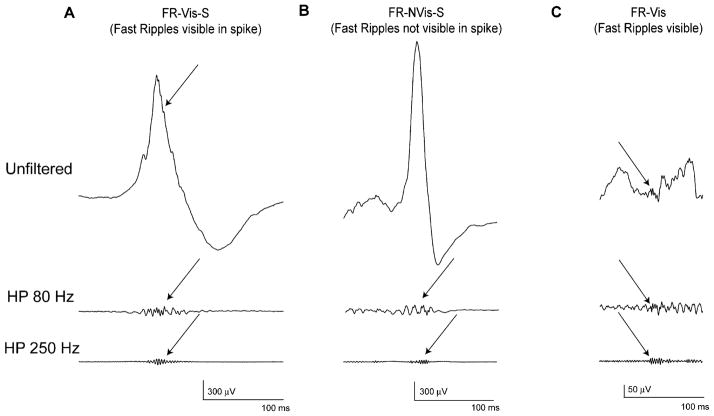
A second important part in the evaluation of HFOs as markers was the comparison with epileptic spikes as the other established interictal marker for epilepsy. The evaluation between both markers was not addressed in microelectrode studies, as recordings in the specific studies had limited spatial coverage due to the use of local reference and the question is less simple to answer even in macroelectrodes than maybe imagined. HFOs are very short and small events and therefore an extended time scale of 0.8 s/page as well as high pass filters are need to visualize the oscillations. Spikes cannot be visualized with this time scale and filter settings. Moreover fast transients, such as spikes, may appear as oscillatory events in a filtered EEG and thus be mistakenly interpreted as a genuine HFO (Benar et al., 2010). HFOs and epileptic spikes are most likely not completely independent events, as they often occur at the same time and over the same brain areas (, Jacobs et al., 2008; Urrestarazu et al., 2007). Urrestarazu and co-workers (Urrestarazu et al., 2007) described three different patterns in which HFOs could occur: (i) Completely independent of epileptic spikes in timing and location, (ii) together with spikes and visible as riding on the spike in the unfiltered EEG, and (iii) together with spikes but invisible in the unfiltered spike (Fig. 3). Subsequent studies provided convincing evidence that HFOs and spikes, even if often co-occurring, are likely to have different neurophysiological mechanisms and clinical relevance. First, HFOs seem to be more specific to the SOZ than spikes (Jacobs et al., 2008; Crepon et al., 2010). Second, HFOs and spikes behave differently to changing conditions: While HFOs directly reflect the epileptogenic potential of the tissue, as they increase in number when epileptic medication is reduced (Zijlmans et al., 2009a), spikes do not show this change; also, spikes increase in the SOZ after recurring seizures, a change that is not observed for HFOs, but, in contrast, a few seconds prior to a seizure, only the rate of HFOs increases (Zijlmans et al., 2011). Closer inspection of the interaction of both biomarkers actually suggests that spikes can be grouped into spikes with and without HFOs and that spikes with HFOs may be more closely related to epileptogenicity than spikes without (Jacobs et al., 2008, 2011). This observation will have to be investigated in the future and might not only reveal insights into HFOs as markers of epileptogenicity but also shed light into the continuing discussion regarding whether spikes themselves are contributing to or inhibiting the occurrence of seizures (Avoli, 2001; Miller and Gotman, 2008). Independently of this discussion, it can be concluded that HFOs can contribute to the understanding of epilepsy in individual patients beyond the contribution of spikes.
Fig. 3.
Co-occurrence between spikes and HFOs. HFO classification. (A) HFO together with spikes and visible as riding on the spike in the unfiltered EEG (B) HFO together with spikes but invisible in the unfiltered spike (C) Completely independent HFO, with no co-occurring spikes. Top: non-filtered EEG; middle: EEG filtered with high pass filter of 80 Hz; bottom: EEG filtered with high-pass filter of 250 Hz.
Source: Figure adapted from Urrestarazu et al. (2007) with permission from Oxford Journals.
The last important measure for a biomarker for the localization of an epileptogenic region is that it can be measured consistently and independently of the unavoidable changes in patient’s state of vigilance and disease. Thus studies were performed during different sleep stages (Staba et al., 2004; Bagshaw et al., 2009), during periods of higher and lower seizure frequency as well as during the tapering of antiepileptic medications (Zijlmans et al., 2009a). All studies revealed similar results: while the actual rate of HFOs might vary with changing external circumstances, the relative difference in rates between areas inside and outside the SOZ did not change. Therefore, the identification of areas relevant to seizure generation was not altered by the changing circumstances.
Most studies described above have not used absolute HFO rates to determine if a region was epileptogenic. They have rather used relative rates and ranked regions from the most to the least epileptogenic. Changing rates caused by sleep and medication and inter-individual differences present a challenge in the use of HFOs as biomarkers of epileptogenicity in the sense that it is not possible to define an absolute rate above which a region can be called epileptogenic. In addition, rates are generally higher in the MTL than in neocortical areas (Fig. 2; Jacobs et al., 2009c). They also might be dependent on the type of electrode and on the underlying pathology of the patient. Thus, clinicians will face the problem of thresholding when deciding whether an area is potentially epileptogenic or not. Akiyama and co-workers applied a threshold using bootstrapping and the resulting individual mean threshold of HFOs rate to distinguish between high and low HFOs channels (2011). With this method they showed a significant correlation between the removal of high rate HFOs channels and seizure free outcome. This might be one way toward the use of HFOs as a reliable clinical marker. Other technical challenges which are discussed more closely in a separate review in the journal have to be met, but the increasing interest in HFOs may lead to fast advances in the field.
2.2. HFOs in different types of epilepsy
Patients with a brain lesion visible on MRI and focal epilepsy tend to be refractory to medication and thus are more likely to need advanced presurgical evaluations. The identification of such lesional changes on MRI often facilitates the identification of the EZ. Nevertheless there is not always a complete overlap between lesional areas and the EZ and some lesions or parts of a lesion might not be epileptogenic (Aubert et al., 2009; Diehl and Luders, 2000). Examples are patients with several tubers in tuberous sclerosis complex in whom only one or two tubers are actually epileptogenic (Major et al., 2009) or patients with large focal cortical dysplasia in whom only parts of the dysplastic tissue contribute to seizure generation (Boonyapisit et al., 2003; Otsubo et al., 2005). Thus it is possible to remove only parts of the lesion and still achieve a seizure free post-surgical outcome. HFOs might help to identify epileptogenic parts within lesional tissue. The occurrence of HFOs is more closely linked to the SOZ within a lesion than to pathological lesional changes (Jacobs et al., 2009a). In patients with nodular heterotopia for example, no HFOs are found in nodules, while HFOs are clearly prominent in the SOZ usually found in the surrounding or overlying neocortex. In patients with focal cortical dysplasia, HFOs are not found in all areas of dysplastic tissue but mainly where is the SOZ (Jacobs et al., 2010c). There are however areas outside the SOZ also active in generating HFOs, and the surgical removal of all the HFOs generating areas (within and outside SOZ) in these patients correlated with a good post-surgical outcome suggesting that HFOs outside the SOZ pointed toward additional epileptogenic areas (Jacobs et al., 2010b). The major gain of evaluating HFOs in lesional neocortical epilepsy might therefore be to identify the EZ independently of lesional boundaries.
In mesial temporal epilepsy, hippocampal sclerosis (HS) is the lesion most often observed. In those patients studied with microelectrodes, structural MRI and histological analysis of resected hippocampal tissue showed that high rates of hippocampal fast ripples and low rates of ripples correlated with reduced hippocampal volumes and neuron densities (Staba et al., 2007). Studies using advance neuroimaging techniques (Three-dimensional (3D) surface maps of hippocampus) provide evidence that links local areas of hippocampal atrophy with fast ripples, but not ripples, as recorded with microelectrodes (Ogren et al., 2009). Evidence from these studies suggest that morphological alterations associated with HS could promote the generation of hippocampal fast ripples and possibly disrupt the generation of ripples. Macroelectrode studies could not find a different behavior for ripples and fast ripples in the mesial temporal structures but rather found an increase of both ripple and fast ripples in areas with mesial temporal sclerosis (Jacobs et al., 2008, 2009c). Again even in macroelectrode studies different electrode types might lead to differing results in regard to the ripple–fast-ripple relationship in the epileptogenic mesial temporal structures. It has been hypothesized that very large electrodes may have the advantage to filter out physiological HFOs, so that pathological HFOs remain preferentially visible (Crepon et al., 2010). Detailed analysis of the local differences in volume changes and neuronal densities are however not possible with macrocontacts due to a lack of spatial resolution. Animal studies however using the intrahippocampal tetanus toxin model suggest that cell loss is not a prerequisite for the presence of fast ripples (Jiruska et al., 2010), as earlier suggested in microelectrode studies. Studies in this non-lesional model of epilepsy found that fast ripples occurred in the focus and surrounding areas suggesting that the occurrence of HFOs may depend on the functional reorganization of cortical circuits rather than cell loss. Thus, the exact relationship between lesional tissue changes and the generation of HFOs is not completely understood and further studies may be required.
In patients with a clear unilateral SOZ, the clinical value of HFOs is questionable as many of these patients directly undergo surgery without implantation. Long-term monitoring with intracranial electrodes, however, remains a consideration when HS coexists with the presence of a neocortical lesion (dual pathology), when electroclinical arguments suggest that the EZ extends outside the boundaries of a standard temporal lobectomy (temporal versus temporal “plus” epilepsy), or when extratemporal ictal onset is suspected (temporal versus “pseudotemporal” epilepsy). In such situations, a SOZ confined to the sclerotic hippocampus is in fact relatively uncommon, provided that spatial sampling is large enough to evaluate all potential seizure generators. Increasing evidence suggests that the spectrum of TLE with HS includes many subdivisions, from the focal mesio-TL subtype to the widely extended temporal “plus” subtype (Kahane and Bartolomei, 2010), but whether HFOs analysis might be helpful in such cases has not been evaluated yet. When the side of mesiotemporal ictal onset is unclear (unilateral versus bitemporal epilepsy), whether analysis of HFOs might be helpful remains also a debatable issue. In such a case, epileptologists face the difficult task to decide which of the two hippocampi is more involved in generating disabling seizures. This is further complicated as some patients have predominantly seizures from one hippocampus during one period and from the contra-lateral hippocampus during another period and thus a count of seizures during 10 days of intracranial investigation might not be reliable (Boling et al., 2009). Unfortunately the use of HFOs in MTL structures is compromised by the inability to differentiate physiological and pathological HFOs, most likely generated over the same areas (Engel et al., 2009). Thus, a high rate of HFOs may on one hand represent an active epileptogenic region or on the other hand one that is involved in active memory processing. A clinical decision using interictal HFOs in the MTL areas may now be premature. The use of ictal HFOs however may be of help with this dilemma (Usui et al., 2011) and should be investigated further.
The last group of patients that might profit from the analysis of intracranial HFOs are MRI negative focal epilepsy cases. As a rule, results of epilepsy surgery in such cases are poorer than those obtained in patients with an abnormal MRI (Tonini et al., 2004), and this group remains the most challenging in terms of presurgical assessment. Invasive recordings are performed in most of the cases but the selection of areas which should be implanted with intracranial electrodes is a major challenge and electrode placement might actually miss the SOZ. Jirsch and co-workers found that an ictal increase in HFOs is only visible if a clear focal seizure onset can be found and not in areas of propagation (Jirsch et al., 2006). Therefore, HFOs might be helpful to identify those patients in whom the SOZ was missed and distinguish whether the first ictal activity seen on the intracranial EEG is actually the seizure onset or a result of fast propagation. This study included a relatively small number of patients and needs validation. In MRI negative patients having a clear seizure onset it could also be shown that HFOs are specific to the SOZ (Andrade-Valenca et al., 2011a). This observation is of special importance as previous studies have shown that HFO rates analyzed from 5 to 10 min of interictal EEG are representative for the spatial distribution of HFOs within one patient (Bagshaw et al., 2009; Zelmann et al., 2009) and sufficient to gain information about the SOZ. Thus, the need for long-term EEG recordings may be reduced. This advantage of course does not only relate to MRI negative cases but applies to all studies using interictal HFOs as epileptologists would not have to wait for spontaneous seizures to identify the SOZ and even intraoperative evaluations without chronic recordings might be possible.
2.3. HFOs in pediatric epilepsy
2.3.1. HFOs in presurgical evaluation for children with focal epilepsy
The largest proportion of pediatric surgical candidates have neocortical epilepsy due to various underlying etiologies (Harvey et al., 1997). In a number of pediatric epilepsy centers, chronic and acute intracranial electrocorticography (ECoG) is commonly used to determine the resection margin. Investigators have recently attempted to determine the diagnostic utility of ictal and interictal HFOs in better identification of the regions responsible for seizure generation by conducting retrospective studies using ECoG sampled via macroelectrodes.
2.3.2. Ictal HFOs in children with medically uncontrolled epilepsy
The first case-series study of children with neocortical epilepsy with ECoG sampled at 1000-Hz reported that ictal discharges began with sustained focal increase of HFOs between 80 and 250 Hz in the majority of neocortical focal seizures with or without secondarily generalized tonic–clonic seizures (Ochi et al., 2007). Such sustained ictal HFOs gradually evolved into another distinct form such as repetitive spike-waves or rhythmic slow waves. The frequency and propagation pattern of ictal HFOs were similar to those reported in a study of adult patients with ECoG sampled at 1000-Hz (Modur et al., 2011). Developmental changes in spectral-frequency band of ictal HFOs across children and adults have not been systematically studied.
A substantial proportion of pediatric surgical candidates have epileptic spasms, which are characterized by periodic and brief muscle contractions of variable intensity. Studies of children with spasms with ECoG sampled at 1000 Hz have shown that ictal discharges consisted of focal and brief neocortical increase of HFOs between 40 and 300 Hz and quick propagation of such HFOs to widespread cortical regions (Nariai et al., 2011; Ramachandrannair et al., 2008). Since epileptic spasms are uniquely characterized by clusters of multiple brief seizures with clinical manifestations resembling each other, the spatial-temporal modulations of ictal HFOs can be statistically determined by time-frequency analysis with a large number of ictal events enrolled into analysis. Statistical analyses on 13 patients aged 1.3–8.8 years revealed that augmentation of ictal HFOs between 80 and 200 Hz was more prominent and earlier than that of HFOs between 200 and 300 Hz (Fig. 1). The severity of ictal motor symptoms was predicted by the amplitude of ictal HFOs in the sensorimotor cortex rather than that by that in the SOZ. Univariate analysis suggested that removal of the site showing initial augmentation of ictal HFOs between 80 and 200 Hz was associated with a better surgical outcome than in patients in whom these areas remained (Nariai et al., 2011). In contrast to the observations that ictal HFOs associated with focal seizures frequently evolved into another form such as repetitive spike-wave discharges, ictal HFOs associated with spasms frequently terminated without evolving into another form (Nariai et al., 2011). A study of a rat model of epileptic spasms with ECoG also showed that ictal events began with increase of HFOs below 200 Hz, which was followed within 100 ms by increase of activity above 200 Hz and ictal HFOs subsequently slowed down in frequency to below 100 Hz (Frost et al., 2011). These observations are consistent with the hypotheses that spasms are of cortical and not of brainstem origin and that the cortical sites showing initial increase of HFOs during ictal events may be located close to the region responsible for seizure generation.
2.3.3. Interictal HFOs in children with medically uncontrolled epilepsy
Studies of children with medically uncontrolled epilepsy have shown that HFOs are spontaneously generated by neocortical regions during the interictal state. A study of 30 children aged 8 months–20 years determined the diagnostic utility of interictal HFOs above 250 Hz on intraoperative ECoG sampled at 2000 Hz; HFOs with a mean duration of 30 ms and a mean frequency of 296 Hz were visually identified in 1–3 sampled neocortical sites in 80% of the patients; complete removal of sites showing interictal HFOs was an independent predictor of good outcome, even after eliminating the effect of a potential confounding factor such as hemispherectomy (Wu et al., 2010). An extraoperative ECoG study of 28 children aged 1–18 years determined the diagnostic utility of interictal HFOs during non-REM sleep; an automatic detector was applied to ECoG sampled at 1000 Hz and identified interictal HFOs between 200 and 300 Hz in 1–69 sampled sites and HFOs between 80 and 200 Hz in 2–81 sites; removal of sites showing interictal HFOs in the higher frequency band was independently associated with a better seizure outcome (Akiyama et al., 2011).
In conclusion, the analysis of HFOs may be an interesting additional tool in pediatric epilepsy surgery. Yet, none of the previous studies have proven that measurement of ictal or interictal HFOs can indeed prospectively predict the post-surgical outcome or that guiding the resection using the information of HFOs analysis might improve the surgical outcome.
2.4. HFOs in the transition from interictal to ictal periods
Considering the clinical use of HFOs as markers of epileptogenicity always poses the question of whether to analyze interictal, pre-ictal, or ictal HFOs. The question also arises whether HFOs cause seizures or are just a by-product of epileptogenic tissue. Several studies have addressed this question for different time periods. Early on, Fisher and Allen described the occurrence of activity around 100 Hz during seizure onset (Allen et al., 1992; Fisher et al., 1992). Later on, HFOs in the 100–500 Hz range were also found in the early part of seizures and as seizures propagate, measured as spectral energy or in number of HFOs, particularly in the SOZ (Jirsch et al., 2006). An increase was also observed during the seconds preceding seizure onset (Khosravani et al., 2009; Nariai et al., 2011). A pre-ictal increase was not consistently found when reviewing the epochs that precede the seizure onset by several minutes (Jacobs et al., 2009b). In the seconds preceding seizure onset, the HFOs are often more frequent than during interictal slow-wave sleep during the night before the seizure (Zijlmans et al., 2011). HFOs also increased during the days preceding seizures, while anti-epileptic medication is lowered (Zijlmans et al., 2009a). This differs from epileptiform spikes, which decrease in the periods before seizures (Zijlmans et al., 2009a, 2011). The increase during seizure onset differs between different seizure types. As already discussed, in epileptic spasms ictal HFOs can emerge and increase immediately before the clinical onset of spasms, suggesting that the ictal HFOs trigger the clinical spasms (Akiyama et al., 2005; Nariai et al., 2011,). In other types of epilepsy, interictal HFOs were neither found to increase before all seizures nor in a predictable manner. This makes it less likely that interictal HFOs are the actual cause of these seizures and maybe they should be seen as a sign of seizure susceptibility; a greater number of interictal HFOs indicate a greater risk of a seizure (Jacobs et al., 2009b; Zijlmans et al., 2009b, 2011). More study is needed to relate the occurrence of HFOs to different types of seizures in humans and in animal models to understand their role in seizure genesis.
A second question is whether ictal HFOs are similar to interictal and pre-ictal HFOs. One could argue that they are, because they occur mainly on the same EEG channels and consist of similar frequencies (Jirsch et al., 2006; Zijlmans et al., 2011). However, ictal HFOs can have a different appearance in time showing a continuous pattern (Jirsch et al., 2006; Zijlmans et al., 2011). Even more, only some of the channels with HFOs during seizure onset still show HFOs during seizure evolution, and these HFOs consist of higher frequencies and seem more specific for the SOZ (Modur et al., 2011; Ochi et al., 2007).
The HFOs that occur during seizure onset and seizure evolution could be helpful to find the EZ in the clinical practice of epilepsy surgery. They are more specific for the SOZ than interictal HFOs or spikes (Zijlmans et al., 2011) and could predict post-surgical outcome (Modur et al., 2011; Ochi et al., 2007). For clinical practice, it can help to review seizures at a different time scale and filter settings than the standard EEG to look for higher frequencies at seizure onset. When doing so, one should be aware of potential artifacts, especially caused by ictal muscle activity. High-frequency muscle activity can be distinguished from epileptic HFOs as muscle is mostly recognizable on the electrode contacts close to the skull with low-voltage simultaneous activity on deeper contacts, and can show a different frequency distribution (Otsubo et al., 2008; Zijlmans et al., 2011). HFOs can also be recorded in the temporal pole due to the contamination of ocular muscles activity (Jerbi et al., 2009b; Nagasawa et al., 2011). More study is needed to establish the clinical value of HFOs during seizure onset. In the mean time, it is worthwhile to build up clinical experience by reviewing seizures for HFOs in addition to the traditional evaluation.
2.5. HFOs on the surface EEG and MEG
The option to review HFOs on scalp EEG and MEG would allow to use HFOs as markers of epileptogenicity in many more patients with epilepsy and not only those investigated intracranially as a result of their medically refractory epilepsy. It could be helpful, for instance, to evaluate the effects of AEDs or to predict the risks or seizure recurrence in newly diagnosed epilepsy.
2.5.1. Surface EEG
HFOs analysis on scalp EEGs not only avoids the surgical risks –although low – of intracranial recordings but also allows safe repeated measures over time. In addition, scalp EEG offers the advantage of being widely available, compared to the limited availability and the significantly more costly MEG. Important emerging data show that HFOs can be detected on scalp EEG. Ictally, HFOs has been reported at the onset of epileptic spasms on scalp recordings in children (50–100 Hz, Kobayashi et al., 2004; 40–120 Hz, Inoue et al., 2008), as well as at the onset of tonic seizures in Lennox–Gastaut Syndrome (50–100 Hz, Kobayashi et al., 2009).
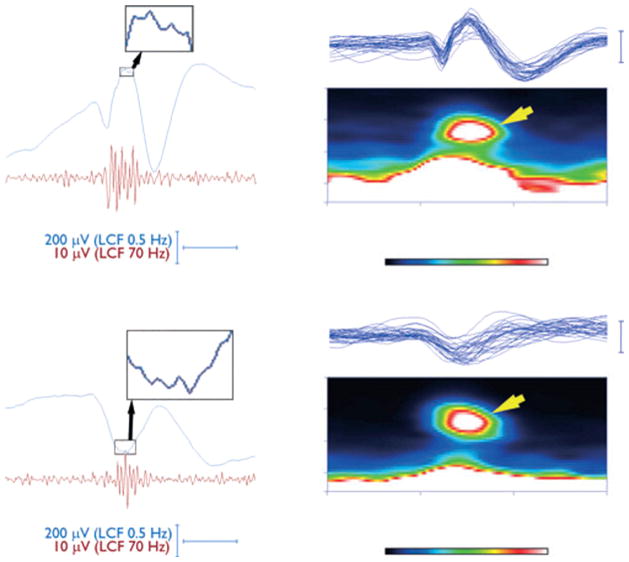
More importantly, interictal fast frequencies have also been detected on scalp EEG. While not frequent, interictal HFOs on scalp recordings during non-REM sleep in children was found to be specific in identifying children with epilepsy and localizing their SOZ in the same recordings (30–70 Hz, Wu et al., 2008). Similarly, in children with continuous spike and wave in slow-wave sleep, interictal HFOs was detected (70–200 Hz, Kobayashi et al., 2010, Fig. 4), as well as in idiopathic partial epilepsy (Kobayashi et al., 2011). Most recently, in an adult population with focal epilepsy, different HFOs were detected interictally during non-REM sleep on scalp recordings (40–80 Hz, 80–200 Hz, Andrade-Valenca et al., 2011a), with higher specificity and accuracy for ripple frequency in identifying the SOZ (Fig. 5). The separation between cerebral activity and EMG artifact requires particular care in scalp recordings (Andrade-Valenca et al., 2011b).
Fig. 4.
Representative time-frequency spectra and the corresponding electroencephalography (EEG) traces. Parts of the temporally expanded and filtered EEG traces are shown in the left column (low-cut filtered at 0.5 Hz in blue and filtered at 70 Hz in red). A ripple with a frequency above 100 Hz occurs in temporal association with the positive peak, the ascending slope, and/or the negative peak of the spike; it is barely visible near the spike-peak in the trace with a 0.5-Hz filter (magnification in box). The resulting time-frequency spectra in the right column show spectral spots (yellow arrows) in association with the spikes in the overlaid traces from the EEGs with CSWS (B corresponding to A with a peak frequency of 128.9 Hz; (D) corresponding to (C) with a peak frequency of 125.0 Hz).
Source: Figure adapted from Kobayashi et al. (2010) with permission from John Wiley & Sons.
Fig. 5.
Ripples recorded with surface EEG. Example of ripples recorded on surface EEG. The upper section shows ripple oscillations co-occurring with a spike, with oscillations visible during the spike. The middle section shows a ripple co-occurring with a spike with oscillations not visible during the spike, but visible after filtering. In the bottom section a ripple independent of any spike can be observed. (A) Raw EEG. (B) Raw EEG with expanded time. (C) EEG filtered with high-pass filter of 80 Hz.
Source: Figure adapted from Andrade-Valenca et al. (2011b) with permission of the Lippincott Williams & Wilkins.
In summary, great strides have been made in detecting HFOs on non-invasive scalp recordings, both ictally and interictally, in children and adults, in different frequency bands, corresponding to the SOZ. More studies are needed in the possibility and the implication of detecting even faster activities on scalp in the fast ripple frequency, and in establishing scalp fast frequencies in the identification and localization of the EZ in epilepsy surgery outcome studies and as a possible marker of epileptogenicity. Methods have to be developed to safely distinguish between high-frequency artifacts and genuine oscillations. It has not been confirmed yet that oscillations in the high frequency range, which can be recorded at the surface have the same generators as those observed with intracranial electrodes. Studies with simultaneous surface and intracranial electrodes may be necessary to further evaluate these issues.
2.5.2. MEG
Several studies have emphasized the value of MEG in identifying patients who are good candidates for epilepsy surgery, guiding intracranial electrode placement and planning the surgical strategy (Agirre-Arrizubieta et al., 2009; Iida et al., 2005; Minassian et al., 1999). In clinical practice, the single equivalent current dipole model remains the most widely used method for analysis of ictal and interictal MEG data (Baumgartner et al., 2000; Knowlton et al., 2006). The equivalent current dipole model is not suitable however to localize sources of low amplitude activity with low signal-to-noise ratio such as HFOs (Sakuma et al., 1999) or in the presence of high background noise (Sekihara et al., 1996) and is considered too simplistic to accurately explain complex epileptic sources and dynamically propagating epileptic discharges (Shiraishi et al., 2005).
Several alternative approaches have been described to avoid the shortcomings of the single dipole model, when trying to analyze HFOs in MEG. These approaches aim to shorten analyses time and minimize dependence on subjective decisions (Kirsch et al., 2006), to demonstrate the propagation of interictal discharges (Shiraishi et al., 2005; Tanaka et al., 2010), or to better account for sources over extended areas of active cortex (Michel et al., 2004; Sperli et al., 2006). Sources of neuromagnetic spike-locked power increase in the beta and gamma (10–60 Hz) band were localized using an adaptive spatial filtering technique (Guggisberg et al., 2008). Spikes were identified visually first; a dual-state adaptive spatial filter was then used to calculate source power changes in the beta and gamma frequency bands during the interictal spikes. In patients with good surgical outcome (Engel class I or II, Engel et al., 1993), the surgically resected area was identified with an accuracy of 85% by sources of spike-locked beta/gamma activity. Xiang and co-workers (Xiang et al., 2009) reported that MEG source localizations of interictal and ictal high-frequency components (100–1000 Hz) were concordant with intracranial recordings for 9 of the 11 children who underwent epilepsy surgery.
More recently, spatiotemporal or “event-related” beamforming methods have been developed for the localization of instantaneous or evoked brain responses (Cheyne et al., 2006; Sekihara et al., 2001). This method involves deriving a beamforming filter from the single trial MEG data with a single optimal current direction at each voxel. 3D images are constructed by mapping the spatial distribution of source power at selected latencies, to produce millisecond-by-millisecond 3D images of brain activity making it suitable for localization of the high-frequency component of interictal spikes. In a study of three children with refractory epilepsy using an event-related beamformer, source localization of beta and gamma oscillations (20–70 Hz) of interictal spikes was concordant with the equivalent current dipole model and the intracranial EEG results (Mohamed et al., 2007).
The above data suggest the feasibility of source localization of HFOs in interictal spikes in MEG recordings. Sources of interictal gamma and beta oscillations were concordant with intracranial recordings in the majority of patients studied. Advances in analysis software development can further enhance the utility of source localization of the high-frequency component of interictal spikes in clinical settings.
3. Conclusion
HFOs seem to be promising biomarkers for epileptogenic tissue. They seem to be more specific to the SOZ than epileptic spikes, and they can also point out epileptogenic areas outside the SOZ (Fig. 6), i.e. areas that have the potential to generate seizures. HFOs may actually represent epileptogenic areas independently of the underlying pathology and type of epilepsy. HFOs may even become a marker for physiologically function or deficit if there could be a simple technique to differentiate physiological from pathological HFOs in the future. In that sense, efforts should be made to understand the relationships between HFOs as recorded spontaneously in epileptic patients, and the so-called gamma-band responses that are observed intracranially in these patients during a variety of cognitive tasks (Jerbi et al., 2009a).
Fig. 6.
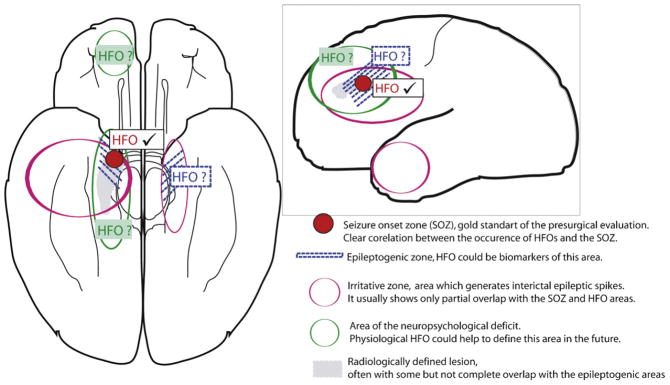
Adaptation of the presurgical model of Rosenow and Lüders (Rosenow and Lüders, 2001). Up to now HFOs are able to identify the SOZ (in red). Studies about the correlation between HFO removal and post-surgical seizure outcome suggest that HFOs may also be able to measure the epileptogenic area (in blue). Whether this is true can only be measured by large prospective studies. Physiological HFOs at some point in the future might be able to help us define the area of the neuropsychological deficit (in green).
As it is not currently possible to differentiate physiological from pathological HFOs (frequency is certainly not sufficient when using macroelectrodes), one may wonder how the studies discussed above can be confident that they are reporting properties of pathological rather than physiological HFOs. There is no formal proof, but circumstantial evidence: HFOs are much more frequent in the SOZ than outside, where they are often totally absent. If many of the reported HFOs were physiological, one would expect that most channels would show some HFO activity and that the separation between epileptogenic and non-epileptogenic regions using HFOs would not be as clear as it is. There is no reason to think that physiological oscillations would be predominant in the epileptogenic region. If physiological HFOs are also present among all HFOs recorded, they are likely to represent a small proportion. One must be cautious in this respect however, as all brain regions may not have the propensity to generate physiological HFOs.
An even braver look into the future allows analyzing HFOs on surface recordings. A clinical gain of this technique could only materialize if surface HFOs were, as intracranial HFOs, more specific than established markers. As a result, a much broader group of patients could profit from the technique (Fig. 7). The biomarker could not only address the question of focus localization but also prognostic questions, and the effectiveness of treatment could be analyzed in long-term studies.
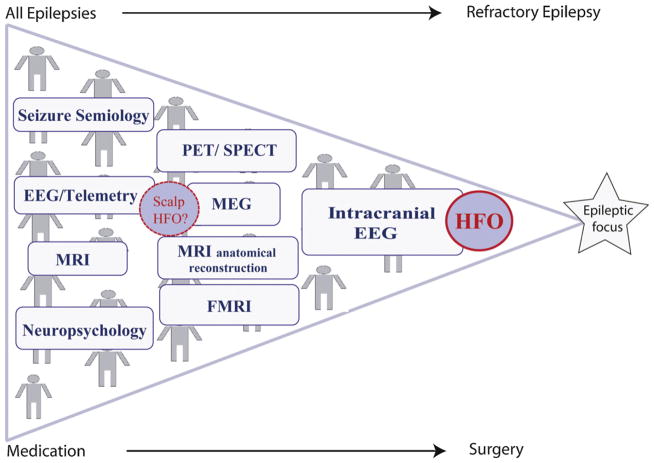
Fig. 7.
Pyramid of the presurgical diagnostic as it is performed in most epilepsy centers. Only patients with complicated and refractory seizures profit from the benefit of HFOs as biomarkers in intracranial EEG. Future identification of HFOs on surface EEG and MEG might allow a use of HFOs as long-term marker for epileptogenicity in a larger group of patients with epilepsy.
HFOs should be seen as a new diagnostic tool which can be used in conjunction with other techniques delineating the SOZ and epileptogenic areas. Another new technique is functional imaging. Little is known about the correlations between the localization of HFOs and those regions delineated by functional imaging. It is most likely that there will be a regional overlap between areas showing metabolic changes in PET and fMRI and those generating HFOs when both are close to the presumed SOZ. It remains however unclear whether additional more unspecific blood flow changes outside the presumed epileptic focus, as they are sometimes described in EEG-fMRI (for review see Gotman, 2008), will also colocalize with regions of HFO generation. It has been suggested that the coupling between blood oxygenation dependent effects (BOLD) and physiological gamma-band EEG is higher than coupling in other frequency bands (Magri et al., 2012). There might also be a close coupling between pathological HFOs and BOLD in patients with epilepsy, but no evidence for this hypothesis has been presented. At the moment we face a substantial number of challenges which have to be met before HFOs can play a significant role in clinical practice. Many of these challenges are technical and will be addressed in the accompanying review (see review in the issue, Worrell et al., 2012: Recording and analysis techniques for HFOs). They include a method for simple and reliable automatic event detection, as well as the question of which electrode types are best adapted to HFO recording. This also includes the development of methods allowing statistical images of HFOs in the patient’s brain to further explore the relationships between ictal and interictal HFOs, to study interactions between physiological and pathological HFOs and to compare these data with other localizing methods (David et al., 2011). Moreover all proposed methods for HFO analysis do not deal with the problem of physiological and pathological HFOs occurring at the same frequency and anatomic region. Last but not the least, studies have shown that not only the presence but also the rate of occurrence of HFOs matters. A large difference in rates of HFOs between anatomical regions as well as a large inter-patient variability has been observed. Therefore, clinicians will face a threshold problem, when it comes to deciding whether a specific tissue is epileptogenic or not, i.e. must be resected or not. Studies to date have only ranked regions according to HFO rates, pointing to the most or the least epileptogenic.
To conclude, the authors would like to propose three suggestions that could help future studies on HFOs, aiming to allow for HFOs to become a reliable biomarker of epileptogenicity:
When recording with macroelectrodes, it appears that the pathological nature of HFOs is not determined by spectral frequency (ripples and fast ripples behave similarly with respect to the SOZ or epileptogenicity). Efforts should therefore be made to separate pathological from physiological HFOs and if this is possible, these terms (pathological and physiological) should be preferred to ripples or fast ripples.
Publications should refer to the frequency range analyzed in the study in the title of the manuscript, as definitions of the gamma, high gamma, ripple, and fast ripple bands are not universal, and may overlap in terms of frequency bands.
The next step to evaluate the clinical value of epileptogenic HFOs should be a multicenter trial combining large patient numbers, different recording techniques and solid statistics.
Acknowledgments
This publication is the outcome of the workshop “High Frequency Oscillations in Epilepsy and Cognition” held at the Montreal Neurological Institute on June 2–4, 2011. The workshop was supported by UCB Pharma, the Canadian Institutes of Health Research, the American Epilepsy Society, the Savoy Foundation for Epilepsy, EISAI, DIXI Medical, Neuralynx, Blackrock Microsystems, and the Montreal Neurological Institute.
Abbreviations
- AED
Antiepileptic drugs
- EC
Entorhinal cortex
- EEG
Electroencephalogram
- EZ
Epileptogenic zone
- HFO
High frequency oscillation
- HS
Hippocampal sclerosis
- MEG
Magnetencephalogram
- MTLE
Mesial temporal lobe epilepsy
- SOZ
Seizure onset zone
References
- Agirre-Arrizubieta Z, Huiskamp GJ, Ferrier CH, van Huffelen AC, Leijten FS. Interictal magnetoencephalography and the irritative zone in the electrocorticogram. Brain. 2009;132:3060–3071. doi: 10.1093/brain/awp137. [DOI] [PubMed] [Google Scholar]
- Allen PJ, Fish DR, Smith SJ. Very high-frequency rhythmic activity during SEEG suppression in frontal lobe epilepsy. Electroencephalography and Clinical Neurophysiology. 1992;82:155–159. doi: 10.1016/0013-4694(92)90160-j. [DOI] [PubMed] [Google Scholar]
- Andrade-Valenca L, Mari F, Jacobs J, Zijlmans M, Olivier A, Gotman J. Interictal high frequency oscillations (HFOs) in patients with focal epilepsy and normal MRI. Clinical Neurophysiology. 2011a Jul;123 doi: 10.1016/j.clinph.2011.06.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Valenca LP, Dubeau F, Mari F, Zelmann R, Gotman J. Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology. 2011b;9:524–531. doi: 10.1212/WNL.0b013e318228bee2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert S, Wendling F, Regis J, McGonigal A, Figarella-Branger D, Peragut JC. Local and remote epileptogenicity in focal cortical dysplasias and neurodevelopmental tumours. Brain. 2009;132:3072–3086. doi: 10.1093/brain/awp242. [DOI] [PubMed] [Google Scholar]
- Avoli M. Do interictal discharges promote or control seizures? Experimental evidence from an in vitro model of epileptiform discharge. Epilepsia. 2001;42 (Suppl 3):2–4. doi: 10.1046/j.1528-1157.2001.042suppl.3002.x. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Otsubo H, Ochi A, Ishiguro T, Kadokura G, Ramachandrannair R, Weiss SK, Rutka JT, Carter Snead O., 3rd Focal cortical high-frequency oscillations trigger epileptic spasms: confirmation by digital video subdural EEG. Clinical Neurophysiology. 2005;116:2819–2825. doi: 10.1016/j.clinph.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Akiyama T, McCoy B, Go CY, Ochi A, Elliott IM, Akiyama M, Donner EJ, Weiss SK, Snead OC, 3rd, Rutka JT, Drake JM, Otsubo H. Focal resection of fast ripples on extraoperative intracranial EEG improves seizure outcome in pediatric epilepsy. Epilepsia. 2011:116. doi: 10.1111/j.1528-1167.2011.03199.x. [DOI] [PubMed] [Google Scholar]
- Babb TL, Carr E, Crandall PH. Analysis of extracellular firing patterns of deep temporal lobe structures in man. Electroencephalography and Clinical Neurophysiology. 1973;34:247–257. doi: 10.1016/0013-4694(73)90252-6. [DOI] [PubMed] [Google Scholar]
- Bagshaw AP, Jacobs J, LeVan P, Dubeau F, Gotman J. Effect of sleep stage on interictal high-frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia. 2009;50:617–628. doi: 10.1111/j.1528-1167.2008.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner C, Pataraia E, Lindinger G, Deecke L. Magnetoencephalography in focal epilepsy. Epilepsia. 2000;3 (Suppl 41):S39–S47. doi: 10.1111/j.1528-1157.2000.tb01533.x. [DOI] [PubMed] [Google Scholar]
- Benar CG, Chauviere L, Bartolomei F, Wendling F. Pitfalls of high-pass filtering for detecting epileptic oscillations: a technical note on false ripples. Clinical Neurophysiology. 2010;121:301–310. doi: 10.1016/j.clinph.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Blanco JA, Stead M, Krieger A, Viventin J, Marsh WR, Lee KH, Worrell GA, Litt B. Unsupervised classification of high-frequency oscillations in human neocortical epilepsy and control patients. Journal of Neurophysiology. 2010;104 (5):2900–2912. doi: 10.1152/jn.01082.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling W, Aghakhani Y, Andermann F, Sziklas V, Olivier A. Surgical treatment of independent bitemporal lobe epilepsy defined by invasive recordings. Journal of Neurology and Neurosurgery and Psychiatry. 2009;80:533–538. doi: 10.1136/jnnp.2008.155291. [DOI] [PubMed] [Google Scholar]
- Boonyapisit K, Najm I, Klem G, Ying Z, Burrier C, LaPresto E, Nair D, Bingaman W, Prayson R, Lüders H. Epileptogenicity of focal malformations due to abnormal cortical development: direct electrocorticographic–histopathologic correlations. Epilepsia. 2003;44:69–76. doi: 10.1046/j.1528-1157.2003.08102.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999a;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999b;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Engel J., Jr Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia. 2000;41:S144–S152. doi: 10.1111/j.1528-1157.2000.tb01573.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. Journal of Neuroscience. 2002a;22:2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, Engel J., Jr Interictal high frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinal cortex. Annals of Neurology. 2002b;52:407–415. doi: 10.1002/ana.10291. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Engel J., Jr Voltage depth profiles of high-frequency oscillations after kainic acid-induced status epilepticus. Epilepsia. 2007;48:35–40. doi: 10.1111/j.1528-1167.2007.01287.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Benassi SK, Kheiri F, Engel J., Jr Further evidence that pathologic high-frequency oscillations are bursts of population spikes derived from recordings of identified cells in dentate gyrus. Epilepsia. 2011;52:45–52. doi: 10.1111/j.1528-1167.2010.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Bakhtazad L, Gaetz W. Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event-related beam-forming approach. Human Brain Mapping. 2006;27:213–229. doi: 10.1002/hbm.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall PH, Walter RD, Rand RW. Clinical applications of studies on stereotactically implanted electrodes in temporal lobe epilepsy. Journal of Neurosurgery. 1963;20:827–840. doi: 10.3171/jns.1963.20.10.0827. [DOI] [PubMed] [Google Scholar]
- Crepon B, Navarro V, Hasboun D, Clemenceau S, Martinerie J, Baulac M, Adam C, Le Van Quyen M. Mapping interictal oscillations greater than 200 Hz recorded with intracranial macroelectrodes in human epilepsy. Brain. 2010;133(Pt. 1):33–45. doi: 10.1093/brain/awp277. [DOI] [PubMed] [Google Scholar]
- David O, Blauwblomme T, Job AS, Chabardès S, Hoffmann D, Minotti L, Kahane P. Imaging the seizure onset zone with stereoelectroencephalography. Brain. 2011;134:2898–2911. doi: 10.1093/brain/awr238. [DOI] [PubMed] [Google Scholar]
- Diehl B, Luders HO. Temporal lobe epilepsy: when are invasive recordings needed? Epilepsia. 2000;41 (Suppl 3):S61–S74. doi: 10.1111/j.1528-1157.2000.tb01536.x. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr Mesial temporal lobe epilepsy: what have we learned? Neuroscientist. 2001;7:340–352. doi: 10.1177/107385840100700410. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Van Ness PC, Rasmussen TB. Outcome with respect to epileptic seizures. In: Engel J, editor. Surgical Treatment of the Epilepsies. 2. Raven Press; New York: 1993. pp. 609–621. [Google Scholar]
- Engel J, Jr, Bragin A, Staba RJ, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Webber WR, Lesser RP, Arroyo S, Uematsu S. High-frequency EEG activity at the start of seizures. Journal of Clinical Neurophysiology. 1992:441–448. doi: 10.1097/00004691-199207010-00012. [DOI] [PubMed] [Google Scholar]
- Fried I, Wilson CL, Maidment NT, Engel J, Jr, Behnke E, Fields TA, MacDonald KA, Morrow JW, Ackerson L. Cerebral microdialysis combined with single-neuron and electroencephalographic recording in neurosurgical patients: technical note. Journal of Neurosurgery. 1999;91:697–705. doi: 10.3171/jns.1999.91.4.0697. [DOI] [PubMed] [Google Scholar]
- Frost JD, Jr, Lee CL, Hrachovy RA, Swann JW. High frequency EEG activity associated with ictal events in an animal model of infantile spasms. Epilepsia. 2011;52:53–62. doi: 10.1111/j.1528-1167.2010.02887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotman J. Epileptic networks studied with EEG-fMRI. Epilepsia. 2008;49 (Suppl 3):42–51. doi: 10.1111/j.1528-1167.2008.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggisberg AG, Kirsch HE, Mantle MM, Barbaro NM, Nagarajan SS. Fast oscillations associated with interictal spikes localize the epileptogenic zone in patients with partial epilepsy. NeuroImage. 2008;39:661–668. doi: 10.1016/j.neuroimage.2007.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AS, Berkovic SF, Wrennall JA, Hopkins IJ. Temporal lobe epilepsy in childhood: clinical, EEG, and neuroimaging findings and syndrome classification in a cohort with new-onset seizures. Neurology. 1997;49:960–968. doi: 10.1212/wnl.49.4.960. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34:453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- Holden EW, Thanh NH, Grossman E, Robinson S, Nelson LS, Von Worley MJ, Gunter A, Thurman DJ. Estimating prevalence, incidence, and disease-related mortality for patients with epilepsy in managed care organizations. Epilepsia. 2005;46:311–319. doi: 10.1111/j.0013-9580.2005.30604.x. [DOI] [PubMed] [Google Scholar]
- Howard MA, Volkov IO, Noh MD, Granner MA, Mirsky R, Garell PC. Chronic microelectrode investigations of normal human brain physiology using a hybrid depth electrode. Stereotactic and Functional Neurosurgery. 1997;68(1–4 Pt 1):236–242. doi: 10.1159/000099931. [DOI] [PubMed] [Google Scholar]
- Iida K, Otsubo H, Mohamed IS, Okuda C, Ochi A, Weiss SK, Chuang SH, Snead OC., 3rd Characterizing magnetoencephalographic spike sources in children with tuberous sclerosis complex. Epilepsia. 2005;46:1510–1517. doi: 10.1111/j.1528-1167.2005.14005.x. [DOI] [PubMed] [Google Scholar]
- Inoue T, Kobayashi K, Oka M, Yoshinaga H, Ohtsuka Y. Spectral characteristics of EEG gamma rhythms associated with epileptic spasms. Brain Development. 2008;30:321–328. doi: 10.1016/j.braindev.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–1907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, LeVan P, Chatillon CE, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009a;132:1022–1037. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Zelmann R, Jirsch J, Chander R, Dubeau CE, Gotman J. High frequency oscillations (80–500 Hz) in the preictal period in patients with focal seizures. Epilepsia. 2009b;50:1780–1792. doi: 10.1111/j.1528-1167.2009.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, LeVan P, Dubeau F, Gotman J. Generation of high frequency oscillations (80–500 Hz) in different anatomical structures and their relation to the seizure onset zone. Clinical Neurophysiology. 2009c;120:e29–e30. [Google Scholar]
- Jacobs J, Zijlmans M, Zelmann R, Olivier A, Hall J, Gotman J, Dubeau F. Value of electrical stimulation and high frequency oscillations (80–500 Hz) in identifying epileptogenic areas during intracranial EEG recordings. Epilepsia. 2010a;51 (4):573–582. doi: 10.1111/j.1528-1167.2009.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Zijlmans M, Zelmann R, Chatillon CE, Hall J, Olivier A, Dubeau F, Gotman J. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Annals of Neurology. 2010b;67:209–220. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Kerber K, Levan P, Dümpelmann M, Korinthenberg R, Schulze-Bonhage A. Occurrence of high frequency oscillations depends on pathology in patients with Focal cortical dysplasia. Epilepsia. 2010c;35(Abst 2) doi: 10.1111/epi.12262. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Kobayashi K, Gotman J. High-frequency changes during interictal spikes detected by time-frequency analysis. Clinical Neurophysiology. 2011;122:32–42. doi: 10.1016/j.clinph.2010.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerbi K, Ossandon T, Hamame CM, Senova S, Dalal SS, Minotti L, Betrand O, Berthoz A, Kahane P, Lachaux JP. Task-related gamma-band dynamics from an intracerebral perspective: review and implications for surface EEG and MEG. Human Brain Mapping. 2009a;30:1758–1771. doi: 10.1002/hbm.20750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerbi K, Freyermuth S, Dalal S, Kahane P, Bertrand O, Berthoz A, Lachaux JP. Saccade related gamma-band activity in intracerebral EEG: dissociating neural from ocular muscle activity. Brain Topography. 2009b;22 (1):18–23. doi: 10.1007/s10548-009-0078-5. [DOI] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–1608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- Jiruska P, Finnerty GT, Powell AD, Lofti N, Cmejla R, Jefferys JG. Epileptic high-frequency network activity in a model of non-lesional temporal lobe epilepsy. Brain. 2010;133(Pt. 5):1380–1390. doi: 10.1093/brain/awq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahane P, Bartolomei F. Temporal lobe epilepsy and hippocampal sclerosis: lessons from depth EEG recordings. Epilepsia. 2010;51 (Suppl 1):59–62. doi: 10.1111/j.1528-1167.2009.02448.x. [DOI] [PubMed] [Google Scholar]
- Khosravani H, Mehrotra N, Rigby M, Hader WJ, Pinnegar CR, Pillay N, Wiebe S, Federico P. Spatial localization and time-dependant changes of electrographic high frequency oscillations in human temporal lobe epilepsy. Epilepsia. 2009;50:605–616. doi: 10.1111/j.1528-1167.2008.01761.x. [DOI] [PubMed] [Google Scholar]
- Kirsch HE, Robinson SE, Mantle M, Nagarajan S. Automated localization of magnetoencephalographic interictal spikes by adaptive spatial filtering. Clinical Neurophysiology. 2006;117:2264–2271. doi: 10.1016/j.clinph.2006.06.708. [DOI] [PubMed] [Google Scholar]
- Knowlton RC, Elgavish R, Howell J, Blount J, Burneo JG, Faught E, Kankirawatana P, Riley K, Morawetz R, Worthington J, Kuzniecky RI. Magnetic source imaging versus intracranial electroencephalogram in epilepsy surgery: a prospective study. Annals of Neurology. 2006;59:835–842. doi: 10.1002/ana.20857. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Oka M, Akiyama T, Inoue T, Abiru K, Ogino T, Yoshinaga H, Ohtsuka Y, Oka E. Very fast rhythmic activity on scalp EEG associated with epileptic spasms. Epilepsia. 2004;45:488–496. doi: 10.1111/j.0013-9580.2004.45703.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Inoue T, Watanabe Y, Oka M, Endoh F, Yoshinaga H, Ohtsuka Y. Spectral analysis of EEG gamma rhythms associated with tonic seizures in Lennox–Gastaut syndrome. Epilepsy Research. 2009;86 (1):15–22. doi: 10.1016/j.eplepsyres.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Watanabe Y, Inoue T, Oka M, Yoshinaga H, Ohtsuka Y. Scalp-recorded high-frequency oscillations in childhood sleep-induced electrical status epilepticus. Epilepsia. 2010;51:2190–2194. doi: 10.1111/j.1528-1167.2010.02565.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Yoshinaga H, Toda Y, Inoue T, Oka M, Ohtsuka Y. High-frequency oscillations in idiopathic partial epilepsy of childhood. Epilepsia. 2011;52 (10):1812–1819. doi: 10.1111/j.1528-1167.2011.03169.x. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JDB, Cobden PM, Buzsaki G, Somogyi P. Brain-state and cell-type specific firing of hippocampal interneurons in vivo. Nature. 2003;42:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. The New England Journal of Medicine. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, Bragin A, Staba R, Crepon B, Wilson CL, Engel J., Jr Cell type-specific firing during ripple oscillations in the hippocampal formation of humans. Journal of Neuroscience. 2008;28:6104–6110. doi: 10.1523/JNEUROSCI.0437-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri C, Schridde U, Murayama Y, Panzeri S, Logothetis NK. The amplitude and timing of the BOLD signal reflects the relationship between local field potential power at different frequencies. Journal of Neuroscience. 2012;32 (4):1395–1407. doi: 10.1523/JNEUROSCI.3985-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major P, Rakowski S, Simon MV, Cheng ML, Eskandar E, Baron J, Leeman BA, Frosch MP, Thiele EA. Are cortical tubers epileptogenic? Evidence from electrocorticography. Epilepsia. 2009;50:147–154. doi: 10.1111/j.1528-1167.2008.01814.x. [DOI] [PubMed] [Google Scholar]
- Michel CM, Lantz G, Spinelli L, De Peralta RG, Landis T, Seeck M. 128-channel EEG source imaging in epilepsy: clinical yield and localization precision. Journal of Clinical Neurophysiology. 2004;21:71–83. doi: 10.1097/00004691-200403000-00001. [DOI] [PubMed] [Google Scholar]
- Miller JW, Gotman J. The meaning of interictal spikes in temporal lobe epilepsy: should we count them? Neurology. 2008;71:392–393. doi: 10.1212/01.wnl.0000324256.00488.69. [DOI] [PubMed] [Google Scholar]
- Minassian BA, Otsubo H, Weiss S, Elliott I, Rutka JT, Snead OC., 3rd Magnetoencephalographic localization in pediatric epilepsy surgery: comparison with invasive intracranial electroencephalography. Annals of Neurology. 1999;46:627–633. doi: 10.1002/1531-8249(199910)46:4<627::aid-ana11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Modur PN, Zhang S, Vitaz TW. Ictal high-frequency oscillations in neocortical epilepsy: implications for seizure localization and surgical resection. Epilepsia. 2011 doi: 10.1111/j.1528-1167.2011.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed IS, Gaetz W, Otsubo H, Snead OC, Cheyne D. Localization of interictal discharges using an event-related beamformer. In: Cheyne D, Ross B, Stroink G, Weinberg H, editors. New Frontiers in Biomagnetism. International Congress Series. Vol. 1300. 2007. pp. 669–672. [Google Scholar]
- Nagasawa T, Matsuzaki N, Juhász C, Hanazawa A, Shah A, Mittal S, Sood S, Asano E. Occipital gamma-oscillations modulated during eye movement tasks: simultaneous eye tracking and electrocorticography recording in epileptic patients. NeuroImage. 2011;58:1101–1109. doi: 10.1016/j.neuroimage.2011.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nariai H, Nagasawa T, Juhász C, Sood S, Chugani HT, Asano E. Statistical mapping of ictal high-frequency oscillations in epileptic spasms. Epilepsia. 2011;52:63–74. doi: 10.1111/j.1528-1167.2010.02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren JA, Wilson CL, Bragin A, Lin JJ, Salamon N, Dutton RA, Luders E, Fields TA, Fried I, Toga AW, Thompson PM, Engel J, Jr, Staba RJ. Three dimensional surface maps link local atrophy and fast ripples in human epileptic hippocampus. Annals of Neurology. 2009;66:783–791. doi: 10.1002/ana.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsubo H, Iida K, Oishi M, Okuda C, Ochi A, Pang E, Weiss SK, Rutka JT, Chuang SH, Snead OC., 3rd Neurophysiologic findings of neuronal migration disorders: intrinsic epileptogenicity of focal cortical dysplasia on electroencephalography, electrocorticography, and magnetoencephalography. Journal of Child Neurology. 2005;20:357–363. doi: 10.1177/08830738050200041501. [DOI] [PubMed] [Google Scholar]
- Otsubo H, Ochi A, Imai K, Akiyama T, Fujimoto A, Go C, Dirks P, Donner EJ. High-frequency oscillations of ictal muscle activity and epileptogenic discharges on intracranial EEG in a temporal lobe epilepsy patient. Clinical Neurophysiology. 2008;119:862–868. doi: 10.1016/j.clinph.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Ochi A, Ostubo H, Donner EJ, Elliott I, Iwata R, Funaki T, Akizuki Y, Akiyama T, Imai K, Rutka JT, Snead OC., 3rd Dynamic changes of ictal high-frequency oscillations in neocortical epilepsy: using multiple band frequency analysis. Epilepsia. 2007;48:286–296. doi: 10.1111/j.1528-1167.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- Ramachandrannair R, Ochi A, Imai K, Benifla M, Akiyama T, Holowka S, Rutka JT, Snead OC, 3rd, Otsubo H. Epileptic spasms in older pediatric patients: MEG and ictal high-frequency oscillations suggest focal-onset seizures in a subset of epileptic spasms. Epilepsy Research. 2008;78:216–224. doi: 10.1016/j.eplepsyres.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain. 2001;124:1683–1700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- Sakuma K, Sekihara K, Hashimoto I. Neural source estimation from a time-frequency component of somatic evoked high-frequency magnetic oscillations to posterior tibial nerve stimulation. Clinical Neurophysiology. 1999;110:1585–1588. doi: 10.1016/s1388-2457(99)00120-0. [DOI] [PubMed] [Google Scholar]
- Schevon CA, Trevelyan AJ, Schroeder CE, Goodman RR, McKhann G, Jr, Emerson RG. Spatial characterization of interictal high frequency oscillations in epileptic neocortex. Brain. 2009;132:3047–3059. doi: 10.1093/brain/awp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekihara K, Abraham-Fuchs K, Stefan H, Hellstrandt E. Suppression of background brain activity influence in localizing epileptic spike sources from biomagnetic measurements. Brain Topography. 1996;8:323–328. doi: 10.1007/BF01184792. [DOI] [PubMed] [Google Scholar]
- Sekihara K, Nagarajan SS, Poeppel D, Marantz A, Miyashita Y. Reconstructing spatiotemporal activities of neural sources using an MEG vector beamformer technique. IEEE Transactions on Biomedical Engineering. 2001;48:760–771. doi: 10.1109/10.930901. [DOI] [PubMed] [Google Scholar]
- Shiraishi H, Stufflebeam SM, Knake S, Ahlfors SP, Sudo A, Asahina N, Egawa K, Hatanaka K, Kohsaka S, Saitoh S, Grant PE, Dale AM, Halgren E. Dynamic statistical parametric mapping for analyzing the magnetoencephalographic epileptiform activity in patients with epilepsy. Journal of Child Neurology. 2005;20:363–369. doi: 10.1177/08830738050200041601. [DOI] [PubMed] [Google Scholar]
- Sperli F, Spinelli L, Seeck M, Kurian M, Michel CM, Lantz G. EEG source imaging in pediatric epilepsy surgery: a new perspective in presurgical workup. Epilepsia. 2006;47:981–990. doi: 10.1111/j.1528-1167.2006.00550.x. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. Journal of Neurophysiology. 2002;88:1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J. High-frequency oscillations recorded in human medial temporal lobe during sleep. Annals of Neurology. 2004;56:108–115. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Frighetto L, Behnke EJ, Mathern GW, Fields TA, Bragin A, Ogren J, Fried I, Wilson CL, Engel J., Jr Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia. 2007;48:2130–2138. doi: 10.1111/j.1528-1167.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Hämäläinen MS, Ahlfors SP, Liu H, Madsen JR, Bourgeois BF, Lee JW, Dworetzky BA, Belliveau JW, Stufflebeam SM. Propagation of epileptic spikes reconstructed from spatiotemporal magnetoencephalographic and electroencephalographic source analysis. NeuroImage. 2010;50 (1):217–222. doi: 10.1016/j.neuroimage.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonini C, Beghi E, Berg AT, Bogliun G, Giordano L, Newton RW, Tetto A, Vitelli E, Vitezic D, Wiebe S. Predictors of epilepsy surgery outcome: a meta-analysis. Epilepsy Research. 2004;62:75–87. doi: 10.1016/j.eplepsyres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Ulbert I, Halgren E, Heit G, Karmos G. Multiple microelectrode-recording system for human intracortical applications. Journal of Neuroscience Methods. 2001;106 (1):69–79. doi: 10.1016/s0165-0270(01)00330-2. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130:2354–2366. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- Usui N, Terada K, Baba K, Matsuda K, Nakamura F, Usui K, Yamaguchi M, Tottori T, Umeoka S, Fujitani S, Kondo A, Mihara T, Inoue Y. Clinical significance of ictal high frequency oscillations in medial temporal lobe epilepsy. Clinical Neurophysiology. 2011;122:1693–1700. doi: 10.1016/j.clinph.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Van Gompel JJ, Stead SM, Giannini C, Meyer FB, Marsh WR, Fountain T, So E, Cohen-Gadol A, Lee KH, Worrell GA. Phase I trial: safety and feasibility of intracranial electroencephalography using hybrid subdural electrodes containing macro- and microelectrode arrays. Journal of Neurosurgery. 2008;25:E23–E29. doi: 10.3171/FOC/2008/25/9/E23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, Meyer FB, Marsh R, Litt B. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–937. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Koh S, Sankar R, Mathern GW. Paroxysmal fast activity: an interictal scalp EEG marker of epileptogenesis in children. Epilepsy Research. 2008;82 (1):99–106. doi: 10.1016/j.eplepsyres.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Sankar R, Lerner JT, Matsumoto JH, Vinters HV, Mathern GW. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology. 2010;75:1686–1694. doi: 10.1212/WNL.0b013e3181fc27d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, Buzsaki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. Journal of Neuroscience. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J, Liu Y, Wang Y, Kirtman EG, Kotecha R, Chen Y, Huo X, Fujiwara H, Hemasilpin N, Lee K, Mangano FT, Leach J, Jones B, DeGrauw T, Rose D. Frequency and spatial characteristics of high-frequency neuromagnetic signals in childhood epilepsy. Epileptic Disorder. 2009;11:113–125. doi: 10.1684/epd.2009.0253. [DOI] [PubMed] [Google Scholar]
- Zelmann R, Zijlmans M, Jacobs J, Châtillon CE, Gotman J. Improving the identification of high frequency oscillations. Clinical Neurophysiology. 2009;120 (8):1457–1464. doi: 10.1016/j.clinph.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009a;72:979–986. doi: 10.1212/01.wnl.0000344402.20334.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High frequency oscillations and seizure frequency in patients with focal epilepsy. Epilepsy Research. 2009b;85:287–292. doi: 10.1016/j.eplepsyres.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Kahn YU, Zelmann R, Dubeau F, Gotman J. Ictal and interictal high frequency oscillations in patients with focal epilepsy. Clinical Neurophysiology. 2011;122:664–671. doi: 10.1016/j.clinph.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]