Abstract
Objective
Nonadherence is a significant health care issue in pediatric inflammatory bowel disease (IBD) that requires intervention to improve outcomes. This pilot randomized controlled trial was designed to evaluate the feasibility, acceptability, and preliminary efficacy of an individually tailored behavioral treatment for nonadherence in adolescents with IBD.
Patients and Methods
Fourteen adolescents ages 14.89 ± 2.01 years were randomly assigned to immediate care or wait list control conditions and received a manualized individually tailored behavioral intervention for nonadherence. Medication adherence, measured by pill count, served as the primary endpoint. Parents provided demographic data and ratings of intervention acceptability and patients provided disease-severity data.
Results
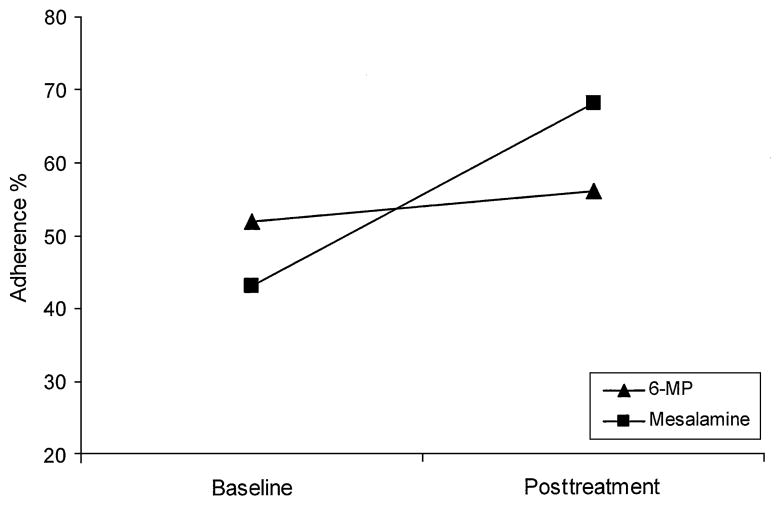
Feasibility of the treatment was demonstrated by 100% treatment session attendance for all of the patients enrolled in the trial. Both parent and patient acceptability ratings were favorable. Comparison of baseline with posttreatment percent adherence across both conditions demonstrated that treatment resulted in a 4% gain in 6-mercaptopurine/azathioprine adherence (52% at baseline; 56% at posttreatment; δ =0.07) and a 25% gain in mesalamine adherence (43% at baseline; 68% at posttreatment; δ =0.57).
Conclusions
Individually tailored treatment of nonadherence in adolescents with IBD is feasible and may result in substantial improvement in medication adherence. Differential effect of the intervention on medications requires further investigation, but it may reflect differences in regimen complexity, concerns about medication adverse effects, and/or patient/parent preference to target more complex regimens. Large-scale testing of this intervention is needed to demonstrate effect on clinical outcomes.
Keywords: adherence, compliance, inflammatory bowel disease, medication, pediatric
Treatment nonadherence is a significant health care issue that has considerable negative effects on disease outcomes, clinical trial research, and clinical decisions by health care teams (1), and the annual cost of nonadherence in the United States is approximately $100 to $300 billion (2–3). The prevalence of medication nonadherence is approximately 50% in children (1) and 65% to 75% in adolescents (4). In inflammatory bowel disease (IBD), comprising both Crohn disease and ulcerative colitis, the prevalence is even higher, with 64% and 88% nonadherence to 6-mercaptopurine (6-MP)/azathioprine and 5-aminosalicyclic acid, the 2 most commonly prescribed medications in pediatric IBD (5). Moreover, there is evidence in adults with IBD that patients who are nonadherent are 5.5 times more likely to relapse (6). Thus, nonadherence negatively affects public health expenditures and patient clinical outcomes. The empirical literature base regarding treatment of nonadherence in pediatrics remains modest, however, and there are no published clinical trials reporting outcomes of medication nonadherence treatment in pediatric IBD.
Rapoff (1) provides a recent review of the extant intervention research for nonadherence across pediatric populations. Although most clinical trials have demonstrated positive results, a wide variety of techniques (eg, educational, behavioral) have been used and the number of sessions and total length of intervention have varied greatly (7–12). In general, both group-based and individually tailored treatment approaches have received empirical support. Only 1 known treatment outcome study has been published that targets treatment adherence in pediatric IBD (13); the present study focused on improving dietary calcium intake in young children. Consequently, the results are not generalizable to the treatment of medication nonadherence in adolescents. In addition, the challenge in implementing interventions for nonadherence in pediatric IBD is that treatment is not standardized for either Crohn disease or ulcerative colitis. Thus, the factors that may contribute to or maintain nonadherence may differ from patient to patient. This suggests that an individually tailored treatment approach may be particularly helpful because it allows unique barriers and behavioral factors to be targeted.
With these issues in consideration, we developed a brief (ie, 4 sessions) individually tailored multicomponent treatment protocol for medication nonadherence in pediatric IBD targeting educational, organizational, behavioral, and family factors that may contribute to or maintain nonadherence in adolescents with IBD. Using this manualized treatment protocol, we tested the preliminary efficacy, feasibility, and acceptability of the intervention in adolescent patients with IBD; moreover, the present study generated effect size data for future statistical power considerations. The present pilot study was a randomized controlled trial (RCT) that evaluated this individually tailored behavioral treatment for nonadherence using immediate care and wait list control conditions. Given the small sample used for the present study, we did not anticipate statistically significant differences in adherence between conditions following treatment completion for the immediate care condition. Conversely, in evaluating the effect of the treatment on the entire sample, we anticipated substantial improvement in adherence from baseline to posttreatment, with small to medium effect sizes for each medication measured. We also anticipated that the intervention would demonstrate feasibility via treatment session attendance rate and acceptability using patient and parent ratings on various aspects of the intervention components.
PATIENTS AND METHODS
Patients
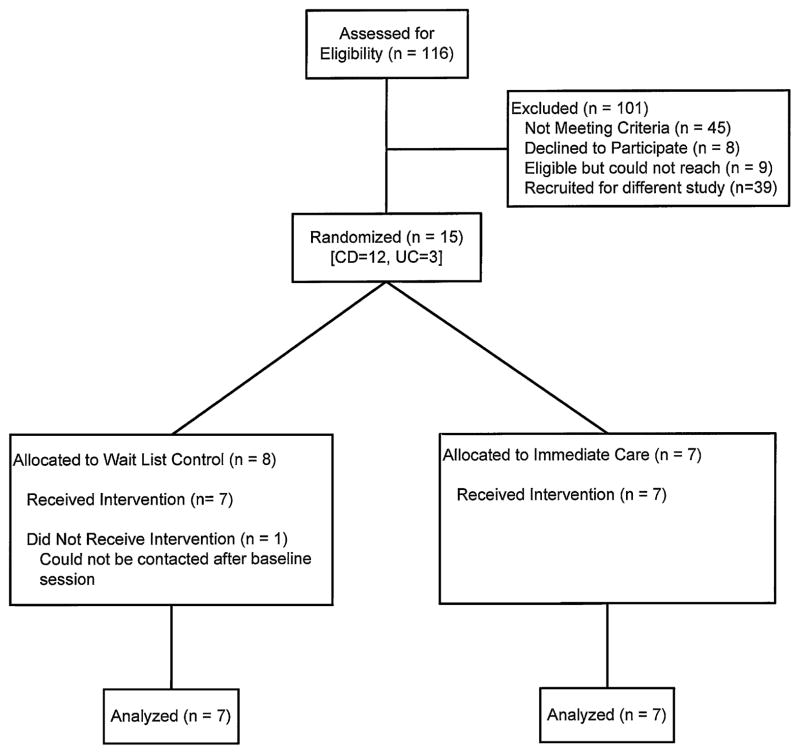
Eligible patients were recruited from an outpatient gastroenterology clinic to a large tertiary care pediatric hospital. Inclusion criteria for patients were diagnosis of IBD (Crohn disease or ulcerative colitis), ages 11 to 18 years (patient must be attending school and currently living at home if age 18), current prescription of 6-MP/azathioprine and/or mesalamine, and English-language fluency for patient and caregivers. Given the fact that the primary endpoint (medication adherence) for this trial was the same and generalizable across IBD subtypes, and that both patients with Crohn disease and ulcerative colitis take medications with varying dosing schedules, both diagnoses were included. The study received approval by the hospital institutional review board. Potential patients were identified through chart reviews and mailed a recruitment letter. An opt-out telephone number was included for families that did not wish to be contacted. The remaining families were contacted via telephone or approached during a regularly scheduled clinic visit. Of the 23 families contacted, 8 declined participation (3: no interest in research; 3: too busy with current activities; 1: no concerns about taking medications; and 1: live too far away from hospital). Of the 15 patients randomized, 1 could not be contacted after baseline to schedule treatment sessions. Thus, the final sample included 14 adolescents (7 per condition) with IBD and their caregivers (Fig. 1).
FIGURE 1.
Participant flowchart. CD =Crohn disease; UC =ulcerative colitis.
Study Design
The present study was a randomized controlled clinical trial. Patients were randomly assigned to an immediate care or wait list control condition using permuted block randomization with a block size of 2. During baseline visits, informed consent/ assent was obtained, patients completed measures of disease severity, and adherence was calculated. Two weeks later, patients in the immediate care condition attended 4 weekly individually tailored, adherence-focused treatment sessions led by doctoral-level clinical psychologists or postdoctoral fellows (see Table 1 for content overview). The 4-session format of the intervention was chosen to provide a brief, focused treatment to enhance feasibility and acceptability for patients and families while providing a substantive intervention targeting nonadherence. There is evidence supporting the feasibility and efficacy of brief interventions targeting health behaviors in the pediatric literature, including IBD (13). Patients consisted of dyads of patients and their caregivers (eg, parent), and each dyad was seen independently (ie, not in a group with other patients and parents). One week after the final treatment session, patients in both conditions completed study assessments. At that time, patients in the wait list control condition completed the aforementioned treatment protocol and posttreatment assessments.
TABLE 1.
Overview of treatment session content
| Session | Duration, min | Session content |
|---|---|---|
| 1 | 60–75 | Educational and organizational intervention: education regarding IBD, medications, and adherence; discussion of barriers/facilitators of adherence; discussion of organizational cues to prompt adherence. |
| 2 | 60–75 | Behavior modification: discussion/problem solving of organizational changes made in home; training in behavioral contracts/goal setting, positive reinforcement, extinction. |
| 3 | 60–75 | Problem-solving skills and monitoring adherence: discussion/problem solving of goal setting/ behavioral contracts; training in problem-solving skills, parental monitoring, and self-monitoring of adherence. |
| 4 | 60–75 | Family functioning: discussion/problem solving using problem-solving skills/monitoring; training in communication, negotiation, and resolving conflict between family members. |
Measures
Caregivers completed a demographic form at the baseline assessment. Demographic data obtained included parent age, education, marital status, patient ethnicity, and annual household income.
Pill Count
Pill counts were conducted by study personnel for both 6-MP/azathioprine and mesalamine at each assessment time point. Data obtained from pill bottles included dosing instructions, date on which the prescription was filled, quantity filled, and present quantity. Adherence was calculated as (doses consumed/doses prescribed) ×100.
Pediatric Ulcerative Colitis Activity Index
The Pediatric Ulcerative Colitis Activity Index (PUCAI) is a 6-item measure of disease severity for patients with ulcerative colitis (14). An interview format allows patients to report on 6 typical symptoms of ulcerative colitis: abdominal pain, rectal bleeding, stool consistency of most stools, number of stools per 24 hours, nocturnal stools, and activity level. A total score is obtained by summing the 6 items, resulting in a range of 0 to 85, with a higher score representing more severe disease (ie, 0–9 =inactive; 10–34 =mild; 35–64 =moderate; ≥65 =severe disease). The PUCAI has demonstrated adequate reliability and validity in earlier research. Internal consistency reliability for this sample was 0.58; however, this lower estimate is likely caused by the low number of patients with ulcerative colitis (n =3) in the present study. The PUCAI was conducted at each assessment time point.
Partial Harvey Bradshaw-Index
The Partial Harvey-Bradshaw Index (PHBI) is a 3-item measure of disease severity for patients with Crohn disease that allows patients to rate 3 categories of symptom severity during the last 7 days (15). Symptom categories include general well-being, abdominal pain, and number of liquid stools. Scores range from 0 to 12, with a higher score indicating more active disease (ie, 0 =inactive disease; 1–4 =mild disease; ≥5 =moderate to severe disease). The PHBI has demonstrated adequate reliability and validity in prior studies and was preferred in the present study because of its feasible administration. Internal consistency was 0.88 for this sample. The PHBI was conducted at each assessment time point.
Feasibility Acceptability Questionnaire
The Feasibility Acceptability Questionnaire (FAQ) is a 22-item patient and parent self-report of the feasibility and acceptability of the intervention that was developed for the present study. Factors assessed include the format, structure, length, number of sessions, total time of intervention, convenience of treatment, extent to which parents used behavioral skills to manage treatment adherence, and perception of effect on adherence. Items were assessed on a 7-point Likert scale. The FAQ was completed at the posttreatment assessment for each condition.
Data Analyses
All of the data analyses were conducted in Predictive Analytics 18.0 (IBM, Armonk, NY). Data were entered and cross-checked for accuracy. Descriptive statistics were calculated for key demographic variables, adherence, disease severity, and feasibility/acceptability variables. Independent-samples t tests were conducted to examine differences in adherence between immediate care and wait list control conditions at baseline and following treatment completion for the immediate care condition. Paired-samples t tests were conducted to examine the mean change in medication adherence rates in both conditions, from baseline to posttreatment for both 6-MP/azathioprine and mesalamine. Cohen δ effect sizes were calculated to examine the magnitude of effect for the treatment because of the small sample recruited for this pilot study.
RESULTS
Descriptive Analyses
The patients were 10 girls and 4 boys ages 14.89 ±2.01 years. All of the patients reported their ethnicity as white. Parent age was 45.87 ±3.79 years. All of the parents reported being married, and 43% reported having at least a college degree. Median annual household income for families was $75,000 to 100,000. Among patients with Crohn disease (n =11), 4 had inactive disease and 7 had mild disease. Of those diagnosed as having ulcerative colitis (n =3), 2 had inactive disease and 1 had moderate disease. The feasibility of the treatment was demonstrated by 100% treatment session attendance for all of the patients enrolled in the trial. Patient and parent ratings on the FAQ are summarized in Table 2.
TABLE 2.
Descriptive data for parent- and patient-report forms of the Feasibility Acceptability Questionnaire
| Parent
|
Child
|
|||
|---|---|---|---|---|
| Rating in ideal range, % | Mean rating | Rating in ideal range, % | Mean rating | |
| I liked the individualized format* | 100 | 6.62 | 93 | 5.86 |
| I thought the individualized format was helpful* | 100 | 6.50 | 71 | 5.43 |
| Amount of information† | 86 | 4.71 | 93 | 4.50 |
| Treatment session length† | 93 | 4.43 | 86 | 4.64 |
| No. sessions† | 86 | 4.14 | 100 | 4.00 |
| Total time commitment for treatment (ie, 4 wk)† | 86 | 4.36 | 86 | 3.86 |
| I thought attending sessions was convenient* | 43 | 4.50 | 36 | 4.14 |
| I used the behavioral skills I learned* | 62 | 5.15 | 50 | 4.79 |
| Treatment helped improve my (child’s) adherence* | 92 | 5.92 | 71 | 5.43 |
Ideal range is based on assumption that ratings in this range represent a high degree of acceptability for respondents.
Ideal range =5–7 on 7-point Likert scale.
Ideal range =3–5 on 7-point Likert scale.
Evaluation of Treatment Effect
Adherence was calculated as (doses consumed/doses prescribed) ×100. Independent-samples t tests revealed no significant differences between conditions at baseline on adherence to 6-MP/azathioprine (t =1.13, P =0.30) or mesalamine (t = −1.42, P = 0.19). Independent-samples t tests examining differences between conditions following completion of treatment for the immediate care condition revealed that those who received the intervention had significantly greater 6-MP/azathioprine adherence compared with controls (t =2.72, P <0.05), and there was a non-significant difference between conditions on mesalamine adherence (t =1.09, P =0.31). Paired-samples t tests examining change in medication adherence across conditions from baseline to post-treatment revealed statistically nonsignificant change for 6-MP/ azathioprine (t =0.19, P =0.85) and mesalamine (t = −1.90, P =0.09). Although the percentage of adherence improvement was 4% for 6-MP/azathioprine (52% at baseline, 56% at posttreatment), a 25% increase (43% at baseline, 68% at posttreatment) in adherence was observed for mesalamine (Fig. 2). These results translated to observed effect sizes of δ =0.07 for 6-MP/azathioprine and δ =0.57 for mesalamine.
FIGURE 2.
Treatment effect on medication adherence following treatment in both conditions. 6-MP =6-mercaptopurine.
DISCUSSION
This pilot study is the first RCT to evaluate the feasibility and preliminary efficacy of an individually tailored behavioral treatment for nonadherence in youth with IBD. In general, data from the present study suggest that an individually tailored approach to adherence promotion is a viable treatment option that requires larger-scale investigation. The 100% retention rate of patients indicates this 4-session treatment intervention is a feasible approach for treating nonadherence. Additionally, the intervention was rated favorably in terms of acceptability by both patients and their parents. Several aspects of this treatment may have increased perceptions of acceptability. For example, treatment sessions were scheduled at times that were convenient for families. In addition, compared with group interventions that focus on issues shared by multiple families, this intervention provided more time for individualized problem solving of families’ unique barriers. Some of the common barriers targeted for intervention in the present study included forgetting to take medications, poor planning for taking medication during other activities, parent–child communication skills regarding adherence, and diffused responsibility for adherence between parents and adolescents.
Overall, improvements in adherence were substantially better for mesalamine than for 6-MP/azathioprine, with a medium effect size and 25% improvement in adherence for mesalamine. This discrepant change in adherence across medications may reflect differences in the regimen complexity between these 2 drugs. Although newer mesalamine preparations can be prescribed as once-daily dosing, mesalamine is often prescribed as several pills taken 2 to 4 times daily and 6-MP/azathioprine is usually prescribed as 1 to 2 pills taken 1 to 2 times daily (16). With a greater number of doses and pills per dose, there is likely an increased variability in adherence and consequently more opportunities to make significant adherence gains. It is also plausible that an individualized treatment protocol allows families to target more complex treatment regimens first because these are more burdensome and difficult to adhere to, resulting in greater improvements over time compared with less-complex treatments. In addition, medication-specific barriers to adherence such as fear of medication adverse effects, which may particularly affect 6-MP/azathioprine, may have resulted in more modest adherence gains compared with mesalamine. Regardless, this finding suggests that an individually tailored, adherence-focused intervention may be particularly useful and beneficial for chronically ill youth following complex medication dosing regimens. Moreover, this finding is especially relevant given that mesalamine is one of the most commonly used maintenance drug therapies to treat pediatric IBD, particularly ulcerative colitis.
There are several noteworthy strengths of the present study. First, the RCT study design provided control over maturation effects at the primary endpoint, thus increasing the likelihood that the observed changes in adherence were the result of the treatment used. The present study also used a validated and objective measure of adherence (ie, pill counts) that is feasible for use in clinical settings (1). Finally, a multifaceted assessment of treatment feasibility and acceptability, which is uncommon in studies of this nature, was an important component of the present study. Indeed, these data are critical to understanding the clinical utility of this treatment approach and support the rationale for developing and testing a larger RCT of an individually tailored, adherence-focused intervention in pediatric IBD.
These findings also should be interpreted in the context of some limitations that carry important implications for future research. First, this was a pilot study and is consequently limited by the small sample size and lack of long-term follow-up, which precludes broad generalization of the findings. Effect sizes were calculated to estimate the effect of the treatment on medication adherence, but large-scale testing is necessary. Also, it is possible that several months after the intervention, adherence will have returned to baseline for some patients. A larger clinical trial with long-term follow-up will be able to provide data on the types of patients who relapse and optimal timing for follow-up intervention. Second, demographic characteristics of the sample (ie, adolescents, white, married caregivers, high household income), although representative of other published pediatric IBD studies (5,17), may not adequately characterize the larger IBD population. Third, multiple measures of adherence were not obtained. Although pill counts are a feasible, reliable, and objective measure of adherence to oral medication (1), future research should incorporate additional measures of adherence to examine patterns of nonadherence, which also will facilitate individual tailoring of this intervention. Moreover, a multimethod assessment approach to adherence may provide more reliable and informative adherence estimates (18,19). Assessment of medications in addition to 6-MP/azathioprine and mesalamine also will provide more comprehensive data regarding adherence difficulties in these patients. Lastly, the scope of the present study precluded an analysis of the differential effect of various intervention components (eg, organization, problem solving, behavior modification) on treatment adherence. Future research that dismantles components of adherence intervention may prove particularly beneficial and may guide subsequent clinical intervention and practice.
The present study provides preliminary evidence for individually tailored treatment of nonadherence to oral medication among chronically ill youth. It is plausible that this type of treatment for nonadherence would be beneficial for other conditions that are treated with oral medications. Because the prevalence of nonadherence among youth with chronic medical conditions is high, it will be important to determine the extent to which individually tailored adherence intervention is useful and effective at improving outcomes. Individually tailored interventions offer important benefits that group-based interventions cannot, including a focus on families’ specific and unique barriers to treatment adherence and the potential for delivery of treatment sessions in conjunction with regular clinic appointments. It is anticipated that further evaluation of this type of intervention in large-scale trials will yield salient findings regarding its efficacy and generalizability and that effectiveness research will demonstrate its clinical utility.
Acknowledgments
Research supported in part by PHS Grant P30 DK 078392, NIDDK K23 DK079037, and Institutional Clinical and Translational Science Award NIH/NCRR Grant No. 1UL1RR026314.
Footnotes
The authors report no conflicts of interest.
References
- 1.Rapoff MA. Adherence to Pediatric Medical Regimens. 2. New York: Springer; 2010. [Google Scholar]
- 2.DiMatteo MR. The role of effective communication with children and their families in fostering adherence to pediatric regimens. Patient Educ Couns. 2004;55:339–44. doi: 10.1016/j.pec.2003.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Berg JS, Dischler J, Wagner DJ, et al. Medication compliance: a healthcare problem. Ann Pharmacother. 1993;279(Suppl):S1–24. [PubMed] [Google Scholar]
- 4.Logan D, Zelikovsky N, Labay L, et al. The Illness Management Survey: identifying adolescents’ perceptions of barriers to adherence. J Pediatr Psychol. 2003;28:383–92. doi: 10.1093/jpepsy/jsg028. [DOI] [PubMed] [Google Scholar]
- 5.Hommel KA, Davis CM, Baldassano RN. Objective versus subjective assessment of oral medication adherence in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:589–93. doi: 10.1002/ibd.20798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kane S, Huo D, Aikens J, et al. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med. 2003;114:39–43. doi: 10.1016/s0002-9343(02)01383-9. [DOI] [PubMed] [Google Scholar]
- 7.Bonner S, Zimmerman BJ, Evans D, et al. An individualized intervention to improve asthma management among urban Latino and African-American families. J Asthma. 2002;39:167–79. doi: 10.1081/jas-120002198. [DOI] [PubMed] [Google Scholar]
- 8.Burkhart PV, Rayens MK, Oakley MG, et al. Testing an intervention to promote children’s adherence to asthma self-management. J Nurs Scholarsh. 2007;39:133–40. doi: 10.1111/j.1547-5069.2007.00158.x. [DOI] [PubMed] [Google Scholar]
- 9.van Es SM, Nagelkerke AF, Colland VT, et al. An intervention programme using the ASE-model aimed at enhancing adherence in adolescents with asthma. Patient Educ Couns. 2001;44:193–203. doi: 10.1016/s0738-3991(00)00195-6. [DOI] [PubMed] [Google Scholar]
- 10.Downs JA, Roberts CM, Blackmore AM, et al. Benefits of an education programme on the self-management of aerosol and airway clearance treatments for children with cystic fibrosis. Chron Respir Dis. 2006;3:19–27. doi: 10.1191/1479972306cd100oa. [DOI] [PubMed] [Google Scholar]
- 11.Smith NA, Seale JP, Ley P, et al. Effects of intervention on medication compliance in children with asthma. Med J Aust. 1986;144:119–122. doi: 10.5694/j.1326-5377.1986.tb112234.x. [DOI] [PubMed] [Google Scholar]
- 12.Koontz KL, Slifer KJ, Cataldo MD, et al. Improving pediatric compliance with positive airway pressure therapy: the impact of behavioral intervention. Sleep. 2003;26:1010–5. doi: 10.1093/sleep/26.8.1010. [DOI] [PubMed] [Google Scholar]
- 13.Stark LJ, Hommel KA, Mackner LM, et al. Randomized trial comparing two methods of increasing dietary calcium intake in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2005;40:501–7. doi: 10.1097/01.mpg.0000157913.32465.45. [DOI] [PubMed] [Google Scholar]
- 14.Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133:423–32. doi: 10.1053/j.gastro.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 15.Markowitz J, Grancher K, Kohn N, et al. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn’s disease. Gastroenterology. 2000;119:895–902. doi: 10.1053/gast.2000.18144. [DOI] [PubMed] [Google Scholar]
- 16.Crohn’s and Colitis Foundation of America. [Accessed July 22, 2010];Treating children and adolescents. 2009 http://www.ccfa.org/info/treatment/kidsmeds.
- 17.Mackner LM, Crandall WV. Oral medication adherence in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:1006–1012. doi: 10.1097/01.mib.0000186409.15392.54. [DOI] [PubMed] [Google Scholar]
- 18.Modi AC, Lim CS, Yu N, et al. A multi-method assessment of treatment adherence for children with cystic fibrosis. J Cyst Fibros. 2006;5:177–85. doi: 10.1016/j.jcf.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Hommel KA, Mackner LM, Denson LA, et al. Treatment regimen adherence in pediatric gastroenterology. J Pediatr Gastroeneterol Nutr. 2008;47:526–43. doi: 10.1097/MPG.0b013e318175dda1. [DOI] [PMC free article] [PubMed] [Google Scholar]