Abstract
Background
Peripheral nociceptive action of the proinflammatory cytokines IL-1β and IL-6 has been implicated in the pathogenesis of numerous pain syndromes. An increase in the level of these cytokines in jugular venous blood has been reported during migraine attacks, suggesting their potential involvement in mediating the intracranial headache of migraine.
Methods
In this work we examined, using in vivo single-unit recording of meningeal nociceptors in the trigeminal ganglion of anesthetized rats, whether the peripheral actions of IL-1β and IL-6 can promote the activation and sensitization of nociceptors that innervate the intracranial meninges, two neural processes that are believed to play a key role in promoting the intracranial throbbing pain of migraine.
Results
We found that meningeal application of IL-1β leads to the activation and mechanical sensitization of about 70% and 45% of the nociceptors respectively. In contrast, IL-6 was a very poor modulator of meningeal nociceptors' response properties affecting overall only about 20% of the nociceptors.
Conclusions
Our study provides for the first time in vivo electrophysiological evidence that meningeal action of IL-1β can promote the activation and increased mechanosensitivity of intracranial meningeal nociceptors and that IL-6 generally lacks these properties. Future studies are required to examine the mechanism that plays a role in mediating the nociceptive effects of IL-1β on meningeal nociceptors, which may serve as a target for migraine therapy.
Keywords: IL-1β, IL-6, Meningeal nociceptor, headache, sensitization, inflammation
Introduction
Activation and enhanced responsiveness to natural stimuli (i.e. sensitization) of primary afferent nociceptors that innervate the intracranial meninges are thought to serve as the neural substrate that underlies the development of migraine as well as other headaches of intracranial origin (1–4). The exact cellular and molecular mechanisms that drive this nociceptor plasticity are incompletely understood, although meningeal action of proinflammatory algesic mediators is likely to play an important role (5).
Among the mediators thought to contribute to the activation and sensitization of nociceptors are the proinflammatory cytokines tumor necrosis factor alpha (TNFα), interleukin 1 beta (IL-1β) and interleukin 6 (IL-6), which are released primarily by resident and infiltrating immune cells. The level of these cytokines increases in the internal jugular blood during the first hours of a migraine attack (6), suggesting their potential ability to interact with meningeal nociceptors and promote migraine headache.
We have shown recently that meningeal action of one of these cytokines (TNFα) can increase the mechanosensitivity of meningeal nociceptors, through a local vascular action (7), and proposed that this cellular mechanism may be important in promoting the painful throbbing sensation during migraine. The aim of the current study was to further examine the potential role of proinflammatory cytokines in headache by determining whether IL-1β and IL-6 can also act upon meningeal nociceptors and promote their activation and increased mechanosensitivity.
Previous in vitro electrophysiological studies have shown that local application of IL-1β to the cell body of small-diameter sensory neurons in dorsal root ganglia can potentiate heat-evoked currents (8), increase sodium currents and evoke action potentials (9). However, whether IL-1β can act upon nociceptors in vivo, particularly at the level of their peripheral nerve endings to promote sensitization and activation has never been tested. Putative pronociceptive effects of IL-6 on sensory nociceptive neurons were also demonstrated in vitro (10). However, in vivo electrophysiological studies indicated that the ability of IL-6 to sensitize nociceptors (at least to heat stimuli) requires the co-presence of its soluble receptor (11–13). Whether IL-6 can act within the intracranial meninges to activate or mechanically sensitize nociceptors is not known.
Given the potential role of IL-1β and IL-6 in mediating migraine headache, we investigated for the first time, using an in vivo preparation, whether these proinflammatory cytokines can activate or increase the mechanosensitivity of meningeal nociceptors.
Materials and methods
Animals
Sprague Dawley male rats (250–350 g) were used in compliance with the experimental protocol approved by the institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center and adhered to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain (14).
Electrophysiological recordings
Rats were deeply anesthetized with urethane (1.5–1.8 g/kg). Single-unit recordings of meningeal nociceptors in the trigeminal ganglion were conduced as previously described (15,16). Briefly, a craniotomy was made to expose the left transverse sinus, as well as the adjacent dura. The dura was kept moist using local perfusion with modified synthetic interstitial fluid (SIF; 135 mM NaCl, 5 mM KCl, 1 mM MgCl2, 5 mM CaCl2, 10 mM glucose and 10 mM HEPES, pH 7.2). SIF was also used as the vehicle for all drug testing. For single-unit recording, a platinum-coated tungsten microelectrode (impedance approximately 150 KOhm; FHC Inc., Bowdoin, Maine, USA) was inserted into the left trigeminal ganglion and meningeal nociceptors were identified by their constant latency responses to electrical stimuli (0.5 ms pulse, 0.5–5 mA, 0.5 Hz) applied to the transverse sinus. Based on their conduction velocity (CV), units were classified as either A delta (CV > 1.5 m/s) or C (CV ≤ 1.5 m/s) units. Action potentials were acquired using a real-time waveform discriminator (Spike 2; Cambridge Electronic Design, Cambridge, UK), which were used for online and offline data analyses. Only one unit was tested in each animal.
Testing of mechanical sensitization
Mechanical receptive fields of meningeal nociceptors were initially mapped using a series of calibrated von Frey monofilaments that exert pressure stimuli in the range of 0.16–2 g/force. Quantitative evaluation of mechanically-evoked neuronal responses were determined using a servo force-controlled stimulator (Series 300B, Aurora Scientific Inc., Aurora, Ontario, Canada) fitted with a flat-ended plastic cylinder (17). Stimulus trials for testing changes in mechanical sensitivity consisted of graded square-wave stimuli (100-ms rise time, 2-s width, 60-s interstimulus interval) delivered in ascending order. Each trial included threshold and suprathreshold stimuli. Neuronal responses to mechanical stimulation of the dura as well as ongoing spontaneous activity, were recorded every 15 min throughout the experiment. Baseline measurements of spontaneous and mechanically evoked activity were obtained in at least three consecutive trials prior to cytokine administration. Only units that exhibited consistent responses were tested further.
Data and statistical analyses
Cumulative data are displayed as the median and interquartile range. For each neuron an increase in threshold, suprathreshold responses or ongoing discharge level were defined as an increase in firing rates that exceeded the mean plus twice the standard deviation of the baseline values and that persisted at least for two consecutive trials (i.e. 30 min). Group changes were analyzed initially by applying the Friedman test on all time points tested during the application. The level of significance was set at p=0.05. The time course of activation/sensitization and their resolution were further analyzed by comparing neuronal responses at baseline to those obtained at 30 and 60 min after application and 60 min of wash using the Wilcoxon matched-pairs signed-ranks test with Bonferroni correction, with the level of significance set at p=0.016 (0.05/3).
Results
Electrophysiological properties of meningeal nociceptors
The effects of IL-1β and IL-6 were tested on 30 units (16 A delta and 14 C units). Only one cytokine was tested in each unit. At baseline, meningeal nociceptors had ongoing discharge ranging between 0.2 and 2 Hz (mean 0.82±0.32 Hz). The mean baseline activity of the C-unit population was higher than that of the A-delta unit population (1.09 vs 0.65 Hz, p<0.05 Mann–Whitney test). All units were mechanosensitive and had receptive fields localized on or near the left transverse sinus. Baseline mechanosensitivity measured using von Frey monofilaments ranged from 0.16–2 g/force (mean 0.52±0.09 g/force).
The effect of IL-1β
The effect of local administration of recombinant rat IL-1β was tested on 19 meningeal nociceptors (11 A delta and 9 C units). In preliminary studies IL-1β (PeproTech Inc., Rocky Hill, New Jersey, USA) was tested at doses of 1–100 ng/cc. Based on these as well as previous studies (9–11), neurons were further tested with 100 ng/cc, which is now reported. IL-1β promoted an increase in threshold responsiveness in 47% of the units (4 A delta, 5 C units, see an example in Figure 1), which was overall significant (p<0.01 Friedman's test). A significant increase in mechanosensitivity was noted already following 15 min of treatment, reached a peak at 30 min and remained heightened following 60 min of treatment (p<0.01 Wilcoxon signed-rank sum test, see Figure 2). Following administration of IL-1β, increased suprathreshold responsiveness was observed in 42% of the units (5 A delta, 3 C units), an effect that was significant overall during the application time (p<0.01 Friedman test). This increase in responsiveness was significant at 45 and 60 min of treatment (p<0.01 Wilcoxon signed-rank sum test). After 60 min of wash with SIF, the enhanced threshold and suprathreshold responses remained heightened when compared to baseline values (p<0.01 Wilcoxon signed-rank sum test).
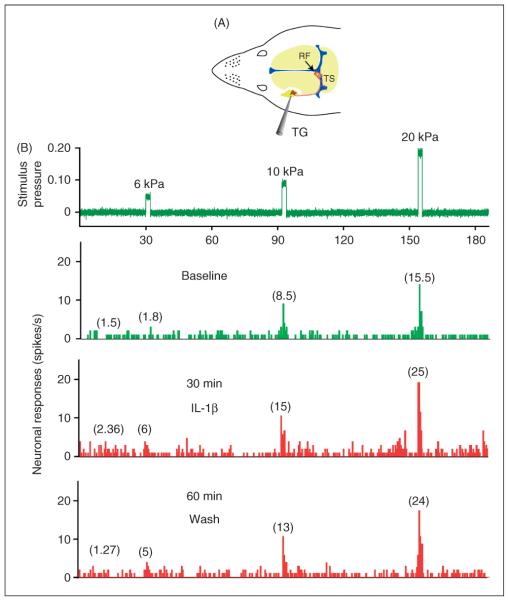
Figure 1.
Experimental arrangement and raw data example of the responses of one C-unit meningeal nociceptor to local application of IL-1β. (A) Schematic localization of the recording site for the meningeal nociceptors in the trigeminal ganglion (TG) and the mechanical receptive field (RF) of the recorded neuron on the left transverse sinus (TS). (B) Peri-stimulus time histograms (0.5 s beans) depicting the responses to threshold and suprathreshold mechanical stimulation of the unit's dural receptive field as well as ongoing discharge rates at baseline (green trace), and during the sensitization stage following 30 min of IL-1β administration and 60 min after wash with SIF (red traces). The numbers in parenthesis indicate mean spikes/s. Note the persistent sensitization to mechanical stimulation despite 60 min of wash. IL-1β: interleukin 1β, IL-6: interleukin 6, SIF: synthetic interstitial fluid.
Figure 2.
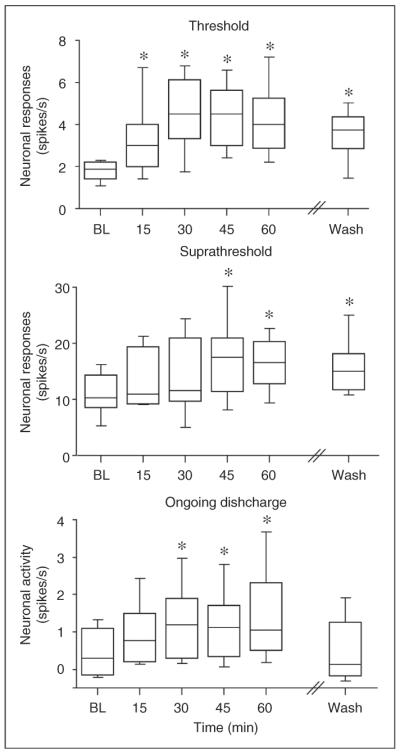
Effects of IL-1β on the mechanosensitivity and activity of meningeal nociceptors. The median and interquartile range of the responses to threshold and suprathreshold mechanical stimuli and the ongoing discharge levels at baseline, during IL-1β application and 60 min after wash with SIF, are shown. *p<0.01, Wilcoxon signed-rank test compared to baseline. BL: baseline, IL-1β: interleukin 1β, IL-6: interleukin 6, SIF: synthetic interstitial fluid.
Following application of IL-1β, increase in ongoing discharge was noted in 68% of the units (8 A delta, 5 C units) and was overall significant (p<0.01 Friedman test). The increase in ongoing discharge rate developed within 30 min of exposure to the cytokine and remained elevated during the treatment time (p<0.01 Wilcoxon signed-rank sum test). After 60 min of wash, the ongoing discharge level declined and was not statistically different than that observed during the baseline period.
The effect of IL-6
The effect of the local application of recombinant rat IL-6 was tested on 11 units (5 A delta and 6 C units). In initial experiments IL-6 (R&D Systems, Inc., Minneapolis, Minnesota, USA) was tested at doses of 1–100 ng/cc, based on these as well as previous nociceptor studies (10, 11). The highest dose was chosen to be further tested and is reported herein. IL-6 had a minimal effect on the mechanosensitivity of meningeal nociceptors and promoted an increase in threshold responsiveness in only three units (27%; 1 A delta, 2 C units; Figure 3). In these units, the maximal increase in firing rate was noted following 30 min of application in two units and following 60 min in another. In one-third of affected units the heightened responsiveness remained during the 60 min of wash with SIF. An increase in suprathreshold firing was noted in only two units (18%, 1 A delta, 1 C unit) and reached maximum values following 60 min of treatment. After 60 min of wash with SIF, the enhanced suprathreshold responses remained heightened in one unit and returned to baseline in another.
Figure 3.
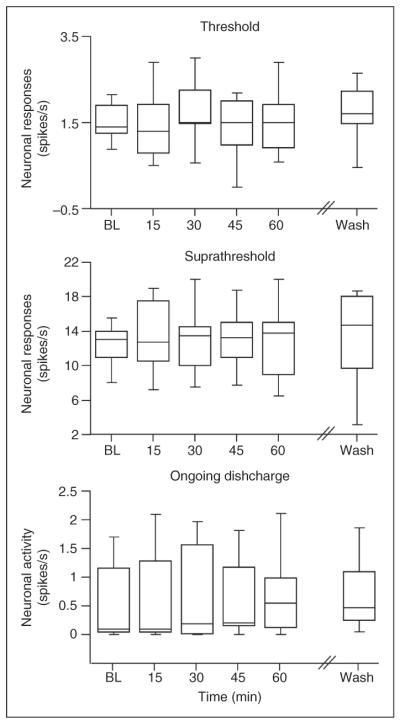
Lack of overall effect of topical application of IL-6 to the receptive field of meningeal nociceptors. The median and interquartile range of the responses to threshold and suprathreshold mechanical stimuli and the ongoing discharge levels at baseline, during IL-6 application and 60 min after wash with SIF, are shown. BL: baseline, IL-1β interleukin 1β, IL-6: interleukin 6, SIF: synthetic interstitial fluid.
The effect of local application of IL-6 on ongoing discharge level was also very minimal and was noted in only two units, (1 A delta and 1 C unit) following 30 or 45 min of application respectively. In these two units, the level of ongoing discharge returned to baseline level during the washout period.
Discussion
A major finding of this study was that local meningeal action of IL-1β led to an increase in the mechanosensitivity of meningeal nociceptors and promoted their activation. To the best of our knowledge this is the first evidence that IL-1β can mechanically sensitize and activate nociceptors in vivo, in particular those that innervate the intracranial meninges. Another important finding of this study was that IL-6, which like IL-1β is considered a pro-algesic cytokine in somatic tissues, had only a minimal effect on the mechanosensitivity of meningeal nociceptors as well as their ongoing discharge level.
The peripheral in vivo nociceptive action of IL-1β we observed is in agreement with previous in vitro electrophysiological studies (8,9) as well as its ability to promote mechanical pain hypersensitivity in animal models upon peripheral administration (9,18). The increase in plasma and internal jugular blood levels of this cytokine during migraine (6,19), together with our current findings, suggest that IL-1β may play a role in the cascade of cytokines that enhance the sensitivity of meningeal nociceptors, cause their activation and thus contribute to migraine headache.
With regard to its modulatory effect on the mechanosensitivity of meningeal nociceptors, IL-1β promoted an increase in both the threshold and suprathreshold responses. The throbbing painful sensation of many intracranial headaches is likely mediated by enhanced mechanosensitivity at the threshold level, by allowing vascular meningeal nociceptors to detect mechanical forces arising as a result of arterial pulsation. We therefore propose that IL-1β could play an important role in mediating this painful sensation. IL-1β-mediated enhanced suprathreshold responses could play a role in mediating the exacerbation of the headache during activities that transiently increase intracranial pressure such as coughing and bending over.
The effect of IL-1β on the threshold responses was achieved relatively fast, following 15 minutes of exposure. This suggests that IL-1β promoted this effect by acting directly on the nerve endings of meningeal nociceptors. Based on recent in vitro data, one potential cellular mechanism that could have mediated this rapid nociceptive response is the enhancement of persistent tetrodotoxin (TTX)-resistant currents near threshold by IL-1β (9). The relatively slower increase in suprathreshold responses (following approximately 45 min of treatment) suggests that this sensory change requires an additional neural process, for example the modulation of ionic currents that underlie sustained neural firing such as hyperpolarization-activated cation currents (20). Whether IL-1β can modulate these ionic currents or promote changes in suprathreshold responses through a different mechanism will require further studies.
The excitatory effect of IL-1β on meningeal nociceptors (i.e. an increase in ongoing discharge level) was even more pronounced than its effect on mechanosensitivity. An increase in ongoing discharge rate is of particular importance because of its contribution to the development of central sensitization of second-order trigeminal dorsal horn neurons. This mechanism is likely to play a role in mediating the long duration of migraine headache as well as the development of cephalic allodynia (21). Future studies will be required to determine whether the level of meningeal nociceptor activity evoked by IL-1β is sufficient to promote such central nociceptive changes.
Of note is that, unlike the changes in mechanosensitivity, the increase in ongoing discharge rate evoked by IL-1β decreased and returned to baseline level after washout. This suggests that the mechanisms by which IL-1β promotes mechanosensitization and activation are likely distinct. For example, it is possible that while changes in mechanosensitivity are mediated by sustained molecular changes that occur at the level of the nociceptor receptive field within the meninges, the increase in ongoing discharge is due to a secondary, IL-1β-evoked, release of algesic mediators from other meningeal constituents such as blood vessels. The return of the ongoing discharge to baseline level after the wash period indicates nevertheless that these mediators, if released, are capable of promoting only an acute nociceptive response.
Previous in vitro (10,12) and in vivo (11) electrophysiological studies of somatic nociceptors documented the sensitizing effects of IL-6 to heat. The ability of IL-6 to promote mechanical sensitization of somatic nociceptors has never been tested, although the induction of mechanical pain hypersensitivity following intradermal or intramuscular administration of IL-6 (22,23) suggests so. Our study, however, indicates that in vivo the ability of IL-6 to increase the mechanosensitivity of meningeal nociceptors as well as promote their activation (i.e. an increase in ongoing discharge rate) is very minor. It is unlikely that the minimal action of IL-6 we documented was due to a dose issue because in previous studies IL-6 modulated nociceptors' responsiveness at similar and even lower doses.
One potential difference that may account for the minimal action we observed is a relative lower sensitivity of meningeal nociceptors to IL-6 compared with that of somatic nociceptors. This may be due to a lower-level meningeal expression of factors that are required for the sensitizing action of IL-6, for example the soluble IL-6 receptor, the neuronal IL-6 receptor glycoprotein 130 (gp130) (11–13,24) or the eukaryotic initiation factor 4 E and its binding protein (23). Despite its potential involvement in mediating the pain associated with chronic inflammatory conditions such as rheumatoid arthritis (25), the poor modulatory effect of IL-6 on meningeal nociceptors' response properties suggests that peripheral meningeal action of IL-6 may not play an important role in mediating migraine headache.
In summary, our data suggest that IL-1β can promote a powerful activation and mechanical sensitization of meningeal nociceptors while IL-6 generally lacks such an effect. Future studies are required to examine the mechanism that plays a role in mediating these nociceptive of IL-1β, which may be a target for migraine therapy.
Acknowledgments
Funding The study was supported by NIH grants NS046502 and NS061116 to D.L. and the National Headache Foundation.
Footnotes
Conflict of interest declaration The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Strassman AM, Raymond SA. On the origin of headaches. Endeavour. 1997;21:97–100. doi: 10.1016/s0160-9327(97)80216-5. [DOI] [PubMed] [Google Scholar]
- 2.Messlinger K. Migraine: where and how does the pain originate? Exp Brain Res. 2009;196:179–193. doi: 10.1007/s00221-009-1756-y. [DOI] [PubMed] [Google Scholar]
- 3.Olesen J, Burstein R, Ashina M, et al. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8:679–690. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- 4.Levy D. Migraine pain and nociceptor activation—where do we stand? Headache. 2010;50:909–916. doi: 10.1111/j.1526-4610.2010.01670.x. [DOI] [PubMed] [Google Scholar]
- 5.Levy D. Migraine pain, meningeal inflammation, and mast cells. Curr Pain Headache Rep. 2009;13:237–240. doi: 10.1007/s11916-009-0040-y. [DOI] [PubMed] [Google Scholar]
- 6.Sarchielli P, Alberti A, Baldi A, et al. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache. 2006;46:200–207. doi: 10.1111/j.1526-4610.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang XC, Kainz V, Burstein R, et al. Tumor necrosis factor- α induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions. Pain. 2011;152:140–149. doi: 10.1016/j.pain.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obreja O, Rathee PK, Lips KS, et al. IL-1 beta potentiates heat-activated currents in rat sensory neurons: involvement of IL-1RI, tyrosine kinase, and protein kinase C. FASEB J. 2002;16:1497–1503. doi: 10.1096/fj.02-0101com. [DOI] [PubMed] [Google Scholar]
- 9.Binshtok AM, Wang H, Zimmermann K, et al. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oprée A, Kress M. Involvement of the proinflammatory cytokines tumor necrosis factor-alpha, IL-1 beta, and IL-6 but not IL-8 in the development of heat hyperalgesia: effects on heat-evoked calcitonin gene-related peptide release from rat skin. J Neurosci. 2000;20:6289–6293. doi: 10.1523/JNEUROSCI.20-16-06289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obreja O, Schmelz M, Poole S, et al. Interleukin-6 in combination with its soluble IL-6 receptor sensitises rat skin nociceptors to heat, in vivo. Pain. 2002;96:57–62. doi: 10.1016/s0304-3959(01)00420-1. [DOI] [PubMed] [Google Scholar]
- 12.Obreja O, Biasio W, Andratsch M, et al. Fast modulation of heat-activated ionic current by proinflammatory interleukin 6 in rat sensory neurons. Brain. 2005;128:1634–1641. doi: 10.1093/brain/awh490. [DOI] [PubMed] [Google Scholar]
- 13.Andratsch M, Mair N, Constantin CE, et al. A key role for gp130 expressed on peripheral sensory nerves in pathological pain. J Neurosci. 2009;29:13473–13483. doi: 10.1523/JNEUROSCI.1822-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Strassman AM, Burstein R, et al. Sensitization and activation of intracranial meningeal nociceptors by mast cell mediators. J Pharmacol Exp Ther. 2007;322:806–812. doi: 10.1124/jpet.107.123745. [DOI] [PubMed] [Google Scholar]
- 16.Levy D, Zhang XC, Jakubowski M, et al. Sensitization of meningeal nociceptors: inhibition by naproxen. Eur J Neurosci. 2008;27:917–922. doi: 10.1111/j.1460-9568.2008.06068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy D, Strassman AM. Mechanical response properties of A and C primary afferent neurons innervating the rat intracranial dura. J Neurophysiol. 2002;88:3021–3031. doi: 10.1152/jn.00029.2002. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira SH, Lorenzetti BB, Bristow AF, et al. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- 19.Perini F, D'Andrea G, Galloni E, et al. Plasma cytokine levels in migraineurs and controls. Headache. 2005;45:926–931. doi: 10.1111/j.1526-4610.2005.05135.x. [DOI] [PubMed] [Google Scholar]
- 20.Ingram SL, Williams JT. Modulation of the hyperpolarization-activated current (Ih) by cyclic nucleotides in guinea-pig primary afferent neurons. J Physiol. 1996;492(Pt 1):97–106. doi: 10.1113/jphysiol.1996.sp021292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burstein R. Deconstructing migraine headache into peripheral and central sensitization. Pain. 2001;89:107–110. doi: 10.1016/s0304-3959(00)00478-4. [DOI] [PubMed] [Google Scholar]
- 22.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152:521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melemedjian OK, Asiedu MN, Tillu DV, et al. IL-6- and NGF-induced rapid control of protein synthesis and nociceptive plasticity via convergent signaling to the eIF4F complex. J Neurosci. 2010;30:15113–15123. doi: 10.1523/JNEUROSCI.3947-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quarta S, Vogl C, Constantin CE, et al. Genetic evidence for an essential role of neuronally expressed IL-6 signal transducer gp130 in the induction and maintenance of experimentally induced mechanical hypersensitivity in vivo and in vitro. Mol Pain. 2011;7:73. doi: 10.1186/1744-8069-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimoto N, Miyasaka N, Yamamoto K, et al. Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis. 2009;68:1580–1584. doi: 10.1136/ard.2008.092866. [DOI] [PMC free article] [PubMed] [Google Scholar]