Abstract
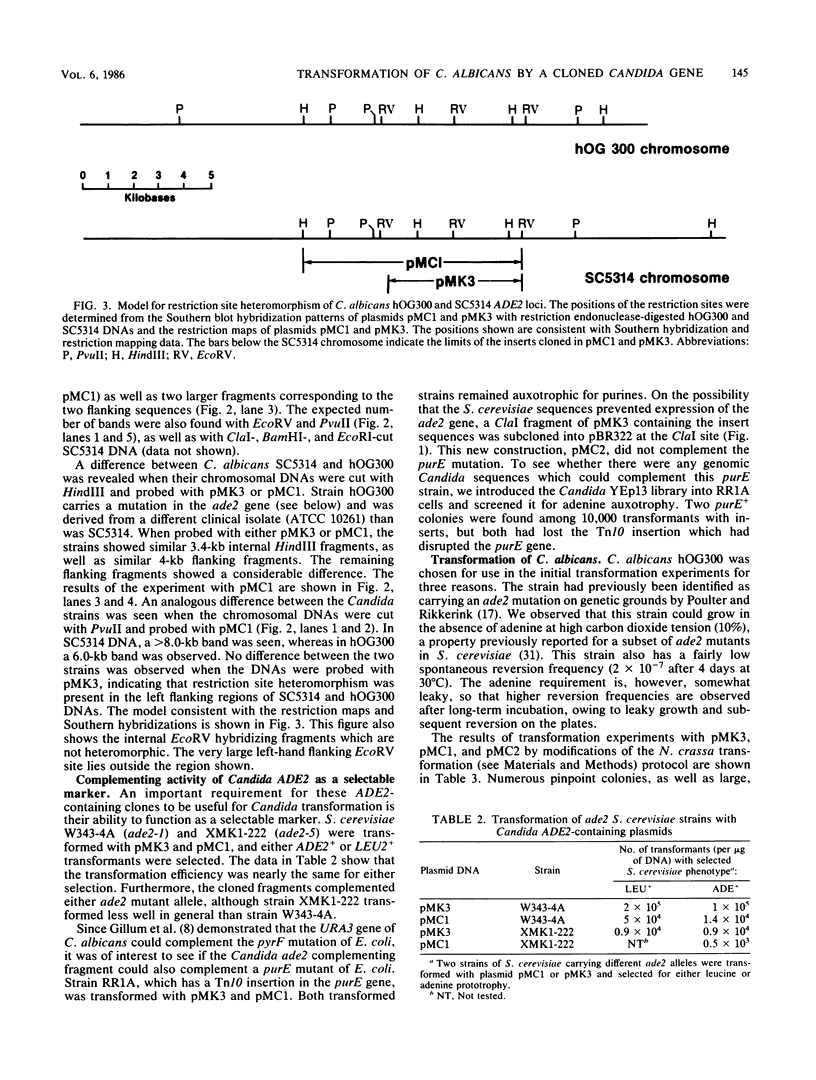
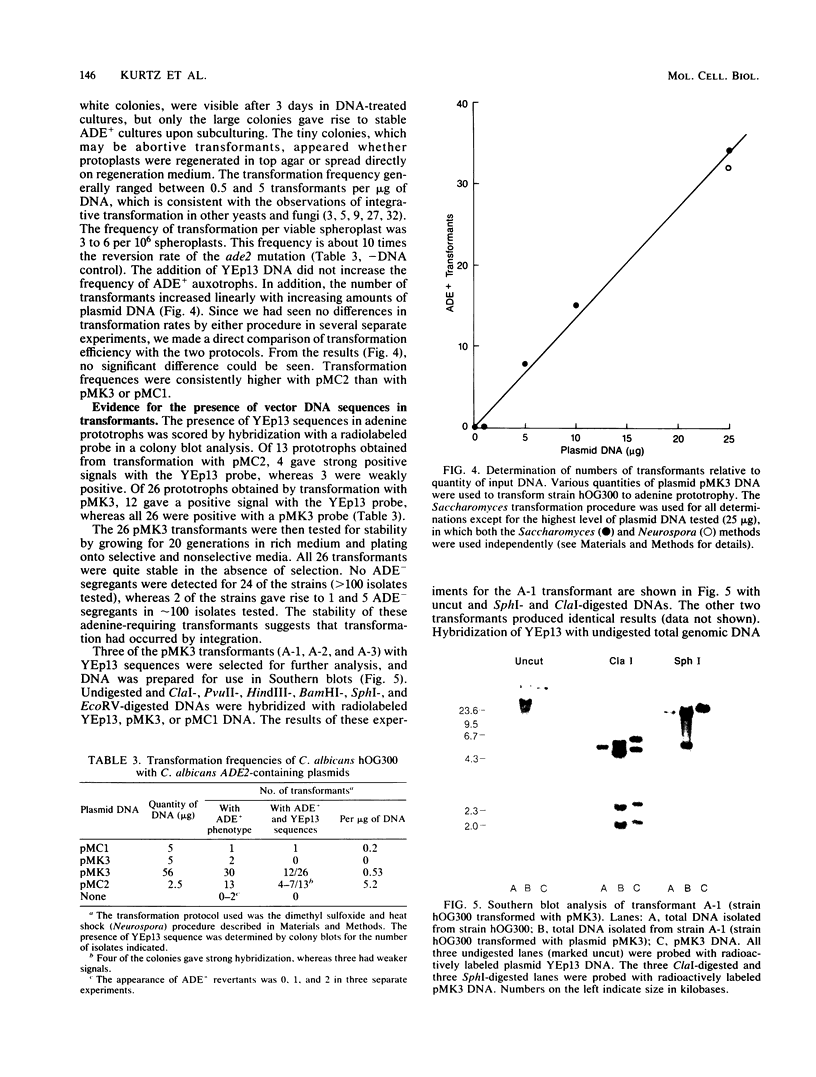
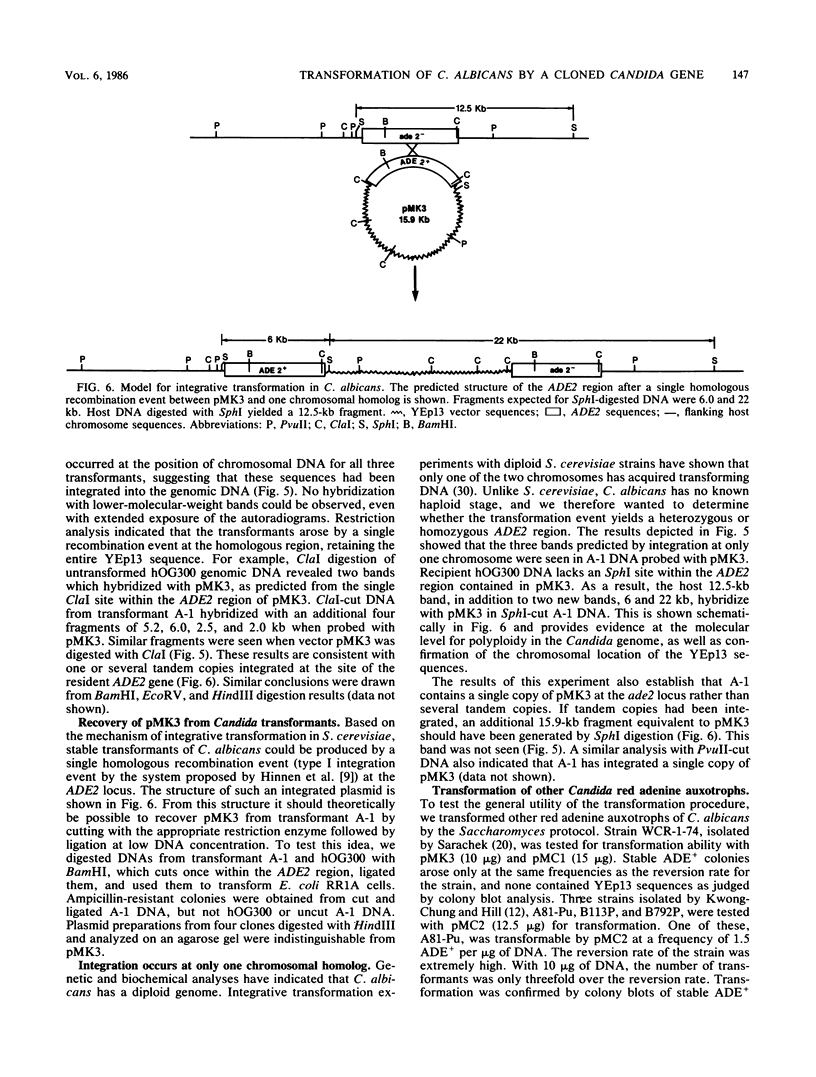
Candida albicans is a diploid dimorphic yeast with no known sexual cycle. The development of a DNA transformation system would greatly improve the prospects for genetic analyses of this yeast. Plasmids were isolated from a Candida Sau3A partial library which complements the ade2-1 and ade2-5 mutations in Saccharomyces cerevisiae. These plasmids contain a common region, part of which, when subcloned, produces ade2 complementation. Among the small number of auxotrophs previously isolated in C. albicans, red adenine-requiring mutants had been identified by several groups. In two of these strains, the cloned Candida DNA transformed the mutants to ADE+ at frequencies of 0.5 to 5 transformants per micrograms of DNA. In about 50% of the transformants, plasmid DNA sequences became stably integrated into the host genome and, in the several cases analyzed by Southern hybridization, the DNA was integrated at the site of the ADE2 gene in one of the chromosomal homologs.
Full text
PDF
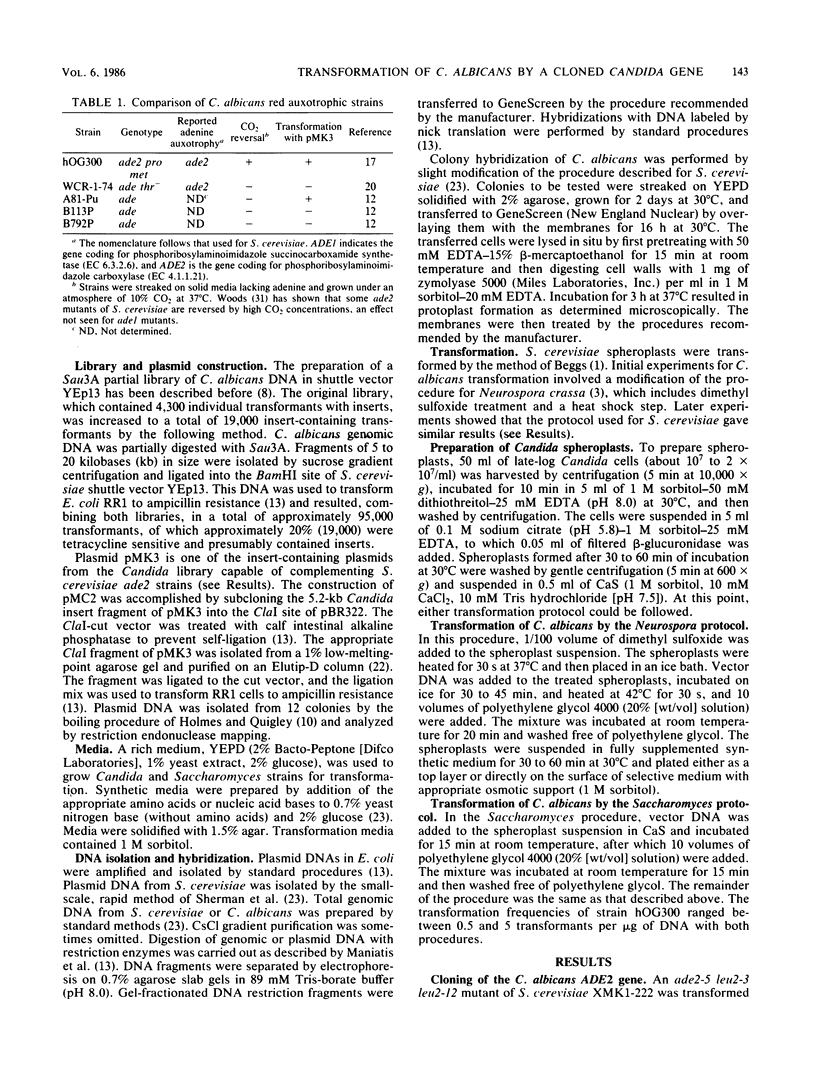
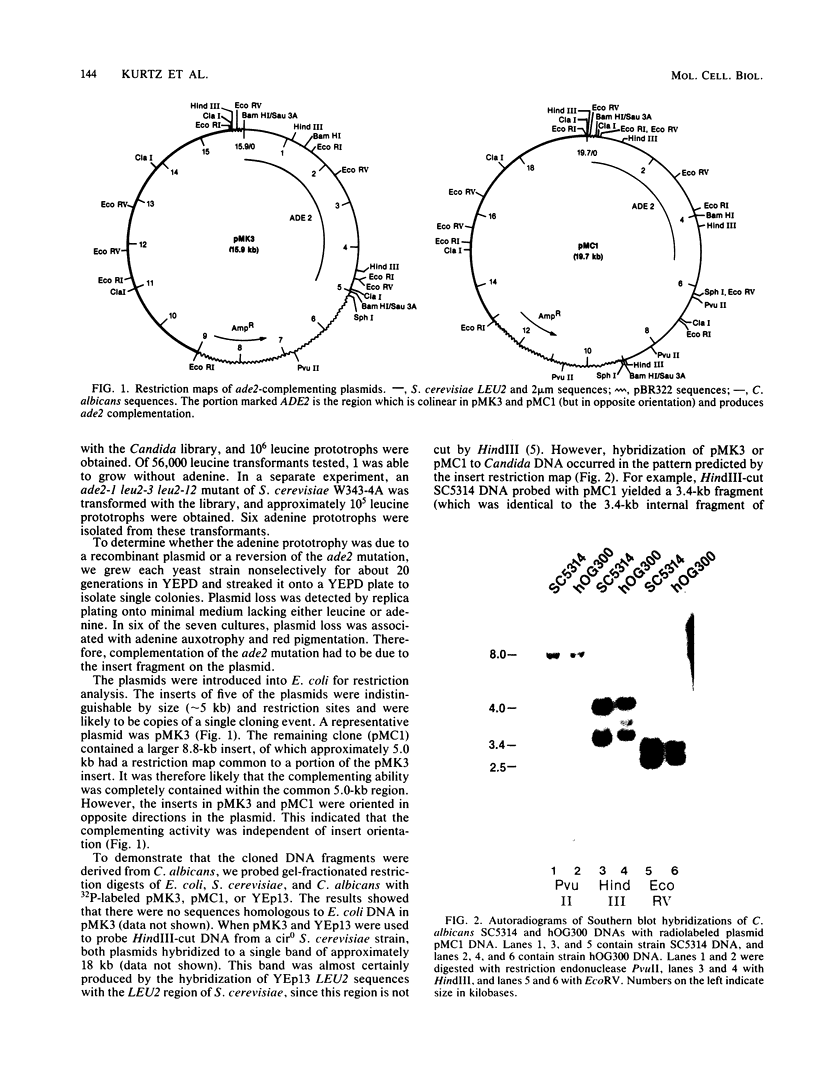


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chung K. J., Hill W. B. Studies on the pink, adenine-deficient strains of Candida albicans. I. Cultural and morphological characteristics. Sabouraudia. 1970 May;8(1):48–59. [PubMed] [Google Scholar]
- Das S., Kellermann E., Hollenberg C. P. Transformation of Kluyveromyces fragilis. J Bacteriol. 1984 Jun;158(3):1165–1167. doi: 10.1128/jb.158.3.1165-1167.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher C. R. Enzymology of the pigmented adenine-requiring mutants of Saccharomyces and Schizosaccharomyces. Biochem Biophys Res Commun. 1969 Feb 7;34(3):306–310. doi: 10.1016/0006-291x(69)90832-8. [DOI] [PubMed] [Google Scholar]
- Fisher C. R. Phosphoribosyl-aminoimidazole-succinocarboxamide synthetase from Neurospora crassa. I. Partial purification and properties. Biochim Biophys Acta. 1969 Apr 22;178(2):380–388. doi: 10.1016/0005-2744(69)90406-9. [DOI] [PubMed] [Google Scholar]
- Gillum A. M., Tsay E. Y., Kirsch D. R. Isolation of the Candida albicans gene for orotidine-5'-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198(2):179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Olaiya A. F., Sogin S. J. Ploidy determination of Canadida albicans. J Bacteriol. 1979 Dec;140(3):1043–1049. doi: 10.1128/jb.140.3.1043-1049.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter R. T., Rikkerink E. H. Genetic analysis of red, adenine-requiring mutants of Candida albicans. J Bacteriol. 1983 Dec;156(3):1066–1077. doi: 10.1128/jb.156.3.1066-1077.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter R., Hanrahan V., Jeffery K., Markie D., Shepherd M. G., Sullivan P. A. Recombination analysis of naturally diploid Candida albicans. J Bacteriol. 1982 Dec;152(3):969–975. doi: 10.1128/jb.152.3.969-975.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggsby W. S., Torres-Bauza L. J., Wills J. W., Townes T. M. DNA content, kinetic complexity, and the ploidy question in Candida albicans. Mol Cell Biol. 1982 Jul;2(7):853–862. doi: 10.1128/mcb.2.7.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M., Grisafi P., Botstein D. Structure and function of the yeast URA3 gene: expression in Escherichia coli. Gene. 1984 Jul-Aug;29(1-2):113–124. doi: 10.1016/0378-1119(84)90172-0. [DOI] [PubMed] [Google Scholar]
- SARACHEK A. PROMOTION OF RETARDATION OF THE GROWTH OF ADENINE AUXOTROPHS OF CANDIDA ALBICANS BY PURINES, PYRIMIDINES AND NUCLEOSIDES. Antonie Van Leeuwenhoek. 1964;30:289–302. doi: 10.1007/BF02046735. [DOI] [PubMed] [Google Scholar]
- Schmitt J. J., Cohen B. N. Quantitative isolation of DNA restriction fragments from low-melting agarose by Elutip-d affinity chromatography. Anal Biochem. 1983 Sep;133(2):462–464. doi: 10.1016/0003-2697(83)90109-4. [DOI] [PubMed] [Google Scholar]
- Silver J. M., Eaton N. R. Functional blocks of the ad-1 and ad-2 mutants of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1969 Feb 7;34(3):301–305. doi: 10.1016/0006-291x(69)90831-6. [DOI] [PubMed] [Google Scholar]
- Sreekrishna K., Webster T. D., Dickson R. C. Transformation of Kluyveromyces lactis with the kanamycin (G418) resistance gene of Tn903. Gene. 1984 Apr;28(1):73–81. doi: 10.1016/0378-1119(84)90089-1. [DOI] [PubMed] [Google Scholar]
- Tilburn J., Scazzocchio C., Taylor G. G., Zabicky-Zissman J. H., Lockington R. A., Davies R. W. Transformation by integration in Aspergillus nidulans. Gene. 1983 Dec;26(2-3):205–221. doi: 10.1016/0378-1119(83)90191-9. [DOI] [PubMed] [Google Scholar]
- Whelan W. L., Markie D. M., Simpkin K. G., Poulter R. M. Instability of Candida albicans hybrids. J Bacteriol. 1985 Mar;161(3):1131–1136. doi: 10.1128/jb.161.3.1131-1136.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Partridge R. M., Magee P. T. Heterozygosity and segregation in Candida albicans. Mol Gen Genet. 1980;180(1):107–113. doi: 10.1007/BF00267358. [DOI] [PubMed] [Google Scholar]
- Winston F., Chumley F., Fink G. R. Eviction and transplacement of mutant genes in yeast. Methods Enzymol. 1983;101:211–228. doi: 10.1016/0076-6879(83)01016-2. [DOI] [PubMed] [Google Scholar]
- Woods R. A. Response of ad-2 mutants of Saccharomyces cerevisiae to carbon dioxide. Mol Gen Genet. 1969;105(4):314–316. doi: 10.1007/BF00277586. [DOI] [PubMed] [Google Scholar]
- Yelton M. M., Hamer J. E., Timberlake W. E. Transformation of Aspergillus nidulans by using a trpC plasmid. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1470–1474. doi: 10.1073/pnas.81.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]