Abstract
Evidence suggests that both the arterial baroreflex and vestibulosympathetic reflex contribute to blood pressure regulation, and both autonomic reflexes integrate centrally in the medulla cardiovascular center. A previous report indicated increased sympathetic baroreflex sensitivity during the midluteal (ML) phase of the menstrual cycle compared with the early follicular (EF) phase. On the basis of this finding, we hypothesize an augmented vestibulosympathetic reflex during the ML phase of the menstrual cycle. Muscle sympathetic nerve activity (MSNA), mean arterial pressure (MAP), and heart rate responses to head-down rotation (HDR) were measured in 10 healthy females during the EF and ML phases of the menstrual cycle. Plasma estradiol (Δ72 ± 13 pg/ml, P < 0.01) and progesterone (Δ8 ± 2 ng/ml, P < 0.01) were significantly greater during the ML phase compared with the EF phase. The menstrual cycle did not alter resting MSNA, MAP, and heart rate (EF: 13 ± 3 bursts/min, 80 ± 2 mmHg, 65 ± 2 beats/min vs. ML: 14 ± 3 bursts/min, 81 ± 3 mmHg, 64 ± 3 beats/min). During the EF phase, HDR increased MSNA (Δ3 ± 1 bursts/min, P < 0.02) but did not change MAP or heart rate (Δ0 ± 1 mmHg and Δ1 ± 1 beats/min). During the ML phase, HDR increased both MSNA and MAP (Δ4 ± 1 bursts/min and Δ3 ± 1 mmHg, P < 0.04) with no change in heart rate (Δ0 ± 1 beats/min). MSNA and heart rate responses to HDR were not different between the EF and ML phases, but MAP responses to HDR were augmented during the ML phase (P < 0.03). Our results demonstrate that the menstrual cycle does not influence the vestibulosympathetic reflex but appears to alter MAP responses to HDR during the ML phase.
Keywords: muscle sympathetic nerve activity, arterial blood pressure, otolith stimulation, head-down rotation, estrogen
fluctuations in female endogenous sex hormones have been linked to baroreflex sensitivity variation (19). Minson et al. (19) reported an augmented sympathetic baroreflex sensitivity during the midluteal (ML) phase of the menstrual cycle compared with the early follicular (EF) phase in young, healthy women. A change in baroreflex sensitivity suggests that estrogen and progesterone may contribute importantly to acute blood pressure regulation in females.
Another autonomic reflex that appears to contribute to the maintenance of arterial blood pressure regulation during orthostasis is the vestibulosympathetic reflex. Doba and Reis (4) demonstrated that transection of the vestibular nerve (VIII cranial nerve) severely attenuated arterial blood pressure during a nose-up tilt in anesthetized cats. Jian et al. (12) reported similar findings in awake cats. Evidence suggests that the attenuation of arterial blood pressure during nose-up tilt after transection of the vestibular nerve may be due to inadequate sympathetic responses (14–16). In humans, head-down rotation (HDR) increases sympathetic neural traffic to the skeletal muscle (30). This maneuver activates gravity-sensitive receptors in the vestibule (i.e., otolith organs) without loading or unloading the baroreflex (23, 30). Furthermore, the latency of the vestibulosympathetic reflex appears to be shorter than that of the baroreflex and thus may be the earliest mechanism to sustain blood pressure during orthostasis (13). Collectively, these studies indicate that the vestibulosympathetic reflex may serve as a feed-forward mechanism in blood pressure regulation.
In a retrospective study, Ray (22) reported no difference in the vestibulosympathetic reflex between men and women, but menstrual cycle phase was not controlled for in the female subjects. Therefore, the purpose of the current study was to monitor neural and cardiovascular responses to HDR during the EF (low estrogen and low progesterone) and ML (high estrogen and high progesterone) phases of the menstrual cycle. On the basis of an augmented sympathetic baroreflex during the ML phase (19), we hypothesized that fluctuations in reproductive hormones would alter the vestibulosympathetic reflex, resulting in augmented muscle sympathetic nerve activity (MSNA) during the ML phase.
METHODS
Subjects.
Ten healthy women (age 26 ± 1 yr, height 163 ± 3 cm, weight 62 ± 2 kg) participated in the study. All subjects were nonsmokers, nondiabetics, and not taking oral contraceptives and/or other hormonal supplementations. They were all instructed to abstain from exercise and caffeine for 12 h prior to laboratory testing. Only subjects with consistent and regular menstrual cycles were included in the study (i.e., 26–30 days in length). The Michigan Technological University Human Subjects Committee approved the experimental protocols, and all participants gave written informed consent.
Experimental design.
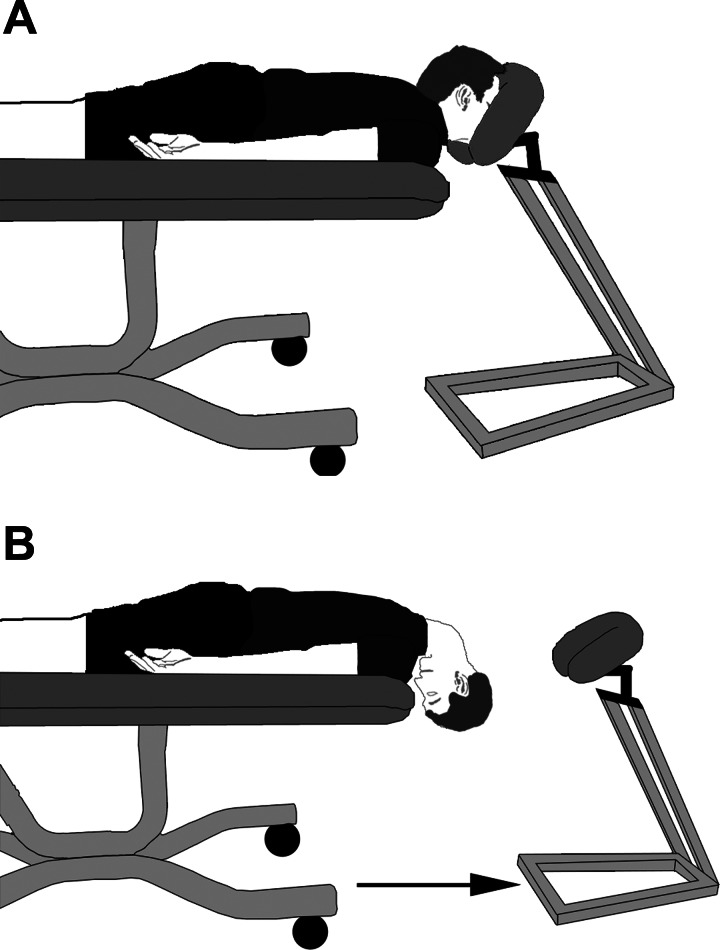
Subjects were tested once during the EF phase (3 ± 0 days after start of menstruation) and again during the ML phase (22 ± 1 days after start of menstruation). The phase of the menstrual cycle was randomized. Four subjects began the study during the EF phase, and six subjects began the study during the ML phase. On the days of testing, the subjects reported for a blood draw to document levels of estradiol and progesterone. All subjects were tested during the same time of day for both trials. The experiment was conducted with subjects lying prone, and subjects were adjusted so that the neck could maximally flex without interference from the table. The neck was then fully extended with a face rest supporting the head to approximate a position of normal gravitational orientation experienced during upright posture (Fig. 1). Baseline values were recorded in this position for 3 min. Following baseline the face rest was removed, and HDR was performed for 3 min. During HDR, the neck was passively flexed (the head moved toward the floor and the chin moved toward the chest). MSNA, heart rate (HR), and beat-to-beat arterial blood pressure were acquired throughout the experiment.
Fig. 1.
Illustration of the head-down rotation (HDR) model. A: the subject's forehead is supported and the neck is fully extended during baseline to approximate the normal gravitational orientation of the otolith organs during upright posture. B: during HDR, the face rest is removed and the head is passively rotated by an investigator (chin to chest). This maneuver stimulates the otolith organs and allows for examination of the vestibulosympathetic reflex.
Measurements.
Multifiber recordings of MSNA were made by inserting a tungsten microelectrode into the peroneal nerve in the popliteal region behind the knee. A reference electrode was inserted subcutaneously 2–3 cm from the recording electrode. Both electrodes were connected to a differential preamplifier and then to an amplifier (total gain of 80,000), where the nerve signal was band-pass filtered (700–2,000 Hz) and integrated (time constant 0.1) to obtain a mean voltage display of the nerve activity. Satisfactory recordings of MSNA were defined by spontaneous pulse synchronous bursts that increased during end-expiratory apnea and did not change during stroking of the skin or auditory stimulation (yell).
Arterial blood pressure was measured using two techniques. Resting arterial blood pressure was measured three consecutive times (separated by 1-min intervals) with an automated sphygmomanometer and reported as a mean value. Beat-to-beat arterial blood pressure was recorded continuously via Finometer (Finapres Medical Systems, Amsterdam, The Netherlands). The Finometer allowed us to determine precise changes in blood pressure that occur during the interventions, whereas the sphygmomanometer allowed us to compare baseline arterial pressure during the EF and ML phases. Continuous HR was recorded using a three-lead electrocardiogram. We were unsuccessful at reproducing MSNA recordings over multiple days for one subject, and thus nine subjects are reported for resting MSNA. Eight subjects are reported for head-down rotation MSNA, as the nerve signal was lost during the head-down rotation in one subject.
Data analysis.
Data were imported and analyzed in a software program (WinCPRS; Absolute Aliens, Turku, Finland) on a Windows-formatted computer. R waves were detected and marked in the time series. Muscle sympathetic bursts were automatically detected on the basis of amplitude using a signal-to-noise ratio of 3:1; within 0.5 s, a search window centered on the 1.3-s expected burst peak latency from the previous R wave. Potential bursts were displayed and edited by one trained investigator. The integral of all sympathetic bursts occurring during the initial 3-min baseline period was calculated and divided by the number of bursts to derive an average control burst area that could be compared across time. Subsequent bursts that were equal to average bursts occurring during baseline were assigned a value of 100. MSNA was expressed as bursts/min and total burst activity (total activity = total number of bursts × the averaged normalized burst area).
All data were analyzed statistically using commercial software (SPSS 15.0; SPSS, Chicago, IL). A two-way repeated-measures ANOVA was calculated for changes in MSNA (bursts/min and total activity), mean arterial pressure (MAP), and HR during each intervention (baseline and head-down rotation) and across trials (menstrual cycle phases). Protected dependent t-test statistics were calculated for post hoc analyses. Resting variables were compared using paired t-tests. Means were considered significantly different when P < 0.05, and results are expressed as means ± SE.
RESULTS
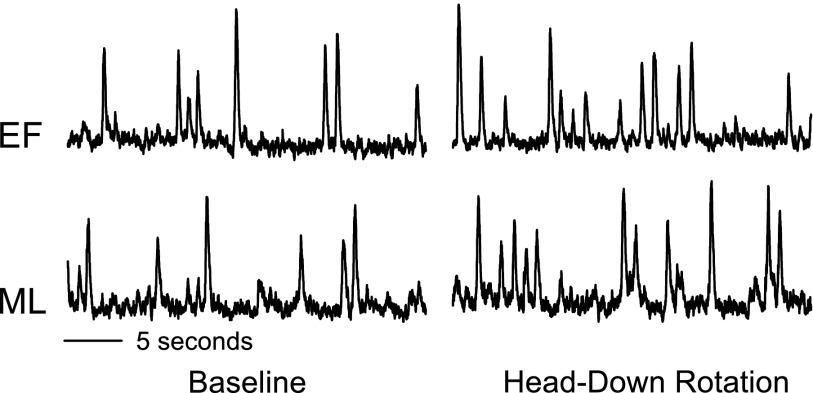
Resting baseline values during the EF and ML phases are presented in Table 1. The levels of plasma estradiol and progesterone were significantly greater during the ML phase compared with the EF phase (P < 0.01). Resting MSNA, MAP, and HR were not different between the EF and ML phases. Representative neurograms of MSNA during the EF and ML phases are illustrated in Fig. 2.
Table 1.
Baseline values for the EF and ML phases of the menstrual cycle
| Variable | EF | ML | P Value |
|---|---|---|---|
| Estrogen, pg/ml | 33±3 | 105±13* | 0.001 |
| Progesterone, ng/ml | 1.09±0.11 | 8.68±2.06* | 0.002 |
| MSNA, bursts/min | 13±2 | 14±3 | 0.799 |
| MAP, mmHg | 80±2 | 81±3 | 0.594 |
| HR, beats/min | 65±2 | 64±3 | 0.360 |
Values are means ± SE. EF, early follicular; ML, midluteal; MSNA, muscle sympathetic nerve activity; MAP, mean arterial blood pressure; HR, heart rate.
Values are significantly different from EF phase.
Fig. 2.
Representative neurograms obtained from 1 subject during baseline and HDR of the early follicular (EF) and midluteal (ML) phases of the menstrual cycle.
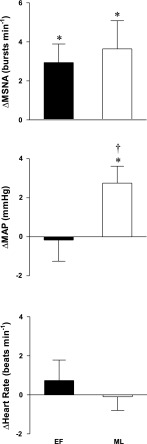
HDR did not change HR during either the EF (65 ± 2 to 65 ± 2 beats/min) or ML (64 ± 3 to 64 ± 2 beats/min) phases. HDR did not change MAP from baseline during the EF phase (Δ0 ± 1 mmHg). However, during the ML phase, MAP increased during HDR (Δ3 ± 1 mmHg, P < 0.02). MAP during HDR of the ML phase was significantly different compared with the EF phase (interaction, P < 0.03; Fig. 3). HDR increased MSNA during the EF (13 ± 3 to 16 ± 2 bursts/min, P < 0.02) and ML (14 ± 3 to 18 ± 2 bursts/min, P < 0.04) phases, and these increases were of similar magnitude (Fig. 3). Similarly, the increases in total MSNA during HDR were not different between the two phases (EF: Δ515 ± 220 arbitrary units, and ML: Δ869 ± 610 arbitrary units; interaction, P > 0.58).
Fig. 3.
Changes in muscle sympathetic nerve activity (ΔMSNA), heart rate, and mean arterial pressure (ΔMAP) during HDR. Subjects were examined during the EF and ML phases of the menstrual cycle. HDR increased MSNA similarly during the EF and ML phases but increased MAP only during the ML phase. Values are reported as means ± SE. *P < 0.05 vs. baseline; †P < 0.05 vs. corresponding EF value.
DISCUSSION
This study investigated the neural and cardiovascular responses to HDR during the EF and ML phases of the menstrual cycle. We hypothesized that HDR during the ML phase would elicit an augmented vestibulosympathetic reflex, based on hypersensitivity of the baroreflex during this phase (19). Although we failed to observe an augmented vestibulosympathetic reflex during the ML phase, our results reveal an augmented MAP response to HDR when levels of progesterone and estrogen are elevated. Because HDR elicited similar increases in MSNA, this finding suggests an increase in sympathetic transduction to the vascular smooth muscle during the ML phase.
Neural and cardiovascular responses to HDR are well documented. HDR consistently increases MSNA in humans, a response commonly referred to as the vestibulosympathetic reflex (2, 30). In contrast, HDR elicits variable MAP responses. In young healthy adults, HDR does not typically change MAP, but some studies report an increase in MAP during HDR (10, 30). In older healthy adults, HDR elicits a decrease in MAP (24). Ray and Monahan (24) have suggested that the failure to maintain or increase MSNA during HDR in the elderly, coupled with the increased prevalence of orthostatic intolerance in the elderly (27), indicates that the vestibulosympathetic reflex may contribute importantly to arterial blood pressure regulation in humans.
Another population susceptible to orthostatic intolerance is women (3, 6, 8, 20, 26, 29, 36). Ray (22) reported no differences in MSNA or MAP responses to HDR between men and women, but one limitation of the study was a failure to control for the menstrual cycle in the female subjects. In the current study, we controlled for the effects of sex hormones at two specific time points of the menstrual cycle, and HDR elicited similar increases in MSNA during the EF (low estrogen, low progesterone) and ML (high estrogen, high progesterone) phases. However, HDR increased MAP during the ML phase but not the EF phase.
Administering estrogen through hormone therapy decreases norepinephrine spillover and vasoconstriction (32) and enhances nitric oxide release in the forearm vasculature (33). Decreasing vasoconstriction and increasing nitric oxide dilates the vasculature, resulting in a decrease of vascular resistance. Thus, a rise in estrogen could potentially provoke vasodilation during the ML phase of the menstrual cycle and trigger a corresponding drop in MAP. However, our study revealed no change in resting MAP and an increase in MAP during HDR of the ML phase. These findings suggest that the vasodilatory effects of estrogen are being masked, possibly by progesterone, which is also at higher levels during the ML phase of the menstrual cycle. Sharkey et al. (28) demonstrated an increase of blood pressure with progesterone supplementation in animals. Therefore, it is possible that there is an increased vascular resistance during HDR due to the increased progesterone that may counteract estrogen's effects on vascular smooth muscle and result in an augmented MAP.
Another possible explanation for our findings is an increased sympathetic transduction to vascular smooth muscle during the ML phase of the menstrual cycle. Increased sympathetic transduction could reflect an increased neurotransmitter release for a given amount of MSNA or greater sensitivity of the vasculature to the given amount of neurotransmitter release. Increases in norepinephrine spillover (31) and MSNA (25) during baroreceptor unloading appear to be greater in black adults compared with white adults, suggesting differences in vascular transduction among races. These studies (25, 31) suggest that vascular transduction is altered by race, but it is unclear whether vascular transduction is altered by sex. Minson et al. (19) observed no differences in vascular responsiveness for a given level of sympathetic outflow during the EF and ML phases of the menstrual cycle but suggested that some forms of sympathoexcitatory maneuvers may be too complex to detect any significant changes between cycle phases. Our study revealed a differential MAP response to HDR during the EF and ML phases but no difference in the MSNA response to HDR between phases. We did not measure vascular blood flow, but it is possible that the augmented MAP response to HDR during the ML phase may be due to increased sympathetic transduction to the vascular smooth muscle.
One proposed mechanism for increased vascular smooth muscle responsiveness is through increased α2C-adrenoceptor expression on vascular smooth muscle cells during periods of high estrogen (5). Eid et al. (5) reported that the treatment of 17β-estradiol on human cultured vascular muscle cells interacted with cell surface receptors to cause an increase in α2C-adrenoceptor transcription. Thus, the activation of the vestibulosympathetic reflex may elicit differences in vascular transduction at times of low and high estrogen due to the expression of postsynaptic α2-adrenoceptors on the smooth muscle cells. If the expression of α2-adrenoceptors is greater during the ML phase, the response to the vestibulosympathetic reflex would result in an increase in vascular resistance. An increase in vascular resistance could contribute to the augmentation of MAP during HDR in the ML phases of the menstrual cycle.
Studies examining MSNA and arterial blood pressure responses to other sympathoexcitatory maneuvers throughout the menstrual cycle are relevant. Ettinger et al. (7) reported a greater rise in MSNA during static handgrip performed during the EF phase compared with the follicular phase (i.e., 10–12 days after menstruation), but changes in arterial blood pressure during static handgrip were not different between phases. MSNA responses to ischemic handgrip were not different across phases, suggesting that blood flow is necessary to induce the menstrual cycle effect observed with static handgrip (7). These results support our current findings and suggest that differences in sympathetic vascular transduction may exist during different phases of the menstrual cycle. Minson et al. (19) also examined MSNA and arterial pressure responses to ischemic handgrip during different phases of the menstrual cycle, but the time frames differed from those of Ettinger et al. (7), and blood flow was measured using venous occlusion plethysmography. Minson et al. (19) reported a similar rise in MSNA and arterial pressure during ischemic handgrip exercise performed during the EF and ML phases of the menstrual cycle but did not observe significant differences in the transduction of sympathetic activity into vascular responsiveness. However, these authors found this finding “surprising” and suggested that the complexity of the ischemic handgrip exercise paradigm or other factors (i.e., progesterone) may have influenced the results. More recently, Meendering et al. (17, 18) reported higher cutaneous vascular conductance during the ML phase of heated head-up tilt compared with the EF phase or follicular phase (18) but failed to observe differences in calf venous compliance during the EF, ovulatory, and ML phases of the menstrual cycle (17). More studies examining the relationship between sympathetic nerve activity, arterial blood pressure, and blood flow appear warranted.
The effects of reproductive hormones on resting MSNA are controversial. It has been reported that estrogen replacement therapy decreases (34, 35) or does not change resting MSNA (11, 21, 34). Some of these inconsistencies can be explained by the mode of delivery or the type of estrogen used (34). Other investigations examining resting MSNA throughout the menstrual cycle are also inconsistent. Two studies report no change in MSNA during high- and low-hormone phases of the menstrual cycle (1, 7), whereas one study reports an increase of resting MSNA during the ML phase of the menstrual cycle (19). The current study reports no differences in resting MSNA during the EF and ML phases of the menstrual cycle, thus supporting the previous findings of Ettinger et al. (7) and Carter and Lawrence (1).
The current study has three limitations. First, blood flow was not recorded. The suggested increase in vascular transduction is based on the significant increase in MAP with no change in MSNA during HDR of the ML phase compared with the EF phase. Second, norepinephrine spillover was not collected. During the luteal phase of the menstrual cycle, higher levels of circulating plasma norepinephrine have been observed (9, 19). Greater norepinephrine release and reduced clearance are possible mechanisms that allow for increased levels of norepinephrine to be available to α-adrenergic receptors. Third, fluctuations of hormones during the menstrual cycle are highly variable. We controlled for the effects of sex hormones at two specific time periods of the menstrual cycle, low estrogen/progesterone and high estrogen/progesterone. By testing at these two extreme hormone levels, we believe these data provide clear evidence of the hormonal changes and their effects on the vestibulosympathetic reflex.
In summary, we examined MSNA, MAP, and HR responses to HDR in healthy females during the EF and ML phases of the menstrual cycle. Our results demonstrate that HDR does not elicit differential MSNA and HR responses during the EF and ML phases in healthy females. However, it appears that HDR causes an augmentation of MAP during the ML phase. We attribute the increase in MAP during HDR of the ML phase to an increase in vascular smooth muscle responsiveness.
GRANTS
This project was supported by grants from the Michigan Technological University Research Excellence Fund (REF-060605) and the National Institutes of Health (HL-088689 and DC-006459).
Acknowledgments
We thank Angela Lucas, John Durocher, and Betty Jaehnig for their assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Carter JR, Lawrence JE. Effects of the menstrual cycle on sympathetic neural responses to mental stress in humans. J Physiol 585: 635–641, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter JR, Ray CA. Sympathetic responses to vestibular activation in humans. Am J Physiol Regul Integr Comp Physiol 294: R681–R688, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Convertino VA Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 275: R1909–R1920, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Doba N, Reis DJ. Role of the cerebellum and the vestibular apparatus in regulation of orthostatic reflexes in the cat. Circ Res 40: 9–18, 1974. [DOI] [PubMed] [Google Scholar]
- 5.Eid AH, Maiti K, Mitra S, Chotani MA, Flavahan S, Bailey SR, Thompson-Torgerson CS, Flavahan NA. Estrogen increases smooth muscle expression of α2C-adrenoceptors and cold-induced constriction of cutaneous arteries. Am J Physiol Heart Circ Physiol 293: H1955–H1961, 2007. [DOI] [PubMed] [Google Scholar]
- 6.el-Bedawi KM, Hainsworth R. Combined head-up tilt and lower body suction: a test of orthostatic tolerance. Clin Auton Res 4: 41–47, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Ettinger SM, Silber DH, Gray KS, Smith MB, Yang QX, Kunselman AR, Sinoway LI. Effects of the ovarian cycle on sympathetic neural outflow during static exercise. J Appl Physiol 85: 2075–2081, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Fritsch-Yelle JM, Charles JB, Jones MM, Beightol LA, Eckberg DL. Spaceflight alters autonomic regulation of arterial pressure in humans. J Appl Physiol 77: 1776–1783, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein DS, Levinson P, Keiser HR. Plasma and urinary catecholamines during the human ovulatory cycle. Am J Obstet Gynecol 146: 824–829, 1983. [DOI] [PubMed] [Google Scholar]
- 10.Hume KM, Ray CA. Sympathetic responses to head-down rotations in humans. J Appl Physiol 86: 1971–1976, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Hunt BE, Taylor JA, Hamner JW, Gagnon M, Lipsitz LA. Estrogen replacement therapy improves baroreflex regulation of vascular sympathetic outflow in postmenopausal women. Circulation 103: 2909–2914, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Jian BJ, Cotter LA, Emanuel BA, Cass SP, Yates BJ. Effects of bilateral vestibular lesions on orthostatic tolerance in awake cats. J Appl Physiol 86: 1552–1560, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann H, Biaggioni I, Voustianiouk A, Diedrich A, Costa F, Clarke R, Gizzi M, Raphan T, Cohen B. Vestibular control of sympathetic activity. An otolith-sympathetic reflex in humans. Exp Brain Res 143: 463–469, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Kerman IA, Emanuel BA, Yates BJ. Vestibular stimulation leads to distinct hemodynamic patterning. Am J Physiol Regul Integr Comp Physiol 279: R118–R125, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Kerman IA, Yates BJ. Regional and functional differences in the distribution of vestibulosympathetic reflexes. Am J Physiol Regul Integr Comp Physiol 275: R824–R835, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Kerman IA, Yates BJ, McAllen RM. Anatomic patterning in the expression of vestibulosympathetic reflexes. Am J Physiol Regul Integr Comp Physiol 279: R109–R117, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Meendering JR, Torgrimson BN, Houghton BL, Halliwill JR, Minson CT. Effects of menstrual cycle and oral contraceptive use on calf venous compliance. Am J Physiol Heart Circ Physiol 288: H103–H110, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Meendering JR, Torgrimson BN, Houghton BL, Halliwill JR, Minson CT. Menstrual cycle and sex affect hemodynamic responses to combined orthostatic and heat stress. Am J Physiol Heart Circ Physiol 289: H631–H642, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Montgomery LD, Kirk PJ, Payne PA, Gerber RL, Newton SD, Williams BA. Cardiovascular responses of men and women to lower body negative pressure. Aviat Space Environ Med 48: 138–145, 1977. [PubMed] [Google Scholar]
- 21.Moreau KL, Donato AJ, Tanaka H, Jones PP, Gates PE, Seals DR. Basal leg blood flow in healthy women is related to age and hormone replacement therapy status. J Physiol 547: 309–316, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ray CA Effect of gender on vestibular sympathoexcitation. Am J Physiol Regul Integr Comp Physiol 279: R1330–R1333, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Ray CA Interaction of the vestibular system and baroreflexes on sympathetic nerve activity in humans. Am J Physiol Heart Circ Physiol 279: H2399–H2404, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Ray CA, Monahan KD. Aging attenuates the vestibulosympathetic reflex in humans. Circulation 105: 956–961, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Ray CA, Monahan KD. Sympathetic vascular transduction is augmented in young normotensive blacks. J Appl Physiol 92: 651–656, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Robertson D The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci 317: 75–77, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension 19: 508–519, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Sharkey LC, Kirchain S, McCune SA, Simpson GI, Archambault EZ, Boatright NK, Hicks E, Fray J. Progesterone increases blood pressure in spontaneous gestational hypertension in rats. Am J Hypertens 18: 36–43, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol 281: H2028–H2035, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Shortt TL, Ray CA. Sympathetic and vascular responses to head-down neck flexion in humans. Am J Physiol Heart Circ Physiol 272: H1780–H1784, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Stein CM, Lang CC, Singh I, He HB, Wood AJ. Increased vascular adrenergic vasoconstriction and decreased vasodilation in blacks. Additive mechanisms leading to enhanced vascular reactivity. Hypertension 36: 945–951, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Sudhir K, Elser MD, Jennings GL, Komesaroff PA. Estrogen supplementation decreases norepinephrine-induced vasoconstriction and total body norepinephrine spillover in perimenopausal women. Hypertension 30: 1538–1543, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Sudhir K, Jennings GL, Funder JW, Komesaroff PA. Estrogen enhances basal nitric oxide release in the forearm vasculature in perimenopausal women. Hypertension 28: 330–334, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Vongpatanasin W, Tuncel M, Mansour Y, Arbique D, Victor RG. Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation 103: 2903–2908, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Weitz G, Elam M, Born J, Fehm HL, Dodt C. Postmenopausal estrogen administration suppresses muscle sympathetic nerve activity. J Clin Endocrinol Metab 86: 344–348, 2001. [DOI] [PubMed] [Google Scholar]
- 36.White DD, Gotshall RW, Tucker A. Women have lower tolerance to lower body negative pressure than men. J Appl Physiol 80: 1138–1143, 1996. [DOI] [PubMed] [Google Scholar]