Abstract
Background
Randomized trials have demonstrated the efficacy of several new therapies for heart failure (HF) with reduced ejection fraction over the preceding two decades. This study investigates whether these therapeutic advances have translated into improvement in outcomes for patients with advanced HF referred to a heart transplant center.
Methods and Results
Patients with HF (n=2507) referred to a single university center were analyzed in three 6-year eras during which medical and device therapies were evolving: 1993-1998 (era 1), 1999-2004 (era 2), and 2005-2010 (era 3). Impaired hemodynamics and comorbidities were more frequent at time of referral in later eras, whereas other HF severity parameters where similar or improved. Successive eras had greater utilization of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, beta-blockers, aldosterone antagonists, implantable cardioverter defibrillators, and cardiac resynchronization therapy, consistent with evolving evidence and guideline-recommendations over the study period. All-cause mortality and sudden death were significantly lower in era 2 and 3 compared to era 1. After multivariable risk adjustment, era 3 had significantly decreased 2- and 3-year all-cause mortality risk and significantly decreased 1- and 3-year sudden death risk compared to era 1. However, progressive HF death and the combined outcome of mortality / urgent transplant / ventricular assist device were modestly increased in the latter eras.
Conclusions
Over the past two decades, patients with advanced HF referred to and managed at a tertiary university referral center have benefited from advances in HF medications and devices, as evidenced by improvements in overall survival and sudden death risk.
Keywords: heart failure, mortality, therapy
In the last two decades, randomized trials have identified several therapies that are efficacious in patients with heart failure (HF) and reduced ejection fraction (EF).1 Angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB), beta blockers, and aldosterone antagonists have been shown to prolong survival in large randomized, placebo-controlled trials, forming the foundation of medical therapy for HF with reduced EF.2-8 Major trials have also demonstrated the efficacy of implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy (CRT) in improving outcomes of select patients with HF and reduced EF.9-14
Implementation of HF medical and device therapies associated with survival benefit in clinical trials is expected to improve survival in real-world HF populations. However, community- and population- based studies that consider temporal trends in outcomes have generally not examined long-term survival rates in advanced referral HF populations after the advent of modern medical and device therapies.15-21 There are also other factors that may impact survival in advanced HF patients including differences in disease severity at time of referral, longer waiting times on heart transplant lists, and the increasing availability of ventricular assist devices (VADs). This study examines trends in treatment and outcomes in patients with advanced HF and reduced EF presenting to a university referral center for HF management and/or transplant/VAD evaluation between 1993 to 2010, a time period during which there were significant advances in medical and device therapies for HF.
Methods
Patients
The study was comprised of consecutive patients referred to the Ahmanson-UCLA Cardiomyopathy Center from 1993 to 2010. All patients were followed in a comprehensive management program for HF, as previously described.22 Patients with left ventricular EF > 40% (n = 1881) were excluded from this study. The remaining patients (n = 2507) were considered in three six-year eras, 1993-1998 (era 1, n = 793), 1999-2004 (era 2, n = 879), and 2005-2010 (era 3, n = 835), a time period during which HF therapies were evolving, specifically with the introduction of beta-blockers, aldosterone antagonists, ICDs, and CRT. A prior publication from our center reported on temporal trends in clinical outcomes from 1986-1993.23 Review of medical records was approved by the University of California-Los Angeles, Medical Institutional Review Board.
Baseline Data
Medications were recorded at time of referral and every visit thereafter. Diuretic doses were converted to furosemide equivalents. The formula used to convert other loop diuretics to furosemide equivalents was as follows: furosemide 80 mg = torsemide 40 mg = bumetanide 3 mg = ethacrynic acid 50 mg. Laboratory testing, echocardiography and cardiopulmonary exercise tests analyzed in this study all occurred within 3 months of initial referral. EF and dimensions were extracted from echocardiography reports; left ventricular end-diastolic dimension index (LVEDDI) was calculated as LVEDDI = left ventricular end-diastolic dimension (LVEDD)/body surface area (BSA). Past medical history was extracted from medical record review. Device therapy (CRT or ICD) in this study was considered “present” if the device had been placed before referral, or within 3 months of referral. Hemodynamic variables used in the analyses were the optimal values recorded after pulmonary artery catheter-tailored medical therapy, as these measurements have been shown to best correlate with survival.24
End Points
The primary endpoint analyzed was all-cause mortality. Secondary endpoints analyzed were sudden death, progressive HF death, urgent transplant (UNOS status IA), VAD, and the combined endpoint of death, urgent transplant and VAD. All endpoints were assessed at one, two, and three years. Non-urgent transplants (status IB and II) were censored and considered as a nonfatal end of follow-up for the combined endpoint. Death was considered sudden if it was unexpected based on the patient's clinical status and if it occurred out of the hospital within 15 minutes of the onset of unexpected symptoms or during sleep, as defined previously.23 Death during hospitalization for worsening congestive symptoms was considered a progressive HF death.
Statistical Analysis
Differences in baseline data between the three eras were analyzed using Pearson chi-square test, one-way analysis of variance (ANOVA), and non-parametric tests, as appropriate. Actuarial event curves for each era were calculated by the Kaplan Meier method, and differences in the curves were assessed by the log-rank statistic. Multivariable analyses adjusting for established predictors of HF outcomes, including age, gender, LVEF, NYHA class, body mass index (BMI), history of coronary artery disease (CAD), history of diabetes, history of hypertension, total cholesterol, serum sodium, serum blood urea nitrogen (BUN) and pulmonary capillary wedge pressure after optimization of therapy, were performed by Cox proportional hazards regression analysis to estimate adjusted hazard ratios and 95% confidence intervals (CIs). The Cox model retained all independent variables with p<0.15. Tests for trend were performed with the Cochran-Armitage test for actuarial and adjusted survival across the eras. Adjusted hazard curves were estimated graphically as 1-survival using the Cox model. To evaluate the severity of illness at time of referral, mortality risk scores and expected 1- and 3-year mortality rates for patients in each of the 3 eras were calculated using the Seattle HF Model (excluding adjustment for medications, other than loop diuretic doses, and devices), a previously developed model for predicting mortality in HF patients.25 Probability values were 2-sided with P<0.05 considered statistically significant. Statistics were calculated using PASW Statistics, version 18.0 (IBM, Somers, NY).
Results
Patient characteristics for each era are shown in Table 1. Patients were 74% male with a mean age of 53 years. The left ventricular EF at time of referral was slightly lower in era 3 compared with the previous two eras (p<0.001). Diabetes and BMI were higher in later eras, and B-type natriuretic peptide (BNP) levels were higher in era 3 compared to era 2 (p<0.001). Lower LVEDDI and lower total cholesterol were also observed in later eras (p<0.001). The proportion of patients that were classified as New York Heart Association (NYHA) class IV functional class was lower in eras 2 and 3, with a concomitantly higher proportion of patients having NYHA class II and III disease (p<0.001). In terms of hemodynamic variables, heart rate was lower while right atrial pressure, systolic pulmonary artery pressure and pulmonary capillary wedge pressure were higher in later eras (p<0.001). The Seattle HF Risk Model score was highest in era 3 (p=0.004) (Table 1).
Table 1. Patient Characteristics in the Successive Treatment Eras.
|
Era 1 1993-1998 (n=793) |
Era 2 1999-2004 (n=879) |
Era 3 2005-2010 (n=835) |
P value* | |
|---|---|---|---|---|
| N | 793 (31.6%) | 879 (35.1%) | 835 (33.3%) | |
| Age (yr) | 53.5±12.2 | 52.8±13.4 | 53.9±12.9 | 0.214 |
| Male (%) | 75.2% | 74.1% | 73.1% | 0.650 |
| Ischemic etiology of HF (%) | 44.8% | 43.6% | 40.5% | 0.201 |
| NYHA class (%) | <0.001 | |||
| I | 0.5% | 4.1% | 5.9% | |
| II | 8.6% | 22.6% | 21.4% | |
| III | 32.5% | 44.0% | 43.9% | |
| IV | 58.4% | 29.3% | 28.7% | |
| LVEF (%) | 23.5±6.62 | 24.1±7.32 | 22.3±7.74 | <0.001 |
| Body Mass Index | 26.1±5.37 | 27.1±5.56 | 27.6±5.96 | <0.001 |
| Diabetes (%) | 25.9% | 25.3% | 30.5% | 0.034 |
| Hypertension (%) | 42.1% | 38.4% | 40.9% | 0.275 |
| Smokers (past or present) (%) | 65.7% | 50.0% | 51.5% | <0.001 |
| LVEDD (mm) | 70.0±10.4 | 66.6±11.0 | 65.4±12.1 | <0.001 |
| LVEDDI (mm/m2) | 36.8±6.45 | 34.3±6.37 | 33.5±6.90 | <0.001 |
| Peak VO2 (ml/kg/min) | 14.2±4.73 | 13.5±4.98 | 13.9±6.24 | 0.149 |
| Severe MR (%) | 29.1% | 15.3% | 15.4% | <0.001 |
| Severe TR (%) | 15.8% | 6.7% | 9.7% | <0.001 |
| Atrial fibrillation (%) | 18.5% | 23.4% | 37.9% | <0.001 |
| Time from first HF symptoms to referral (months)‡ | 39 (8, 88) | 17 (4, 57) | 28 (6, 80) | <0.001 |
| Laboratories | ||||
|
|
||||
| Sodium (mmol/L) | 136±4.71 | 136±4.64 | 136±6.52 | 0.703 |
| Creatinine (mg/dL) | 1.40±0.760 | 1.51±1.37 | 1.55±1.66 | 0.057 |
| Blood urea nitrogen (mg/dL) | 27.4±17.1 | 28.5±20.1 | 30.0±20.7 | 0.037 |
| Hemoglobin (g/dL) | 13.4±1.93 | 13.1±1.93 | 13.1±2.68 | 0.003 |
| Total cholesterol (mg/dL) | 177±55.6 | 166±55.3 | 147±50.6 | <0.001 |
| HDL (mg/dL) | 35.4±14.5 | 38.7±15.1 | 37.6±17.8 | <0.001 |
| LDL (mg/dL) | 112±42.8 | 97.4±39.3 | 83.8±36.8 | <0.001 |
| Triglycerides (mg/dL)‡ | 101 (67, 170) | 114 (74, 183) | 107 (74, 165) | 0.014 |
| B-type natriuretic peptide (pg/mL)‡ | 472 (154, 1150) | 661 (253, 1540) | <0.001 | |
| Hemodynamics† | ||||
|
|
||||
| Heart Rate (beats/min) | 86±17 | 81±16 | 82±16 | <0.001 |
| Systolic Blood Pressure (mm Hg) | 102±17 | 109±20 | 104±18 | <0.001 |
| Diastolic Blood Pressure (mm Hg) | 56±12 | 65±14 | 65±13 | <0.001 |
| Right Atrial Pressure (mm Hg) | 7±4 | 9±6 | 9±5 | <0.001 |
| Systolic Pulmonary Artery Pressure (mm Hg) | 38±11 | 40±13 | 42±13 | <0.001 |
| Diastolic Pulmonary Artery Pressure (mm Hg) | 18±6 | 20±7 | 21±8 | <0.001 |
| Pulmonary Capillary Wedge Pressure (mm Hg) | 15±6 | 17±7 | 18±7 | <0.001 |
| Cardiac Output (L/min) | 4.7±1.1 | 4.5±2.2 | 4.4±2.7 | 0.010 |
| Cardiac Index (L/mm2) | 2.5±0.56 | 2.3±0.97 | 2.3±1.6 | 0.001 |
| Systemic Vascular Resistance (dyn*s/cm5) | 1110±290 | 1300±425 | 1320±496 | <0.001 |
| Clinical Risk Score | ||||
|
|
||||
| Seattle HF Model Score | 2.17±0.75 | 2.07±0.80 | 2.28±1.09 | 0.004 |
| Expected 1-year mortality | 29.9% | 27.5% | 32.7% | |
| Expected 3-year mortality | 65.5% | 61.8% | 69.5% |
NYHA, New York Heart Association; LVEDD, left ventricular end-diastolic dimension; LVEDDI, left ventricular end-diastolic dimension index; VO2, oxygen consumption; MR, mitral regurgitation; TR, tricuspid regurgitation; HDL, high-density lipoprotein; LDL, low-density lipoprotein. Categorical variables are presented as percentage of patients, and continuous variables are presented as mean ± standard deviation.
P values reflect Pearson chi-square test for categorical variables, one-way analysis of variance for normally distributed continuous variables and
non-parametric tests for non-normally distributed continuous variables.
After optimization of medical therapy.
Management of HF
As expected, use of HF therapies varied strikingly across the eras (Table 2). The proportion of patients receiving aldosterone antagonists, beta blockers, ICD, and CRT was substantially higher in the later eras (p<0.001). ACEI/ARB use remained at a high level in all eras with a small decrease seen in era 3 compared to era 2. Nitrates, hydralazine and loop diuretics were used by fewer patients in later eras. The proportion of patients receiving anticoagulation was unchanged over the study period.
Table 2. Heart Failure Therapies in the Successive Treatment Eras.
|
Era 1 1993-1998 (n=793) |
Era 2 1999-2004 (n=879) |
Era 3 2005-2010 (n=835) |
P value* | |
|---|---|---|---|---|
| Drugs | ||||
|
|
||||
| Angiotensin converting enzyme inhibitor or angiotensin receptor blocker, % | 86.5 | 89.5 | 78.8 | <0.001 |
| Beta blocker, % | 15.5 | 75.9 | 87.1 | <0.001 |
| Aldosterone antagonist, % | 6.5 | 49.0 | 57.9 | <0.001 |
| Loop diuretic, % | 87.9 | 83.5 | 82.0 | 0.004 |
| Mean dose of loop diuretic (mg) (furosemide equivalent) | 119 | 89 | 88 | <0.001 |
| Coumadin, % | 43.7 | 41.9 | 41.4 | 0.611 |
| Hydralazine, % | 13.8 | 5.9 | 7.4 | <0.001 |
| Metolazone, % | 9.0 | 9.8 | 12.8 | 0.067 |
| Nitrate, % | 62.1 | 23.7 | 19.7 | <0.001 |
| Statin, % | 22.1 | 50.2 | 49.2 | <0.001 |
| Device therapies | ||||
|
|
||||
| Cardiac resynchronization therapy, %† | 0.0 | 10.2 | 39.2 | <0.001 |
| Implantable cardioverter defibrillator, %† | 11.1 | 35.2 | 65.7 | <0.001 |
Therapy usage is presented as percentage of patients.
P values reflect Pearson chi-square test.
Percentages reflect patients' year of referral.
Temporal Trends in Outcomes
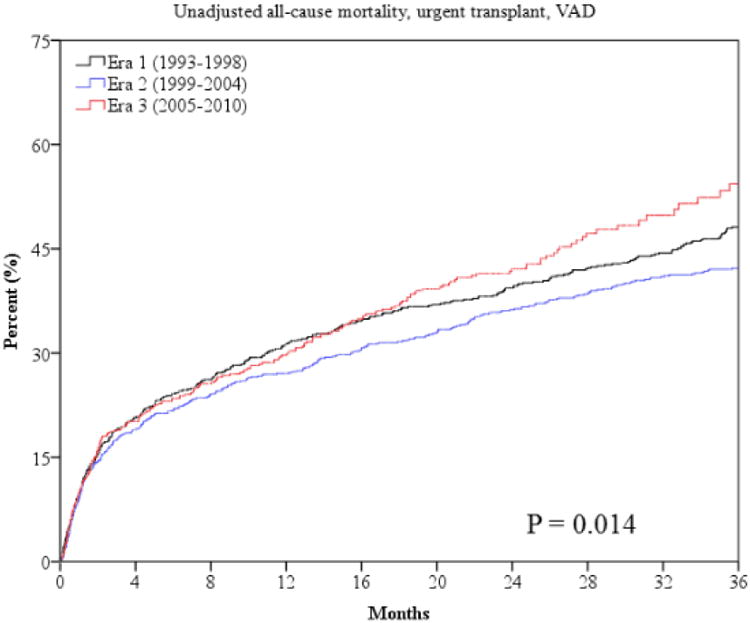
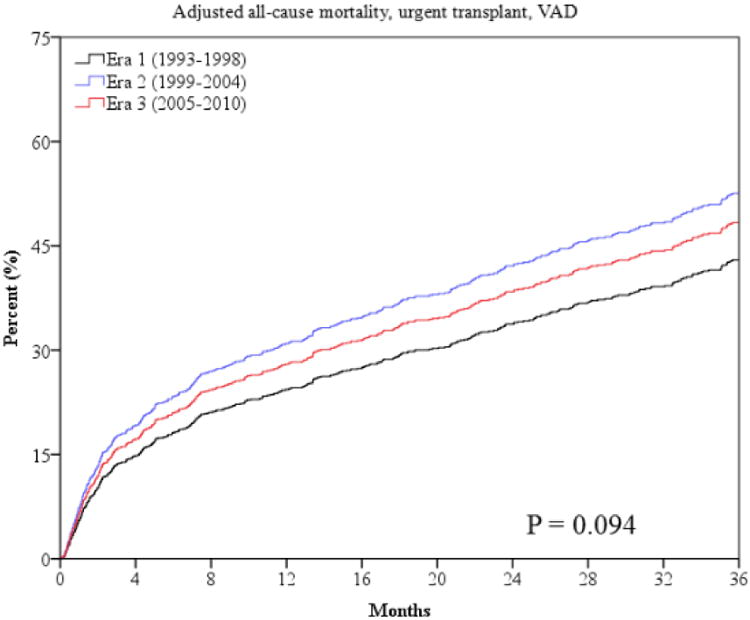
The primary outcome of all-cause mortality was significantly lower at one and three years in era 2 and 3 (Table 3 and Figure 1). Sudden death was lower in later eras (Table 3 and Figure 2). However, mortality from progressive HF death was higher in era 3 compared to era 1 (Table 3 and Figure 2). The combined endpoint of all-cause mortality, urgent transplant, or VAD was lower in era 2 but was higher in era 3 (Figure 1). This is largely accounted for by the higher rate of urgent heart transplantation (UNOS status 1a) and VAD implantation in the last era (Table 3). There were lower rates of non-urgent heart transplantation in era 2 and 3 (Table 3). The median times from referral to urgent transplantation were similar in all 3 eras (2.1, 2.4, and 2.1 months, p=0.929). The time from referral to non-urgent transplantation also did not statistically differ in the 3 eras (8.5, 11.9, and 7.8 months, p=0.091).
Table 3. Event Rates During the Successive Treatment Eras (Unadjusted).
|
Era 1 1993-1998 (n=793) |
Era 2 1999-2004 (n=879) |
Era 3* 2005-2010 (n=835) |
P value† | P trend‡ | |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| 1 yr | 20.6% | 15.3% | 17.8% | 0.042 | 0.114 |
| 2 yr | 27.9% | 23.8% | 25.5% | 0.121 | 0.109 |
| 3 yr | 36.4% | 29.4% | 31.5% | 0.022 | 0.029 |
| Sudden death | |||||
| 1 yr | 6.9% | 3.1% | 2.0% | <0.001 | <0.001 |
| 2 yr | 8.1% | 5.1% | 4.7% | 0.002 | 0.001 |
| 3 yr | 10.1% | 6.4% | 4.6% | 0.001 | <0.001 |
| Progressive HF death | |||||
| 1 yr | 7.1% | 7.0% | 12.6% | 0.002 | 0.002 |
| 2 yr | 9.6% | 9.4% | 16.9% | 0.004 | 0.006 |
| 3 yr | 11.6% | 11.3% | 19.9% | 0.004 | 0.007 |
| Urgent transplant or VAD | |||||
| 1 yr | 13.9% | 14.0% | 19.9% | 0.002 | 0.002 |
| 2 yr | 16.7% | 16.5% | 23.9% | 0.002 | 0.003 |
| 3 yr | 18.9% | 18.5% | 33.4% | <0.001 | 0.001 |
| Urgent transplant | |||||
| 1 yr | 13.9% | 14.0% | 18.7% | 0.020 | 0.014 |
| 2 yr | 16.7% | 16.5% | 23.7% | 0.002 | 0.003 |
| 3 yr | 18.9% | 18.5% | 33.4% | <0.001 | 0.001 |
| VAD | |||||
| 1 yr | 0% | 0% | 1.4% | <0.001 | 0.038 |
| 2 yr | 0% | 0% | 0.2% | 0.291 | 0.498 |
| 3 yr | 0% | 0% | 0% | N/A | N/A |
| Non-urgent transplant | |||||
| 1 yr | 11.2% | 6.7% | 8.1% | 0.015 | 0.039 |
| 2 yr | 16.7% | 11.7% | 14.1% | 0.029 | 0.053 |
| 3 yr | 19.4% | 14.8% | 15.9% | 0.065 | 0.097 |
| Mortality, urgent transplant or VAD | |||||
| 1 yr | 31.6% | 27.2% | 34.2% | 0.018 | 0.311 |
| 2 yr | 40.0% | 36.4% | 45.3% | 0.059 | 0.426 |
| 3 yr | 48.5% | 42.5% | 53.4% | 0.014 | 0.669 |
P values reflect the log rank (Mantel-Cox) test.
2005-2009 and 2005-2008 for 2 and 3 year analyses, respectively.
P value for trend from the Cochran-Armitage test.
Figure 1.
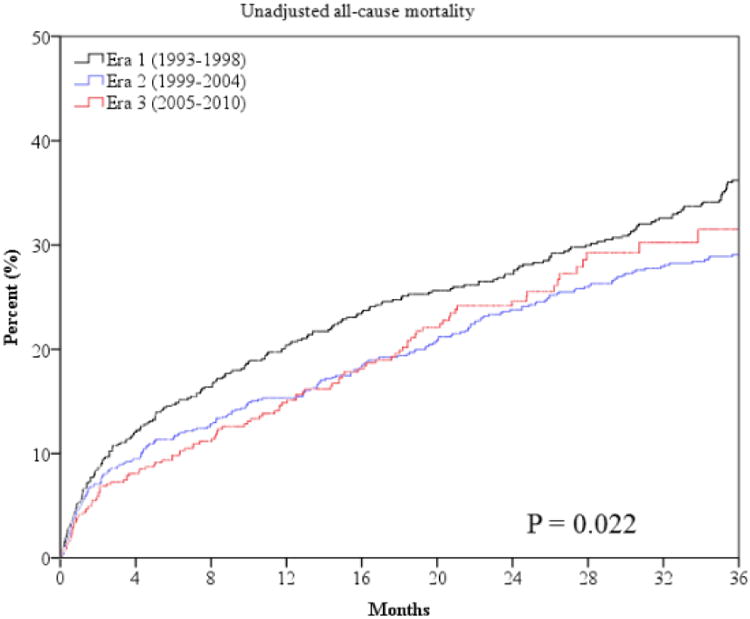
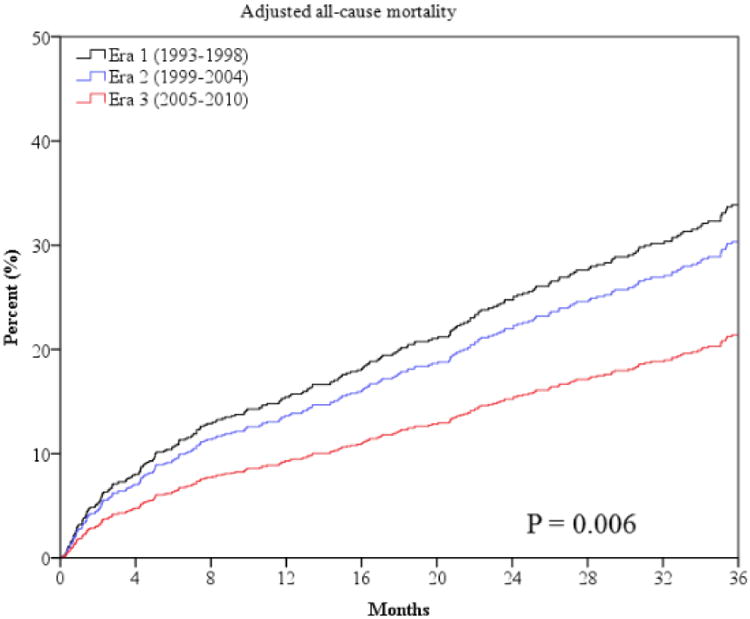
Unadjusted and risk-adjusted survival curves for the outcome of all-cause mortality (panels a and b) and all-cause mortality, urgent transplant and VAD (panels c and d).
Risk adjusted survival curves are adjusted for patients' age, gender, LVEF, NYHA class, body mass index, history of CAD, history of diabetes, history of hypertension, total cholesterol, serum sodium, serum BUN and pulmonary capillary wedge pressure after optimization of therapy. P values for unadjusted curves (panels a and c) are derived from log-rank statistic. P values for adjusted curves (panels b and d) are derived from Cochran-Armitage trend test.
Figure 2.
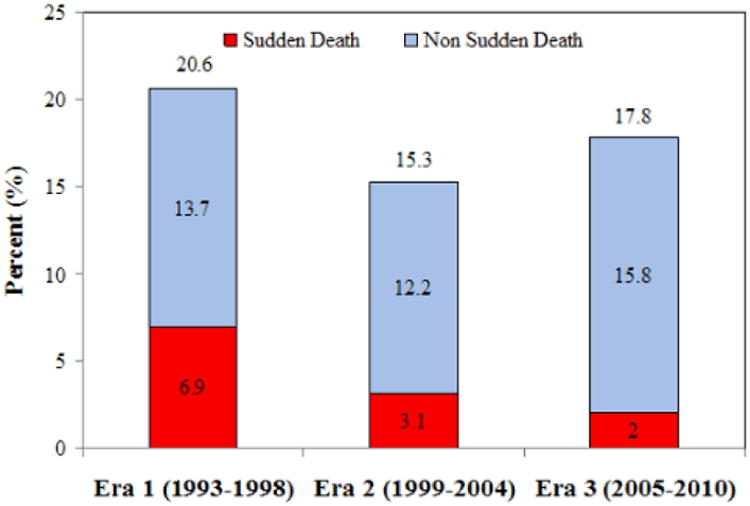
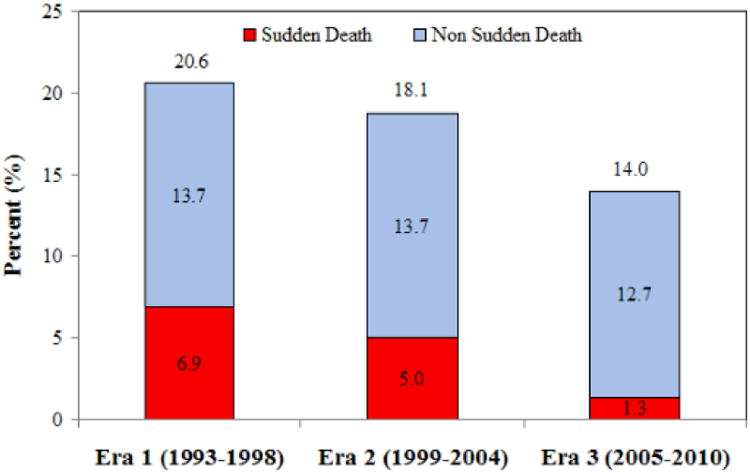
Sudden death, non sudden death, and total mortality at one year in the three eras, (a) unadjusted and (b) adjusted rates.
See figure 1 legend for adjustment variables.
Risk-Adjusted Analysis of Outcomes
After adjusting for known predictors of HF mortality, era 3 was independently associated with decreased risk of all-cause mortality compared to era 1 at two and three years, with a borderline p value at one-year follow-up (Table 4 and Figure 1). Compared to era 1, era 3 was associated with a 42% lower adjusted relative risk of mortality during 3-year follow-up (hazard ratio [HR] 0.58, 95% confidence intervals [CI] 0.40-0.85). Era 3 was independently associated with decreased risk of sudden death compared to era 1 at one and three years, with a borderline p value (p=0.057) for decreased risk at two years (Table 4 and Figure 2). In contrast, after multivariable adjustment, latter eras were not independently associated with lower or higher risk of progressive HF death with the exception of a higher risk seen for era 2 at one year. After multivariable analysis era 2 had a higher risk of the combined endpoint of mortality, urgent transplant, or VAD (Table 4). Unadjusted and risk-adjusted hazard curves for the outcome of all-cause mortality and the composite of mortality, urgent transplant, or VAD are shown in Figure 1.
Table 4. Risk-Adjusted All-Cause Mortality, Cause Specific Mortality, and Composite Hazard Ratios in Successive Treatment Eras.
|
Era 1 1993-1998 |
Era 2 1999-2004 |
Era 3† 2005-2010 |
Era 1 1993-1998 |
Era 2 1999-2004 |
Era 3† 2005-2010 |
P trend‡ | |
|---|---|---|---|---|---|---|---|
| Unadjusted hazard ratios | Adjusted hazard ratios* | ||||||
| 1-year HR (95% CI) | |||||||
| All-cause mortality | 1.00 | 0.74 (0.58-0.94) p=0.013 | 0.83 (0.65-1.06) p=0.141 | 1.00 | 0.91 (0.63-1.32) p=0.618 | 0.68 (0.45-1.04) p=0.072 | 0.080 |
| Sudden death | 1.00 | 0.45 (0.27-0.74) p=0.002 | 0.24 (0.12-0.48) p<0.001 | 1.00 | 0.73 (0.36-1.48) p=0.384 | 0.19 (0.05-0.64) p=0.007 | 0.006 |
| Progressive HF death | 1.00 | 1.01 (0.69-1.48) p=0.969 | 1.70 (1.19-2.42) p=0.004 | 1.00 | 1.95 (1.07-3.56) p=0.029 | 1.65 (0.88-3.11) p=0.122 | 0.124 |
| Mortality, urgent transplant or VAD | 1.00 | 0.85 (0.71-1.02) p=0.078 | 1.10 (0.92-1.31) p=0.305 | 1.00 | 1.61 (1.25-2.07) p<0.001 | 1.37 (1.04-1.80) p=0.023 | 0.013 |
| 2 year HR (95% CI) | |||||||
| All-cause mortality | 1.00 | 0.82 (0.67-1.00) p=0.053 | 0.85 (0.67-1.06) p=0.144 | 1.00 | 0.95 (0.69-1.30) p=0.738 | 0.67 (0.46-0.97) p=0.035 | 0.047 |
| Sudden death | 1.00 | 0.57 (0.37-0.88) p=0.012 | 0.42 (0.24-0.72) p=0.002 | 1.00 | 0.97 (0.53-1.77) p=0.909 | 0.43 (0.18-1.03) p=0.057 | 0.085 |
| Progressive HF death | 1.00 | 0.99 (0.71-1.40) p=0.972 | 1.59 (1.14-2.22) p=0.006 | 1.00 | 1.58 (0.95-2.64) p=0.081 | 1.27 (0.72-2.26) p=0.410 | 0.354 |
| Mortality, urgent transplant or VAD | 1.00 | 0.89 (0.76-1.04) p=0.141 | 1.08 (0.92-1.28) p=0.359 | 1.00 | 1.48 (1.18-1.85) p=0.001 | 1.28 (0.99-1.64) p=0.057 | 0.027 |
| 3-year HR (95% CI) | |||||||
| All-cause mortality | 1.00 | 0.78 (0.65-0.94) p=0.008 | 0.82 (0.65-1.02) p=0.075 | 1.00 | 0.87 (0.66-1.16) p=0.355 | 0.58 (0.40-0.85) p=0.005 | 0.006 |
| Sudden death | 1.00 | 0.58 (0.39-0.86) p=0.007 | 0.41 (0.23-0.72) p=0.002 | 1.00 | 0.95 (0.54-1.65) p=0.842 | 0.35 (0.14-0.87) p=0.024 | 0.042 |
| Progressive HF death | 1.00 | 0.99 (0.72-1.37) p=0.973 | 1.62 (1.16-2.26) p=0.005 | 1.00 | 1.34 (0.83-2.16) p=0.232 | 1.02 (0.57-1.90) p=0.955 | 0.822 |
| Mortality, urgent transplant or VAD | 1.00 | 0.85 (0.74-0.99) p=0.033 | 1.08 (0.91-1.27) p=0.391 | 1.00 | 1.33 (1.08-1.64) p=0.008 | 1.18 (0.92-1.51) p=0.197 | 0.094 |
P values reflect the likelihood ratio test by the Cox model.
Multivariate analysis is adjusted for patients' age, gender, LVEF, NYHA class, body mass index, history of CAD, history of diabetes, history of hypertension, total cholesterol, serum sodium, serum BUN and pulmonary capillary wedge pressure after optimization of therapy.
2005-2009 and 2005-2008 for 2 and 3 year analyses, respectively.
P value for trend from Cochran-Armitage test for adjusted models.
Discussion
The major findings of this study are that over successive time periods from 1993 to 2010, there has been a marked increase in the use of evidence-based therapies in advanced HF patients with reduced EF referred to a tertiary university HF center, with a corresponding improvement in overall survival in this patient population. Sudden cardiac death was also significantly lower in later eras. Concomitant with a substantial decrease in sudden death was a modest increase in progressive HF death, urgent transplants, and VADs. After risk adjustment, there was a lower risk of all-cause mortality and sudden death for patients treated in the 2005-2010 time frame compared to 1993 to 1998. Although rates of progressive HF death were high in the later eras, this is not surprising given that sudden deaths were significantly lower in later eras and likely reflects a shifting mode of death. These findings confirm the successful translation of evidence-based care in treatment of advanced referral patients with HF and reduced EF from clinical trials to patients in real-world clinical practice.
The severity of HF disease state at time of referral to our university center seems to be worsened in later eras in our HF population by some, but not other, measures. Over the past two decades as documented in this study, patients in the later eras had modest but significantly worse hemodynamic status, as indicated by higher pulmonary artery pressure, higher pulmonary capillary wedge pressure, and lower cardiac index, measured after medical optimization. Furthermore, Seattle HF Model risk score and predicted mortality were highest in the last era. However, other individual parameters including NYHA class, peak VO2, left ventricular end-diastolic dimension, systolic blood pressure, and heart rate did not indicate increased disease state severity at time of referral. A prior publication reporting on temporal trends in clinical outcomes at our center from an earlier time period 1986-1993 found that patients in the different time periods had similar hemodynamic variables after tailored therapy.23 This study cohort also has increased comorbidities in later eras as indicated by higher rates of diabetes and atrial fibrillation, lower hemoglobin levels, and higher creatinine levels in the later eras; this increase in comorbidities over time is congruent with findings from other studies of HF populations. In an epidemiological study of HF in Western Australia, Teng et al found that prevalence of ischemic heart disease fell while prevalence of hypertension, diabetes, atrial fibrillation and renal failure rose from 1990-2003.19 One study of Medicare beneficiaries hospitalized for HF found increased prevalence of hypertension, diabetes, anemia and pneumonia from 1993-2006, while another study described increased prevalence of hypertension and renal failure;16, 18 similar trends in prevalence of comorbid conditions are noted in our population.
The successful translation of life-prolonging medications and devices for HF and reduced EF, from clinical trial to real-world populations, seen in this study is echoed in other publications of HF trends over the last decades. Jhund et al showed significant increases in prescribing rates of ACEI, beta blockers and spironolactone from 1986-2003 for patients with HF in Scotland.26 More recently, Cubbon et al found increased usage of medications, ICD and CRT in a 2006-2009 cohort compared to a 1993-1995 cohort of patients with stable HF in the United Kingdom.27 Our study extends this finding to a population of advanced HF patients referred to a tertiary center for heart transplant or VAD evaluation, with higher rates of beta blocker and aldosterone antagonist use as well as device implantation seen in the later eras of treatment.
Community, population and cohort studies have shown improved survival in HF populations over the past two decades, similar to the trends observed in the present analysis. An older study from a community cohort in Olmstead County, Minnesota showed reduced mortality in men after onset of HF from 26% in 1985-1990 to 21% in 1996-2000.21 Several additional studies have shown general improvement in survival after hospitalization for HF;16, 18, 19, 26, 28 US Medicare beneficiaries had modest increases in thirty-day and one-year survival after hospitalization for HF, with the number of hospitalizations falling from 1998 to 2008.16, 18 Jhund et al also showed that mortality after hospitalization for HF fell in Scotland from 1995 to 2003, coinciding with the increase in prescribing rates for ACEIs, beta blockers and spironolactone during this time period.26 A larger 38% decrease in all-cause mortality at one year between the 1993 –1995 cohort and the 2006 – 2009 cohort of stable HF patients in the United Kingdom was demonstrated by Cubbon et al.27 Singh et al found that the risk of dying while listed for heart transplant or becoming too sick for transplant has decreased in the US after the implementation of a new sharing algorithm for these organs in 2006.29 Our study of an advanced HF population referred to a tertiary center for heart transplant or VAD evaluation demonstrates a 13% and 42% lower risk of all-cause mortality at three years for eras 2 and 3 respectively (p trend = 0.006) compared to era 1 among patients with reduced EF treated at our center after adjustment for multiple known predictors of HF mortality.
Outcomes in patients with advanced HF over the past 20 years may also have been influenced by differences in waiting times for transplantation, rates of transplantation, and use of VADs in the three eras. In our cohort, waiting times for urgent and non-urgent transplantation did not significantly change during the 3 eras, suggesting differences in transplantation waiting times are unlikely to account for the improved survival in latter eras. There were, however, higher rates of urgent transplantation in era 3 which may contribute to the lower mortality rates observed. However, there was also a decrease in non-urgent transplants, which may in part account for the higher rate of urgent transplants. Lastly, the use of VADs remained low across the eras, even in era 3, suggesting access to this new technology did not significantly affect the differences in outcomes.
Study Limitations
We acknowledge some limitations to our study. This study is observational and evaluates a cohort of patients with advanced HF referred to a single university center. As it was observational in nature, we cannot establish causality between increased use of evidence-based therapies and decreased mortality observed over the study period. Also, we cannot exclude that other factors, such as increased understanding of and compliance with lifestyle modifications and HF disease management, may be reflected in our results. Our findings reflect a single center referral experience and findings may not be able to be generalized to other settings. While we adjusted for multiple variables of established prognostic significance in patients with advanced HF, there are other established markers that were not included. The assessment of NYHA class potentially shifted over time with differing clinician experience expectations; in era 1 the majority of the patients (58%) were classified as NYHA IV while in era 2 and 3 only 29% of patients were classified as NYHA IV, even though other measures of HF severity were similar or worse. There may be residual measured and unmeasured confounding variables that account for some or all of these findings. There are also well-recognized limitations in distinguishing between different modes of death in patients with HF so the findings regarding different time trends in mode of death should be interpreted with these limitations in mind. Lastly, the time period of our study predates a more recent increase in VAD usage, and so we cannot determine how higher rates of VAD usage may affect long term HF outcomes.
Conclusions
In a cohort of patients with advanced HF and reduced EF referred to and managed at a tertiary university referral center from 1993 to 2010, there has been successive application of evidence-based, guideline-recommended therapies, coincident with improvement in overall survival and lower sudden death risk over time. Increased rates of HF deaths, transplants, and VADs in the later eras were seen, and are potentially attributable to different risk profiles at time of referral and substantial reductions in sudden death in later eras;. Lastly, despite improvement in survival over the past two decades, the all-cause mortality rate for advanced HF with reduced EF at three years remains at approximately 30%, indicating the need to continue seeking new treatment options and management strategies for this prevalent problem with significant relevance to public health.
Clinical Perspective
Heart failure is a major public health problem affecting close to 6 million men and women in the United States. In the last 20 years, randomized trials have demonstrated the efficacy of several new therapies for heart failure with reduced ejection fraction and changed treatment paradigms. However, the impact that this has had on real-world patient outcomes is still largely unknown. Using data from 2507 patients with heart failure and reduced ejection fraction referred to and followed at a single university tertiary referral center, this study examines trends in clinical characteristics, management, and outcomes across therapeutic eras in the last two decades. Comorbidities and impaired hemodynamics were more frequent at referral in the later eras, whereas other parameters of heart failure severity were similar to the first era or improved. Management of heart failure with use of beta-blockers, aldosterone antagonists, implantable cardioverter defibrillators and cardiac resynchronization therapy increased significantly, consistent with evolving evidence and guideline-recommendations during the study period. At one-, two- and three-year follow-up, all-cause mortality and sudden death were significantly lower in the later eras of treatment, and the most recent era was independently associated with a decreased risk of all-cause mortality and sudden death in multivariable analysis. The waiting time to heart transplantation did not change significantly in the study period. Taken together, this study demonstrates patients with advanced heart failure have benefited from recent advances in evidence-based, guideline-recommended heart failure therapies, with improvement in survival and lower sudden death risk over time.
Acknowledgments
Sources of Funding: This work was supported by the Ahmanson Foundation (Los Angeles, CA). Dr. Horwich was supported by NIH 1K23HL085097. Dr. Fonarow holds the Eliot Corday Chair in Cardiovascular Medicine and Science.
Footnotes
Disclosures: Fonarow: Research – Medtronic (modest); Gambro (modest); Novartis (significant).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. ACCF/AHA guidelines for the diagnosis and management of heart failure in adults. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 2.SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 3.Hjalmarson Å, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, Wikstrand J, El Allaf D, Vítovec J, Aldershvile J, Halinen M, Dietz R, Neuhaus KL, Jánosi A, Thorgeirsson G, Dunselman PH, Gullestad L, Kuch J, Herlitz J, Rickenbacher P, Ball S, Gottlieb S, Deedwania P. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure. JAMA. 2000;283:1295–1302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 4.Poole-Wilson PA, Swedberg K, Cleland JGF, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp-Pedersen C, Scherhag A, Skene A. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 5.Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, Klein M, Lamas GA, Packer M, Rouleau J, Rouleau JL, Rutherford J, Wertheimer JH, Hawkins CM. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 6.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 7.CIBIS II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 8.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 9.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 10.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NAM, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 11.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 12.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 13.Cleland JGF, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 14.McAlister FA, Ezekowitz J, Hooton N, Vandermeer B, Spooner C, Dryden DM, Page RL, Hlatky MA, Rowe BH. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction. JAMA. 2007;297:2502–2514. doi: 10.1001/jama.297.22.2502. [DOI] [PubMed] [Google Scholar]
- 15.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 16.Bueno H, Ross JS, Wang Y, Chen J, Vidán MT, Normand S, Curtis JP, Drye EE, Lichtman JH, Keenan PS, Kosiborod M, Krumholz HM. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA. 2010;303:2141–2147. doi: 10.1001/jama.2010.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosiborod M, Lichtman JH, Heidenreich PA, Normand S, Wang Y, Brass LM, Krumholz HM. National trends in outcomes among elderly patients with heart failure. Am J Med. 2006;119:616.e1–616.e17. doi: 10.1016/j.amjmed.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Normand S, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teng T, Finn J, Hobbs M, Hung J. Incidence, case fatality, and hospitalization rates in Western Australia between 1990 and 2005. Circ Heart Fail. 2010;3:236–243. doi: 10.1161/CIRCHEARTFAILURE.109.879239. [DOI] [PubMed] [Google Scholar]
- 20.Laribi S, Aouba A, Nikolaou M, Lassus J, Cohen-Solal A, Plaisance P, Pavillon G, Jois P, Fonarow GC, Jougla E, Mebazaa A. Trends in death attributed to heart failure over the past two decades in Europe. Eur J Heart Fail. 2012;14:234–239. doi: 10.1093/eurjhf/hfr182. [DOI] [PubMed] [Google Scholar]
- 21.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 22.Fonarow GC, Stevenson LW, Walden JA, Livingston NA, Steimle AE, Hamilton MA, Moriguchi J, Tillisch JH, Woo MA. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol. 1997;30:725–732. doi: 10.1016/s0735-1097(97)00208-8. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson WG, Stevenson LW, Middlekauff HR, Fonarow GC, Hamilton MA, Woo MA, Saxon LA, Natterson PD, Steimle A, Walden JA, Tillisch JH. Improving survival for patients with advanced heart failure: a study of 737 consecutive patients. J Am Coll Cardiol. 1995;26:1417–1423. doi: 10.1016/0735-1097(95)00341-x. [DOI] [PubMed] [Google Scholar]
- 24.Stevenson LW, Tillisch JH, Hamilton M, Luu M, Chelimsky-Fallick C, Moriguchi J, Kobashigawa J, Walden J. Importance of hemodynamic response to therapy in predicting survival with ejection fraction ≤ 20% secondary to ischemic or nonischemic dilated cardiomyopathy. Am J Cardiol. 1990;66:1348–1354. doi: 10.1016/0002-9149(90)91166-4. [DOI] [PubMed] [Google Scholar]
- 25.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle heart failure model. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 26.Jhund PS, MacIntyre K, Simpson CR, Lewsey JD, Stewart S, Redpath A, Chalmers JW, Capewell S, McMurray JJ. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003. Circulation. 2009;119:515–523. doi: 10.1161/CIRCULATIONAHA.108.812172. [DOI] [PubMed] [Google Scholar]
- 27.Cubbon RM, Gale CP, Kearney LC, Schechter CB, Brooksby WP, Nolan J, Fox KA, Rajwani A, Baig W, Groves D, Barlow P, Fisher AC, Batin PD, Kahn MB, Zaman AG, Shah AM, Byrne JA, Lindsay SJ, Sapsford RJ, Wheatcroft SB, Witte KK, Kearney MT. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction. Circ Heart Fail. 2011;4:396–403. doi: 10.1161/CIRCHEARTFAILURE.110.959882. [DOI] [PubMed] [Google Scholar]
- 28.Schaufelberger M, Swedberg K, Köster M, Rosén M, Rosengren A. Decreasing one-year mortality and hospitalization rates for heart failure in Sweden. Eur Heart J. 2004;25:300–307. doi: 10.1016/j.ehj.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Singh TP, Almond CS, Taylor DO, Graham DA. Decline in heart transplant wait list mortality in the United States following broader regional sharing of donor hearts. Circ Heart Fail. 2012;5:249–258. doi: 10.1161/CIRCHEARTFAILURE.111.964247. [DOI] [PubMed] [Google Scholar]


