Abstract
Background
Dengue displays a broad spectrum of clinical manifestations that may vary from asymptomatic to severe and even fatal features. Plasma leakage/hemorrhages can be caused by a cytokine storm induced by monocytes and dendritic cells during dengue virus (DENV) replication. Plasmacytoid dendritic cells (pDCs) are innate immune cells and in response to virus exposure secrete IFN-α and express membrane TRAIL (mTRAIL). We aimed to characterize pDC activation in dengue patients and their function under DENV-2 stimulation in vitro.
Methods & Findings
Flow cytometry analysis (FCA) revealed that pDCs of mild dengue patients exhibit significantly higher frequencies of mTRAIL compared to severe cases or healthy controls. Plasma levels of IFN-α and soluble TRAIL are increased in mild compared to severe dengue patients, positively correlating with pDC activation. FCA experiments showed that in vitro exposure to DENV-2 induced mTRAIL expression on pDC. Furthermore, three dimension microscopy highlighted that TRAIL was relocalized from intracellular compartment to plasma membrane. Chloroquine treatment inhibited DENV-2-induced mTRAIL relocalization and IFN-α production by pDC. Endosomal viral degradation blockade by chloroquine allowed viral antigens detection inside pDCs. All those data are in favor of endocytosis pathway activation by DENV-2 in pDC. Coculture of pDC/DENV-2-infected monocytes revealed a dramatic decrease of antigen detection by FCA. This viral antigens reduction in monocytes was also observed after exogenous IFN-α treatment. Thus, pDC effect on viral load reduction was mainly dependent on IFN-α production
Conclusions
This investigation characterizes, during DENV-2 infection, activation of pDCs in vivo and their antiviral role in vitro. Thus, we propose TRAIL-expressing pDCs may have an important role in the outcome of disease.
Author Summary
Dengue is an important endemic tropical disease to which there are no specific therapeutics or approved vaccines. Currently several aspects of pathophysiology remain incompletely understood. A crucial cellular population for viral infections, the plasmacytoid dendritic cells (pDCs) was analyzed in this study. The authors found an in vivo association between the activation state of pDCs and the disease outcome. Membrane TNF-related apoptosis inducing ligand (TRAIL) expressing pDCs, representing activated pDCs, were found in higher frequency in milder cases of dengue than severe cases or healthy individuals. Detection of antiviral cytokine interferon-alpha (IFN-α) and soluble TRAIL positively correlated with pDC activation. Dengue virus (DENV) serotype-2 was able to directly activate pDCs in vitro. Under DENV stimulation TRAIL was relocalized from intracellular to pDC plasma membrane and IFN-α was highly produced. The authors suggest an endocytosis-dependent pathway for DENV-induced pDC activation. It is also highlighted here a role for exogenous IFN-α and pDCs in reducing viral replication in monocytes, one of DENV main target cells. These findings may contribute in the future to the establishment of good prognostic immune responses together with clinical manifestations/warning signs.
Introduction
Dengue is the most important arthropod-borne emerging viral disease in tropical countries due to its high morbidity and risk of mortality [1]. For example, in Brazil, dengue is a major public health problem and about two million cases were reported during 2010–2012 [2]. Dengue virus (DENV) is a single-stranded RNA virus belonging to genus Flavivirus [3], [4]. All DENV serotypes (DENV-1 to -4) may induce a broad spectrum of clinical manifestations from asymptomatic to severe clinical features, characterized by hemorrhagic manifestations and a shock syndrome [5], [6], [7]. High viral load may cause an exacerbated cytokine production that plays a key role in the generation of important physiopathological processes [8], [9]. Human monocytes/macrophages and dendritic cells are susceptible to viral replication [10], [11], [12], [13] and can release soluble mediators involved in vascular permeability and plasma leakage besides coagulation disorders [14], [15], [16], [17].
Dendritic cells link innate and adaptive immunity and play a key role in shaping effective immune responses. Two major subpopulations are described: myeloid or conventional dendritic cells (cDCs) and plasmacytoid dendritic cells (pDCs) [18], [19], [20], [21]. In contrast to cDCs, pDC are not found in homeostatic tissues but mainly in circulating blood and in lymphoid tissues [21], [22], [23]. Despite being rare cells, pDCs produce up to 1,000-fold more IFN-α than other cell types in response to virus exposure [24]. Viral activation of pDCs can be regulated by either one of the two Toll-like receptors (TLR), TLR-7 or TLR-9 [25], which are considered to be the pattern recognition receptors (PRR) for RNA [26] and DNA [27], respectively. It has been shown that cDC are efficiently infected by DENV and that viral replication blocked cDC maturation [28], [29]. However, unlike cDCs, it has been reported that pDCs are not supporting productive DENV replication [30]. Indeed, DENV can activate pDCs through cell endosomal activity and TLR-7 pathway [31]. Furthermore, dengue-infected patients had impaired pDC activation features. Indeed, absolute numbers of blood pDC were decreased [32], [33] and low levels of serum IFN-α [34] were reported.
TNF-related apoptosis-inducing ligand (TRAIL) is a pro-apoptotic molecule, which induces death of cells that express its death receptors (DR), DR4 and DR5 [35], [36]. Furthermore, IFN-α regulates TRAIL expression by several cell types [37]. Soluble or membrane TRAIL mediates apoptosis on cells that are selectively expressing DR4 and DR5, mainly killing virus-infected cells and leaving intact normal cells [38], [39]. Additionally an antiviral role was proposed for TRAIL. DENV-infected monocytes and dendritic cells display reduced viral replication when TRAIL is exogenously administered [40]. Soluble TRAIL (sTRAIL) was found in sera from dengue patients [41], but mTRAIL role and expression by DENV-2 exposed pDC to has not been investigated yet.
In this report we studied pDC activation by DENV and its consequences on viral infection. The clinical study showed that during acute phase of DF, pDCs are activated characterized by TRAIL and IFN-α markers. Indeed, the more pDC are activated the less the disease is severe. We found that DENV-2 efficiently activated TRAIL expression and IFN-α production by pDC. The microscopy study revealed that TRAIL was intracellularly stocked in resting pDC and was relocalized to plasma membrane when pDC were exposed to DENV-2. Furthermore, we showed that pDC could decrease DENV infection in monocytes mainly due to the effects of IFN-α produced. Thus pDC activation constitutes a host defense against DENV-2 infection strongly suggesting that these cells are likely beneficiating the disease outcome.
Materials and Methods
Ethics statement
Experimental procedures with human blood have been approved by Necker Hospital Ethical Committees for human research and were done according to the European Union guidelines and the Declaration of Helsinki. Procedures were also approved by the ethical committee at Instituto de Pesquisas Clinicas Evandro Chagas, FIOCRUZ (CAAE 3723.0.000.009-08). All patients were informed of procedures and gave written consent.
Patient and blood samples
Blood from HIV-1-seronegative blood bank donors was obtained anonymously from “Etablissement Français du Sang” (convention # 07/CABANEL/106), Paris, France. Forty three patients with confirmed dengue fever (Table 1) from two Brazilian Health Centers at Campo Grande, MS and Campos de Goytacases, RJ, Brazil were studied. All patients presented clinical diagnosis of dengue infection.
Table 1. Demographic information about the study population with dengue fever (DF)1.
| Characteristics | DF ± WS | Severe DF2 | ||
| (N) | (N) 3 | |||
| Age (median years, 25–75%) | 43, 26–58 | (33) | 42, 24–50 | (10) |
| Sex (M:F; patient number) | 14:19 | 5:5 | ||
| Fever | 87% | (31) | 90% | (10) |
| Hospitalization | 52% | (31) | 100% | (8) |
| Hemorrhagic manifestations (mucosal)4 | 16% | (31) | 30% | (10) |
| Constant vomits | 8% | (25) | 50% | (6) |
| Persistent abdominal pain | 8% | (25) | 60% | (5) |
| Hypotension5 | 4% | (26) | 25% | (8) |
| Effusions6 | 0% | (33) | 40% | (10) |
| Platelet counts (×103/mm3)7 | 172±37 | (33) | 40±12 | (9) |
| Thrombocytopenia (<50.000×103/mm3) | 18% | (33) | 78% | (9) |
| Hematocrit | 41±1% | (30) | 42±2% | (9) |
| Hemoconcentration8 | 33% | (30) | 56% | (9) |
| Previous dengue (IgG positive) | 79% | (30) | 100% | (8) |
| Rapid hematocrit increase and platelet decrease | 13% | (31) | 60% | (10) |
| Leukocyte counts (×103/mm3)7 | 4028±522 | (27) | 3818±571 | (8) |
| ALT (U)7 | 52±10 | (23) | 2784±2685 | (8) |
| AST (U)7 | 73±16 | (23) | 670±611 | (9) |
Study population with 43 patients.
DF ± WS dengue fever without or with warning signs; Severe DF, dengue fever with severe clinical manifestations according to WHO criteria [43].
Number of patients with the available information during hospitalization.
Hemorrhagic manifestations (epistaxis, gengivorrhagia, metrorrhagia, bleeding after coughing).
Postural hypotension with decrease in systolic arterial pressure in 20 mmHg in supine position or systolic arterial pressure <90 mm Hg.
Pleural, pericardial effusion or ascites.
Average ± standard error from minimal recorded platelet, leukocyte/maximal hematocrit counts/ALT or AST values.
Elevated hematocrit (20% during course of illness and recovery; or >45%, men and >41%, women).
Criteria for dengue fever severity and laboratorial diagnosis
Dengue fever was considered mild when no warning signs (WS) or severe clinical manifestations were observed as follows. Dengue fever with WS was considered if patients presented any of the following warnings: (1) abdominal pain or tenderness; (2) persistent vomiting; (3) Clinical fluid accumulation; (4) mucosal bleeding; (5) lethargy; (6) liver enlargement more than 2 cm associated to laboratory parameters as increase in hematocrit (HCT) concurrent with rapid decrease in platelet counts (hemoconcentration or significant increase in hematocrit together with platelet counts bellow 50,000/mm3). Severe DF was considered if patient displayed fever of 2–7 days plus any of the following: (1) Evidence of plasma leakage, such as high or progressively rising hematocrit evidenced by hemoconcentration; pleural effusions or ascites; circulatory compromise or shock (tachycardia, cold and clammy extremities, capillary refill time greater than three seconds, weak or undetectable pulse, narrow pulse pressure or, in late shock, unrecordable blood pressure); (2) Significant (internal) bleeding. [42], [43]. Dengue virus infection was confirmed either by anti-dengue-IgM ELISA, serotype specific reverse transcription-polymerase chain reaction (RT-PCR) or by virus isolation as described earlier [44]. Predominant serotypes was Dengue-2 identified in DF±WS (N = 10) and Severe DF (N = 3) but Dengue-1 was also identified in DF±WS patients (N = 6).
Virus strain and viral stock
Dengue virus type 2 (strain Thailand/16681/1984) [45] was used for virus stock preparation as described elsewhere [46]. Briefly, Aedes albopictus cell clone C6/36 (CRL-1660, ATCC) were maintained at 28°C in Dulbecco's modified Eagle Medium (Gibco/Life Technologies, Foster City, CA, USA) with sodium bicarbonate (Sigma-Aldrich, St. Louis, MO, USA) and supplemented with 5% fetal bovine serum (Hyclone, Logan, UT, USA), 1% penicillin-streptomycin-glutamine (Gibco), 0,5% non-essential amino acids (Gibco) and 10% tryptose phosphate broth (Sigma). C6/36 cell monolayers were infected with DENV-2 and cell culture supernatants were harvested 8 days later when cytopathic effect was observed. A purified DENV-2 stock was obtained by ultracentrifugation at 100,000 g for 1 h and set to a final volume 20 times smaller than initial (see also Fig. S1) [47], [48]. Titration was performed in C6/36 cells using a standard TCID50 (50% tissue culture infective dose) assay as described elsewhere [49]. Uninfected flasks were maintained, also purified and used as negative control (MOCK). Infectivity of ultracentrifuged virus inoculum (UC) was comparable with the original C6/36 supernatant (SNDT) because infection rates obtained with the dilution 1/100 (UC) is similar to the dilution 1/5 (SNDT) as shown in Fig. S1.
Human cell isolation
Cryopreserved peripheral blood mononuclear cells (PBMC) from patients or healthy donors were obtained from density gradient centrifugation of heparinized blood with lymphocyte separation medium (StemCell Technologies, Grenoble, FR). In vitro experiments were performed using fresh PBMC, which were obtained from blood bank donors and isolated as mentioned above. PDCs and monocytes were purified using Human plasmacytoid DC Negative Isolation Kit and Human CD14+ monocytes Isolation Kit, respectively (StemCell Technologies). Cells were cultured in RPMI 1640 (Invitrogen, Gaithersburg, MD, USA) containing 10% fetal bovine serum (Hyclone) and 1% penicillin-streptomycin-glutamine (Gibco) at 37°C in a humidified 5% CO2 chamber according to protocol.
PDC stimulation and coculture with monocytes
Freshly purified pDCs were cultured with DENV-2 at approximately MOI 4 to 20, mock for 18 hours (overnight). Chloroquine (Sigma-Aldrich) was used at 5 µM/well and added before viral stimulation. Cells were harvested and assessed for pDC cell markers and membrane TRAIL expression or plated on coated slides for 3D microscopy. Supernatant was stored at −70°C for cytokine detection. Monocyte infection was performed as already described [46]. Briefly, freshly isolated monocytes were plated overnight followed by infection with DENV-2 at MOI 10, mock or not infected for 48 hours. Soluble human recombinant IFN-α (PBL International, Piscataway, NJ, USA) was added 18 hours before viral infection at 100 IU/mL. For autologous coculture assay, monocytes were cultured overnight in media, meanwhile pDCs were chloroquine-treated or not and then stimulated overnight with CpG A 2216 (InvivoGen, San Diego, CA, USA) at 5 µM or DENV-2 at MOI 20, or not stimulated. Monocytes were then infected with DENV-2 (MOI 10) and pDCs were added at ratio 1∶5 pDC/monocytes as explained in Fig. S2. Cells were harvested and assessed for intracellular DENV antigens.
Flow cytometry
Antibodies for fluorescein isothiocyanate (FITC)-conjugated anti-CD123 or BDCA-2 (Miltenyi Biotec, Auburn, CA), Phycoerythrin (PE)- conjugated CD11c (IOTest/Beckman Coulter, Marseille, FR), Allophycocyanin (APC)-conjugated anti-BDCA-4 (Miltenyi Biotec) and Allophycocyanin-Cy7 (APC-Cy7)-conjugated anti-CD14 (BD Biosciences, San Jose, CA), Vioblue-conjugated anti-CD4 (Miltenyi Biotec), V500 anti-CD3 (BD Biosciences) or with appropriate isotype-matched control antibodies (at 5 mg/mL each) in PBS containing 2% fetal bovine serum (Hyclone) and 2 mM EDTA (Gibco). Human PBMCs or isolated monocytes/pDCs were incubated for 20 min at 4°C with antibody cocktails. Cells were washed twice in ice-cold PBS and flow cytometry acquisition was performed on FACSCanto 7 colors or FACS Aria 13 colors flow cytometers using FACSDiva software (BD Biosciences). CD3− CD4+ CD14− CD123+/BDCA-2+ BDCA-4+ gated cells were then tested for the expression of surface markers using PE-labeled anti-TRAIL (BD Biosciences). Mosquito C6/36 cell line monolayers were washed with PBS-1% bovine serum albumin (Sigma) and incubated for 60 min at 4°C with purified anti-DENV-complex (Millipore, Billerica, MA, USA) then 30 min with goat anti-mouse Alexafluor647 (Molecular Probes/Life Technologies) and fixed. Intracellular antigen staining for C6/36 or cocultures was performed using 2% paraformaldehyde (Sigma) followed by antibodies staining steps with 0,1% saponin (Sigma) buffer. Cells were analyzed by C6 Cytometer (Accuri/BD Biosciences). FlowJo software (Treestar, Ashland, OR, USA) was used to analyze flow cytometry data.
Three dimension (3D) microscopy and immunofluorescence
Cells were plated on poly-L-lysine (Sigma)-coated slides and then fixed in 4% paraformaldehyde (Sigma), quenched with 0.1 M glycine (Sigma). Cells were blocked and incubated in permeabilizing buffer containing 0.1% saponin (Sigma) with mouse anti-TRAIL (clone RIK-2, eBioscience, San Diego, CA) or mouse anti-DENV (clone D3-2H2-9-21, Millipore). TRAIL and DENV staining were revealed using a secondary donkey anti-mouse IgG-Cy3 (Jackson ImmunoResearch, West Grove, PA, USA). Nucleus was stained using DAPI (Molecular Probes/Life Technologies). Mounted slides were scanned with a Nikon Eclipse 90i Upright microscope (Nikon Instruments Europe, Badhoevedorp, The Netherlands) using a 100× Plan Apo VC piezo objective (NA 1.4) and Chroma bloc filters (ET-DAPI, ET-Cy3) and were subsequently deconvoluted with a Meinel algorithm and 8 iterations and analyzed using Metamorph (MDS Analytical Technologies, Winnersh, UK). Overlays were: TRAIL or DENV/DAPI/Trans. ImageJ (NIH, Bethesda, MD, USA) plugin 3D interactive surface plot was used on overlay stack on pDC stained with TRAIL or DENV/DAPI. Quantity of TRAIL and DENV-2 were determined using the measure and label plugin (ImageJ). C6/36 mosquito cell line were plated on slides and fixed with cold acetone. Mosquito cells were stained with mouse anti-DENV complex (Millipore) in PBS-1%bovine serum albumin (Sigma), washed twice with PBS. DENV E protein was revealed with goat anti-mouse Alexafluor488 (Molecular Probes/Life Technologies). Slides were mounted with ProLong Gold with DAPI (Molecular Probes/Life Technologies) and visualized at Evosfl Microscope (AMG, Bothell, WA, USA).
Cytokine detection
Supernatants of pDCs/monocytes or cocultures in presence of DENV-2 or negative controls as well as acute phase plasma from dengue patients were tested for multispecies soluble IFN-α by ELISA (PBL International) according to the manufacturer's instructions. Plasma samples were also tested for soluble TRAIL by ELISA (R&D Systems, Minneapolis, MN, USA)
Statistical analysis
Experiments were repeated at least four times. P values (P) were determined using a two-tailed Student's t test for in vitro data and nonparametric Mann-Whitney test for patient data. P<0.05 was considered statistically significant. Univariate distributions of flow cytometric data were performed by probability binning, in 300 bins using FlowJo software [50].
Results
Dengue patients differentially exhibit TRAIL+ pDCs, soluble IFN-α and TRAIL levels
We studied a cohort of DENV infected patients and classified them regarding the severity of the disease. Detailed demographic, clinical, and laboratorial data from dengue patients are summarized in Table 1. From 43 patients enrolled, 10 were classified as severe DF and the remaining as DF including those with warning signs for severity (WS), according to latest WHO classification [42], [43]. In order to explore pDC activation by DENV infection, we first characterized the CD4+/CD14−/BDCA-2/4+/CD123+ pDC frequency/profile in 40 patients compared to 20 healthy controls (figure 1A).
Figure 1. TRAIL and IFN-α expression on dengue fever (DF) patients.
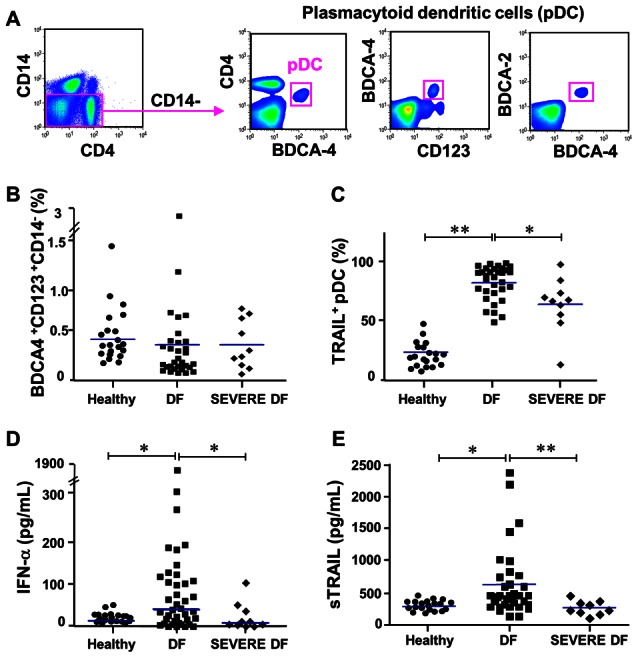
PBMCs from acute dengue fever patients were analyzed for mTRAIL expression on pDCs gated as CD14−, CD3−, CD4+, CD123+ and BDCA-4+ (A). (B) Blood pDC percentages and (C) mTRAIL expressing pDCs were assessed for healthy donors, DF and severe DF patients. (D) IFN-α and (E) Soluble TRAIL were analyzed by ELISA in plasma samples from healthy donors, DF and severe DF patients. Each dot represents one individual and median values are shown as blue bars. Data values were submitted to Mann-Whitney statistical test in which * p<0.05 and **p<0.005.
As described by others [51], pDC frequencies in healthy individuals range from 0.2% to 0.8% of peripheral blood mononuclear cells (PBMCs). We observed no significant differences in pDC frequencies among healthy donors, DF±WS patients or Severe DF patients (figure 1B). We then observed that mTRAIL expression on pDC was increased in DF±WS patients compared to healthy controls or severe DF cases (figure 1C). Therefore, pDCs become activated in dengue patients with regard to mTRAIL expression.
Although pDCs are not the only IFN-α producers, activated pDCs can support a 1000-fold greater production of this factor than other cell types. We next sought a correlation of IFN-α with severity. Soluble IFN-α level in plasma samples from the studied population was determined by ELISA. Similarly to TRAIL+ pDC frequency, we found that DF patients exhibit higher levels of IFN-α compared to healthy controls or Severe DF patients (figure 1D). Indeed, we found a positive correlation between IFN-α levels and TRAIL+ pDCs (Spearman r = 0.36, p<0.05). To further determine the IFN-α role, we quantified soluble TRAIL (sTRAIL) levels that is produced by immune cells and is induced by type I IFN. Similarly to previous data, DF±WS patients displayed elevated sTRAIL in contrast to healthy controls or severe DF patients (figure 1E). Moreover, a strong positive correlation between TRAIL+ pDCs and sTRAIL was determined (Spearman r = 0.60 p<0.005). PDC activation during dengue fever, elevated IFN-α and TRAIL levels is therefore associated with mild dengue fever.
DENV-2 activates pDC leading to TRAIL display at cell surface and IFN-α secretion
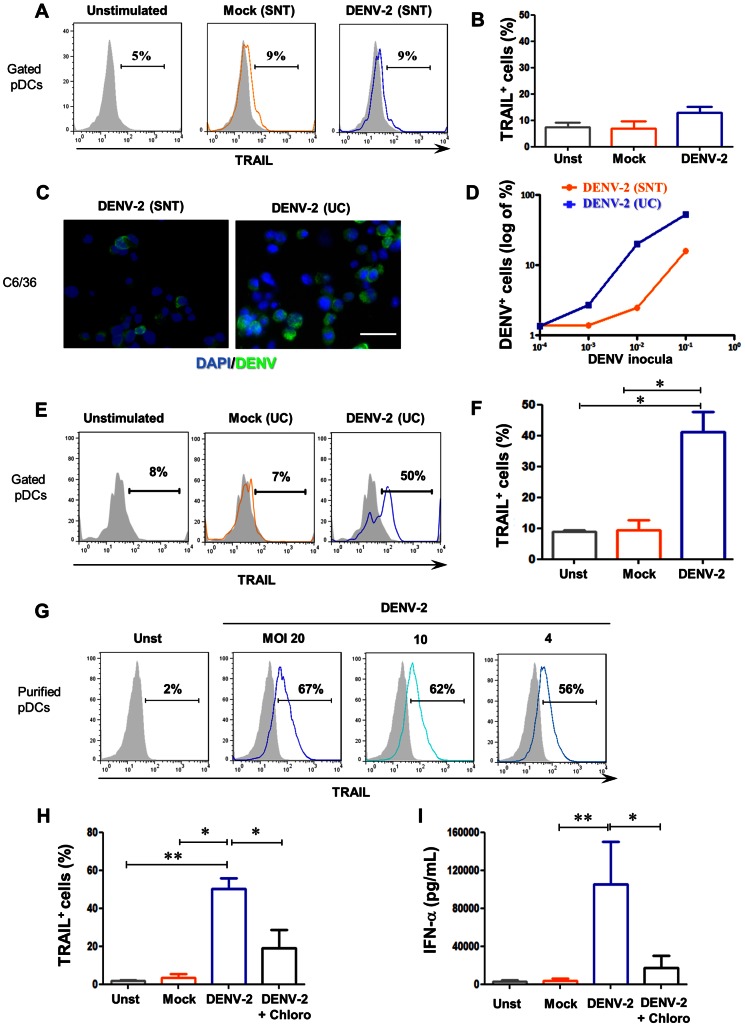
PDC activation by DENV-2 was shown to occur by TLR-7 stimulation after endocytosis [31] and this pathway was crucial for IKpDC transformation by HTLV-1 [48]. To assess pDC activation by DENV-2, peripheral blood mononuclear cells (PBMCs) from healthy donors were stimulated overnight with virus. Initially, we observed that DENV-2 from mosquito cell line supernatant (SNT) promoted a trend, however not statistically significant, in TRAIL detection on pDC surface after viral stimulation in PBMCs, compared to unstimulated or mock-stimulated pDCs (figure 2A and B). Thereafter, an ultracentrifugation of DENV-2 viral stock was performed in order to concentrate viral particles, increasing MOI (figure S1). The DENV-2 infectivity was assayed for both viral stocks by infecting the mosquito cell line C6/36 and comparing them in serial dilutions. Viral antigens were detected inside cells inoculated with concentrated DENV-2 (UC) as early as 48 hours and at higher frequencies than the non-concentrated supernatant indicating that the concentrated virus had enhanced replication rates and it was intracellularly present as detected by immunofluorescence microscopy and flow cytometry (figure 2C and D). This viral stock (DENV-2 UC) was therefore adopted for assessing DENV-2 induced pDC activation in all experiments described in the present work.
Figure 2. Purified DENV-2-induced in vitro mTRAIL expression and IFN-α production by purified plasmacytoid dendritic cells.
PBMCs from healthy donors were stimulated overnight with DENV-2, mock or none (unstimulated). (A) mTRAIL expression profile on pDCs gated from PBMCs (overlay) and (B) mTRAIL positive pDCs for three donors induced by mock SNT (orange) or DENV-2 SNT (blue) using unstimulated (grey fill) pDCs as negative control. DENV positive C6/36 cells infected for 48 h with supernatant of DENV-2-infected C6/36 cells (DENV-2 SNT) or ultracentrifuged DENV-2 SNT (DENV-2 UC) as described in M&M and figure S1. (C) DENV antigens/AlexaFluor488 (green) and nucleus/DAPI (blue) of C6/36 cells infected with DENV-2 SNT (left) and UC (right) at the same inocula dilution (10−3). (D) DENV positive C6/36 cells by flow cytometry in which cells were infected with SNT (orange) or UC (blue) DENV-2 inocula at different dilutions. PBMCs from healthy donors were stimulated overnight with DENV-2 UC, mock UC or none (unstimulated). (E) mTRAIL expression profile on pDCs gated from PBMCs (overlay) and (F) mTRAIL positive pDCs for four donors induced by mock UC (orange) or DENV-2 UC (blue) using unstimulated (grey fill) pDCs as negative control. Freshly purified pDCs were stimulated overnight with DENV-2 UC, mock UC or not (unstimulated). (G) TRAIL expression induced by different MOIs of DENV-2 UC (blue) using unstimulated cells (grey) as negative control. (H) Purified pDCs positive for mTRAIL expression and (I) IFN-α secretion by unstimulated (grey), mock UC (orange), DENV-2-UC-stimulated pDCs pre-treated (black) or not (blue) with chloroquine, for four donors. Values were submitted to paired t test in which * p<0.05 and ** p<0.005.
Therefore, using purified virus in PBMC cultures we observed an increase of mTRAIL detection (figure 2E and F) in 41%±6% of pDCs (CD4+ CD14− BDCA4+ CD123+) compared with less than 10% TRAIL+ pDCs on mock or unstimulated conditions (p<0.05). To exclude pDC bystander activation and to confirm that DENV-2 is directly inducing mTRAIL on pDCs, we assayed purified pDC for TRAIL and IFN-α production. Purified pDC were exposed to different multiplicities of infection (MOI) for DENV-2 and we observed an increased inoculum-dependency of mTRAIL detection by virus-activated pDCs (figure 2G). The mTRAIL displayed on cell surface was mostly blocked when pDCs were pre-treated with chloroquine, an endosomal blocker of TLR activation, supporting the concept of an endocytosis-TLR-dependent TRAIL activation (figure 2H). To further characterize DENV-2-induced activation of pDCs, we measured IFN-α production in purified pDC cultures supernatants. DENV-2-stimulated pDCs produced approximately 10,000-fold more IFN-α than mock-treated or not stimulated pDCs. Chloroquine pre-incubation abrogated most DENV-2-induced IFN-α production (figure 2I). These results confirm that DENV-2 is able to activate pDCs in vitro through endocytosis pathway, responding by TRAIL expression and IFN-α production.
DENV-2 and TRAIL location within pDCs by 3-dimension microscopy
To better characterize DENV-2-activated pDCs, we analyzed them by 3-dimension (3D) microscopy. Focal plane analysis revealed the presence of intracellular TRAIL expression in unstimulated pDC (figure 3A, upper panels), confirming our cytometry data and our previous study [48]. Images also revealed some ‘peripheral’ TRAIL expression that did not seem to be localized in the cytoplasm but rather on the membrane (figure 3A, middle panels). TRAIL expression profile in DENV-2-stimulated pDC did not seem to differ from unstimulated cells, even if TRAIL appeared to be decreased in the cytoplasm at the expense of “peripheral” TRAIL (figure 3A, middle panels). However, it remained hard to distinguish between intracellular and membrane TRAIL profile expression in both conditions without the use of a membrane marker. The blocking of endosomal acidification by chloroquine use revealed the same profile as mock-stimulated pDCs.
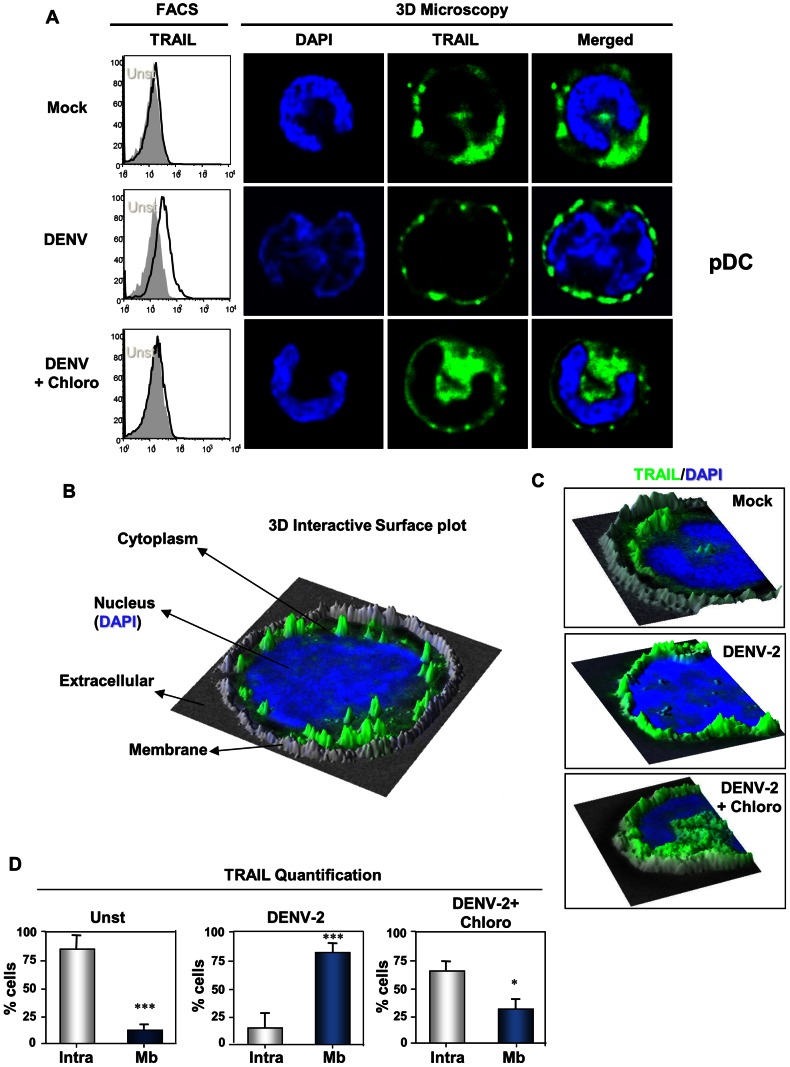
Figure 3. TRAIL localization in DENV-2-activated pDCs by 3D microscopy analysis.
Freshly purified pDCs stimulated with DENV-2 pre-treated or not with chloroquine (Chloro), or mock infected. TRAIL expression was analyzed by flow cytometry or by a 3D microscope. (A) Membrane TRAIL flow cytometry profiles (left column) on pDC stimulated by different stimuli -mock, DENV-2 or DENV-2+Chloro overlaid by unstimulated (grey). Microscopic images from pDC cultured with the mock, DENV-2 or DENV-2+Chloro showing DAPI-colored nucleus, TRAIL staining (green) and overlay. (B) 3D interactive surface plots analysis of 3D microscopic image. Overlay of nucleus (blue), TRAIL (green) and phase contrast (grey) as seen in (C) for different stimuli: DENV-2-stimulated pDC (DENV-2) exhibits membrane TRAIL localization in contrast to DENV-2+Chloro or unstimulated, where TRAIL is detected only intracellularly. (D) Percentage of pDCs expressing intracellular TRAIL only (Intra) or on the membrane (Mb) is shown as percentage of total analyzed cells. Values were submitted to paired t test in which * p<0.05 and *** p<0.0005.
Thus, to better characterize TRAIL localization in pDCs, 3D reconstruction (focal plan, XZ and YZ-stacks) analysis was performed (figure 3B–D). 3D interactive surface plot plugin of ImageJ software combined with phase contrast acquisition allowed us to visualize with precision internal or external localization of TRAIL (membrane delimitation) (figure 3B). This combined analysis clearly showed intracytoplasmic TRAIL repartition of mock stimulated pDC (figure 3B and C upper panel). DENV-2-stimulated pDC (figure 3C, middle panel) mainly harbored membrane TRAIL localization in contrast to the restrictive intracellular TRAIL expression of pDC from mock stimulated cells (figure 3A–B, right panels). The addition of the endocytosis-TLR pathway inhibitor chloroquine induced an intracellular blocking of TRAIL by DENV-2 exposed pDC. Quantification of membrane vs. intracellular TRAIL in pDC by 3D microscopy in independent assay sets demonstrates a clear shift from intracellular to membrane TRAIL location under DENV-2 exposure (figure 3D). We observed that almost all mock stimulated pDCs express only intracellular TRAIL. Chloroquine-treatment of DENV-2-stimulated pDC cultures prevented most TRAIL membrane co-localization on pDCs. Considered together, these results demonstrate that DENV-2 induces TRAIL relocalization from intracellular compartment to pDC plasma membrane.
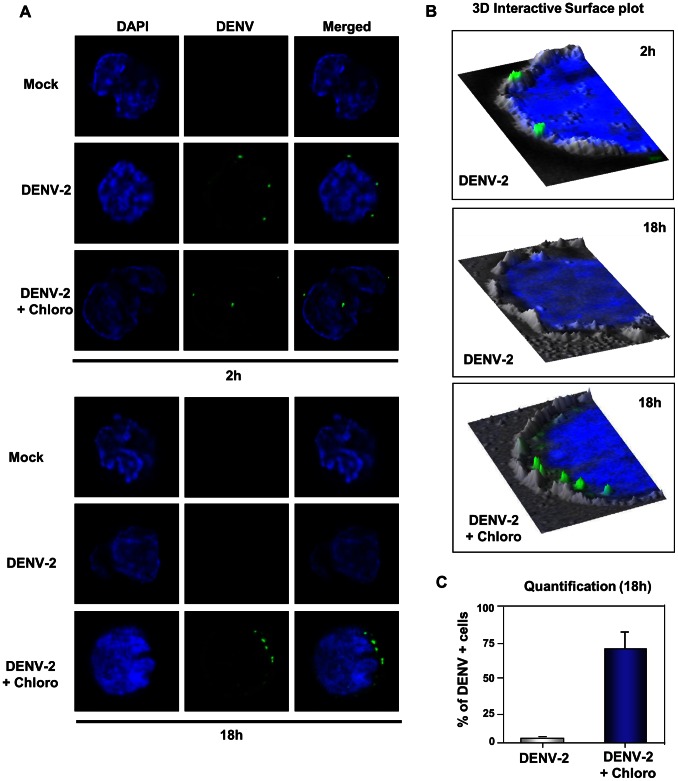
We also attempted to detect virus inside pDCs by 3D microscopy. Because virus is rapidly degraded in endosomes by acid-activated proteases, we analyzed DENV-2 localization as early as 2 hours of viral stimulation. Focal plane images revealed that DENV-2 envelope protein was detected in close proximity to pDC periphery. In contrast, DENV-2 seemed to be intracellular in chloroquine treated pDC (figure 4A, upper panel). However, after overnight culture, DENV-2 labeling was exclusively detected in chloroquine-treated cells (figure 4A, lower panel). As described above for TRAIL detection, a 3D interactive surface plot analysis was performed and clearly showed that DENV-2 was co-localized in the cell membrane after 2 hours of stimulation (figure 4B, upper panel). We did not detect any virus in pDCs, suggesting a complete viral degradation within lysosomes either overnight (figure 4B, middle panel) or after a 2 hour-stimulation. However, DENV-2 particles were detected inside chloroquine-treated pDCs, indicating that chloroquine would probably neutralize acid proteases allowing viral antigen detection within most pDCs (figure 4B, lower panel and 4C). Therefore, within the same stimulus, pDCs exhibit TRAIL relocalization at the time point when no virus was detected, supporting our data for endosomal activation of TRAIL pathway.
Figure 4. 3D microscopy of DENV-2 particles in purified plasmacytoid dendritic cells.
Freshly purified pDCs cultured with DENV-2 pre-treated or not with chloroquine (Chloro), or mock infected were stained with anti-DENV (green) and nucleus was colored with DAPI (blue). (A) pDC images (nucleus, virus and overlay) for mock, DENV-2 and chloroquine-treated plus DENV-2. Inhibition of endosomal acidification (chloroquine) allowed easier detection of DENV particles (DENV-2+Chloro at 2 h or 18 h stimulation). 2 h pDC incubation with DENV-2 was sufficient to detect viral proteins in contrast to the overnight (18 h) DENV-2-incubated pDCs when no virus was detected. (B) pDCs cultured with mock, DENV-2 or DENV-2+chloro were observed by 3D microscope. DENV staining (green) was merged with DAPI (blue)-colored nucleus and with phase contrast (grey). DENV particles were co-localized with pDC cell membrane at 2 h stimulation. Chloroquine allowed DENV-2 detection inside pDCs after 18 h of culture whereas DENV-2 alone did not. Panels shown microscopic images analyzed by 3D interactive surface plot. (C) Quantification of PDCs expressing DENV antigens without (DENV-2) and with chloroquine pre-treatment (DENV-2+Chloro) is shown as percentage of total analyzed cells.
DENV-2 infection is impaired in monocytes during coculture with activated pDCs
Because viral load is considered to be an important factor in dengue severity [8], we next studied the role of pDCs in viral replication. For that purpose, we used primary autologous human monocytes that allow efficient DENV-2 replication in order to assess whether pDC could inhibit viral replication or not. Analysis of purified monocytes infected for 48 hours revealed in 2D microscopy a robust intracellular but not nuclear staining of DENV proteins (figure 5A, lower panel), consistent with flavivirus replication cycle [4]. Considering that pDCs produce high levels of IFN-α upon DENV-2 stimulation, we evaluated its antiviral effect. Monocytes that were pre-treated with IFN-α 24 hours before DENV-2 incubation showed a great reduction in viral antigen detection compared to untreated cells (figure 5B). Quantification by microscopy of DENV-2 positive/negative cells showed that IFN-α treatment reduced by 80% (p<0.001) the number of DENV-2 positive cells (figure 5C) and the same reduction was observed by flow cytometry (figure 5D). We also observed a low production of IFN-α by DENV-infected monocytes and confirmed IFN-α on supernatants of monocytes pre-treated with the cytokine (figure S2). These data are supporting that IFN-α has a restricting antiviral role during DENV infection.
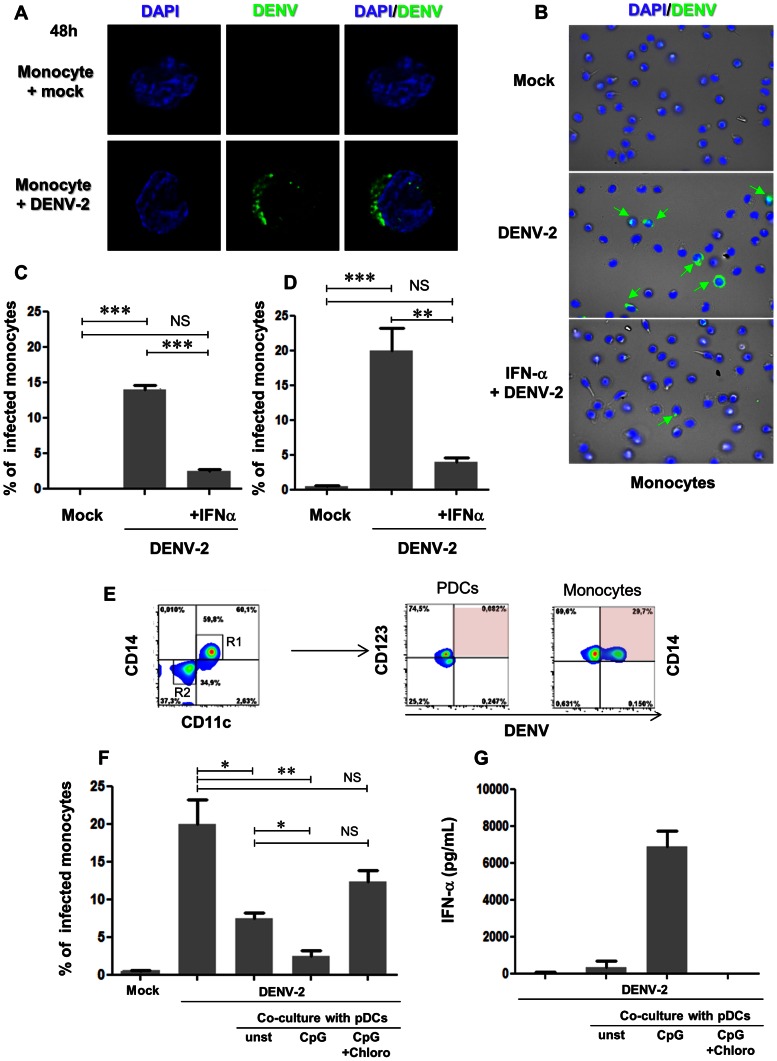
Figure 5. IFN-α treatment and activated pDC coculture in DENV-2-infected monocytes.
Freshly purified monocytes were infected with DENV-2 (MOI 10) for 48 hours, pre-treated or not with IFN-α. (A) Nucleus/DAPI (blue) and virus (green) of monocytes mock- (upper panels) or DENV-2-infected (lower panels). Viral particles were detected in the cytoplasm only in DENV-2-infected monocytes. (B) Nucleus/DAPI (blue), virus (green) and phase contrast (grey) for different stimuli (mock – top, DENV-2 only – middle, IFN-α+DENV-2 – bottom) in monocytes. Green arrows show intracellular detection of DENV particles in DENV-2 infected monocytes, whereas pre-treatment with IFN-α strongly reduced viral antigen detection. Quantification of DENV antigens in mock, DENV-2 and IFN-α pre-treated DENV-2-infected monocytes using microscopy (C) or flow cytometry (D). (E) Freshly purified monocytes were DENV-2-infected then immediately cocultured with DENV-2-stimulated pDCs. DENV antigen detection in CD14-CD11c-CD123+ pDCs or CD14+CD11c+ monocytes after 48 h of culture. Monocytes were DENV-2-infected then immediately cocultured with CpG-stimulated only (CpG), Chroloquine pre-treated CpG-stimulated (CpG+Chloro) or unstimulated (unst) pDCs (figure S2). Cocultures were analysed 48 h later for viral antigens (F) and IFN-α production (G). Data represent independent experiments from two different donors and values were submitted to paired t test in which * p<0.05; ** p<0.005 and *** p<0.0005.
Thus, to determine the potential effect of pDC on DENV infection within monocytes, we cocultured pDCs with infected monocytes. First, we confirmed that viral antigens were only detected in monocytes during cocultures, as only CD14+CD11c+ DENV-2-infected monocytes display DENV antigens compared to CD14−CD11c−CD123+ DENV-2-stimulated pDCs (figure 5E). To activate pDC in a non-viral way, we stimulated them with the TLR-9 agonist CpG, which was reported to induce IFN-α production and TRAIL expression by pDC [48]. DENV-2 detection on monocytes was significantly diminished when cells were incubated with CpG-stimulated pDCs including supernatants (figure 5F). Importantly, non-pre-activated pDCs also diminished DENV-2 infection in monocytes, although to lesser extent. Chloroquine, which is an inhibitor of TLR-9 pathway, blocked pDC activation and partially restored DENV+ cell detection within monocytes. Indeed, IFN-α was highly detected in the cocultures of infected monocytes with CpG-activated pDCs compared to non-pre-activated pDCs (figure 5G). Chloroquine completely blocked IFN-α secretion, suggesting the more IFN-α produced by activated pDCs the less viral antigens are detected. These findings are strongly supportive for an important role for pDC on DENV replication in monocytes.
Finally, we also tested whether IKpDC-mediated apoptosis was involved in reduced DENV-2 detection in monocyte-pDC coculture. Monocytes were infected with DENV-2 or mock and then co-cultured with or without CpG-stimulated with or without pDCs (figure S3). After 48 hours of infection, cultures were collected and stained for AnnexinV and TOPRO3. We observed that the addition of unstimulated pDC to DENV-infected monocytes had no major impact on cell death during co-culture, remarkably, when compared to DENV only. Furthermore, CpG-activated pDCs addition caused an increase survival of monocyte during co-cultures, meanwhile reducing viral antigens. Therefore, we could rule out the killing effect of IKpDCs and once more attribute an antiviral role for IFN-α (and/or TRAIL) in the supernatant.
Discussion
The present work describes features of pDC activation during DENV-2 infection and discusses its importance for disease outcome. We characterized, for the first time, an activated profile of pDCs from dengue patients using membrane TRAIL expression as a marker. Moreover, we observed that, in vitro, activated pDCs exerted an antiviral activity in infected human primary monocytes. Thus, pDCs may contribute to the control of viral clearance and to diminish the severity of the disease.
Upon challenge by viral particles, pDC activation takes place, characterized by upregulation of co-stimulatory markers, and by very high levels of IFN-α secretion [52]. Simultaneously to IFN production, we previously demonstrated that viral-activated pDC also expressed the pro-apoptotic ligand TRAIL on their membrane, which transforms them into IFN-producing Killer pDC (IKpDCs) [48], [53], [54]. For instance, in HIV-1 infection, the number of IKpDCs was correlated to CD4 depletion and disease progression [53]. However, the pDC function depends on the etiology of viral infection. During dengue disease, pDC activation by membrane TRAIL expression was found associated with less severe clinical manifestations. Other studies have also assessed blood pDCs from DENV-infected patients. Reduced absolute numbers of these cells were associated with a poor outcome, because severe cases of dengue disease exhibit a lower number of blood pDCs [32] and low levels of blood pDCs were correlated with high viral loads [33]. Nevertheless, treatment with TLR-3 and -7/8 agonists enhanced pDC activation and reduced viral replication in non-human primate model during DENV infection [55]. Supposedly, a blunted pDC response would allow viral replication to take place. Therefore, we also decided to characterize plasma levels of pDC-related cytokines.
Viral activation of PDC leads to production of IFN-α. Although pDC does not produce sTRAIL, pDC-produced IFN-α leads to production of soluble or membrane bound TRAIL by several cell types including monocytes [56]. Because IFN-α and TRAIL were reported to be antiviral in vitro for DENV, we analyzed the soluble levels in dengue patients. Indeed, plasma levels of both factors were statistically correlated with pDC activation in our cohort. Regarding blood cytokine levels in dengue patients, we find discrepancies in literature [9]. Inflammatory cytokines are increased in severe cases compared to mild forms. Even though, IFN-α was reported in DF and DF severe cases [57], Chen et al. detected higher IFN-α levels in DF compared to severe cases [34], supporting our data. Soluble TRAIL levels were not associated to severe forms but to febrile period and to primary infections [41]; however, we found a negative association between soluble TRAIL and severity. Because TRAIL is a downstream IFN stimulated gene, reduced IFN-α levels could explain low levels of soluble TRAIL in severe patients. Moreover, a weak type I IFN response in severe DF patients could represent a viral escape pathway. Others have reported that some viruses can evade TLR-induced IFN-α production, by inhibiting pDC function through the binding to BDCA-2, a cell surface molecule that functions as IFN secretion inhibitor [58], [59]. Indeed, BDCA-2 attachment can also abolish TRAIL-mediated cytotoxicity of pDCs [60]. It remains to be investigated whether DENV proteins can downregulate pDC function. This could explain why some patients respond efficiently to DENV infection and show high levels of IFN-α and sTRAIL, while others do not produce sufficient levels of the factors (severe cases). Furthermore, elevated numbers of activated pDCs could, by releasing high levels of IFN-α, protect target cells and activate other innate immunity actors, like Natural Killer cells that are associated with mild DF [61]. Therefore we suggest a protective role of activated pDCs during acute phase of dengue virus infection.
We asked whether pDCs could acquire IKpDC phenotype and have a protective role against DENV infection in vitro. DENV-2 induced IFN-α production and TRAIL relocalization from the intracellular compartment (in resting pDC) to pDC membrane (activated pDC) soon after viral exposure, supporting the idea of a rapid response to viruses. A high viral load was necessary to activate pDCs that was only achieved after a concentration procedure using ultracentrifugation protocols [47], [48]. Although purification and ultra-centrifugation protocols may decrease infectious-to-particle ratio [62] our concentrated inoculum displayed improved infectious features. However, we cannot rule out that both non-infectious and infectious particles are activating pDCs in synergism, as it was shown for HIV. Indeed, infectious and AT-2-treated HIV (non-infectious) were both able to activate TLR-7 pathway in pDCs [63]. Apparently, it seems that pDCs need large quantities of virus to be activated or high frequency of viral receptor [64]. HTLV-1 also required high viral loads to activate IKpDCs [48]. Indeed, some discrepancies of IFN-α production by DENV-stimulated pDC are reported that may be result from using low viral loads [65] [66]. Flaviviruses may have acquired intrinsic mechanisms to avoid pattern recognition receptors [67] and consequently pDC activation.
To better elucidate pDC activation by DENV, we studied endocytosis pathway in pDC. Lysosomal acidification was crucial for TRAIL expression and IFN-α production by DENV-2 activated pDC. Another report showed that TLR-7 was the endosomal recognition receptor for DENV-2 by using specific inhibitors and acidification blockers [31] and that endocytosis pathway was crucial for co-stimulatory markers upregulation and IFN-α production [30]. Indeed, DENV-2 particles are detectable in pDC in the early stage (2 h) before viral degradation in lysosomes. However, after 18 h we did not detect viral antigens suggesting an absence of viral replication into pDC. Furthermore, lysosomal acidification impairment allowed detection of DENV-2 in pDC, contrasting with non-treated pDCs, suggesting that viruses are not disassembling. We did not observe an increase of non-structural protein 1 in culture supernatants (data not shown) after viral adsorption, supporting the incapacity for virus replication in pDCs. Our data is in accordance with others, as low levels of replicative negative strand RNA were found inside pDCs [30]. Therefore, we suggest that DENV-2 particle sensing occurs in endosomal compartments. Recognition but not infection of DENV-2 is responsible for IKpDC activation, whereas it leads to TRAIL relocalization and IFN-α production.
We next wonder whether IKpDC and IFN-α could inhibit DENV-2 replication in human monocytes, one of main target cells for DENV. Type I interferon have a crucial role during innate immune responses inhibiting viral replication and spreading of many viruses [68]. Binding and activation of IFN receptors triggers transcription of interferon stimulated genes, which induce products that are able to inhibit several steps of virus replication [69]. We found that DENV-2 infection was strongly diminished by treatment with IFN-α in human monocytes. In accordance with these data, other reports demonstrated that pre-treatment of several susceptible cell lines with type I interferon blocked DENV-2 replication through a protein kinase R (PKR)-dependent mechanism [70], [71], [72]. Indeed, recently, several interferon-stimulated genes such as interferon-inducible trans-membrane (IFITM) proteins were able to inhibit dengue infection in cell lines [73], [74]. However, type I interferon pathway is also subject to interference by many viruses that directly target pathways required for type I interferon response. Monocytes and monocyte-derived dendritic cells can produce IFN-α once they are infected by DENV, however at much lower levels compared to other viruses [66], [75], [76]. Moreover, several reports show degradation of downstream [77], [78], [79] and upstream [65], [80] interferon signaling pathways by DENV non-structural proteins. Although DENV blocks type I IFN pathway, the cytokine still remains protective for other uninfected cells reducing viral spreading during infection as described by others [81].
In our study, infected monocytes co-cultured with IKpDCs displayed a dramatic reduction in viral load that could be partially reversed by lysosomal blockage. Viral detection was negatively related to IFN-α detection in cocultures of monocytes and pDCs. IKpDC activation may play an important role for a rapid viral clearance. TRAIL has been reported as a potential antiviral factor for DENV replication [40]. Because TRAIL expression and production by monocytes is induced by IFN-α [37], we tested several concentrations of recombinant TRAIL on monocytes, and we confirmed the antiviral function as published before [40]. However, membrane TRAIL blockage on IKpDC had minimal effect on viral load or apoptosis during cocultures (data not shown). Moreover, IKpDC had no significant effect on DENV-2-infected monocyte apoptosis, suggesting that the anti-viral effect of pDC is mainly due to IFN-α and/or TRAIL on viral replication and not to cell death. Although, both TRAIL and IFN-α were fundamental in reducing viral load in HIV-infected CD4+ T cell/pDC co-cultures [82], [83]. For DENV, type I interferon was sufficient to largely reduce viral infection rates. Therefore, because we could not demonstrate that IKpDCs have a role in killing infected monocytes, this population may modify the outcome of the disease by producing massive quantities of IFN-α that would in turn block dengue replication in monocytes before adaptive immune responses ensues.
Finally, we showed in this work that DF patients harbored higher frequencies of circulating activated pDC and higher IFN-α/TRAIL levels compare to severe cases. DENV is activating pDC response in terms of IFN-α production and membrane TRAIL expression. We demonstrated that DENV mainly activates the endocytosis pathway and not the infection pathway, as we did not detect viral infection in pDC. Furthermore, our in vitro co-cultures data strongly support a crucial antiviral role for activated pDC and IFN-α by dramatically reducing viral spread. Even though, studies on DENV evasion from pDC response are still needed, we believe that pDC activation in patients' blood may contribute in the future to the establishment of good prognostic immune response together with clinical manifestations/warning signs.
Supporting Information
DENV-2 viral particles concentration by ultracentrifugation and infectivity assays on C6/36 mosquito cells. C6/36 mosquito cell line was DENV-2 infected, supernatant were collected 10 days later and clarified by 1,000 g centrifugation. Cell-depleted supernatant was either stored (DENV-2 SNT) or ultracentrifugated at 100,000 g for 1 hour (DENV-2 UC) and stored (A). C6/36 cells were infected with equivalent dilutions (1–5 of SNT and 1–100 of UC) or not infected (mock) for 48 hours. (B) DENV envelope proteins (green) and DAPI-colored nucleus of C6/36 infected cells with equivalent dilutions of DENV-2 SNT and DENV-UC. White bar represents 25 µm. (C) DENV antigens detected by flow cytometry of C6/36 infected with DENV-2 SNT (orange) and DENV-2 UC (blue) 48 h after infection. (D) DENV antigens extra- and intracellular detection in DENV-2-UC-infected C6/36 cells after viral adsorption (0 h) or at 48 hours (48 h) of infection. Overlay histograms for DENV-2 UC (blue) and uninfected cells (grey). (E) DENV positive C6/36 according to time of infection. All data represents one out of two independent experiments.
(TIF)
Monocytes and pDCs cocultures. Freshly purified monocytes were infected with DENV-2 (MOI 10) for 48 hours, pre-treated or not with IFN-α. (A) IFN-α detection in DENV-2-infected pre-treated or not with IFN-α from three donors. (B) CD14+ purified monocytes were pre-treated or not with IFN-α, as pDCs were differently stimulated. After overnight incubation, monocytes were DENV-infected for 2 h, and then virus inoculum was removed. Whole pDCs cultures were added to infected monocytes during 48 h. DENV positive cells and IFN-α level were analyzed. (C) Isotipic DENV detection in CD14+ CD11c+ DENV-infected monocytes +CD14-CD11c-CD123+ DENV-activated pDCs after 48 h incubation. Data represents one out of two donors.
(TIF)
Apoptosis assay on co-cultures of DENV-infected monocytes and pDCs. Monocytes were infected with DENV-2 or mock and then co-cultured with or without CpG-stimulated with or without pDCs. After 48 hours of infection, cultures were collected and stained for AnnexinV (y axis) and TOPRO3 (x axis). Dot plots represent flow cytometry profiles for one representative donor.
(TIF)
Acknowledgments
We thank Amanda Torrentes de Carvalho, Cintia Marinho, Débora Batista de Oliveira, Ana Rita Motta Castro for their assistance with patient recruitment and sample collection. We also thank Iris Peixoto Alvim and Geraldo Pereira for the help in the FACS Aria Facility, FIOCRUZ. We acknowledge Plinio Barcelar Laboratory, in Campos de Goytacazes, RJ for laboratory confirmation of dengue patients.
Funding Statement
This work was financed by FIOCRUZ/CNRS Agreement, CNPq, FAPERJ, CAPES/COFECUB Program and Agence Nationale de la Recherche sur le Sida. MG is a PhD student financed by CNPQ. NS is a PhD student partially financed by COFECUB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1. Gubler DJ (2002) The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res 33: 330–342. [DOI] [PubMed] [Google Scholar]
- 2.Brazilian-Health-Ministry (2012) VIGILÂNCIA EM SAÚDE. Número de mortes por dengue reduz em 84% http://portalsaude.saude.gov.br/portalsaude/noticia/5106/162/brasil-reduz-em-84-o-numero-%3Cbr%3Ede-mortes-por-dengue-em-2012.html. Portal da Saúde Por Jorge Alexandre Araújo, da Agência Saúde – Ascom/MS.
- 3. Lindenbach BD, Rice CM (2003) Molecular biology of flaviviruses. Advances in virus research 59: 23–61. [DOI] [PubMed] [Google Scholar]
- 4. Mukhopadhyay S, Kuhn RJ, Rossmann MG (2005) A structural perspective of the flavivirus life cycle. Nat Rev Micro 3: 13–22. [DOI] [PubMed] [Google Scholar]
- 5. Gibbons RV, Vaughn DW (2002) Dengue: an escalating problem. Bmj 324: 1563–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martina BE, Koraka P, Osterhaus AD (2009) Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev 22: 564–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kyle JL, Harris E (2008) Global Spread and Persistence of Dengue. Annual Review of Microbiology 62: 71. [DOI] [PubMed] [Google Scholar]
- 8. Wang WK, Chen HL, Yang CF, Hsieh SC, Juan CC, et al. (2006) Slower rates of clearance of viral load and virus-containing immune complexes in patients with dengue hemorrhagic fever. Clin Infect Dis 43: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 9. Srikiatkhachorn A, Green S (2010) Markers of dengue disease severity. Curr Top Microbiol Immunol 338: 67–82. [DOI] [PubMed] [Google Scholar]
- 10. Neves-Souza PC, Azeredo EL, Zagne SM, Valls-de-Souza R, Reis SR, et al. (2005) Inducible nitric oxide synthase (iNOS) expression in monocytes during acute Dengue Fever in patients and during in vitro infection. BMC Infect Dis 5: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu SJ, Grouard-Vogel G, Sun W, Mascola JR, Brachtel E, et al. (2000) Human skin Langerhans cells are targets of dengue virus infection. Nat Med 6: 816–820. [DOI] [PubMed] [Google Scholar]
- 12. Halstead SB (1989) Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Reviews of infectious diseases 11 Suppl 4: S830–839. [DOI] [PubMed] [Google Scholar]
- 13. Durbin AP, Vargas MJ, Wanionek K, Hammond SN, Gordon A, et al. (2008) Phenotyping of peripheral blood mononuclear cells during acute dengue illness demonstrates infection and increased activation of monocytes in severe cases compared to classic dengue fever. Virology 376: 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pang T, Cardosa MJ, Guzman MG (2007) Of cascades and perfect storms: the immunopathogenesis of dengue haemorrhagic fever-dengue shock syndrome (DHF/DSS). Immunol Cell Biol 85: 43–45. [DOI] [PubMed] [Google Scholar]
- 15. Luplertlop N, Misse D, Bray D, Deleuze V, Gonzalez JP, et al. (2006) Dengue-virus-infected dendritic cells trigger vascular leakage through metalloproteinase overproduction. EMBO Rep 7: 1176–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Srikiatkhachorn A (2009) Plasma leakage in dengue haemorrhagic fever. Thromb Haemost 102: 1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dalrymple NA, Mackow ER (2012) Endothelial cells elicit immune-enhancing responses to dengue virus infection. J Virol 86: 6408–6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steinman RM, Granelli-Piperno A, Pope M, Trumpfheller C, Ignatius R, et al. (2003) The interaction of immunodeficiency viruses with dendritic cells. Curr Top Microbiol Immunol 276: 1–30. [DOI] [PubMed] [Google Scholar]
- 19. Steinman RM (2011) Decisions About Dendritic Cells: Past, Present, and Future. Annual review of immunology 30: 1–22. [DOI] [PubMed] [Google Scholar]
- 20. Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, et al. (1997) The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med 185: 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, et al. (1999) The nature of the principal type 1 interferon-producing cells in human blood. Science 284: 1835–1837. [DOI] [PubMed] [Google Scholar]
- 22. Herbeuval JP, Nilsson J, Boasso A, Hardy AW, Kruhlak MJ, et al. (2006) Differential expression of IFN-alpha and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc Natl Acad Sci U S A 103: 7000–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Colonna M, Trinchieri G, Liu YJ (2004) Plasmacytoid dendritic cells in immunity. Nat Immunol 5: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 24. Swiecki M, Colonna M (2010) Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev 234: 142–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crozat K, Beutler B (2004) TLR7: A new sensor of viral infection. Proc Natl Acad Sci U S A 101: 6835–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C (2004) Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303: 1529–1531. [DOI] [PubMed] [Google Scholar]
- 27. Coccia EM, Severa M, Giacomini E, Monneron D, Remoli ME, et al. (2004) Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur J Immunol 34: 796–805. [DOI] [PubMed] [Google Scholar]
- 28. Nightingale ZD, Patkar C, Rothman AL (2008) Viral replication and paracrine effects result in distinct, functional responses of dendritic cells following infection with dengue 2 virus. J Leukoc Biol 84: 1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palmer DR, Sun P, Celluzzi C, Bisbing J, Pang S, et al. (2005) Differential effects of dengue virus on infected and bystander dendritic cells. J Virol 79: 2432–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun P, Fernandez S, Marovich MA, Palmer DR, Celluzzi CM, et al. (2009) Functional characterization of ex vivo blood myeloid and plasmacytoid dendritic cells after infection with dengue virus. Virology 383: 207–215. [DOI] [PubMed] [Google Scholar]
- 31. Wang JP, Liu P, Latz E, Golenbock DT, Finberg RW, et al. (2006) Flavivirus activation of plasmacytoid dendritic cells delineates key elements of TLR7 signaling beyond endosomal recognition. J Immunol 177: 7114–7121. [DOI] [PubMed] [Google Scholar]
- 32. Pichyangkul S, Endy TP, Kalayanarooj S, Nisalak A, Yongvanitchit K, et al. (2003) A blunted blood plasmacytoid dendritic cell response to an acute systemic viral infection is associated with increased disease severity. J Immunol 171: 5571–5578. [DOI] [PubMed] [Google Scholar]
- 33. De Carvalho Bittencourt M, Martial J, Cabie A, Thomas L, Cesaire R (2011) Decreased peripheral dendritic cell numbers in dengue virus infection. J Clin Immunol 32: 161–172. [DOI] [PubMed] [Google Scholar]
- 34. Chen RF, Yang KD, Wang L, Liu JW, Chiu CC, et al. (2007) Different clinical and laboratory manifestations between dengue haemorrhagic fever and dengue fever with bleeding tendency. Trans R Soc Trop Med Hyg 101: 1106–1113. [DOI] [PubMed] [Google Scholar]
- 35. Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, et al. (1997) Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277: 818–821. [DOI] [PubMed] [Google Scholar]
- 36. Wu GS, Burns TF, McDonald ER 3rd, Jiang W, Meng R, et al. (1997) KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nature genetics 17: 141–143. [DOI] [PubMed] [Google Scholar]
- 37. Ehrlich S, Infante-Duarte C, Seeger B, Zipp F (2003) Regulation of soluble and surface-bound TRAIL in human T cells, B cells, and monocytes. Cytokine 24: 244–253. [DOI] [PubMed] [Google Scholar]
- 38. Gura T (1997) How TRAIL kills cancer cells, but not normal cells. Science 277: 768. [DOI] [PubMed] [Google Scholar]
- 39. Cummins N, Badley A (2009) The TRAIL to viral pathogenesis: the good, the bad and the ugly. Curr Mol Med 9: 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Warke RV, Martin KJ, Giaya K, Shaw SK, Rothman AL, et al. (2008) TRAIL is a novel antiviral protein against dengue virus. J Virol 82: 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Becerra A, Warke RV, Martin K, Xhaja K, de Bosch N, et al. (2009) Gene expression profiling of dengue infected human primary cells identifies secreted mediators in vivo. J Med Virol 81: 1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barniol J, Gaczkowski R, Barbato EV, da Cunha RV, Salgado D, et al. (2011) Usefulness and applicability of the revised dengue case classification by disease: multi-centre study in 18 countries. BMC Infect Dis 11: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WHO/TDR. (2009) Dengue: guidelines for diagnosis, treatment, prevention and control – New edition. World Health Organization (WHO) and the Special Programme for Research and Training in Tropical Diseases (TDR) http://whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf downloaded in Oct 2nd, 2012.
- 44. de Azeredo EL, Kubelka CF, Alburquerque LM, Barbosa LS, Damasco PV, et al. (2010) Tissue factor expression on monocytes from patients with severe dengue fever. Blood cells, molecules & diseases 45: 334–335. [DOI] [PubMed] [Google Scholar]
- 45. Halstead SB, Marchette NJ (2003) Biologic properties of dengue viruses following serial passage in primary dog kidney cells: studies at the University of Hawaii. Am J Trop Med Hyg 69: 5–11. [DOI] [PubMed] [Google Scholar]
- 46. Torrentes-Carvalho A, Azeredo EL, Reis SR, Miranda AS, Gandini M, et al. (2009) Dengue-2 infection and the induction of apoptosis in human primary monocytes. Mem Inst Oswaldo Cruz 104: 1091–1099. [DOI] [PubMed] [Google Scholar]
- 47. Shresta S, Kyle JL, Snider HM, Basavapatna M, Beatty PR, et al. (2004) Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical. J Virol 78: 2701–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Colisson R, Barblu L, Gras C, Raynaud F, Hadj-Slimane R, et al. (2010) Free HTLV-1 induces TLR7-dependent innate immune response and TRAIL relocalization in killer plasmacytoid dendritic cells. Blood 115: 2177–2185. [DOI] [PubMed] [Google Scholar]
- 49. Miagostovich MP, Ramos RG, Nicol AF, Nogueira RM, Cuzzi-Maya T, et al. (1997) Retrospective study on dengue fatal cases. Clin Neuropathol 16: 204–208. [PubMed] [Google Scholar]
- 50. Roederer M, Treister A, Moore W, Herzenberg LA (2001) Probability binning comparison: a metric for quantitating univariate distribution differences. Cytometry 45: 37–46. [DOI] [PubMed] [Google Scholar]
- 51. Hosmalin A, Lichtner M, Louis S (2008) Clinical analysis of dendritic cell subsets: the dendritogram. Methods Mol Biol 415: 273–290. [DOI] [PubMed] [Google Scholar]
- 52. Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V (2011) Plasmacytoid dendritic cells: recent progress and open questions. Annual review of immunology 29: 163–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hardy AW, Graham DR, Shearer GM, Herbeuval JP (2007) HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-alpha. Proc Natl Acad Sci U S A 104: 17453–17458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chaperot L, Blum A, Manches O, Lui G, Angel J, et al. (2006) Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. Journal of immunology 176: 248–255. [DOI] [PubMed] [Google Scholar]
- 55. Sariol CA, Martinez MI, Rivera F, Rodriguez IV, Pantoja P, et al. (2011) Decreased dengue replication and an increased anti-viral humoral response with the use of combined Toll-like receptor 3 and 7/8 agonists in macaques. PLoS One 6: e19323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Herbeuval JP, Boasso A, Grivel JC, Hardy AW, Anderson SA, et al. (2005) TNF-related apoptosis-inducing ligand (TRAIL) in HIV-1-infected patients and its in vitro production by antigen-presenting cells. Blood 105: 2458–2464. [DOI] [PubMed] [Google Scholar]
- 57. Kurane I, Innis BL, Nimmannitya S, Nisalak A, Meager A, et al. (1993) High levels of interferon alpha in the sera of children with dengue virus infection. Am J Trop Med Hyg 48: 222–229. [DOI] [PubMed] [Google Scholar]
- 58. Martinelli E, Cicala C, Van Ryk D, Goode DJ, Macleod K, et al. (2007) HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{alpha} secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A 104: 3396–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xu Y, Hu Y, Shi B, Zhang X, Wang J, et al. (2009) HBsAg inhibits TLR9-mediated activation and IFN-alpha production in plasmacytoid dendritic cells. Molecular immunology 46: 2640–2646. [DOI] [PubMed] [Google Scholar]
- 60. Riboldi E, Daniele R, Cassatella MA, Sozzani S, Bosisio D (2009) Engagement of BDCA-2 blocks TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. Immunobiology 214: 868–876. [DOI] [PubMed] [Google Scholar]
- 61. Azeredo EL, De Oliveira-Pinto LM, Zagne SM, Cerqueira DI, Nogueira RM, et al. (2006) NK cells, displaying early activation, cytotoxicity and adhesion molecules, are associated with mild dengue disease. Clin Exp Immunol 143: 345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van der Schaar HM, Rust MJ, Waarts BL, van der Ende-Metselaar H, Kuhn RJ, et al. (2007) Characterization of the early events in dengue virus cell entry by biochemical assays and single-virus tracking. J Virol 81: 12019–12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Herbeuval JP, Hardy AW, Boasso A, Anderson SA, Dolan MJ, et al. (2005) Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: role of type I IFN-producing plasmacytoid dendritic cells. Proc Natl Acad Sci U S A 102: 13974–13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Barblu L, Machmach K, Gras C, Delfraissy JF, Boufassa F, et al. (2012) Plasmacytoid Dendritic Cells (pDCs) From HIV Controllers Produce Interferon-alpha and Differentiate Into Functional Killer pDCs Under HIV Activation. J Infect Dis 206: 790–801. [DOI] [PubMed] [Google Scholar]
- 65. Aguirre S, Maestre AM, Pagni S, Patel JR, Savage T, et al. (2012) DENV inhibits type I IFN production in infected cells by cleaving human STING. PLoS pathogens 8: e1002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rodriguez-Madoz JR, Bernal-Rubio D, Kaminski D, Boyd K, Fernandez-Sesma A (2010) Dengue virus inhibits the production of type I interferon in primary human dendritic cells. J Virol 84: 4845–4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Munoz-Jordan JL, Fredericksen BL (2010) How flaviviruses activate and suppress the interferon response. Viruses 2: 676–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, et al. (2007) Interferons at age 50: past, current and future impact on biomedicine. Nature reviews Drug discovery 6: 975–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang BX, Fish EN (2012) The yin and yang of viruses and interferons. Trends in immunology 33: 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Diamond MS, Roberts TG, Edgil D, Lu B, Ernst J, et al. (2000) Modulation of Dengue virus infection in human cells by alpha, beta, and gamma interferons. J Virol 74: 4957–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Diamond MS, Harris E (2001) Interferon inhibits dengue virus infection by preventing translation of viral RNA through a PKR-independent mechanism. Virology 289: 297–311. [DOI] [PubMed] [Google Scholar]
- 72. Hotta H, Hotta S, Homma M (1984) Effect of interferons on dengue virus multiplication in cultured monocytes/macrophages. Biken J 27: 189–193. [PubMed] [Google Scholar]
- 73. Brass AL, Huang IC, Benita Y, John SP, Krishnan MN, et al. (2009) The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell 139: 1243–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jiang D, Weidner JM, Qing M, Pan XB, Guo H, et al. (2010) Identification of five interferon-induced cellular proteins that inhibit west nile virus and dengue virus infections. J Virol 84: 8332–8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Reis SR, Sampaio AL, Henriques MG, Gandini M, Azeredo EL, et al. (2007) An in vitro model for dengue virus infection that exhibits human monocyte infection, multiple cytokine production and dexamethasone immunomodulation. Mem Inst Oswaldo Cruz 102: 983–990. [DOI] [PubMed] [Google Scholar]
- 76. Gandini M, Reis SR, Torrentes-Carvalho A, Azeredo EL, Freire Mda S, et al. (2011) Dengue-2 and yellow fever 17DD viruses infect human dendritic cells, resulting in an induction of activation markers, cytokines and chemokines and secretion of different TNF-alpha and IFN-alpha profiles. Mem Inst Oswaldo Cruz 106: 594–605. [DOI] [PubMed] [Google Scholar]
- 77. Ashour J, Laurent-Rolle M, Shi PY, Garcia-Sastre A (2009) NS5 of dengue virus mediates STAT2 binding and degradation. J Virol 83: 5408–5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jones M, Davidson A, Hibbert L, Gruenwald P, Schlaak J, et al. (2005) Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J Virol 79: 5414–5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mazzon M, Jones M, Davidson A, Chain B, Jacobs M (2009) Dengue virus NS5 inhibits interferon-alpha signaling by blocking signal transducer and activator of transcription 2 phosphorylation. J Infect Dis 200: 1261–1270. [DOI] [PubMed] [Google Scholar]
- 80. Yu CY, Chang TH, Liang JJ, Chiang RL, Lee YL, et al. (2012) Dengue virus targets the adaptor protein MITA to subvert host innate immunity. PLoS pathogens 8: e1002780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ho LJ, Hung LF, Weng CY, Wu WL, Chou P, et al. (2005) Dengue virus type 2 antagonizes IFN-alpha but not IFN-gamma antiviral effect via down-regulating Tyk2-STAT signaling in the human dendritic cell. J Immunol 174: 8163–8172. [DOI] [PubMed] [Google Scholar]
- 82. Machmach K, Leal M, Gras C, Viciana P, Genebat M, et al. (2012) Plasmacytoid dendritic cells reduce HIV production in elite controllers. J Virol 86: 4245–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Barblu L, Machmach K, Gras C, Delfraissy JF, Boufassa F, et al. (2012) Plasmacytoid Dendritic Cells (pDCs) From HIV Controllers Produce Interferon-alpha and Differentiate Into Functional Killer pDCs Under HIV Activation. J Infect Dis 206: 790–801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DENV-2 viral particles concentration by ultracentrifugation and infectivity assays on C6/36 mosquito cells. C6/36 mosquito cell line was DENV-2 infected, supernatant were collected 10 days later and clarified by 1,000 g centrifugation. Cell-depleted supernatant was either stored (DENV-2 SNT) or ultracentrifugated at 100,000 g for 1 hour (DENV-2 UC) and stored (A). C6/36 cells were infected with equivalent dilutions (1–5 of SNT and 1–100 of UC) or not infected (mock) for 48 hours. (B) DENV envelope proteins (green) and DAPI-colored nucleus of C6/36 infected cells with equivalent dilutions of DENV-2 SNT and DENV-UC. White bar represents 25 µm. (C) DENV antigens detected by flow cytometry of C6/36 infected with DENV-2 SNT (orange) and DENV-2 UC (blue) 48 h after infection. (D) DENV antigens extra- and intracellular detection in DENV-2-UC-infected C6/36 cells after viral adsorption (0 h) or at 48 hours (48 h) of infection. Overlay histograms for DENV-2 UC (blue) and uninfected cells (grey). (E) DENV positive C6/36 according to time of infection. All data represents one out of two independent experiments.
(TIF)
Monocytes and pDCs cocultures. Freshly purified monocytes were infected with DENV-2 (MOI 10) for 48 hours, pre-treated or not with IFN-α. (A) IFN-α detection in DENV-2-infected pre-treated or not with IFN-α from three donors. (B) CD14+ purified monocytes were pre-treated or not with IFN-α, as pDCs were differently stimulated. After overnight incubation, monocytes were DENV-infected for 2 h, and then virus inoculum was removed. Whole pDCs cultures were added to infected monocytes during 48 h. DENV positive cells and IFN-α level were analyzed. (C) Isotipic DENV detection in CD14+ CD11c+ DENV-infected monocytes +CD14-CD11c-CD123+ DENV-activated pDCs after 48 h incubation. Data represents one out of two donors.
(TIF)
Apoptosis assay on co-cultures of DENV-infected monocytes and pDCs. Monocytes were infected with DENV-2 or mock and then co-cultured with or without CpG-stimulated with or without pDCs. After 48 hours of infection, cultures were collected and stained for AnnexinV (y axis) and TOPRO3 (x axis). Dot plots represent flow cytometry profiles for one representative donor.
(TIF)