Abstract
Gene transfer and drug selection systems that enforce ongoing transgene expression in vitro and in vivo which are compatible with human pharmaceutical drugs are currently underdeveloped. Here, we report on the utility of incorporating human enzyme muteins that confer resistance to the lymphotoxic/immunosuppressive drugs methotrexate (MTX) and mycophenolate mofetil (MMF) in a multicistronic lentiviral vector for in vivo T lymphocyte selection. We found that co-expression of human dihydrofolate reductase (DHFRFS; L22F, F31S) and inosine monophosphate dehydrogenase II (IMPDH2IY; T333I, S351Y) conferred T cell resistance to the cytocidal and anti-proliferative effects of these drugs at concentrations that can be achieved clinically (up to 0.1 µM MTX and 1.0 µM MPA). Furthermore, using a immunodeficient mouse model that supports the engraftment of central memory derived human T cells, in vivo selection studies demonstrate that huEGFRt+DHFRFS+IMPDH2IY+ T cells could be enriched following adoptive transfer either by systemic administration of MTX alone (4.4 -fold), MMF alone (2.9-fold), or combined MTX and MMF (4.9-fold). These findings demonstrate the utility of both DHFRFS/MTX and IMPDH2IY/MMF for in vivo selection of lentivirally transduced human T cells. Vectors incorporating these muteins in combination with other therapeutic transgenes may facilitate the selective engraftment of therapeutically active cells in recipients.
Introduction
A continuing unmet need for genetically engineered cellular therapies is the development of drug selection platforms that are non-immunogenic, and, that enable selection to occur either in vitro or in vivo in humans. While a number of drug-resistance enzymes have been employed for selection of gene modified cells, including O6-mehtylguanine-DNA-methyltransferease (MGMT), multidrug resistance associated protein 1 (MDR1), bacterial hygromycin resistance gene (Hy) and neomycin phosphotransferase (neo) variants [1], many of these selective transgenes have proven disadvantageous in the clinic. For example, transgenes of non-human origin used for in vitro selection (e.g., Hy, neo, and Herpes simplex thymidine kinase, HSV-tk), often lead to immunological rejection of gene-modified cells [2]–[6]. Additionally, when MDR1-transduced autologous CD34+ cells were transferred into cancer patients, no enrichment of transduced cells was detected following etoposide treatment [7], [8]. Previous clinical trials of MGMT-, neoR- and Hy- mediated selection have also been halted due to safety concerns with long-term administration of selection drugs, (i.e., with DNA-alkalizing agents, neomycin, and hygromycin respectively) [1], [9]. Thus, there is a need for alternative strategies that will enable drug selection of gene modified cells with a tolerable toxicity profile in human patients.
Genetically engineered T cells expressing scFv chimeric receptors or TCR transgenes hold significant promise for the treatment of infectious and malignant diseases [10]–[14]. The therapeutic responses have been shown to correlate with the levels of long-term T cell persistence following adoptive transfer of gene-engineered T cells to patients [10]. While depletion of lymphocytes and exogenous cytokine administration can improve T cell persistence, their effects are not uniform [15]. One potential approach to further improve T cell persistence is to develop more effective in vivo selection strategies for gene-engineered cells in humans. One strategy would be the inclusion of a drug-resistance gene that would provide a selective proliferative advantage to the gene-modified cells upon drug administration to patients.
Two drugs of potential utility in such a strategy are methotrexate (MTX) and mycophenolate mofetil (MMF), which competitively inhibit dihydrofolate reductase (DHFR), involved in synthesis of thymidylate nucleotides [16], and inosine-5′- monophosphate dehydrogenase II (IMPDH2), a rate-limiting enzyme in the de novo synthesis of guanosine nucleotides [17], [18] respectively. Proliferation of T and B cells is dependent on the activity of both DHFR and IMPDH2 [19], and thus MTX and MMF are known to inhibit the proliferation and survival of T lymphocytes [20]. Previous studies demonstrate that a double point mutation in the human IMPDH2 gene, substituting both Thr333 to Ile, and Ser351 to Tyr (IMPDH2IY) [8] confers resistance to mycophenolic acid (MPA), an active metabolite of MMF. Likewise, a double point mutant of human DHFR with substitutions of Leu22 to Phe, and Phe31 to Ser (DHFRFS) [16], confers resistance to MTX. The products of these two mutant transgenes decrease binding to MTX and MMF (prodrug of MPA) [21], while retaining enzymatic activity in synthesizing purine and pyramidine nucleotides [20]. Expression of the trans-dominant DHFRFS/IMPDH2IY genes is therefore hypothesized to permit the in vivo selection of transduced cells with MTX/MMF without disabling nucleotide synthesis.
The objective of this study was to confer dual resistance of primary human T cells to MTX and MMF for the purpose of mediating selection of gene-modified T cells in vivo when treated with either drug alone or both drugs. Here, we investigated the ability of DHFRFS and IMPDH2IY to confer resistance of primary human T cells to MTX and MMF both in vitro, and in an in vivo mouse xenograft model. Overall, we found that the expression of DHFRFS and IMPDH2IY supported the preferential expansion and selection of transduced over non-transduced T cells following administration of MTX and MMF at dosing schedules that were minimally toxic to animals.
Results
Gene Modification of Human Central Memory Derived T cells for MTX and MMF Resistance
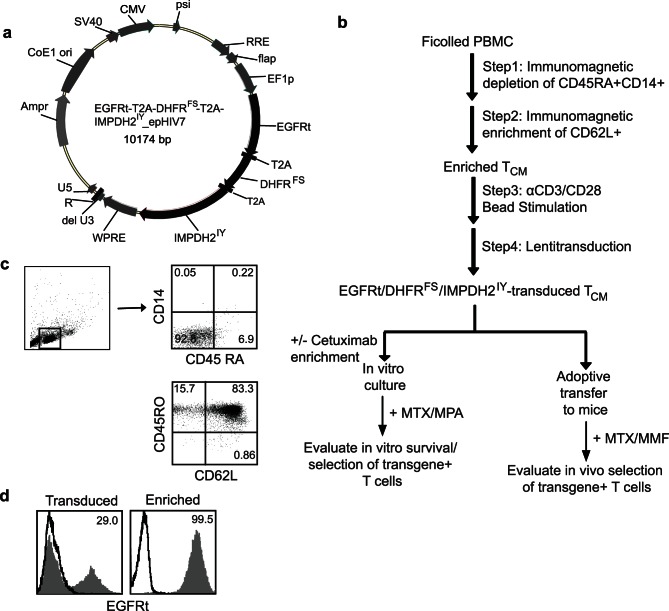
To compare MTX- and MMF-mediated cell selection strategies, either singly or in combination, we designed a lentiviral vector to direct the co-expression of DHFRFS, IMPDH2IY and a truncated human EGF receptor (huEGFRt) [22]. The transgenes are expressed from a single EF-1α promoter, with each polypeptide sequence separated by the ribosomal skip T2A sequence [23] for translation of three proteins from one transcribed message ( Fig. 1a ). EGFRt functions as a way to mark gene modified cells and allows for alternative cell selection via Erbitux™ [22]. We chose to evaluate MTX and MMF drug selection in central memory derived T (TCM) cells, a sub-population of CD62L+CD45RO+ T cells, which have been shown to have favorable properties for therapeutic application including the capacity for self renewal, proliferation, long-term persistence, and an ability to differentiate into effector T cells [10], [15], [24]. As previously published, we consistently enrich TCM cells to greater than 70% purity from peripheral blood by first depleting naïve T cells (CD45RA) and monocytes (CD14), then positively selecting for CD62L+ cells ( Fig. 1b and 1c ) [25]. Here, the enriched TCM (83% CD62L+CD45RO+, Fig. 1c ) were then transduced with an EGFRt-T2A-DHFRFS-T2A-IMPDH2IY_epHIV lentiviral vector ( Fig. 1a ), which typically yields greater than 20% cell transduction as assessed by cell surface expression of huEGFRt ( Fig. 1d ). These engineered cells could then be further immunomagnetically enriched to greater than 98% purity with cetuximab based on expression of huEGFRt ( Fig. 1d ).
Figure 1. Experimental system for evaluating selection of gene-modified T cells in NSG mice.
(a), Plasmid map of construct containing drug resistance genes DHFRFS and IMPDH2IY, huEGFRt and T2A gene sequences (black) that was used to genetically alter primary human T cells. Lentiviral vector backbone (epHIV7) - related sequences are depicted in grey. (b), Schematic for isolation, genetic modification and selection of primary human T cells. (c), CD45RA and CD14 staining of mononuclear cells after sorting from PBMC (top), and CD62L and CD45RO staining of T cells after enriching from PBMC (bottom). The percent positive cells in each quadrant are indicated. (d), Primary human T cells were transduced with above lentiviral vector; transduced T cells and immunomagnetic sorted EGFRt+ T cells were stained for EGFRt expression and analyzed for transduction efficiency by flow cytometry. The percent EGFRt+ T (grey histogram; isotype control-dark line) cells are depicted.
huEGFRt/DHFRFS/IMPDH2IY-engineered Primary Human TCM are Resistant to Cytocidal Concentrations of MTX and MPA
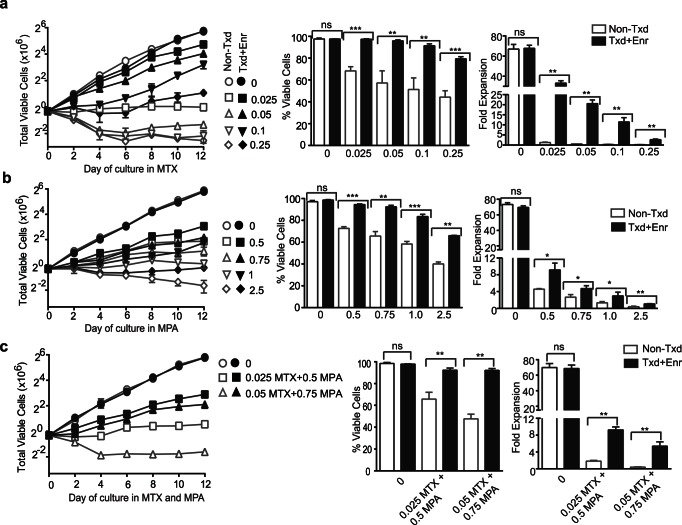
To examine the resistance of huEGFRt/DHFRFS/IMPDH2IY -transduced TCM to MTX and MPA, we first examined engineered cells that had been immunomagnetically enriched for huEGFRt+ expression (>98% purity; Fig. 1d ). As expected, huEGFRt-enriched cells maintained a T cell phenotype as assessed by surface expression of CD4, CD8, CD28, CD45, TCRαβ and CD127 (Fig. S1), and displayed equally potent functional activity, as compared to non-transduced control TCM (Fig. S1). These uniformly positive huEGFRt (>98%) -expressing populations enabled us to determine the optimal drug selection concentrations for huEGFRt+/DHFRFS/IMPDH2IY-engineered TCM. To this end, non-transduced vs. transduced and EGFRt-enriched (EGFRt+) cells were plated in MTX (0–0.25 µM) ( Fig. 2a ), MPA (0–2.5 µM) ( Fig. 2b ), and combinations of both ( Fig. 2c ). In the absence of MTX and MPA, the non-transduced and EGFRt+ T cells expanded at an equivalent rate (70.0±4.6 and 68.6±3.0 fold). Following incubation with MTX for 12 days, non-transduced T cells did not expand at concentrations ≥0.05 µM MTX and a decrease in viability from 92% to 57.1±11.2% ( Fig. 2a ). In contrast, huEGFRt+/DHFRFS/IMPDH2IY T cells expanded 20.5±1.8-fold at 0.05 µM and 11.4±2.1-fold at 0.1 µM MTX with 93.6±2.7% viable cells. ( Fig. 2a ). In the presence of MPA, huEGFRt+/DHFRFS/IMPDH2IY T cells expanded 4.7±0.6-fold at 0.75 µM and 3±0.9-fold at 1 µM MPA over 12 days with 83.4±2.1–92.3±1.4% viable cells, whereas non-transduced cells did not expand and cell viability decreased to 65.6±4.1% and 58.3±2.3% at these concentrations ( Fig. 2b ). Furthermore, we analyzed the survival and expansion of huEGFRt+/DHFRFS/IMPDH2IY T cells in media containing combinations of 0.025 µM MTX +0.5 µM MPA and 0.05 µM MTX +0.75 µM MPA over 12 days. The transduced, huEGFRt-enriched T cells expanded 9.2±0.7 and 5.4±1.0-fold, respectively, with 92.2±1.6% viability, whereas non-transduced cells did not expand and cell viability decreased to 65.7±6.3% and 47.6±4.3% ( Fig. 2c ). These data indicate that both DHFRFS and IMPDH2IY can confer drug resistance to primary human T cells, allowing them to expand and maintain high cell viability (≥90%) upon culture in MTX and MPA. The growth mediated by DHFRFS in the presence of MTX was more robust than that mediated by IMPDH2IY in MPA over the short-term two-week culture period.
Figure 2. Primary human T cells transduced to DHFRFS/IMPDH2IY transgenes are resistant to MTX and MPA.
Non-transduced T cells (non-Txd; grey line/bar) and immunomagnetically-enriched EGFRt+ T cells (99.5% EGFRt+; Txd+Enr; black line/bar) were plated on day 8 at the indicated concentrations of MTX (a), MPA (b), and a combination of MTX+ MPA (c), cells were followed for total viable cell number, percentage of viable cells, and fold expansion for12 days. Equal numbers of cells were plated in triplicate wells of 24-well plates. The data represent the mean ± S.D. There was a significant difference in viability and fold expansion at day 12 between the non-transduced (Non-Txd) and the transduced, EGFRt-enriched T cells (Txd+Enr) at each drug concentration. ***, p≤0.0002; **, p≤0.001; *, p≤0.01. The data are representative of three separate experiments.
huEGFRt/DHFRFS/IMPDH2IY Primary Human T cells Maintain their Phenotype and Function Following Growth in MTX and MMF
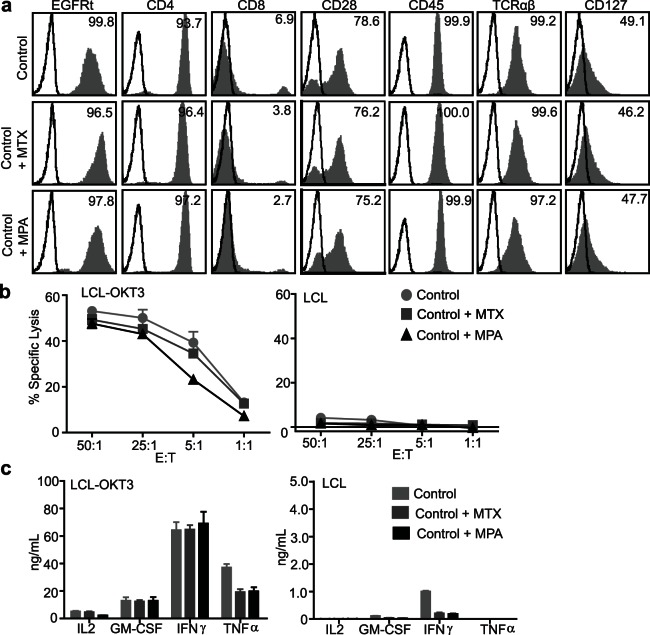
MTX and MPA are lymphotoxic drugs known for their cytocidal activity as well as effects to suppress lymphocyte proliferation [16]–[20]. We therefore assessed whether MTX or MPA, at drug concentrations optimized above for proliferation and viability, alter the phenotype or function of huEGFRt/DHFRFS/IMPDH2IY TCM. For these studies, transgene expressing huEGFRt/DHFRFS/IMPDH2IY TCM was EGFRt-enriched and then treated with or without 0.1 µM MTX or 1 µM MPA for 14 days. The effect of DHFRFS and IMPDH2IY expression and MTX and MPA selection on skewing the phenotype and function of primary human TCM is unknown. In the presence or absence of either MTX or MPA, huEGFRt/DHFRFS/IMPDH2IY TCM expressed comparable levels of CD4, CD8, CD28, CD45, TCRαβ and CD127 surface expression ( Fig. 3a ). Further, using lymphoblastoid cells that express the CD3 agonist OKT3 (LCL-OKT3) as stimulators, the cytolytic potency and activation-dependent cytokine production (IL-2, GM-CSF, IFN-γ and TNF-α) of enriched huEGFRt/DHFRFS/IMPDH2IY T cells were identical in the presence or absence of either MTX or MPA ( Fig. 3b, c ). Together, these results suggest that MTX and MPA exposure of transduced T cells does not overtly alter T cell surface phenotype, cytolytic function or cytokine levels.
Figure 3. Immunomagnetically enriched huEGFRt/DHFRFS/IMPDH2IY T cells maintained their cell surface phenotype and effector function upon culture in MTX and MPA.
(a), Cell surface expression of EGFRt, CD4, CD8, CD28, CD45, TCRαβ and CD127 (grey histogram) vs. isotype control antibody (open histogram) on EGFRt-enriched T cells after 14 days culture +/−0.1 µM MTX or 1 µM MPA were analyzed by flow cytometry. Percentage of positive cells is indicated. (b), Cytotoxic activity of the same cells in (a) after 4 hr co-culture with 51Cr- labeled OKT3-expressing LCL (LCL-OKT3) or negative control LCL targets. Mean percent of 51Cr release ± S.D. of triplicate wells is depicted. (c), Production of cytokines IL-2, GM-CSF, IFN-γ and TNF-α by the same cells as described in (a). Supernatants were collected after overnight co-culture of the same cells in (a) with LCL-OKT3 or negative control LCL stimulators, and mean (±S.D. of triplicate wells) cytokine levels were quantified by cytometric bead array.
MTX/MPA-mediated in vitro Selection of huEGFRt/DHFRFS/IMPDH2IY Transduced Primary Human T cells
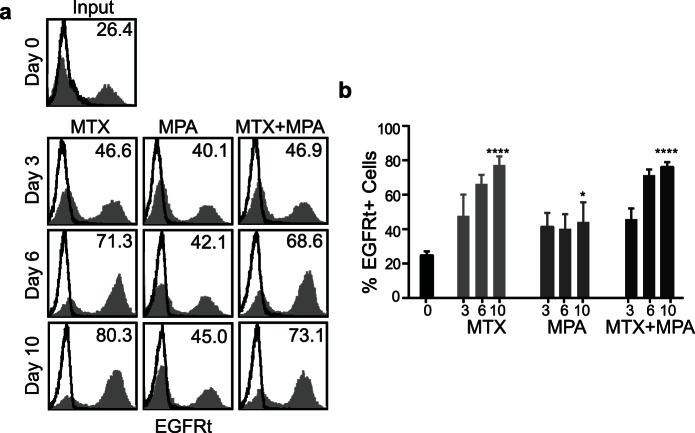
To determine to what degree the gene modified T cells could be selected in vitro, transduced primary human T cells were evaluated following short-term culture with either MTX alone, MPA alone or dual drug-selection with MTX and MPA. For these studies we used huEGFRt/DHFRFS/IMPDH2IY TCM that were 24.8±2.3% transgene positive based on EGFRt staining ( Fig. 4 ). Cells were then cultured for 10 days in 0.1 µM MTX, 1 µM MPA, or 0.05 µM MTX +0.75 µM MPA. Comparison of huEGFRt expression in 0.1 µM MTX revealed a 3.1±0.3-fold DHFRFS-mediated selection from a baseline of 24.8±2.3% to 77.3±5.0% at day 10. ( Fig. 4 ). Transduced T cells cultured in MPA were enriched to 44.2±9.2% (a 1.8±0.4-fold selection), while treatment with MTX and MPA enriched the transduced T cells to 76.1±2.8% (a 3.0±0.3-fold selection). Taken together, these findings demonstrate that both DHFRFS and IMPDH2IY can confer drug-mediated selection of T cells in short-term (10-day) in vitro cultures, with that mediated by DHFRFS in the presence of MTX apparently being more robust that that mediated by IMPDH2IY in MPA.
Figure 4. Primary human T cells transduced to express huEGFRt/DHFRFS/IMPDH2IY can be selected in vitro with MTX and MPA.
(a), Representative flow cytometric evaluation of EGFRt transgene expression (grey histograms) on transduced T cells over 10 days of culture in 0.1 µM MTX, 1 µM MPA, or 0.05 µM MTX +0.75 µM MPA. Percentage of positive cells above control staining (open histograms) is indicated in each histogram. (b), Graphical depiction of the percentages of EGFRt+ cells shown in (a). Equal numbers of three different gene-modified T cell lines were each plated in a 6-well plate at the indicated drug concentrations. The data represent means ± S.D. There was a significant difference between the cells on D0 vs. D10 at either 0.1 µM MTX, 1 µM MPA, or 0.05 µM MTX +0.75 µM MPA (****, p≤0.0001; *, p≤0.05).
Development of a Non-toxic Drug Regimen for in vivo Selection of T cells
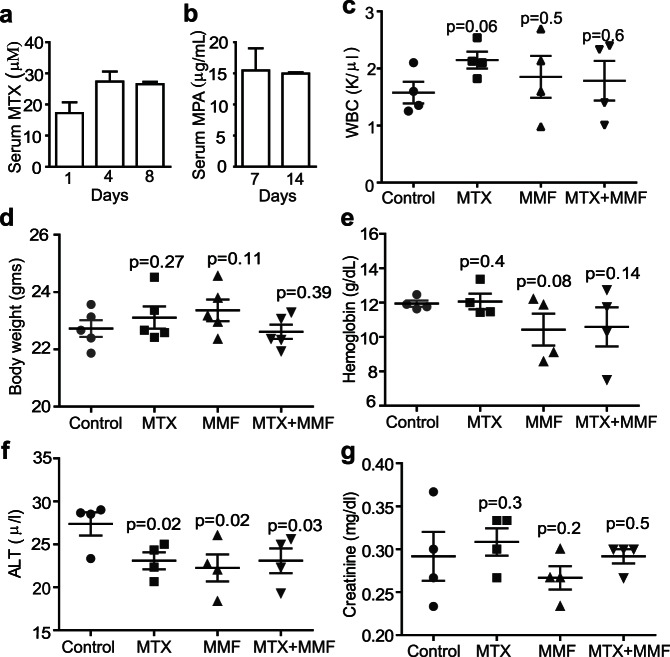
To assess the potential of DHFRFS and IMPDH2IY to allow for in vivo selection of gene-modified T cells, we first optimized the dosage regimens of MTX and MMF (i.e., the prodrug of MPA) that could be tolerated by NSG mice without deleterious effects. Intraperitoneal administration of MTX 3 times over a 2-week period at 25 mg/kg body weight was found to result in average MTX serum concentrations of 17.3±6.0 µM, 27.4±5.5 µM, and 26.6±1.3 µM when blood was harvested 30 minutes post i.p. injection on days 1, 4, and 8 respectively ( Fig. 5a ). For MMF, mice received medicated feed (0.563% MMF) for two consecutive weeks ( Fig. 5b ) in order to maintain more continuous active serum MPA levels, since MPA is known to convert over time to the inactive metabolite glucuronide via uridine 5′ diphosphoglucuronyltransferase [26]. Serum concentrations of MPA achieved with this delivery regimen were 15.5±6.2 µg/ml at day 7, 15.0±0.35 µg/ml at day 14, and then undetectable 7 days after the medicated feed was withdrawn (day 21). Pilot experiments established that these MTX and MMF dosing regimens were sufficient for controlling the proliferation of a murine lymphoblast cell line (CTLL2) in NSG mice (data not shown). Importantly, we found that these drug regimens were well tolerated by mice. There was no significant decrease or increase in white blood cell counts ( Fig. 5c ), mouse bodyweight ( Fig. 5d ), hemoglobin ( Fig. 5e ), alanine aminotransferase (ALT) as a measure of liver function ( Fig. 5f ), or creatinine levels as a measure of kidney function ( Fig. 5g ) (p≥0.05) compared to control mice. Collectively, these findings suggested that these drug dose concentrations are of minimal of toxicity and enabled the assessment of our DHFRFS/IMPDH2IY platform for in vivo selection of transduced primary human T cells in NSG mice.
Figure 5. Establish non-toxic MTX and MMF dose regimens for in vivo selection.
6–8 week old NSG mice (n = 6) were (a), administered MTX by i.p. injection at 25 mg/kg/day twice a week the first week (days 1 and 4) and only once the second week (day 8), and/or (b), fed 0.563% MMF medicated feed for 2 weeks. Serum MTX and MPA levels in each case were analyzed by HPLC. Drug toxicity was then monitored by measuring (c), white blood cell counts, (d), body weight, (e), Hemoglobin, (f), ALT, and (g), creatinine levels. (c–g), Mean levels of each measurement (± S.D) after the last treatment (i.e., at day 14) are depicted. There was no significant difference between control and treatment mice (p≥0.05). Data were evaluated between control and treatment mice using an unpaired, two-tailed Student’s t - test.
In vivo Selection of Primary Human T cells Transduced with huEGFRt/DHFRFS/IMPDH2IY
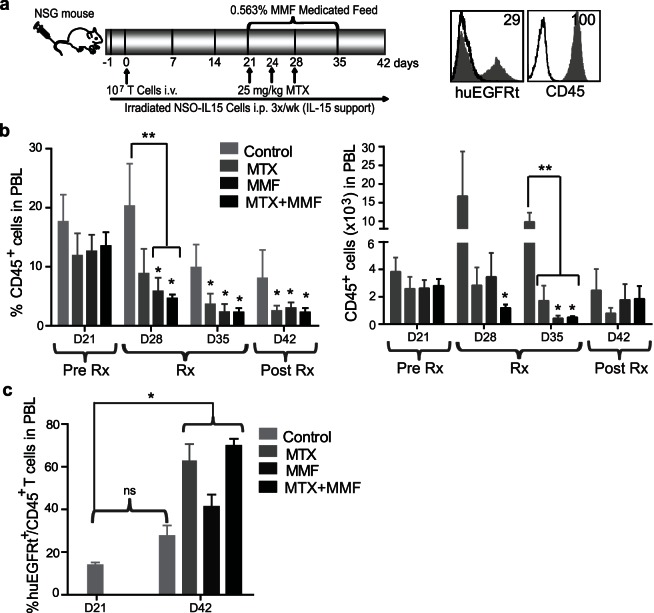
To examine the utility of the DHFRFS/MTX and IMPDH2IY/MPA selection platforms for selecting genetically engineered T cells in vivo, we used a human IL-15 reconstituted NSG mouse model [24] to engraft a mixed population of transduced T cells (29% EGFRt positive) ( Fig. 6a ). The engrafted human cells in mouse peripheral blood were tracked by flow cytometric analysis of human CD45 and EGFRt. Prior to drug treatment, the mean percentage of CD45 cells in the peripheral blood of all groups of mice was 12.6±2.8 at day 21. MTX and/or MPA were then administered to the mice for two weeks ( Fig. 6a ), and seven days after the drug dosing regimens were completed (day 42), the percentage of engrafted CD45+ cells significantly decreased to 2.1±0.9 (p = 0.015) for MTX, 3.0±0.9 (p = 0.005) for MMF, and 1.9±0.7 (p = 0.0004) after combined MTX and MMF administration. This is in contrast to that seen in the control group (6.7±4.1; p = 0.133), which exhibited CD45+ T cell engraftment that was approximately 2–3 fold (p≥0.05) higher than that observed in the drug-administered mice ( Fig. 6b ). Numbers of engrafted CD45+ cells were also significantly decreased compared to controls at the end of the dosing regimen (day 35), i.e., from 10.3×103±2.4×103 for the controls, to 1.7×103±1.1×103 (p = 0.01) for MTX, 0.4×103±0.19×103 (p = 0.008) for MMF, and 0.49×103±0.08×103 (p = 0.009) for combined MTX and MMF.
Figure 6. Transduced T cells that express DHFRFS/IMPDH2IY exhibit an in vivo engraftment advantage upon treatment with MTX and MMF.
(a), Model system for testing the in vivo selection of transduced T cells using MTX, MMF and a combination of both. Depicted is the CD45 cell surface expression (filled grey histogram) and EGFRt expression (filled grey histogram) of transduced T cells prior to adoptive transfer analyzed by flow cytometry. Percentage of positive cells above isotype control staining (open histogram) is indicated in each histogram. 6 to 8 week old NSG (n = 6) mice were injected with 107 T-EGFRt (29% EGFRt+) cells i.v. (Day 0). Drugs were administered 21 days post engraftment and analyzed for CD45+ cells in peripheral blood. Experimental groups were administered either MTX i.p. 2x in week 1 and 1x in week 2 (25 mg/kg), 0.563% MMF feed for 2 weeks, or a combination of both drugs (i.e., from D21 to D35). (b), The average percentage (left) or number (right) of CD45+ peripheral blood cells on day 21 before the initiation of treatment, and 7 (D28), 14 (D35), and 21 (D42) days during and after treatment are shown. P-value ≤0.05 using unpaired, two-tailed Student’s t-tests as compared to that in D21 (*) or as compared to the Control in the same day (**). (c), Percentage of EGFRt+ cells in the CD45+ population on day 21 before the initiation of treatment and one week after treatment (42) are shown. *, p≤0.0006 when each group was compared the control on D21. There was no significant difference between control mice on D21 and D42 (ns). Mean percentage of EGFRt ± S.E.M. of 6 mice are depicted.
To evaluate the impact of drug administration on the engraftment of huEGFRt/DHFRFS/IMPDH2IY TCM versus unengineered T cells, the mean percentages of EGFRt+ expressing T cells within the CD45+ human T cell populations were compared for each treatment group. The EGFRt+ T cells detected in the peripheral blood before initiation of treatment were comparable for all groups at 15.0±8.4% on day 21 after adoptive transfer of transduced T cells. At day 42, 3 weeks after the initiation of drug administration, the mean percentage of EGFRt+ expressing T cells was increased from this baseline to 63±7.7% (p = 0.0002) in mice treated with MTX, and 70±3.2% (p<0.0001) in combination MTX+MMF-treated mice, but only to 41±5.7% (p = 0.0006) in mice treated with MMF alone. Each drug dosing regimen exhibited higher percentages of EGFRt+ cells than that seen in control animals, which was 27.6±4.9% (p = 0.26 vs. control animals on D21) ( Fig. 6c ). The increased percentages of transgene expressing T cells within the human CD45+ populations in mouse peripheral blood indicated that the huEGFRt/DHFRFS/IMPDH2IY-expressing T cells were able to resist lymphotoxic drug concentrations and persist in vivo with a selection advantage over the non-transduced T cells.
Discussion
In human gene therapy, the selection of genetically modified cells is a methodological challenge when potency and safety are linked to the purity of ex vivo manufactured cell products. Selection platforms based on non-human drug resistance genes often lead to immune-based rejection of the therapeutic product [2]–[6], whereas those employing immunomagnetic or sorting methods are technically cumbersome and limited by expense and availability of clinical-grade reagents. Alternately, cell selection can be achieved by chemical means on the basis of human enzymes that confer resistance to cytotoxic selection drugs. It has been previously shown that MTX resistant hDHFR variants have an excellent potential as selectable markers for gene transfer in hematopoietic stem cells (HSCs), resulting in improved protection of HSCs from MTX cytotoxicity [27]. Furthermore, ex vivo selection studies on bone marrow cells transduced with various hDHFR variants demonstrated that the DHFRFS variant displayed decreased MTX affinity [28], with retention of enzymatic activity to allow for cell proliferation [29]. Nonetheless, ex vivo MTX selection of murine or human hematopoietic cells with DHFRFS did not lead to an increased reconstitution of transduced cells in myelo-ablated animal recipients [30], [31], which might be due to the time required for efficient selection exceeded the ex vivo life span of HSCs. Similarly, previous studies have also shown that IMPDH2IY expressing lymphocytes can be selected in vitro using MPA [8]. Here, we now demonstrate the utility of both human mutant enzymes DHFRFS and IMPDH2IY as a strategy to enrich transduced T cells in vitro and in vivo for adoptive T cell therapy.
Our results demonstrate that DHFRFS/IMPDH2IY co-expression renders lentivirally transduced primary human CD45RO+CD62L+ central memory T cells resistant to lymphotoxic concentrations of MTX and MPA (up to 0.1 µM and 1.0 µM, respectively) in vitro. Further, our data provide evidence that MTX, MMF and dual drug selection efficiently enriches gene-modified T cells in vitro within a short expansion time (12 days) (DHFRFS/MTX 11.4±2.1-fold; IMPDH2IY/MMF 3±0.9-fold; and MTX/MMF dual drug selection 5.4±1.0-fold), without compromising the cytolytic or cytokine production capabilities of the gene-modified T cells. Our recent in vitro work with primary human T cells lentitransduced to coordinately express just the DHFRFS with huEGFRt as well as a CD19-specific chimeric receptor revealed a similar DHFRFS/MTX mediated selection and expansion profile [32]. Now, based on the in vitro analysis presented here, we speculate that the dual expression of DHFRFS/IMPDH2IY by drug selected T cells will render adoptively transferred cells resistant to clinically relevant serum concentrations of both MTX (0.1–1 µM ) [33] and MMF (1–10 µM) [34], [35].
We also demonstrate the survival advantage and enrichment of DHFRFS/IMPDH2IY engineered T cells upon drug selection in vivo. After establishing MTX and MMF dosing regimens that are well tolerated by NSG mice, we were able to observe the enrichment of huEGFRt+DHFRFS+IMPDH2IY+ T cells following either administration of MTX alone (4.4 -fold), MMF alone (2.9-fold), or combined MTX and MMF (4.9-fold).
We originally incorporated two drug-resistance transgenes in our selection approach with the idea that it would reduce the necessity for utilizing large doses of either drug individually to drive selection of gene-modified cells. However, the in vivo results suggest that the DHFRFS/MTX strategy alone may be sufficient for potent selection, in that it appears more robust than the IMPDH2IY/MMF strategy alone, and is comparable to dual selection with DHFRFS/MTX and IMPDH2IY/MMF together. This correlates with the observed in vitro expansion of DHFRFS/IMPDH2IY-transduced T cells, which was less robust in MPA compared to that seen in MTX. We presume that MMF inhibition of the guanosine nucleotide pools, which is notably difficult for cells to recover from compared to inhibition of other nucleotides [36]–[38], is responsible for the reduced selection-potential of IMPDH2IY/MMF over that of DHFRFS/MTX in our T cells.
Another appealing attribute of the DHFRFS/IMPDH2IY-mediated selection system includes the ability of MTX and MMF administration to induce lymphopenia while reinforcing transgene expression, with the potential outcome of selective homeostatic cytokine-driven engraftment of transferred gene-modified T cells over that of endogenous T cells. Further, not only would these lymphotoxic drugs favor the proliferation of the transduced cells, but they might also substantially prevent immune responses toward transgene-encoded proteins by suppressing immunological responses. Thus, this platform may be particularly useful in allogeneic applications of adoptive T cell therapy. Lastly, MTX and MMF are used clinically against a variety of hematologic malignancies and may thus provide additive or synergistic anti-tumor effects when used in the adoptive T cell therapy setting.
In summary, this is the first study to demonstrate the feasibility of DHFRFS/IMPDH2IY mediated in vitro and in vivo enrichment of primary human T cells with MTX and MPA and to facilitate selective engraftment of gene-modified cells following transfer in vivo. We are currently evaluating additional studies in animal models to assess the DHFRFS/MTX-mediated selection of therapeutic (i.e., chimeric antigen receptor expressing) primary human T cells in vivo [32].
Materials and Methods
Cell Lines and Maintenance
The human peripheral blood mononuclear cells (PBMCs) were isolated as described [24] from heparinized peripheral blood obtained from discard kits containing residual blood components of healthy donors that had undergone apheresis at the City of Hope National Medical Center (COHNMC). Because this was de-identified discard blood material, informed consent was waived with the approval of the COHNMC Internal Review Board (IRB protocol #09025), and the COHNMC Office of Human Subjects Protection. TCM-derived T cell isolation, stimulation, expansion (Rapid expansion method; REM) and lentiviral-mediated transduction were then done as previously described by our laboratory [24].
EBV-transformed lymphoblastoid cell lines (LCL) and LCL that expressed OKT3 (LCL-OKT3) cells [24] were cultured in RPMI 1640 (Irvine Scientific, Santa Ana, CA) supplemented with 10% heat-inactivated fetal calf serum (FCS, Hyclone, Logan, UT) 2 mM L-glutamine (Irvine Scientific), and 25 mM HEPES (Irvine Scientific).
Mouse myeloma cells secreting human homeostatic IL-15 cytokine (NSO-IL15) cells were generated by transfecting the NSO cells with GFP-IMPDH2IY-2A-IL15_pcDNA3.1. The transfected NSO cells were cultured and expanded as previously described by our laboratory [24]. The transfected cells were selected under MPA, analyzed for the GFP expression by flow cytometry and human IL-15 secretion by cytometric bead array (Bio-Rad Laboratories, Hercules, CA, USA) [24].
Lentiviral Vector
For gene-modification of T cells (i.e., the TCM-derived T cells as described in Cell lines and maintenance section above), the huEGFRt-T2A-DHFRFS-T2A-IMPDH2IY_epHIV7 vector was made and tested for resistance to both MMF and MTX. We generated double mutant DHFRFS and IMPDH2IY transgenes by site-directed mutagenesis, inserting two point mutations in wild type human DHFR and IMPDH2. The DHFRFS [16] and IMPDH2IY [8] selection genes along with a truncated EGFR [22] marker gene (EGFRt expression reflected drug resistance in transduced cells) was incorporated into a lentiviral vector epHIV_7 under transcriptional control of the human elongating factor 1-α (EF-1α) promoter. In the vector, the EGFRt, DHFRFS and IMPDH2IY coding sequences were separated by the ribosomal skip T2A [23] sequence for translation of the three proteins from one transcribed message. The EGFRt gene was placed upstream of DHFRFS, and the IMPDH2IY gene(s) was placed downstream of the T2A sequence in a plasmid ( Fig. 1c ). The proper assembly of the genes was verified by DNA sequence analysis.
EGFRt-T2A-DHFRFS-T2A-IMPDH2IY_epHIV7 lentiviral vector was produced by transient transfection of 293T cells with four plasmids (pCgp, pCMV-G, pCMV-Rev2 and vector construct) using the CaPO4 method. The supernatant containing secreted lentiviral vector was harvested 24, 48 and 72 hours after transfection and ultra-concentrated following polyethylene glycol precipitation and titered on H9 cells for EGFRt expression by FACS analysis. Replication-competent lentivirus (RCL) assays were performed using the HIV-1 p24 antigen ELISA kit (Perkin Elmer Life Sciences, California) to detect p24 viral antigen. RCL negative viral supernatant was released for use.
Flow Cytometry
Cell surface phenotypes of transduced and non-transduced purified CD62L+CD45RO+ healthy donor peripheral blood T cells were analyzed with fluorochrome-conjugated streptavidin and antibodies specific for CD4, CD8, CD62L, CD45 RA, CD45RO, CD45, CCR7, TCRαβ, CD127, and CD28 and isotype matched controls (BD Biosciences) following manufacture’s recommendations. The generation of the biotinylated-cetuximab has been previously described [22]. The percentage of immunofluorescent cells were analyzed by a FACScalibur system (BD Biosciences), and the percentage of cells in a region of analysis were calculated using FCS Express V3 (De Novo Software, CA, USA).
In vitro Selection of Gene-modified T cells
The concentration for in vitro selection of gene-modified T cells in the presence of MTX (Parenta Pharmaceuticals, Yardley, PA), MPA (Sigma-Aldrich, St. Louis, MO) and a combination of MTX and MPA was determined by dose-response curve. The selection experiment was initiated on day 8 of REM stimulation cycle by plating 0.8×106 transduced primary human T cells in 24-well tissue culture plates and culturing for 12 days with MTX, MPA or a combination of both drugs. Drug-selected or treated T cells were analyzed for transgene selection (EGFRt+) efficiency by flow cytometry. Transduced T cells with or w/o cetuximab were immunomagnetically selected for EGFRt expression [22] and were evaluated for their resistance to the indicated concentrations of MTX and MPA. The total viable cell number and percentage of viable cells were enumerated by Guava Personal Cell Analysis (Guava Technologies-Millipore, Hayward, CA) as per the manufacturer’s instructions. The working concentrations of MTX and MPA were diluted in PBS.
Chromium-release Assays
The cytolytic activity of T cells was determined by a 4-hour chromium-release assay (CRA) previously described [24] using the indicated effector/target cell ratios.
Measurement of Cytokine Production
T cells (5×104) were co-cultured with 5×104 LCL-OKT3, LCL, or K562 cells in triplicate wells of a 96-well tissue culture plate overnight. The supernatants were then analyzed for the secreted cytokines by cytometric bead array using a bioplex human cytokine Panel (Bio-Rad Laboratories, Hercules, CA, USA), according to the manufacturer’s instructions.
Determination of Plasma MTX/MPA Levels and Effect on NSG Mice
NOD-scid IL2γc −/− (NSG) mice (Jackson Laboratory) were housed and maintained in a breeding colony in individually ventilated conditions under pathogen-free conditions at the COH animal resources center. All procedures were reviewed and approved by the COH Institute Animal Care and Use Committee (#08004). All mice were on a standard rodent diet.
To determine plasma MTX/MPA levels in NOD-scid IL2Rγnull (NSG) mice, MTX (Parenta Pharmaceuticals, Yardley, PA) was administered as an intraperitoneal (i.p.) injection at 25 mg/kg/day twice a week the first week, and only once the second week. MMF (Sandoz Ltd, India) was administered in 0.563% medicated feed pellets (from pulverized tablets) for 2 weeks. MMF medicated pellets were manufactured (TestDiet, Richmond, IN) with the addition of 5.625 mg MMF per 1 gm of standard rodent diet. The blood samples were collected for the determination of plasma levels of MTX in heparinized microcapillary tubes from the retro-orbital venous plexus 30 minutes after each i.p. administration of 25 mg MTX/kg (this was based on t1/2 [39]), or 7 days after initiation of 0.563% MMF feed. Measured plasma levels of MTX were performed at the Molecular Pharmacology laboratory at COH and plasma levels of MPA were determined at the Transplant Pharmacokinetic Laboratory Dumont (UCLA Liver Transplant Center) by standard high-performance liquid chromatography. Further effects of these drugs were monitored by measuring animal body weights and observing general health condition on a weekly basis. Complete blood counts were determined (HEMAVET 950FS) and serum chemistry was analyzed (Charles River Research Diagnostic Services, Wilmington, MA) before, during and after drug administration.
In vivo T cell Engraftment
Six- to ten-week old NSG mice were injected intravenously on day 0 with 107 T cells. The systemic supply of human IL-15 was provided by injecting the irradiated (8000 cGy) 2×107 NS0-IL15 cells i.p. on Monday, Wednesday and Friday starting on day -1 to support human T cell expansion in vivo. Mouse peripheral blood was collected by retro-orbital bleeding, and analyzed by flow cytometry to monitor human T cell engraftment. Flow cytometric analysis included the use of CountBright™ Absolute Counting Beads (Molecular Probes, Eugene, OR) for normalization of samples according to the manufacturer’s directions. In the case of selection studies, the transferred T cells were allowed to engraft for 21 days. MTX was then administered i.p. at a 25 mg/kg/day twice a week the first week and only once in the second week; MMF was administered as 0.563% medicate feed pellets (from pulverized tablets) for 2 consecutive weeks starting from day 21.
Statistical Analysis
Data are expressed as mean ± S.D. To evaluate the differences between non-transduced and transduced cells in terms of total percentage of viable cells and fold expansion were analyzed using an unpaired, two-tailed Student’s t-test. As well, to test for differences between treatment and control mice, data were evaluated using an unpaired, two-tailed Student’s t - test in Graph Pad Prism5. Mean percentage of EGFRt ± S.E.M. of 6 mice are depicted. Differences were considered statistically significant if p values were less than 0.05.
Supporting Information
(EPS)
Acknowledgments
The authors thank Renate Starr, Araceli Hamlett, Winnie Wong, Brenda Aguilar, and Seon Cho for helpful technical assistance, Xiuli Wang for the helpful discussions and Lara Ausubel for critical reading of our manuscript. We also thank Theodore Sievers (Transplant Pharmacokinetic Laboratory Dumont-UCLA Liver Transplant Center) and Tim Synold (COHNMC) for serum drug analysis. MCJ is an inventor of licensed patents and equity holder in ZetaRx, Inc., a licensee of these patents. All other authors have no conflicts of interest to disclose.
Funding Statement
Funding provided by National Institutes of Health P50 CA107399, PO1 CA030206, the Lymphoma Research Foundation, the Tim Nesvig Lymphoma Research Foundation, the Neidorf Foundation, the Marcus Foundation, and Timothy Lindenfelser. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1. Volpato JP, Mayotte N, Fossati E, Guerrero V, Sauvageau G, et al. (2011) Selectively weakened binding of methotrexate by human dihydrofolate reductase allows rapid ex vivo selection of mammalian cells. J Mol Recognit 24: 188–198. [DOI] [PubMed] [Google Scholar]
- 2. Milsom MD, Fairbairn LJ (2004) Protection and selection for gene therapy in the hematopoietic system. J Gene Med 6: 133–146. [DOI] [PubMed] [Google Scholar]
- 3. Riddell SR, Elliott M, Lewinsohn DA, Gilbert MJ, Wilson L, et al. (1996) T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat Med 2: 216–223. [DOI] [PubMed] [Google Scholar]
- 4. Berger C, Flowers ME, Warren EH, Riddell SR (2006) Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood 107: 2294–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walker RE, Carter CS, Muul L, Natarajan V, Herpin BR, et al. (1998) Peripheral expansion of pre-existing mature T cells is an important means of CD4+ T-cell regeneration HIV-infected adults. Nat Med 4: 852–856. [DOI] [PubMed] [Google Scholar]
- 6. Muul LM, Candotti F (2007) Immune responses to gene-modified T cells. Curr Gene Ther 7: 361–368. [DOI] [PubMed] [Google Scholar]
- 7. Abonour R, Williams DA, Einhorn L, Hall KM, Chen J, et al. (2000) Efficient retrovirus-mediated transfer of the multidrug resistance 1 gene into autologous human long-term repopulating hematopoietic stem cells. Nat Med 6: 652–658. [DOI] [PubMed] [Google Scholar]
- 8. Yam P, Jensen M, Akkina R, Anderson J, Villacres MC, et al. (2006) Ex vivo selection and expansion of cells based on expression of a mutated inosine monophosphate dehydrogenase 2 after HIV vector transduction: effects on lymphocytes, monocytes, and CD34+ stem cells. Mol Ther 14: 236–244. [DOI] [PubMed] [Google Scholar]
- 9. Sorg UR, Kleff V, Fanaei S, Schumann A, Moellmann M, et al. (2007) O6-methylguanine-DNA-methyltransferase (MGMT) gene therapy targeting haematopoietic stem cells: studies addressing safety issues. DNA Repair (Amst) 6: 1197–1209. [DOI] [PubMed] [Google Scholar]
- 10. Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, et al. (2011) T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 3: 95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. June CH (2007) Adoptive T cell therapy for cancer in the clinic. J Clin Invest 117: 1466–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, et al. (2008) Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 14: 1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morgan RA, Dudley ME, Yu YY, Zheng Z, Robbins PF, et al. (2003) High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol 171: 3287–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jena B, Dotti G, Cooper LJ (2010) Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood 116: 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, et al. (2008) Adoptive transfer of effector CD8 T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest 118: 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ercikan-Abali EA, Mineishi S, Tong Y, Nakahara S, Waltham MC, et al. (1996) Active site-directed double mutants of dihydrofolate reductase. Cancer Res 56: 4142–4145. [PubMed] [Google Scholar]
- 17. Shimmura H, Tanabe K, Habiro K, Abe R, Toma H (2006) Combination effect of mycophenolate mofetil with mizoribine on cell proliferation assays and in a mouse heart transplantation model. Transplantation 82: 175–179. [DOI] [PubMed] [Google Scholar]
- 18. Allison AC, Eugui EM (2000) Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 47: 85–118. [DOI] [PubMed] [Google Scholar]
- 19. Sangiolo D, Lesnikova M, Nash RA, Jensen MC, Nikitine A, et al. (2007) Lentiviral vector conferring resistance to mycophenolate mofetil and sensitivity to ganciclovir for in vivo T-cell selection. Gene Ther 14: 1549–1554. [DOI] [PubMed] [Google Scholar]
- 20. Quemeneur L, Gerland LM, Flacher M, Ffrench M, Revillard JP, et al. (2003) Differential control of cell cycle, proliferation, and survival of primary T lymphocytes by purine and pyrimidine nucleotides. J Immunol 170: 4986–4995. [DOI] [PubMed] [Google Scholar]
- 21. McIvor RS (1996) Drug-resistant dihydrofolate reductases: generation, expression and therapeutic application. Bone Marrow Transplant 18 Suppl 3S50–54. [PubMed] [Google Scholar]
- 22. Wang X, Chang WC, Wong CW, Colcher D, Sherman M, et al. (2011) A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 118: 1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, et al. (2004) Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol 22: 589–594. [DOI] [PubMed] [Google Scholar]
- 24. Wang X, Berger C, Wong CW, Forman SJ, Riddell SR, et al. (2011) Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood 117: 1888–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang X, Naranjo A, Brown CE, Bautista C, Wong CW, et al. (2012) Phenotypic and Functional Attributes of Lentivirus-modified CD19-specific Human CD8+ Central Memory T Cells Manufactured at Clinical Scale. J Immunother 35: 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pankiewicz KW, Lesiak-Watanabe K, Watanabe KA, Malinowski K (1999) Novel mycophenolic adenine bis(phosphonate)s as potential immunosuppressants. Curr Med Chem 6: 629–634. [PubMed] [Google Scholar]
- 27. Fossati E, Volpato JP, Poulin L, Guerrero V, Dugas DA, et al. (2008) 2-tier bacterial and in vitro selection of active and methotrexate-resistant variants of human dihydrofolate reductase. J Biomol Screen 13: 504–514. [DOI] [PubMed] [Google Scholar]
- 28. Lewis WS, Cody V, Galitsky N, Luft JR, Pangborn W, et al. (1995) Methotrexate-resistant variants of human dihydrofolate reductase with substitutions of leucine 22. Kinetics, crystallography, and potential as selectable markers. J Biol Chem 270: 5057–5064. [DOI] [PubMed] [Google Scholar]
- 29. Flasshove M, Banerjee D, Mineishi S, Li MX, Bertino JR, et al. (1995) Ex vivo expansion and selection of human CD34+ peripheral blood progenitor cells after introduction of a mutated dihydrofolate reductase cDNA via retroviral gene transfer. Blood 85: 566–574. [PubMed] [Google Scholar]
- 30. Flasshove M, Banerjee D, Bertino JR, Moore MA (1995) Increased resistance to methotrexate in human hematopoietic cells after gene transfer of the Ser31 DHFR mutant. Leukemia 9 Suppl 1S34–37. [PubMed] [Google Scholar]
- 31. Gatlin J, Douglas J, Evans JT, Collins RH, Wendel GD, et al. (2000) In vitro selection of lentivirus vector-transduced human CD34+ cells. Hum Gene Ther 11: 1949–1957. [DOI] [PubMed] [Google Scholar]
- 32.Jonnalagadda M, Brown CE, Chang WC, Ostberg JR, Forman SJ, et al.. (2013) Efficient selection of genetically modified human T cells using methotrexate-resistant human dihydrofolate reductase. Gene Ther. [DOI] [PMC free article] [PubMed]
- 33. Treon SP, Chabner BA (1996) Concepts in use of high-dose methotrexate therapy. Clin Chem 42: 1322–1329. [PubMed] [Google Scholar]
- 34.Mycophenolate mofetil (CellCept) package insert (2005). Roche, USA.
- 35. Dubus I, Vendrely B, Christophe I, Labouyrie JP, Delmas Y, et al. (2002) Mycophenolic acid antagonizes the activation of cultured human mesangial cells. Kidney Int 62: 857–867. [DOI] [PubMed] [Google Scholar]
- 36. Franklin TJ, Morris WP (1994) Pharmacodynamics of the inhibition of GTP synthesis in vivo by mycophenolic acid. Adv Enzyme Regul 34: 107–117. [DOI] [PubMed] [Google Scholar]
- 37. Nguyen BT, El Sayed YM, Sadee W (1984) Interaction among the distinct effects of adenine and guanine depletion in mouse lymphoma cells. Cancer Res 44: 2272–2277. [PubMed] [Google Scholar]
- 38. Catapano CV, Dayton JS, Mitchell BS, Fernandes DJ (1995) GTP depletion induced by IMP dehydrogenase inhibitors blocks RNA-primed DNA synthesis. Mol Pharmacol 47: 948–955. [PubMed] [Google Scholar]
- 39. Lobo ED, Balthasar JP (2003) Pharmacokinetic-pharmacodynamic modeling of methotrexate-induced toxicity in mice. J Pharm Sci 92: 1654–1664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(EPS)