Abstract
Cilia are protrusions on the surface of cells. They are frequently motile and function to propel cells in an aqueous environment or to generate fluid flow. Equally important is the role of immotile cilia in detecting environmental changes or in sensing extracellular signals. The structure of cilia is supported by microtubules, and their formation requires microtubule-dependent motors, kinesins, which are thought to transport both structural and signaling ciliary proteins from the cell body into the distal portion of the ciliary shaft. In multicellular organisms, multiple kinesins are known to drive ciliary transport, and frequently cilia of a single cell type require more than one kinesin for their formation and function. In addition to kinesin-2 family motors, which function in cilia of all species investigated so far, kinesins from other families contribute to the transport of signaling proteins in a tissue-specific manner. It is becoming increasingly obvious that functional relationships between ciliary kinesins are complex, and a good understanding of these relationships is essential to comprehend the basis of biological processes as diverse as olfaction, vision, and embryonic development.
Keywords: C. elegans, cilia, flagella, intraflagellar, kinesin, motor, mouse, photoreceptor, zebrafish
The structure of cilia and their close relatives, flagella, is supported by a characteristic configuration of microtubules. In most cases, nine evenly spaced parallel microtubule doublets run along most of the ciliary length (reviewed in ref. 1). On cross sections, they are arranged in a circle, and two additional single microtubules are frequently found in the center of the ciliary shaft (Fig. 1). Although most cilia are characterized by a simple antenna-like appearance, some differentiate into much more complex structures that feature wing- or finger-shaped membrane extensions thought to aid their sensory functions. Such elaborate morphology characterizes some of the chemosensory cilia in C. elegans, for example.2 Even more structurally complex cilia are found in vertebrate photoreceptor cells. These differentiate hundreds of membrane folds harboring light-sensitive receptor molecules, opsins and other components of the phototransduction apparatus.3,4 A billion or so of opsin molecules is estimated to reside in the ciliary membrane of a single photoreceptor cell in some vertebrate species.5 Receptors of many other signaling pathways, and the components of their respective signal transduction cascades are found in cilia of numerous tissues.5-8
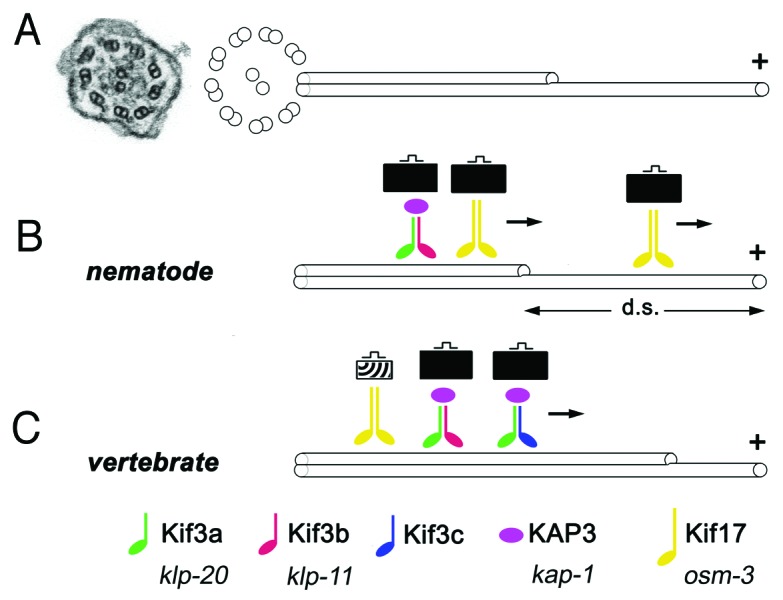
Figure 1. Some key features of ciliary kinesins. (A) An electron micrograph of a cross section through a cilium in zebrafish (left, from ref. 64) and a schematic representation of ciliary microtubules (right). Inside the ciliary shaft, microtubules form nine doublets that point their plus ends toward the tip of the cilium. These doublets frequently continue as single microtubules in the distal region, sometimes referred to as the “distal segment” of the cilium (d. s.). The portion of the cilium occupied by single microtubules is highly variable in different cells. (B and C) Kinesins are microtubule-dependent motors that transport cargo toward plus ends of microtubules. In all species examined so far, the heterotrimeric kinesin-2 (also known as kinesin-II) plays a major role in ciliary transport. It consists of two distinct motor subunits and an accessory subunit (KAP3). In C. elegans, two genes encode kinesin-II motor subunits (B). In vertebrates, on the other hand, these motor subunits are encoded by at least three loci (C). Their protein products are thought to assemble into complexes containing the Kif3a subunit, and either Kif3b or Kif3c. Thus at least two distinct Kinesin-II complexes appear to function in vertebrates. In addition to the heterotrimeric kinesin-2, the homodimeric kinesin-2, in vertebrates encoded by the kif-17 gene, contributes to ciliary transport. In C. elegans, this kinesin is frequently redundant with Kinesin-II and plays a major role in cilia formation. In at least one group of cells, the homodimeric kinesin-2, but not the heterotrimeric one, is necessary for the formation of the distal segment. In vertebrates, the homodimeric kinesin-2 appears to play a less significant role. It may transport unique cargos and/or kinesin-II cargos. There is no evidence that the formation of single microtubules in the distal portion of vertebrate cilia requires kinesins that differ from these that function in the formation of microtubule doubles. Kinesins of other families also contribute to ciliary transport (not shown). Verterbrate and C. elegans nomenclature is provided at the bottom of the figure.
As cilia do not conduct protein synthesis on their own, all protein components, from the most basic blocks of cilia structure, such as tubulin, to transmembrane receptors and channels that mediate signaling functions, must be delivered from the cell’s cytoplasm. This is a multistep process that first involves cytoplasmic transport to the base of the cilium, subsequently crossing of the diffusion barrier that separates the ciliary compartment from the rest of the cell, and finally, so-called intraflagellar transport (IFT) along ciliary microtubules.1 Intraflagellar transport has received ample attention in recent years, and advances in the understanding of this process have been reviewed.9-11 Briefly, IFT involves a movement of protein complexes, so-called IFT particles, which consist of about 20 polypeptides, along ciliary microtubules. In mutants of many IFT genes, cilia are absent.12-14 The IFT particle is thought to engage in two functionally distinct types of molecular interactions: on the one hand it binds microtubule-dependent motors that drive its movement, and on the other it forms transient complexes with molecules that are transported along the cilium and considered its cargo. Kinesins and dyneins move the IFT particle in the anterograde (toward the tip of the cilium) and retrograde directions, respectively. Recent studies reveal an unexpected complexity in the function of ciliary kinesins, and as it frequently happens provoke new questions.
The genetics of ciliary kinesins has been studied in several model systems, most notably in Chlamydomonas and C. elegans, but also in the mouse, zebrafish, Tetrahymena, and the fruit fly (reviewed in refs. 15 and 16). These studies led to the conclusion that the heterotrimeric kinesin-2 (known also as kinesin-II) is the main anterograde motor that drives cilia formation. In Chlamydomonas, mutations that affect each of its three subunits lead to the loss of flagella.17,18 Similarly strong phenotypes are observed in Tetrahymena and in fly Johnston organs.19,20
In C. elegans, ciliary kinesin function involves multiple motors that act redundantly. Thus the loss of kinesin-II does not produce a mutant phenotype in amphidial channel cilia, due to redundancy with the homodimeric kinesin-2, encoded by the osm-3 gene (Fig. 1B). In this group of cilia, microtubules form doublets in the proximal (here known as the “middle segment”) region and singlets in the distal region. Mutations in osm-3 result in a loss of the distal ciliary segment only, which contains microtubule singlets. Double mutants of both kinesins display, however, a complete absence of cilia.21 The heterotrimeric and homodimeric kinesins are also redundant or partially redundant in the cilia of neighboring AWC and AWB neurons.22,23 An even more complex interplay of kinesin function has been described in nematode CEM (cephalic male) cilia, where three kinesins: kinesin-II, osm-3, and klp-6, the latter a member of the kinesin-3 family, contribute to ciliogenesis. In this class of cilia, klp-6 appears to inhibit the activity of the two kinesin-2 family motors. In addition, the loss of kinesin-II alone leads to an unusual phenotype, a lengthening of CEM cilia.24
So far, the majority of studies on vertebrate ciliary kinesins have been performed in mice. The mouse has three genes that encode the heterotrimeric kinesin 2 motor subunits: kif3a, kif3b and kif3c (Fig. 1C). Genetic analysis in this model organism is complicated by mid-gestation lethality associated with knockout phenotypes of both kif3a and kif3b.25,26 To circumvent this difficulty, a number of studies relied on conditional mutants that eliminate kif3a function in several cell types or tissues of the kidney, skin, pancreas, ear, retina and the brain.27-30 In agreement with invertebrate data, all of these reported a cilia loss, and thus confirmed the importance of kinesin-II in ciliogenesis. As discussed below in more detail, the only exception to this so far may be the vertebrate photoreceptor cell.
In contrast to kif3a and kif3b, the ablation of kif3c in the mouse does not produce any obvious phenotype.31,32 The genetic analysis of Kif3 subunits in the mouse was supplemented with biochemical studies, which revealed that Kif3c dimerizes with Kif3a.33,34 Similarly, early studies reported that Kif3b dimerizes with Kif3a.35 Kif3c and Kif3b do not, however, appear to bind each other (Fig. 1C). Based on these observations, a hypothesis was formulated that kif3c and kif3b are functionally redundant.34 This idea has not been, however, genetically tested in the mouse so far.
In addition to the kinesin-II, the mouse genome encodes a homolog of the homodimeric kinesin-2, kif17. Unlike heterotrimeric kinesin-2 mutants, kif17 knockout mice display very subtle phenotypes only, such as hippocampus-dependent memory impairment.36 Morphological abnormalities have not been reported in the cilia of these animals. Such a weak phenotype could be a consequence of genetic redundancy. So far, there is no evidence, however, that kif17 acts redundantly with the heterotrimeric kinesin in any of the mouse cilia, and thus C. elegans remains the only model organism in which a redundancy of these two kinesins has been reported so far.
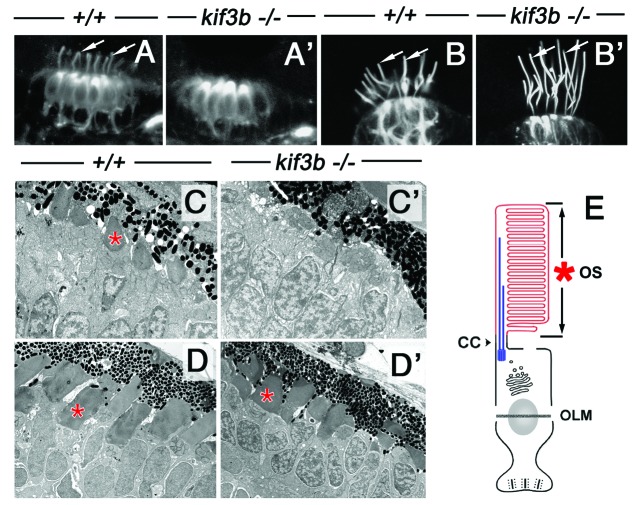
The zebrafish is the most recent model used to study the genetics of vertebrate ciliary kinesins.37,38 The analysis of kinesin-2 family genes in this organism has recently revealed that, surprisingly, cilia of several tissues persist in kif3b mutants. These include the cilia of two sensory cell types, auditory hair cells and cone photoreceptors, as well as some cilia in the spinal canal (ref. 37, and unpublished results). (In the context of the photoreceptor cell, I will use the term “cilium” as meaning the connecting cilium and the outer segment, Figure 2E). At least two aspects of mutant phenotypes in these cell types deserve additional commentary. First, although all wild-type auditory hair cells grossly display the same morphological features, such as the presence of the apical kinocilium and an array of stereocilia, it is only the hair cells of auditory cristae that retain cilia in kif3b mutants (Fig. 2B and B’). In the neighboring maculae, cilia of mutant hair cells are not maintained (Fig. 2A and A’). The second intriguing observation is that cilia of kif3b mutant cone photoreceptors differentiate after a delay (Fig. 2C, C’, D and D’). This has not been observed in mouse conditional mutants,39 perhaps due to a late onset of Cre expression, which may preclude the analysis of kif3a function at the earliest stages of photoreceptor differentiation. A delay of cilia formation in mutant zebrafish suggests the existence of a redundant transport mechanism that is initially absent and becomes active only at a later stage of cell differentiation. The evidence for the presence of such a mechanism is outlined below.
Figure 2. A subset of cilia differentiate in kif3bjj203 mutant embryos. In (A, A’, B and B’), shown are wild-type (A and B) and mutant (A’ and B’) embryos stained with anti-acetylated tubulin antibody at 3 (B and B’) and 7 (A and A’) dpf. (A and A’) Confocal images of a macula in the zebrafish ear. Cilia (arrows) are present in both wild type and mutant. (B and B’) Confocal images of a crista of the zebrafish ear. Cilia (arrows) are absent in the mutant. (C, C’ and D’) Electron micrographs of sections through wild-type (C and D) and mutant (C’ and D’) photoreceptor cells at 3.5 (C and C’) and 5 (D and D’) dpf. Photoreceptor outer segments [OS, asterisks in (C?E)] are initially absent in the mutant. (E) A schematic drawing of the vertebrate photoreceptor cell (after Kennedy and Malicki, 2009). The outer segment membrane is in red. Microtubules that support its structure are in blue. In this work, term “cilium” is used to mean the structure that includes the connecting cilium and the outer segment. The outer segment (OS) forms in the distal part of photoreceptor cilia, which differentiates membrane folds. The connecting cilium (CC), on the other hand, is the proximal region of photoreceptor cilia, and displays characteristics of the ciliary transition zone. OLM, outer limiting membrane.
In addition to kif3b, a kif17 mutant has also become available in zebrafish. This mutant allele, kif17sa0119, obtained through the Sanger Institute TILLING project, contains a stop codon that eliminates the tail region of the Kif17 protein, including the binding domain for at least one of its cargo adaptors in the mouse, the mLin-10/Mint1 protein.40,41 Such a defect should render the Kif17 protein entirely dysfunctional, unless additional cargo binding regions are present in the central portion of the Kif17 polypeptide. In addition, the phenotype of kif17sa0119 homozygotes is not exacerbated by morpholino injections, further suggesting that this allele is either null or close to that.37 The zebrafish kif17sa0119 mutant homozygotes are viable and grossly indistinguishable from the wild type. Apart from a weak shortening of olfactory cilia, no morphological defects have been observed in other types of cilia investigated in this mutant so far.37 Rigorous behavioral testing of kif17sa0119 animals is yet to be performed, and may reveal additional phenotypes.
The C. elegans homolog of kif17 is one of the two major kinesins involved in cilia formation. Is there then a broader role for the kif17 kinesin in vertebrate cilia? One possibility could be that this motor increases the throughput or the stability of transport in cilia that translocate a particularly heavy load of protein cargo. In such a case, the loss of kif17 could produce subtle functional changes in some specialized cilia only. This could happen in photoreceptors, for example, which based on outer segment membrane renewal rate are estimated to transport in the order of 1,000 opsin molecules and many other polypeptides every second.42 To evaluate opsin transport in photoreceptor cells, a pulse of opsin-GFP fusion protein can be expressed from a heat-shock promoter. The time required for opsin-GFP to translocate from the cell body into the outer segment reflects transport efficiency.43 In kif17 zebrafish mutants, such a test does not reveal any transport defects, however. Similarly, no morphological abnormalities, cell loss, or a mislocalization of the endogenous opsin are observed in photoreceptors of adult kif17 homozygotes.37 Thus based on all the above studies, the function of kif17 appears to be very limited. This conclusion is also in agreement with tissue culture experiments showing that a loss of kif17 affects the transport of a ciliary CNG channel, but not cilia morphology.8
The presence of cilia in zebrafish kif3b mutants and the limited scope of the kif17 phenotype may result from the functional redundancy between these two genes. This idea is attractive, given what is known about the function of nematode ciliary kinesins. Nonetheless, contrary to such expectations, in kif17/kif3b double mutants cone opsin continues to localize properly.37 Similarly, cristae cilia are present in the double mutant. The formation of cilia in kif3b mutant cone photoreceptors and hair cells appears then to rely on yet another transport mechanism.
Another potential reason for the absence of defects in some kif3b mutant cilia is a redundancy of function with kif3c. As already discussed above, this possibility is supported by biochemical studies.34 It is not supported, however, by protein localization reports that did not detect Kif3c at the photoreceptor cilium.33,34 Nonetheless, to test this possibility, kif3b mutants were treated with anti-kif3c morpholinos. The zebrafish genome contains two kif3c-related genes, kif3c and kif3c-like (kif3cl). A knockdown of one of these, kif3c, in the kif3b mutant strain does result in a severe loss of cilia both in cone photoreceptors and in hair cells of ear cristae.37 This leads to the conclusion that indeed, as predicted more than a decade ago, kif3b and kif3c do function redundantly in some cilia.
Two additional experiments were performed to test the role of kif3c in photoreceptor cilia. First, the opsin-GFP transport assay was conducted in kif3c morphants at two stages: 3 and 5 d postfertilization (dpf). This revealed that ciliary transport proceeds at a normal speed in morphant photoreceptors at 3 dpf. By 5 dpf, however, transport appears to be slower, compared with control animals. The difference is small but statistically significant. The small magnitude of the slowdown appears nonetheless noteworthy given that antisense morpholinos lose much of their activity by that stage. In the second experiment, kif3c mRNA was overexpressed in kif3b mutant homozygotes, which differentiate photoreceptor cilia with a delay of at least 24 h, compared with the wild type. This resulted in an earlier differentiation of kif3b mutant cilia, more in line with the time course observed in the wild type.37 This observation suggests that kif3c function, although capable of substituting for kif3b, is initially absent during photoreceptor ciliogenesis. kif3c presumably becomes active at a later stage of photoreceptor cilia assembly, and is responsible for partial recovery of outer segment formation in kif3b mutant homozygotes (summarized in Figure 3).
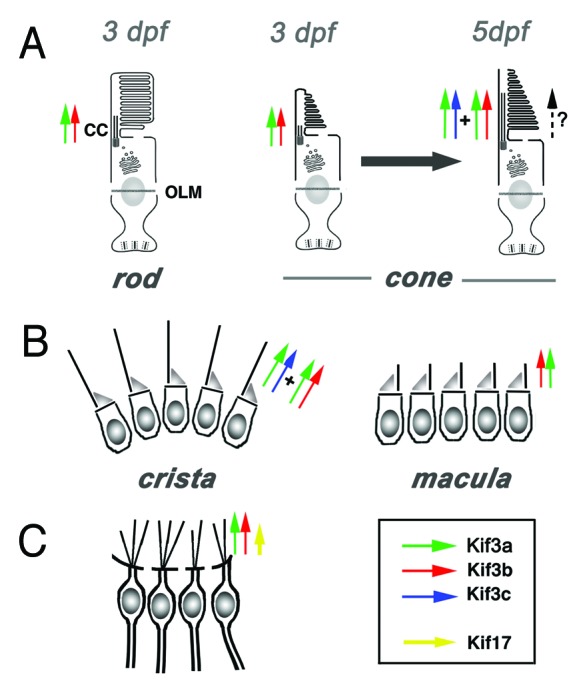
Figure 3. Current understanding of functional relationships between kinesin-2 family motors in vertebrate ciliogenesis. (A) In rod photoreceptors, kif3b is necessary for cilia differentiation and cell survival. In cone photoreceptors, on the other hand, kif3b is necessary for cilia formation at 3 dpf. Somewhat later, at 5 dpf, kif3b and kif3c function largely redundantly in cone cilia formation. kif3c does not, however, entirely compensate for the loss of kif3b function, and vice versa kif3b does not seem to entirely substitute for the loss of kif3c. (B) In the ear, two populations of mechanosensory hair cells display very different requirements for kinesin function: while kif3b is required for ciliogenesis in auditory maculae, kif3b and kif3c function redundantly in the cilia of cristae; either is sufficient to drive ciliogenesis in these cells. (C) In olfactory placodes, both kif3b and kif17 are necessary for normal ciliogenesis. The contribution of kif3b is much more significant, compared with kif17. Based on mouse knockout data, Kif3a is necessary for the differentiation of all cilia, except perhaps in photoreceptor cells, where yet another mechanism may be operational (dashed arrow).
The current understanding of kinesin function in vertebrate cilia leaves many questions unanswered. One is why kif3c functions in some cilia but not in others. This issue is puzzling especially because cells that do deploy kif3c appear morphologically and functionally similar to those that do not. This is the case, for example, in hair cells of maculae and cristae, which display few differences. The most obvious disparity between cells of these two populations, which differentiate in neighboring regions of the inner ear, is the length of kinocilia, which are substantially longer in cristae. This, again, brings to mind the possibility that longer or more bulky cilia, such as these in cristae or in photoreceptor cells, would require a particularly robust transport mechanism, and consequently employ multiple and functionally redundant kinesins.
The biggest number of unsolved questions surrounds, however, transport mechanisms that contribute to cilium formation in the photoreceptor cell. According to the kif3c/kif3b redundancy model, protein products of both genes require Kif3a as a binding partner. If so, kif3a mutants should not differentiate any cilia at all. It is not clear, however, whether this is the case. Following a conditional knockout of kif3a specifically in mouse rod photoreceptors, opsin, peripherin, as well as certain phototransduction cascade components continue to localize to the apical region of the cell at least until postnatal day 21.39 Similarly, following a cone-specific knockout, peripherin continues to be detected in the apical region of the cell until day p30. This suggests that rudimentary outer segments may be maintained in these animals despite the absence of kif3a. Ultrastructural data are needed to determine with certainty whether or not outer segments are present in mutant photoreceptors.
A trivial explanation for the persistence of outer segments in kif3a mutant retinae is that the Kif3a protein perdures for a long time, following conditional knockout of the kif3a gene at about postnatal day 7. If, however, and this has been already suggested in literature, such a scenario is not correct, a kif3a-independent transport mechanism must function in photoreceptor cilia. What could this mechanism involve? Several speculative ideas can be offered. One possibility is that kif3c alone is sufficient to drive the differentiation of some cilia. Kif3c protein appears to exist in two complexes, only one of which contains Kif3a.34 It is thus possible that a from of the Kif3c complex that does not contain Kif3a is sufficient to facilitate at least some aspects of ciliary transport into the photoreceptor outer segment. Another possibility is that an entirely different transport mechanism is involved: one based on actin-dependent motors. Actin has been detected at the junction of the connecting cilium and the outer segment,44,45 and further support for this idea is provided by observations that in Myo7a mutant mice, rod opsin accumulates in the connecting cilium.46,47 This phenotype is much weaker, compared with defects observed in mouse kinesin mutants, perhaps due to redundancy between myo7a and kinesins. Double mutants are needed to investigate this possibility.
In addition to VIIa, several other myosins, including IIa, IIIa, IIIb, VI, and IXb have been detected in vertebrate photoreceptors. Myosin VI appears to be present in photoreceptor inner segments, and its mutation affects photoreceptor function as evidenced by a decrease of ERG a-wave amplitude.48 No structural abnormalities have been reported, however, in mouse Myo6 mutant photoreceptors. Similarly, Myosin IIIa appears to be expressed in the inner segments of mouse photoreceptors.49 Although human mutations in this gene are known to exist, they do not affect vision, possibly due to functional redundancy with Myo IIIB, which is also found in the inner segment.49,50 Finally, an immunohistochemical analysis of several myosins resulted in an intriguing finding that three of them, IIA, VIIA, and IXB, are enriched at the basal bodies of photoreceptor cilia.51 Functional tests of these myosins may lead to interesting findings. One has to keep in mind, however, that even if actin-based transport contributes to ciliogenesis, its importance may be limited to cilia that feature particularly elaborate morphologies, such as the one found in photoreceptor cells.
The significance of a delay in the onset of kif3c function during cilia differentiation in cone cells is also not clear. Zebrafish photoreceptor cilia start to differentiate the complex array of membrane folds that characterizes outer segment morphology at ca. 2.5 dpf.52 By 3 dpf, outer segments of wild-type photoreceptors are prominent, and the zebrafish retina is functional as tested by behavioral criteria.53 By contrast, outer segments are completely absent in kif3b mutants at this stage, and start to form only between 3 and 4 dpf. This is presumably due to the late onset of kif3c activity. What is difficult to explain is that the onset of kif3c activity does not correlate with any obvious morphogenetic event in the differentiation of the wild-type photoreceptor. Perhaps then kif3c activity becomes necessary once the outer segment attains a certain critical volume? In the fully differentiated photoreceptor, the outer segment is one of the most voluminous features of the cell,54 and thus it appears likely that once it reaches a certain size, additional transport mechanisms may have to become operational in order to maintain its structure or function. The lack of an obvious mutant phenotype in kif3c homozygous mutant mice suggests, however, the kif3c-mediated transport is not necessary to maintain outer segment structure. Perhaps then mouse kif3c mutant photoreceptors display functional abnormalities that remain to be characterized? Apart from the above scenario, kif3c function may become important under stress, such as light damage to photoreceptor cells, or in aging animals. These possibilities could be tested in kif3c mutant mice. It also remains to be seen whether developmental changes in kinesin repertoire occur in cilia of other cells.
The studies of kif3b and kif3c lead to another question of a more general nature: to what extent are different kinesins functionally interchangeable? While kif3b appears to almost fully compensate for the loss of kif3c (opsin transport is a bit slower in the fish morphant, but mice do not display any obvious phenotype), the opposite is not true, as zebrafish kif3b mutant homozygotes display outer segment defects.37 In one scenario, the loss of kif3b could be perhaps entirely compensated by the overexpression of kif3c at a sufficiently high level, implying that both kinesins transport the same cargo. If, however, Kif3c can transport only a subset of Kif3b cargos, it will not fully compensate for the loss of the kif3b gene, regardless of its expression level.
To test the idea that vertebrate kinesin-2 family members are interchangeable, kif17 was overexpressed in kif3b mutant animals. This treatment resulted in a rescue of cilia formation in the spinal canal.37 Thus despite the absence of functional redundancy in wild-type development, vertebrate homodimeric kinesin-2 can substitute for kinesin-II function in at least some cilia when overexpressed, and so must be capable of transporting cargos similar to these transported by kinesin-II. At first glance, this is inconsistent with tissue culture studies showing that the loss of kif17 function impairs the transport of an olfactory channel subunit, CNGB1b, but does not affect cilia morphology, implying that kif17 makes little contribution to the transport of structural ciliary proteins, such as tubulin.8 These observations can be reconciled, however, provided that Kif17 and Kif3 display different affinities to overlapping sets of cargo molecules. The kif17 phenotype in vertebrates brings to mind observations of nematode AWC cilia, where the loss of the heterotrimeric kinesin-2, but not homodimeric osm-3, results in impaired chemosensation but not in cilia morphology defects.23 It appears then that different albeit overlapping cargo specificities are a common feature of kinesin-2 family motors. Mechanisms that mediate the binding interactions between kinesins and their cargos are an interesting subject of research. A question that should be addressed is whether such mechanisms provide an avenue for tissue- or developmental stage specific regulation of cargo specificity.
In addition to kinesin-2 motors, members of other kinesin families, such kif28, a homolog of nematode klp-6, as well as two costal2-related genes, kif7 and kif27, may contribute to transport in vertebrate cilia. The best studied in this regard is kif7, a kinesin-4 family member, which localizes to cilia and plays a role in hedgehog signaling but not in cilia morphogenesis (reviewed in ref. 55). Although its fly homolog, Costal2, has been hypothesized to function primarily as a scaffolding factor,56 several lines of evidence suggest that Kif7 may contribute to the transport of cargo along the ciliary axoneme. Support for this idea is provided by crystal structure studies, showing that the Kif7 motor domain is highly related to that of conventional kinesins.57 Moreover, although mutations in the mouse kif7 gene do not affect cilia morphology, they do impair the translocation of Gli proteins to cilia tips.58 In addition, cell culture assays reveal that fly Costal2 displays microtubule-dependent mobility.59 Given that most fly tissues do not differentiate cilia, and IFT is hypothesized to have its evolutionary origins in the cytoplasm,60,61 the cytoplasmic mobility of Costal2 may be related to Kif7-mediated ciliary transport. To put it differently, some cilia-related transport phenomena may take place in the cytoplasm of cells that do not differentiate cilia. Finally, in planarians, the single homolog of kif7/kif27 displays a much broader function as it is essential not only for cilia-related signaling but also for cilia formation.62 Thus in broad terms, vertebrate kif7 and kif17 display related functional characteristics as both of these kinesins are necessary to localize components of signal transduction pathways, a CNG channel and Gli3 respectively, but are largely dispensable for cilia morphogenesis. In other phyla, however, homologs of kif7 and kif17 contribute to cilia formation and thus are likely to transport a much broader assortment of protein cargo.
Finally, it has to be noted that apart from their role in intraflagellar transport, kinesins may regulate cilia formation, maintenance, and function via other mechanisms, such as centriole assembly, for example. This is the case for Kif24 which interacts with centrosomal proteins CP110 and Cep97, remodels centriolar microtubules, and thereby affects the formation of cilia.63 It is safe to assume that future studies will reveal additional kinesins that function in various aspects of vertebrate ciliogenesis. If C. elegans data are of any predictive value, as they should be, the functional relationships between vertebrate ciliary kinesins are likely to be even more complex than these already described for kinesin-II in zebrafish. The interactions of ciliary kinesins may turn out to be as diverse as cilia themselves, and although a common theme may emerge in the function of all ciliary transport mechanisms, multiple models will be required to explain protein transport in vertebrate cilia. There are then many drivers on diverse ciliary highways, and it will still take some time to learn who they all are and what exactly each of them does.
Acknowledgments
The author is thankful to Drs Tomer Avidor-Reiss, David Strutt, Freek van Eeden, Tanya Whitfield, Ching-Hwa Sung and Andrew Grierson for critical reading of earlier versions of this manuscript and helpful suggestions. This work was supported in part by EY018176 and EY016859 grant awards from the National Eye Institute.
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/21101
References
- 1.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–25. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 2.Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans.?2UU. J Comp Neurol. 1975;160:313–37. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy B, Malicki J. What drives cell morphogenesis: a look inside the vertebrate photoreceptor. Dev Dyn. 2009;238:2115–38. doi: 10.1002/dvdy.22010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodieck RW. The Vertebrate Retina. Principles of Structure and Function. San Francisco, California: W. H. Freeman & Co, 1973. [Google Scholar]
- 5.Pugh E, Lamb T. Phototransduction in Vertebrate Rods and Cones. Handbook of Biological Physics: Elsevier Science B. V., 2000:183-255. [Google Scholar]
- 6.Ezratty EJ, Stokes N, Chai S, Shah AS, Williams SE, Fuchs E. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell. 2011;145:1129–41. doi: 10.1016/j.cell.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ocbina PJ, Eggenschwiler JT, Moskowitz I, Anderson KV. Complex interactions between genes controlling trafficking in primary cilia. Nat Genet. 2011;43:547–53. doi: 10.1038/ng.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins PM, Hurd TW, Zhang L, McEwen DP, Brown RL, Margolis B, et al. Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr Biol. 2006;16:1211–6. doi: 10.1016/j.cub.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 10.Baldari CT, Rosenbaum J. Intraflagellar transport: it’s not just for cilia anymore. Curr Opin Cell Biol. 2010;22:75–80. doi: 10.1016/j.ceb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverman MA, Leroux MR. Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends Cell Biol. 2009;19:306–16. doi: 10.1016/j.tcb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–18. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin H, Rosenbaum JL, Barr MM. An autosomal recessive polycystic kidney disease gene homolog is involved in intraflagellar transport in C. elegans ciliated sensory neurons. Curr Biol. 2001;11:457–61. doi: 10.1016/S0960-9822(01)00122-1. [DOI] [PubMed] [Google Scholar]
- 14.Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004;42:703–16. doi: 10.1016/S0896-6273(04)00268-5. [DOI] [PubMed] [Google Scholar]
- 15.Scholey JM. Intraflagellar transport motors in cilia: moving along the cell’s antenna. J Cell Biol. 2008;180:23–9. doi: 10.1083/jcb.200709133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verhey KJ, Dishinger J, Kee HL. Kinesin motors and primary cilia. Biochem Soc Trans. 2011;39:1120–5. doi: 10.1042/BST0391120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller J, Perrone CA, Bower R, Cole DG, Porter ME. The FLA3 KAP subunit is required for localization of kinesin-2 to the site of flagellar assembly and processive anterograde intraflagellar transport. Mol Biol Cell. 2005;16:1341–54. doi: 10.1091/mbc.E04-10-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller MS, Esparza JM, Lippa AM, Lux FG, 3rd, Cole DG, Dutcher SK. Mutant kinesin-2 motor subunits increase chromosome loss. Mol Biol Cell. 2005;16:3810–20. doi: 10.1091/mbc.E05-05-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown JM, Marsala C, Kosoy R, Gaertig J. Kinesin-II is preferentially targeted to assembling cilia and is required for ciliogenesis and normal cytokinesis in Tetrahymena. Mol Biol Cell. 1999;10:3081–96. doi: 10.1091/mbc.10.10.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarpal R, Todi SV, Sivan-Loukianova E, Shirolikar S, Subramanian N, Raff EC, et al. Drosophila KAP interacts with the kinesin II motor subunit KLP64D to assemble chordotonal sensory cilia, but not sperm tails. Curr Biol. 2003;13:1687–96. doi: 10.1016/j.cub.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Snow JJ, Ou G, Gunnarson AL, Walker MR, Zhou HM, Brust-Mascher I, et al. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol. 2004;6:1109–13. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- 22.Mukhopadhyay S, Lu Y, Qin H, Lanjuin A, Shaham S, Sengupta P. Distinct IFT mechanisms contribute to the generation of ciliary structural diversity in C. elegans. EMBO J. 2007;26:2966–80. doi: 10.1038/sj.emboj.7601717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans JE, Snow JJ, Gunnarson AL, Ou G, Stahlberg H, McDonald KL, et al. Functional modulation of IFT kinesins extends the sensory repertoire of ciliated neurons in Caenorhabditis elegans. J Cell Biol. 2006;172:663–9. doi: 10.1083/jcb.200509115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morsci NS, Barr MM. Kinesin-3 KLP-6 regulates intraflagellar transport in male-specific cilia of Caenorhabditis elegans. Curr Biol. 2011;21:1239–44. doi: 10.1016/j.cub.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci U S A. 1999;96:5043–8. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, et al. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–37. doi: 10.1016/S0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 27.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, et al. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci U S A. 2003;100:5286–91. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–84. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- 29.Cano DA, Sekine S, Hebrok M. Primary cilia deletion in pancreatic epithelial cells results in cyst formation and pancreatitis. Gastroenterology. 2006;131:1856–69. doi: 10.1053/j.gastro.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 30.Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, et al. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- 31.Yang Z, Roberts EA, Goldstein LS. Functional analysis of mouse kinesin motor Kif3C. Mol Cell Biol. 2001;21:5306–11. doi: 10.1128/MCB.21.16.5306-5311.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jimeno D, Lillo C, Roberts EA, Goldstein LS, Williams DS. Kinesin-2 and photoreceptor cell death: requirement of motor subunits. Exp Eye Res. 2006;82:351–3. doi: 10.1016/j.exer.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Muresan V, Abramson T, Lyass A, Winter D, Porro E, Hong F, et al. KIF3C and KIF3A form a novel neuronal heteromeric kinesin that associates with membrane vesicles. Mol Biol Cell. 1998;9:637–52. doi: 10.1091/mbc.9.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Z, Goldstein LS. Characterization of the KIF3C neural kinesin-like motor from mouse. Mol Biol Cell. 1998;9:249–61. doi: 10.1091/mbc.9.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamazaki H, Nakata T, Okada Y, Hirokawa N. KIF3A/B: a heterodimeric kinesin superfamily protein that works as a microtubule plus end-directed motor for membrane organelle transport. J Cell Biol. 1995;130:1387–99. doi: 10.1083/jcb.130.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin X, Takei Y, Kido MA, Hirokawa N. Molecular motor KIF17 is fundamental for memory and learning via differential support of synaptic NR2A/2B levels. Neuron. 2011;70:310–25. doi: 10.1016/j.neuron.2011.02.049. [DOI] [PubMed] [Google Scholar]
- 37.Zhao C, Omori Y, Brodowska K, Kovach P, Malicki J. Kinesin-2 family in vertebrate ciliogenesis. Proc Natl Acad Sci U S A. 2012;109:2388–93. doi: 10.1073/pnas.1116035109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Insinna C, Pathak N, Perkins B, Drummond I, Besharse JC. The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development. Dev Biol. 2008;316:160–70. doi: 10.1016/j.ydbio.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avasthi P, Watt CB, Williams DS, Le YZ, Li S, Chen CK, et al. Trafficking of membrane proteins to cone but not rod outer segments is dependent on heterotrimeric kinesin-II. J Neurosci. 2009;29:14287–98. doi: 10.1523/JNEUROSCI.3976-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288:1796–802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- 41.Guillaud L, Wong R, Hirokawa N. Disruption of KIF17-Mint1 interaction by CaMKII-dependent phosphorylation: a molecular model of kinesin-cargo release. Nat Cell Biol. 2008;10:19–29. doi: 10.1038/ncb1665. [DOI] [PubMed] [Google Scholar]
- 42.LaVail MM. Kinetics of rod outer segment renewal in the developing mouse retina. J Cell Biol. 1973;58:650–61. doi: 10.1083/jcb.58.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao C, Malicki J. Nephrocystins and MKS proteins interact with IFT particle and facilitate transport of selected ciliary cargos. EMBO J. 2011;30:2532–44. doi: 10.1038/emboj.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaitin MH. Actin filaments in the photoreceptor cilium of the rds mutant mouse. Exp Eye Res. 1991;53:107–13. doi: 10.1016/0014-4835(91)90152-5. [DOI] [PubMed] [Google Scholar]
- 45.Obata S, Usukura J. Morphogenesis of the photoreceptor outer segment during postnatal development in the mouse (BALB/c) retina. Cell Tissue Res. 1992;269:39–48. doi: 10.1007/BF00384724. [DOI] [PubMed] [Google Scholar]
- 46.Jacobson SG, Cideciyan AV, Aleman TS, Sumaroka A, Roman AJ, Gardner LM, et al. Usher syndromes due to MYO7A, PCDH15, USH2A or GPR98 mutations share retinal disease mechanism. Hum Mol Genet. 2008;17:2405–15. doi: 10.1093/hmg/ddn140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X, Udovichenko IP, Brown SD, Steel KP, Williams DS. Myosin VIIa participates in opsin transport through the photoreceptor cilium. J Neurosci. 1999;19:6267–74. doi: 10.1523/JNEUROSCI.19-15-06267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitamoto J, Libby RT, Gibbs D, Steel KP, Williams DS. Myosin VI is required for normal retinal function. Exp Eye Res. 2005;81:116–20. doi: 10.1016/j.exer.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 49.Katti C, Dalal JS, Dosé AC, Burnside B, Battelle BA. Cloning and distribution of myosin 3B in the mouse retina: differential distribution in cone outer segments. Exp Eye Res. 2009;89:224–37. doi: 10.1016/j.exer.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walsh T, Walsh V, Vreugde S, Hertzano R, Shahin H, Haika S, et al. From flies’ eyes to our ears: mutations in a human class III myosin cause progressive nonsyndromic hearing loss DFNB30. Proc Natl Acad Sci U S A. 2002;99:7518–23. doi: 10.1073/pnas.102091699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin-Jones J, Sohlberg L, Dosé A, Breckler J, Hillman DW, Burnside B. Identification and localization of myosin superfamily members in fish retina and retinal pigmented epithelium. J Comp Neurol. 2009;513:209–23. doi: 10.1002/cne.21958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Branchek T, Bremiller R. The development of photoreceptors in the zebrafish, Brachydanio rerio. I. Structure. J Comp Neurol. 1984;224:107–15. doi: 10.1002/cne.902240109. [DOI] [PubMed] [Google Scholar]
- 53.Easter SS, Jr., Nicola GN. The development of vision in the zebrafish (Danio rerio) Dev Biol. 1996;180:646–63. doi: 10.1006/dbio.1996.0335. [DOI] [PubMed] [Google Scholar]
- 54.Townes-Anderson E, Dacheux RF, Raviola E. Rod photoreceptors dissociated from the adult rabbit retina. J Neurosci. 1988;8:320–31. doi: 10.1523/JNEUROSCI.08-01-00320.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ingham PW, McMahon AP. Hedgehog signalling: Kif7 is not that fishy after all. Curr Biol. 2009;19:R729–31. doi: 10.1016/j.cub.2009.07.060. [DOI] [PubMed] [Google Scholar]
- 56.Kalderon D. Hedgehog signaling: Costal-2 bridges the transduction gap. Curr Biol. 2004;14:R67–9. doi: 10.1016/j.cub.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 57.Klejnot M, Kozielski F. Structural insights into human Kif7, a kinesin involved in Hedgehog signalling. Acta Crystallogr D Biol Crystallogr. 2012;68:154–9. doi: 10.1107/S0907444911053042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Endoh-Yamagami S, Evangelista M, Wilson D, Wen X, Theunissen JW, Phamluong K, et al. The mammalian Cos2 homolog Kif7 plays an essential role in modulating Hh signal transduction during development. Curr Biol. 2009;19:1320–6. doi: 10.1016/j.cub.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 59.Farzan SF, Ascano M, Jr., Ogden SK, Sanial M, Brigui A, Plessis A, et al. Costal2 functions as a kinesin-like protein in the hedgehog signal transduction pathway. Curr Biol. 2008;18:1215–20. doi: 10.1016/j.cub.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jékely G, Arendt D. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. Bioessays. 2006;28:191–8. doi: 10.1002/bies.20369. [DOI] [PubMed] [Google Scholar]
- 61.Carvalho-Santos Z, Azimzadeh J, Pereira-Leal JB, Bettencourt-Dias M. Evolution: Tracing the origins of centrioles, cilia, and flagella. J Cell Biol. 2011;194:165–75. doi: 10.1083/jcb.201011152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rink JC, Gurley KA, Elliott SA, Sánchez Alvarado A. Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science. 2009;326:1406–10. doi: 10.1126/science.1178712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kobayashi T, Tsang WY, Li J, Lane W, Dynlacht BD. Centriolar kinesin Kif24 interacts with CP110 to remodel microtubules and regulate ciliogenesis. Cell. 2011;145:914–25. doi: 10.1016/j.cell.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 64.Zhao C, Malicki J. Genetic defects of pronephric cilia in zebrafish. Mech Dev. 2007;124:605–16. doi: 10.1016/j.mod.2007.04.004. [DOI] [PubMed] [Google Scholar]