Abstract
The unique innovation of the layered neocortex in mammalian evolution is believed to facilitate adaptive radiation of mammalian species to various ecological environments by furnishing high information processing ability. There are no transitional states from the non-mammalian simple brain to the mammalian multilayered neocortex, and thus it is totally a mystery so far how this brain structure has been acquired during evolution. In our recent study, we found the evidence showing that the evolutionary origin of the neocortical neuron subtypes predates the actual emergence of layer structure. Our comparative developmental analysis of the chick pallium, homologous to the mammalian neocortex, revealed that mammals and avians fundamentally share the neocortical neuron subtypes and their production mechanisms, suggesting that their common ancestor already possessed a similar neuronal repertory. We further demonstrated that the neocortical layer-specific neuron subtypes are arranged as mediolaterally separated domains in the chick, but not as layers in the mammalian neocortex. These animal group-specific neuronal arrangements are accomplished by spatial modulation of the neurogenetic program, suggesting an evolutionary hypothesis that the regulatory changes in the neurogenetic program innovated the mammalian specific layered neocortex.
Keywords: bird, brain patterning, evolution, layer, mammal, neocortex, neural progenitor, neuron subtype, pallium, stem cell
A Mystery About Evolution of the Mammalian Layered Neocortex
All mammalian species have a layered neocortex that plays central roles in cognitive functions (Fig. 1A). The neocortex is the dorsal part of the mammalian telencephalon containing a huge variety of neurons in the characteristic multilayered organization. The complete conservation of this cellular arrangement among all mammalian species suggests that the layered neocortex has been greatly advantageous for the mammalian species to perform efficient information processing and thereby to accomplish adaptation to various environmental conditions.
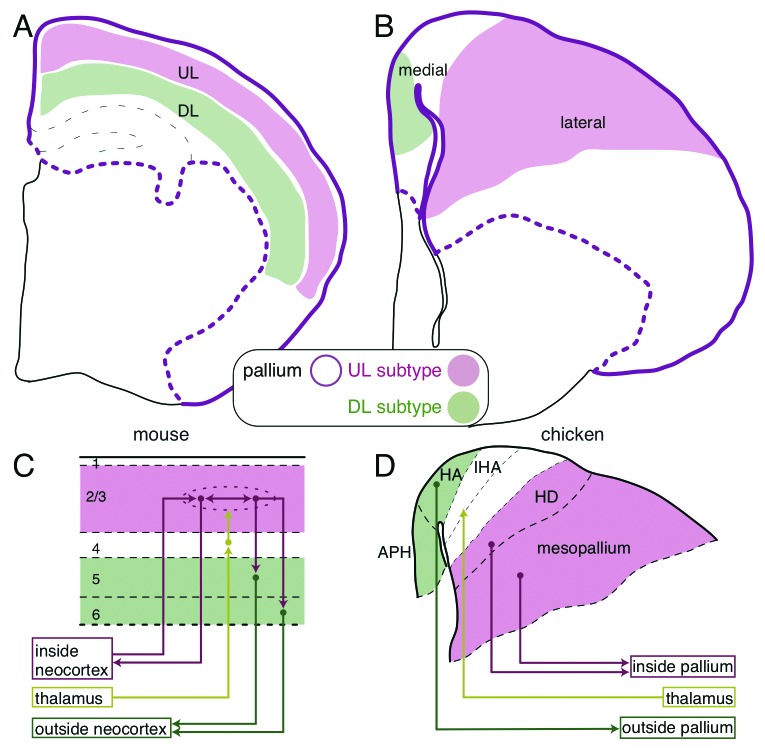
Figure 1. Comparison of the mammalian and avian pallial architectures. (A) The layered neocortex of the mouse. The upper and deep layer (UL and DL) neuron subtypes are tangentially arranged in the pallium. (B) UL and DL neuron subtypes are arranged separately in the medial and lateral domains of the chick pallium, respectively. (C and D) Similarity of the neural circuits in the mammalian neocortex and the avian pallium. (C) The columnar neural circuit in the mammalian neocortex. The input from the thalamus terminates in the layer 4. The information is transferred to and processed in the UL (layer 2/3) neurons that are connected with each other inside the neocortex, and finally output by the DL (layer 5 and 6) neurons to extracortical targets. (D) In the avian pallium, the thalamic input is received by the neurons in the central domain of the hyperpallium (IHA), which is sandwiched by the medial (HA and APH) and lateral domains (HD and mesopallium). The medial and lateral domains project to the intrapallial and extrapallial targets, respectively.
By contrast, non-mammalian species have no similar bioarchitecture in the dorsal telencephalon.1-3 Most of the reptile groups, such as turtles and lizards, have a single neuronal layer sandwiched by two axon-rich layers. Birds have a more complex architecture, in which neuronal cell bodies are clustered into several domains (Fig. 1B). Because of this huge morphological divergence, it had been debated for a long time whether or not non-mammals have a brain region homologous to the mammalian neocortex.2,4 One of the most popular views was that non-mammalian brains only contain the ventral part of the mammalian telencephalon and that the dorsal layered neocortex of the mammalian telencephalon was newly added to the old ventral part during mammalian evolution.5 However, this classical view has been overturned by recent gene expression studies.4,6 Based on expression patterns of neocortical marker genes, such as Emx and Pax6,7,8 a dorsal telencephalic region called the pallium in non-mammals includes the true homolog of the mammalian neocortex.
Therefore, we now know that non-mammalian species have the evolutionary conserved brain region homologous to the mammalian neocortex, but this region, the pallium, somehow lacks the layered bioarchitecture. Curiously enough, no extent animal species represent a transitional state toward the layered neocortex. Thus, this sophisticated layered architecture in the neocortex seems to have suddenly emerged before the mammalian diversification, leaving the evolutionary process a great mystery.
Development of the Mammalian Layered Neocortex
The mammalian neocortex consists of two major classes of neurons, the excitatory and inhibitory neurons. The excitatory neurons are further classified into several neuron subtypes.9 Each neuron subtype shares particular functional properties and occupies specific layers (Fig. 1A and C). For example, the upper layer (UL, layer 2/3) neurons connect with each other inside the neocortex, whereas the deep layer (DL, layer 5 and 6) neurons project outside the neocortex and connect to the subcortical targets. The neurons in the intermediate layer (layer 4), between the UL and DL, receive axonal inputs from outside the neocortex in most mammalian species.1,9 The layered organization of these specific subtypes should have a benefit for local vertical information flow among the subtypes. Indeed, these subtypes are densely connected with each other across the layers and form a vertical columnar unit (Fig. 1C). According to their functional properties, the incoming information that is received by layer 4 neurons is subsequently transferred to and processed by the UL neurons located immediately above, and then conveys to the downstairs DL neurons, which finally output the processed information from the neocortex (Fig. 1C). Such columnar information processing across the layers is the functional foundation of the mammalian neocortex and critically relies on the layered neuronal arrangement.10
The layer-specific properties of the neocortical neurons are allocated according to their differentiation timing;11 A single neural progenitor cell in the mammalian neocortex asymmetrically divides and sequentially generates multiple subtypes one by one from the DL to the UL in an inside-out order. This chronologically sequential production of neuron subtypes is commonly observed in the mammalian species12-14 and also can be recapitulated in culture of mammalian neocortical progenitors.15
Although molecular mechanisms for the sequential subtype production remain largely unclear, there is a unique set of genes known to be specifically expressed in each subtype.9 As might be expected, some of the genes, in fact, have a fate determining role that assigns layer-specific phenotypes to the neurons.9 For example, Satb2, a transcription factor specific to late-generated UL neurons, is responsible for directing axons toward the interhemispheric neocortical targets,16,17 the characteristic connectivity of the UL neurons. On the other hand, a transcription factor specific to the early-generated DL neuron, Ctip2, drives DL-specific brainstem projection.18 These layer-specific transcription factors mutually regulate each other’s expression and make a genetic network,19,20 which can underlie the switch of subtype fate decision. In the example above, Satb2 suppresses the expression of Ctip2, and thereby prohibits late-generated neurons from taking the deep layer fate.16,17 The findings of these functional marker genes for individual layer-specific neuron subtypes, have greatly contributed to our understanding of mammalian neocortical development.
Existence of DL and UL Neuron Subtypes in the Avian Pallium
Because only mammals have a layered neocortex, many people naturally believed that the layer-specific neuron subtypes were first introduced in the neocortex of ancestral mammals accompanied by the emergence of layer structure.3,21 On the contrary, we found that neocortical layer-specific neuron subtypes exist in the non-layered pallium of the non-mammalian chick.22 A combinatorial expression of layer-specific marker genes demonstrated that the medial and lateral domains in the chick pallium harbor the DL and UL subtypes, respectively (Fig. 1B and D). More specifically, in the chick pallium, the DL marker genes, Ctip2, Fezf2 and Er81, are commonly expressed in the parahipocampal region (APH), and the UL marker genes, Satb2, Cux2, Mef2c and Foxp1, are expressed mainly in the mesopallium (Fig. 1D). Furthermore, axonal connections confirmed that the chick DL and UL subtypes are indeed functional homologs of the mammalian subtypes; chick DL subtypes project to the subcortical targets, and chick UL subtypes are locally connected inside the pallium, as are the mammalian neocortical neuron subtypes
Although this work was the first indication of molecular expression similarities between the mammalian and the avian neuron subtypes, the functional similarities have, in fact, been considered previously. Originally, Karten proposed a hypothesis that medio-lateral subdivisions of the avian hyperpallium, previously called the visual wulst, functionally correspond to the neocortical layers of mammals.2,23 More precisely, he mapped the medial, intermediate, and lateral domains of the avian hyperpallium as the functional homologs of the DL (layer 5 and 6), intermediate layer (layer 4), and UL (layer 2/3) of the mammalian neocortex, principally based on the connection patterns (Fig. 1D). Our gene expression data agree well with his hypothesis and provide further molecular support for it. Nevertheless, a big question has remained. Anatomically, the mediolateral axis of the chick pallial domains is orthogonal to the mammalian layer axis. Considering the developmental axis, the homologous neuronal domains between mammals and birds cannot be constructed in the same developmental mechanism.
Spatially Segregated Production of the Neuron Subtypes Underlying the Avian-Type Neuronal Arrangement
The neural progenitors reside in the ventricular zone surrounding the ventricle in all vertebrate brain regions. In the whole mammalian neocortex, the neuron subtypes sequentially generated from progenitors radially migrate from the ventricular zone toward the brain surface and are piled up chronologically in an inside-out fashion (Fig. 2A). Consequently, the neuronal layers are constructed in parallel with the ventricular zone over the entire neocortex. In the chick pallium, neuronal subtypes are also generated from the progenitors in the ventricular zone and migrate radially.2,24 How then is this irregular mediolateral arrangement of DL and UL subtypes achieved?
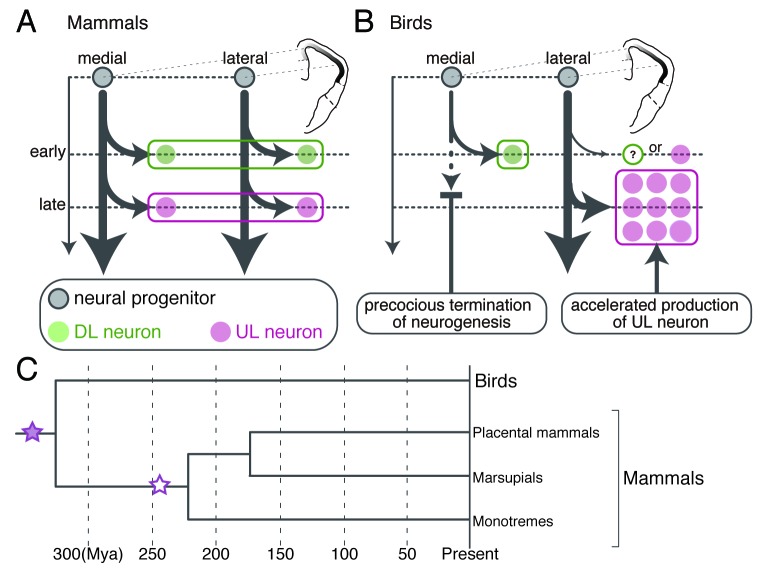
Figure 2. Animal group-specific neurogenesis in the pallium and the potential evolutionary history of pallial bioarchitecture during the evolution. (A and B) Comparison of the pallial neurogenesis between mammals and birds. The gray, green and magenta circles indicate the neural progenitor, DL and UL subtypes, respectively. The arrows indicate the neurogenetic process. (A) Spatially unbiased progression of neurogenesis constructs homogeneous layers of neuron subtypes across the mammalian neocortex. (B) The spatially biased neurogenesis constructs the mediolaterally separated subtype arrangement in the avian pallium. (C) Hypothetical evolutionary scenario of the emergence of the neocortical layer architecture. Neocortical layer-specific neuron subtypes originated from the common ancestor of the mammals and birds (purple filled star). The layered arrangement of neuron subtypes newly emerged (white star) in the mammalian lineage before the branch leading to the monotremes. Illustrations in (A and B) are reproduced with permission from reference 22.
We found that the chick DL and UL subtypes are generated from the mediolaterally separated sources of neural progenitors (Fig. 2B).22 The focal labeling of progenitors in the medial and lateral ventricular zone revealed that they selectively produce the DL and UL subtypes, respectively. These differentiated subtypes then in turn migrate radially and eventually settle down in the medial and lateral separate domains immediately close to their generation sites. Therefore, this spatially segregated production of the neuronal subtypes is the cause of their mediolaterally separated localization in the chick pallium and is the most significant difference from the neuron production in the mammalian neocortex. In mammals, neocortical progenitors are spatially homogeneous throughout the neocortex. They are multipotent and produce multiple layer-specific subtypes sequentially in a time-dependent manner. The time-dependent rule of subtype production is, however, still followed even in the chick system; the DL subtype differentiate earlier than the UL subtype as observed in the mammalian neocortex.22
The Hidden Mammalian-Type Neurogenetic Potential in Avian Neural Progenitors
At first glance, the chick neural progenitors appeared critically different from those of the mammalian neural progenitors; depending on the spatial position, the progenitors seemed to be committed to only produce DL or UL subtypes, but not both. Surprising enough, however, the chick neural progenitors unveil their multipotency and produce both the DL and UL subtypes once they are isolated from the intact brain environment and sparsely cultured in vitro.22 Both the medial and lateral progenitors have almost the same multipotency and sequentially produce the DL and then UL subtypes in the same chronological sequence as that of mammalian neocortical progenitors.15 Therefore, the chick neural progenitors intrinsically have the mammalian type neurogenetic potential, but the potential is somehow restricted by some extrinsic factors in the chick pallium in vivo.
Spatial Modulation of the Neocortical Neurogenetic Program in the Avian Pallium
One remaining question is how the intrinsically multipotent progenitors selectively produce either one of the neuron subtypes in the chick pallium in vivo. We found that the neurogenetic activity of the neural progenitors is spatio-temporally regulated in the chick pallium.22 Early on, when the DL subtype is generated, neurons are homogeneously generated from the ventricular zone across the entire chick pallium. However, later, when the UL subtype is generated, neurogenesis is almost terminated in the medial ventricular zone, but conversely, explosively accelerated in the lateral ventricular zone (Fig. 2B). This spatio-temporally biased neurogenesis enables selective production of the late-generated UL subtype in the lateral region and can contribute to construction of the avian-type mediolateral subtype arrangement. The molecular background of the extrinsic control of neurogenesis still remains unclear and deserves future study toward elucidation of evolutionary mechanisms that create animal group-specific neuronal arrangements in the pallium.
A Novel Model for Evolution of the Layered Neocortex
Because only mammals have the layered neocortex, it is generally accepted that the neocortical, neurogenetic program that sequentially produces multiple layer-specific subtypes is specific to mammals and co-evolved with a layered bioarchitecture.2,25 However, our study challenges this general belief and proposes an alternative evolutionary scenario. The avian pallium possesses the repertoire of neuronal subtypes comparable to that of the mammalian neocortical layer-specific subtypes, which are produced by a conserved neurogenetic program. This means that the neocortical, neurogenetic program originated even before the emergence of mammals and can date back more than 300 million years to the common ancestor of amniote lineages. Thus, both mammals and non-mammals must have utilized the neurogenetic program inherited from the common ancestor in a differently regulated manner to materialize the animal group-specific pallial architectures (Fig. 2C).
The deep conservation of the neocortical neurogenetic program between the anciently diverged mammals and birds suggests a strong selective constraint for this program. It should have been beneficial for various animal groups regardless of the pallial architecture. One obvious benefit is that it can diversify cell types from a limited number of progenitor cells. Indeed, chronological production of multiple neuron subtypes from a single neural progenitor is a common strategy in both vertebrate and invertebrates.26 In addition to this benefit, this neurogenetic program might provide an evolutionary capacity to create novel brain architecture because only a small modification of the program can cause a large impact in the final brain architecture. For example, the extraordinarily rapid expansion of neocortical volume in primate evolution can be caused by a modulation of the neurogenetic program, such as a drastic increase of progenitor proliferation over differentiation.27-29
Because of the lack of information about the neuronal arrangement in the pallium of outgroup species, such as amphibians, it is technically impossible to conclude whether the mediolateral subtype arrangement in the avian pallium is ancestral to the mammalian layered arrangement. Nevertheless, based on comparisons of pallial bioarchitecture of non-mammalian groups, it is pretty obvious that the critical change leading to the layered bioarchitecture occurred in the mammalian lineage after the divergence from the avian and other reptilian lineages. Our model explains this evolutionary event as follows. Before the divergence of the mammals and birds, the common ancestor possessed the avian-type mediolateral subtype arrangement in the pallium, which was constructed by a spatially modulated form of the neurogenetic program. After the divergence, in the mammalian lineage, the neurogenetic program was unleashed from the extrinsic spatial restriction and allowed to produce all layer-specific neuron subtypes in the entire neocortical field. This event must have occurred before the radiation of various mammalian groups (Fig. 2C).30 Since then, the layered neocortex has been retained in all the descendant mammalian species from the monotremes to placental mammals, probably for its functional benefit, and further expanded and complexified in primate and some other lineages.
Acknowledgments
We are grateful to T. Kawasaki, T. Gojobori, Y. Murakami, T. Fukagawa, Y. Hiromi, K. Emoto, K. Sumiyama, T. Nomura and S. Aizawa for comments and encouragement. I.K.S. was a NIG Postdoctoral Research Fellow. This work was supported by Grants-in-Aid for Creative Scientific Research from the Japan Society for the Promotion of Science (grant number 18GS0320 and 23657151 to T.H.) and the Center for the Promotion of Integrated Sciences (CPIS) of Sokendai.
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/21032
References
- 1.Butler AB, Hodos W. Comparative vertebrate neuroanatomy: evolution and adaptation. Hoboken, N.J.: Wiley-Interscience, 2005. [Google Scholar]
- 2.Medina L, Reiner A. Do birds possess homologues of mammalian primary visual, somatosensory and motor cortices? Trends Neurosci. 2000;23:1–12. doi: 10.1016/S0166-2236(99)01486-1. [DOI] [PubMed] [Google Scholar]
- 3.Northcutt RG, Kaas JH. The emergence and evolution of mammalian neocortex. Trends Neurosci. 1995;18:373–9. doi: 10.1016/0166-2236(95)93932-N. [DOI] [PubMed] [Google Scholar]
- 4.Jarvis ED, Güntürkün O, Bruce L, Csillag A, Karten H, Kuenzel W, et al. Avian Brain Nomenclature Consortium Avian brains and a new understanding of vertebrate brain evolution. Nat Rev Neurosci. 2005;6:151–9. doi: 10.1038/nrn1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLean PD. The triune brain in evolution: role in paleocerebral functions. New York: Plenum Press, 1990. [DOI] [PubMed] [Google Scholar]
- 6.Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, et al. Avian Brain Nomenclature Forum Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez AS, Pieau C, Repérant J, Boncinelli E, Wassef M. Expression of the Emx-1 and Dlx-1 homeobox genes define three molecularly distinct domains in the telencephalon of mouse, chick, turtle and frog embryos: implications for the evolution of telencephalic subdivisions in amniotes. Development. 1998;125:2099–111. doi: 10.1242/dev.125.11.2099. [DOI] [PubMed] [Google Scholar]
- 8.Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, et al. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424:409–38. doi: 10.1002/1096-9861(20000828)424:3<409::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–37. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- 10.Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. New York: McGraw-Hill, Health Professions Division, 2000. [Google Scholar]
- 11.McConnell SK. The generation of neuronal diversity in the central nervous system. Annu Rev Neurosci. 1991;14:269–300. doi: 10.1146/annurev.ne.14.030191.001413. [DOI] [PubMed] [Google Scholar]
- 12.Luskin MB, Pearlman AL, Sanes JR. Cell lineage in the cerebral cortex of the mouse studied in vivo and in vitro with a recombinant retrovirus. Neuron. 1988;1:635–47. doi: 10.1016/0896-6273(88)90163-8. [DOI] [PubMed] [Google Scholar]
- 13.Kornack DR, Rakic P. Radial and horizontal deployment of clonally related cells in the primate neocortex: relationship to distinct mitotic lineages. Neuron. 1995;15:311–21. doi: 10.1016/0896-6273(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 14.Reid CB, Tavazoie SF, Walsh CA. Clonal dispersion and evidence for asymmetric cell division in ferret cortex. Development. 1997;124:2441–50. doi: 10.1242/dev.124.12.2441. [DOI] [PubMed] [Google Scholar]
- 15.Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, et al. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–51. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- 16.Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Fariñas I, Grosschedl R, et al. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–77. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57:378–92. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 18.Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–21. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 19.Leone DP, Srinivasan K, Chen B, Alcamo E, McConnell SK. The determination of projection neuron identity in the developing cerebral cortex. Curr Opin Neurobiol. 2008;18:28–35. doi: 10.1016/j.conb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwan KY, Sestan N, Anton ES. Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex. Development. 2012;139:1535–46. doi: 10.1242/dev.069963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiner A. A comparison of neurotransmitter-specific and neuropeptide-specific neuronal cell types present in the dorsal cortex in turtles with those present in the isocortex in mammals: implications for the evolution of isocortex. Brain Behav Evol. 1991;38:53–91. doi: 10.1159/000114379. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki IK, Kawasaki T, Gojobori T, Hirata T. The temporal sequence of the mammalian neocortical neurogenetic program drives mediolateral pattern in the chick pallium. Dev Cell. 2012;22:863–70. doi: 10.1016/j.devcel.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Karten HJ, Hodos W, Nauta WJ, Revzin AM. Neural connections of the “visual wulst” of the avian telencephalon. Experimental studies in the piegon (Columba livia) and owl (Speotyto cunicularia) J Comp Neurol. 1973;150:253–78. doi: 10.1002/cne.901500303. [DOI] [PubMed] [Google Scholar]
- 24.Striedter GF, Marchant TA, Beydler S. The “neostriatum” develops as part of the lateral pallium in birds. J Neurosci. 1998;18:5839–49. doi: 10.1523/JNEUROSCI.18-15-05839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marin-Padilla M. Dual origin of the mammalian neocortex and evolution of the cortical plate. Anat Embryol (Berl) 1978;152:109–26. doi: 10.1007/BF00315920. [DOI] [PubMed] [Google Scholar]
- 26.Jacob J, Maurange C, Gould AP. Temporal control of neuronal diversity: common regulatory principles in insects and vertebrates? Development. 2008;135:3481–9. doi: 10.1242/dev.016931. [DOI] [PubMed] [Google Scholar]
- 27.Kriegstein A, Noctor S, Martínez-Cerdeño V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–90. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- 28.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–9. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 29.Sahara S, O’Leary DD. Fgf10 regulates transition period of cortical stem cell differentiation to radial glia controlling generation of neurons and basal progenitors. Neuron. 2009;63:48–62. doi: 10.1016/j.neuron.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowe TB, Macrini TE, Luo Z-X. Fossil evidence on origin of the mammalian brain. Science. 2011;332:955–7. doi: 10.1126/science.1203117. [DOI] [PubMed] [Google Scholar]