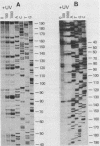
Abstract
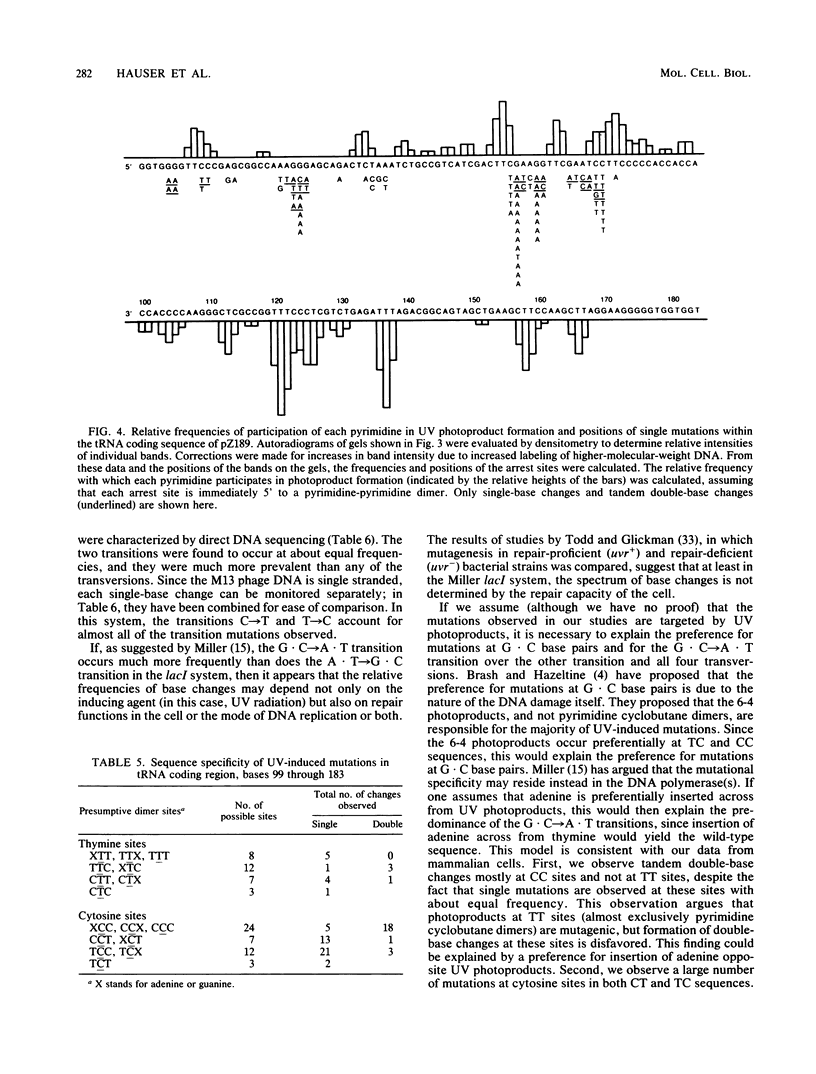
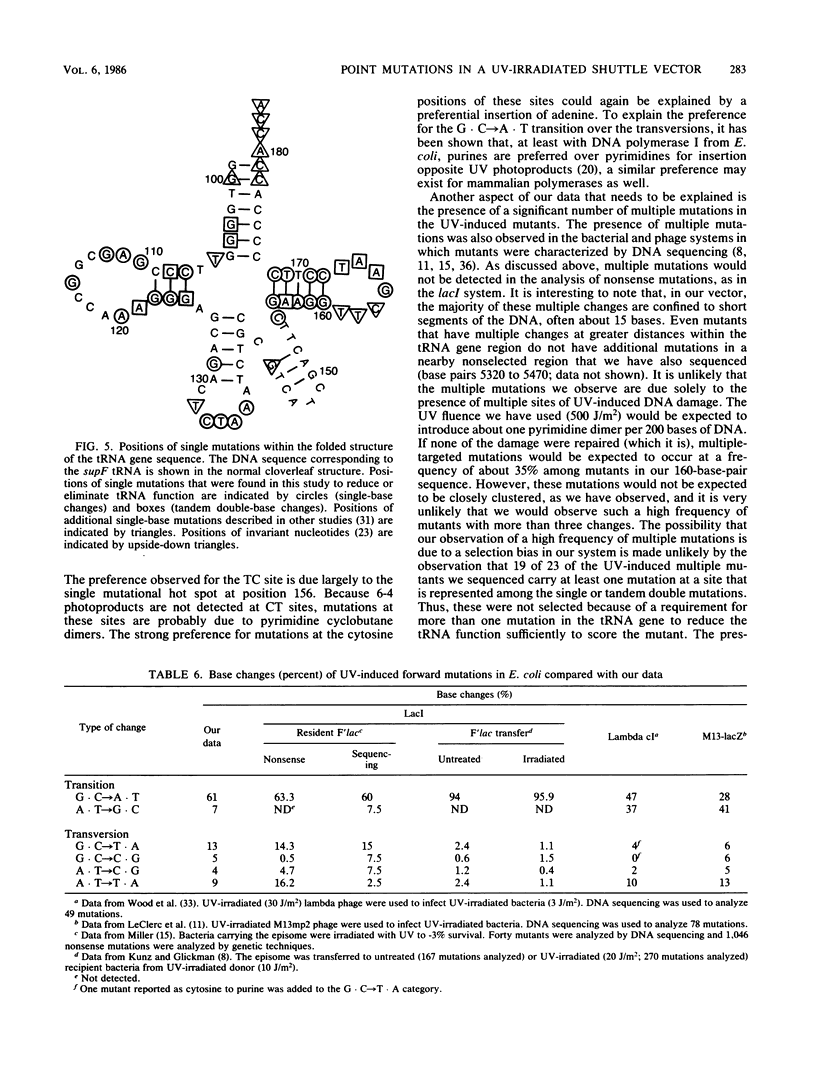
A simian virus 40-based shuttle vector was used to characterize UV-induced mutations generated in mammalian cells. The small size and placement of the mutagenesis marker (the supF suppressor tRNA gene from Escherichia coli) within the vector substantially reduced the frequency of spontaneous mutations normally observed after transfection of mammalian cells with plasmid DNA; hence, UV-induced mutations were easily identified above the spontaneous background. UV-induced mutations characterized by DNA sequencing were found primarily to be base substitutions; about 56% of these were single-base changes, and 17% were tandem double-base changes. About 24% of the UV-induced mutants carried multiple mutations clustered within the 160-base-pair region sequenced. The majority (61%) of base changes were the G . C----A . T transitions; the other transition (A . T----G . C) and all four transversions occurred at about equal frequencies. Hot spots for UV mutagenesis did not correspond to hot spots for UV-induced photoproduct formation (determined by a DNA synthesis arrest assay); in particular, sites of TT dimers were underrepresented among the UV-induced mutations. These observations suggest to us that the DNA polymerase(s) responsible for mutation induction exhibits a localized loss of fidelity in DNA synthesis on UV-damaged templates such that it synthesizes past UV photoproducts, preferentially inserting adenine, and sometimes misincorporates bases at undamaged sites nearby.
Full text
PDF
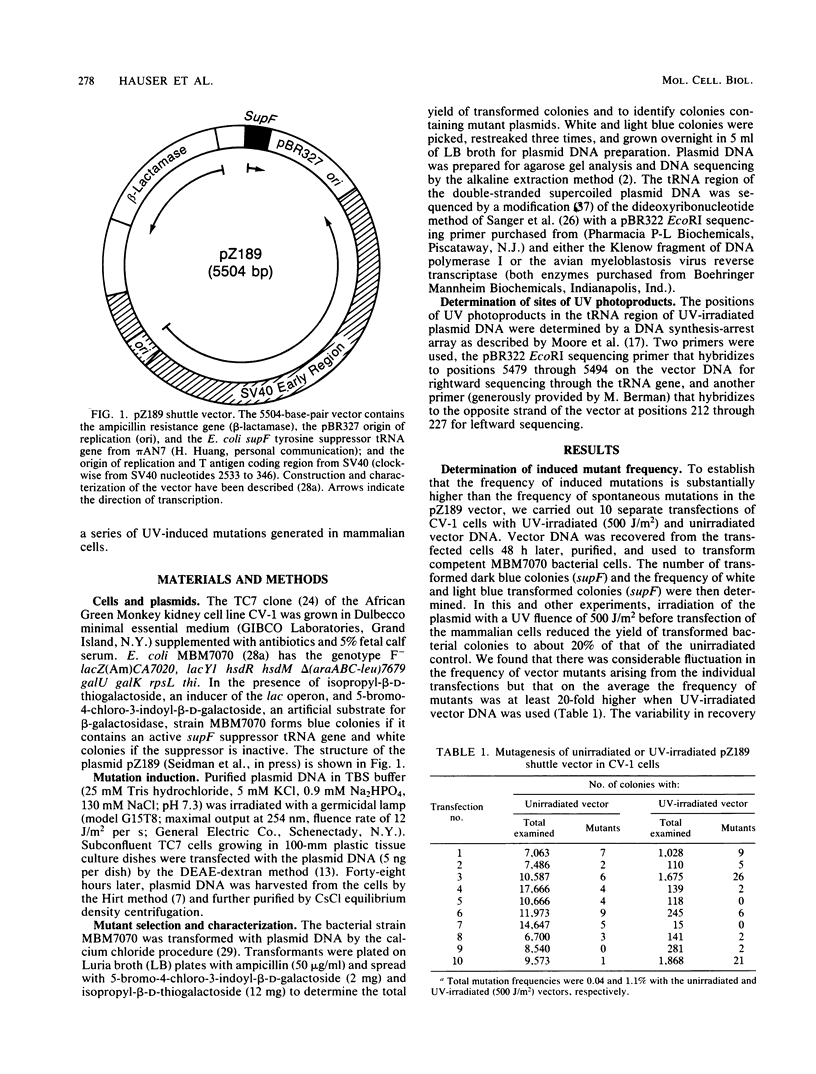

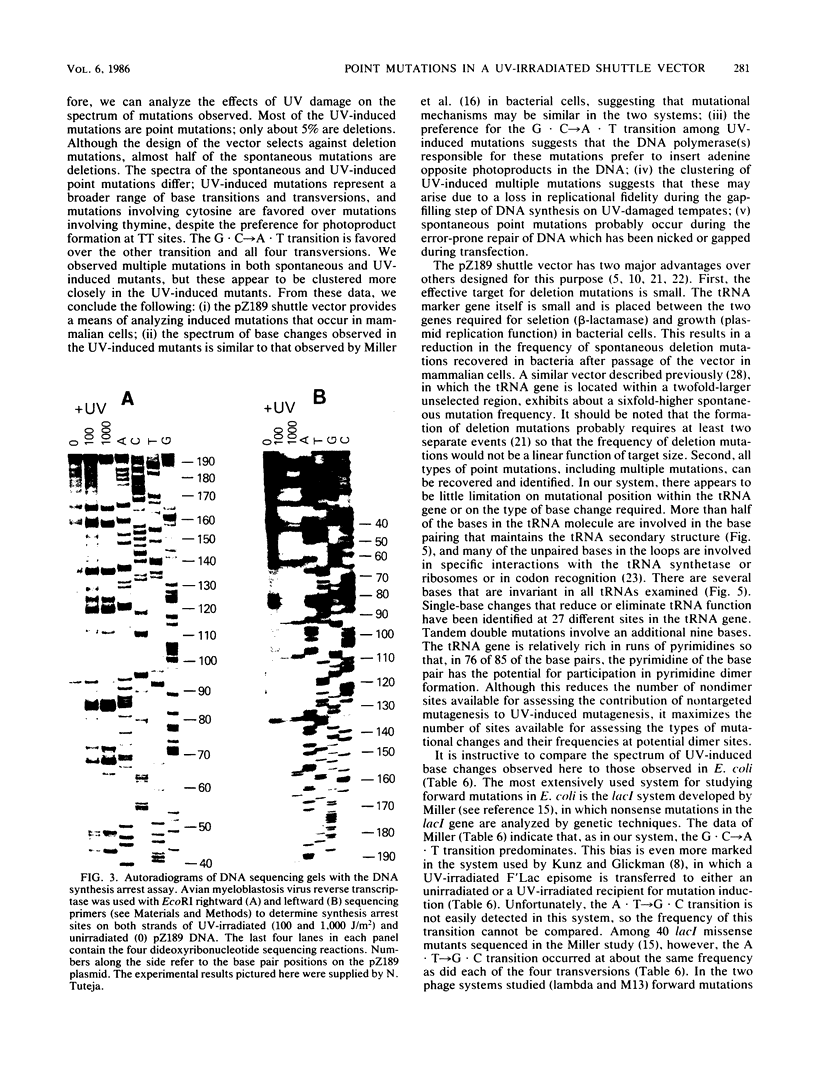


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett S. W., Landaw E. M., Dixon K. Test of models for replication of simian virus 40 DNA following ultraviolet irradiation. Biophys J. 1984 Sep;46(3):307–321. doi: 10.1016/S0006-3495(84)84027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Cancer genes come of age. Cell. 1983 Apr;32(4):1018–1020. doi: 10.1016/0092-8674(83)90284-2. [DOI] [PubMed] [Google Scholar]
- Brash D. E., Haseltine W. A. UV-induced mutation hotspots occur at DNA damage hotspots. Nature. 1982 Jul 8;298(5870):189–192. doi: 10.1038/298189a0. [DOI] [PubMed] [Google Scholar]
- Calos M. P., Lebkowski J. S., Botchan M. R. High mutation frequency in DNA transfected into mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3015–3019. doi: 10.1073/pnas.80.10.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kunz B. A., Glickman B. W. The role of pyrimidine dimers as premutagenic lesions: a study of targeted vs. untargeted mutagenesis in the lacI gene of Escherichia coli. Genetics. 1984 Mar;106(3):347–364. doi: 10.1093/genetics/106.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClerc J. E., Istock N. L., Saran B. R., Allen R., Jr Sequence analysis of ultraviolet-induced mutations in M13lacZ hybrid phage DNA. J Mol Biol. 1984 Dec 5;180(2):217–237. doi: 10.1016/s0022-2836(84)80001-7. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., DuBridge R. B., Antell E. A., Greisen K. S., Calos M. P. Transfected DNA is mutated in monkey, mouse, and human cells. Mol Cell Biol. 1984 Oct;4(10):1951–1960. doi: 10.1128/mcb.4.10.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Miller J. H. Carcinogens induce targeted mutations in Escherichia coli. Cell. 1982 Nov;31(1):5–7. doi: 10.1016/0092-8674(82)90398-1. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Lebkowski J. S., Greisen K. S., Calos M. P. Specificity of mutations induced in transfected DNA by mammalian cells. EMBO J. 1984 Dec 20;3(13):3117–3121. doi: 10.1002/j.1460-2075.1984.tb02267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H. Mutagenic specificity of ultraviolet light. J Mol Biol. 1985 Mar 5;182(1):45–65. doi: 10.1016/0022-2836(85)90026-9. [DOI] [PubMed] [Google Scholar]
- Moore P. D., Bose K. K., Rabkin S. D., Strauss B. S. Sites of termination of in vitro DNA synthesis on ultraviolet- and N-acetylaminofluorene-treated phi X174 templates by prokaryotic and eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1981 Jan;78(1):110–114. doi: 10.1073/pnas.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L., Sherman F. Mutagenic specificity: reversion of iso-1-cytochrome c mutants of yeast. J Mol Biol. 1973 Sep 5;79(1):65–82. doi: 10.1016/0022-2836(73)90270-2. [DOI] [PubMed] [Google Scholar]
- Rabkin S. D., Moore P. D., Strauss B. S. In vitro bypass of UV-induced lesions by Escherichia coli DNA polymerase I: specificity of nucleotide incorporation. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1541–1545. doi: 10.1073/pnas.80.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque A., Chakrabarti S., Joffee S., Seidman M. Mutagenesis of a shuttle vector plasmid in mammalian cells. Mol Cell Biol. 1984 Mar;4(3):435–441. doi: 10.1128/mcb.4.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque A., Mizusawa H., Seidman M. M. Rearrangement and mutagenesis of a shuttle vector plasmid after passage in mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3010–3014. doi: 10.1073/pnas.80.10.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Huebner K. Effect of cell chromosome number on simian virus 40 replication. Exp Cell Res. 1973 Sep;81(1):120–126. doi: 10.1016/0014-4827(73)90118-3. [DOI] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos E., Reddy E. P., Pulciani S., Feldmann R. J., Barbacid M. Spontaneous activation of a human proto-oncogene. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4679–4683. doi: 10.1073/pnas.80.15.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Dasgupta U. B., Summers W. C. Error-prone mutagenesis detected in mammalian cells by a shuttle vector containing the supF gene of Escherichia coli. Mol Cell Biol. 1984 Oct;4(10):2227–2230. doi: 10.1128/mcb.4.10.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman M. M., Dixon K., Razzaque A., Zagursky R. J., Berman M. L. A shuttle vector plasmid for studying carcinogen-induced point mutations in mammalian cells. Gene. 1985;38(1-3):233–237. doi: 10.1016/0378-1119(85)90222-7. [DOI] [PubMed] [Google Scholar]
- Smith J. D. Gentics of transfer RNA. Annu Rev Genet. 1972;6:235–256. doi: 10.1146/annurev.ge.06.120172.001315. [DOI] [PubMed] [Google Scholar]
- Stacks P. C., White J. H., Dixon K. Accommodation of pyrimidine dimers during replication of UV-damaged simian virus 40 DNA. Mol Cell Biol. 1983 Aug;3(8):1403–1411. doi: 10.1128/mcb.3.8.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd P. A., Glickman B. W. Mutational specificity of UV light in Escherichia coli: indications for a role of DNA secondary structure. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4123–4127. doi: 10.1073/pnas.79.13.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. H., Dixon K. Gap filling and not replication fork progression is the rate-limiting step in the replication of UV-damaged simian virus 40 DNA. Mol Cell Biol. 1984 Jul;4(7):1286–1292. doi: 10.1128/mcb.4.7.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. I., Cleaver J. E. Excision repair of ultraviolet damage in mammalian cells. Evidence for two steps in the excision of pyrimidine dimers. Biophys J. 1978 May;22(2):265–279. doi: 10.1016/S0006-3495(78)85488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. D., Skopek T. R., Hutchinson F. Changes in DNA base sequence induced by targeted mutagenesis of lambda phage by ultraviolet light. J Mol Biol. 1984 Mar 5;173(3):273–291. doi: 10.1016/0022-2836(84)90121-9. [DOI] [PubMed] [Google Scholar]