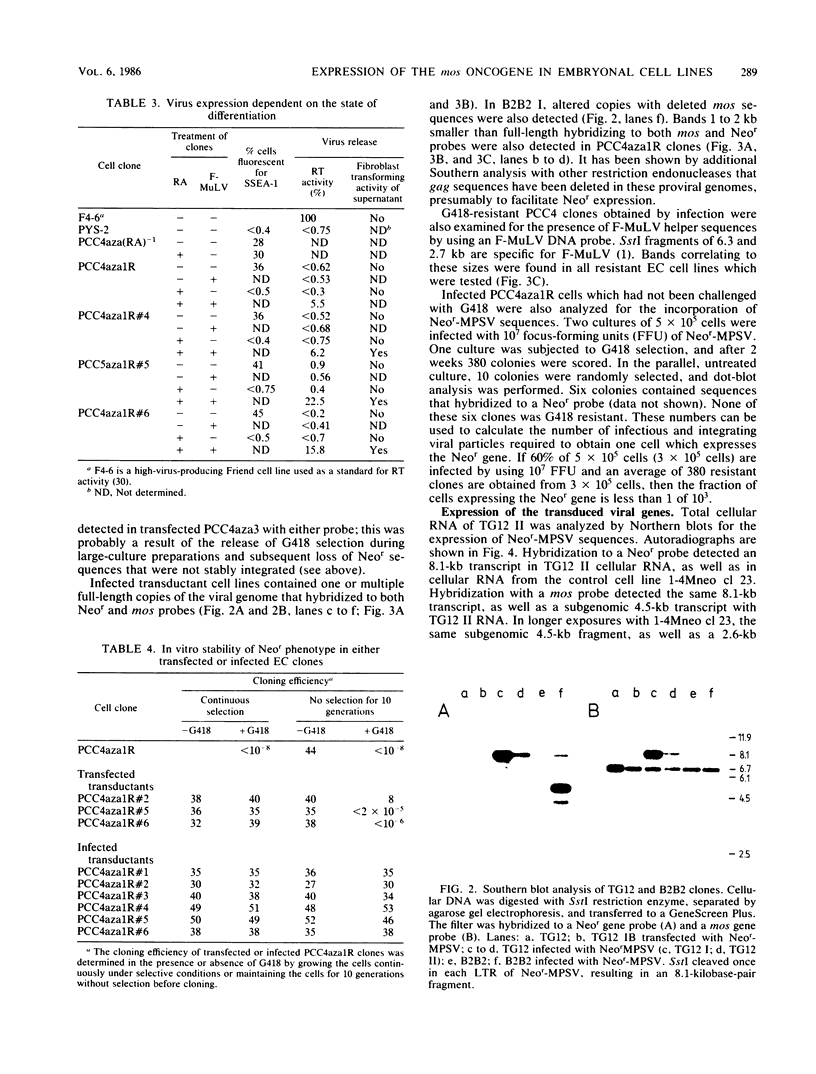
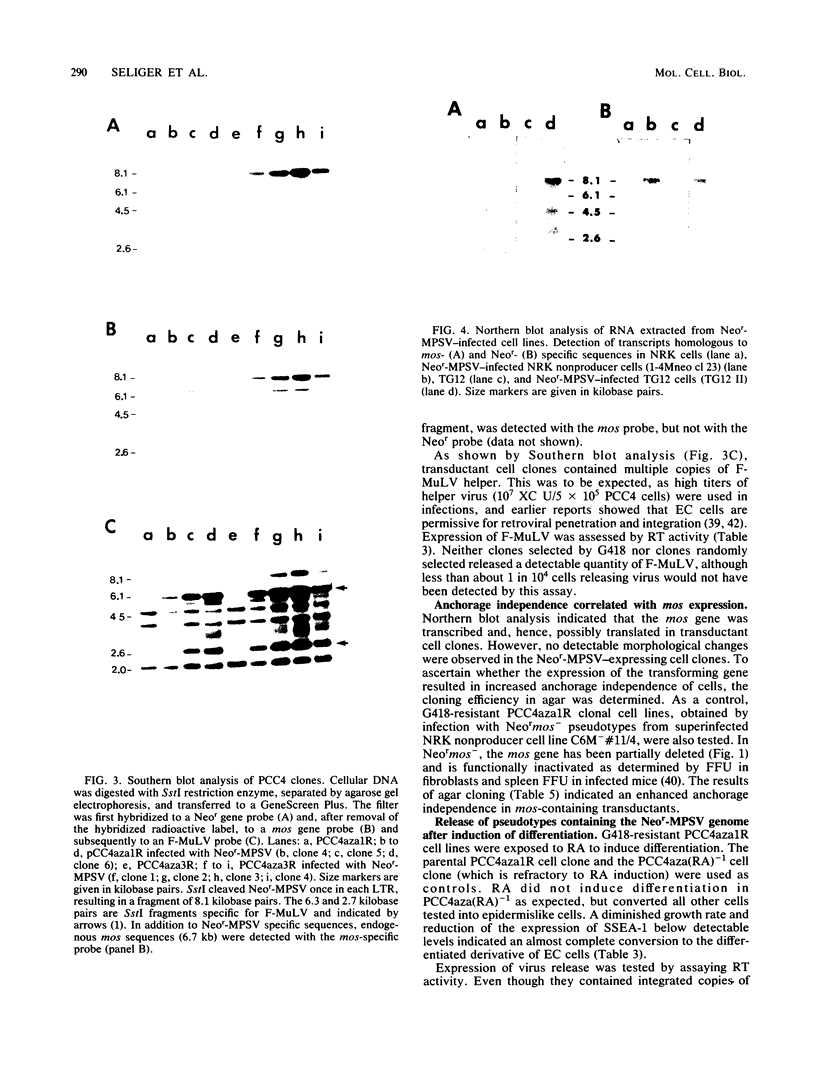
Abstract
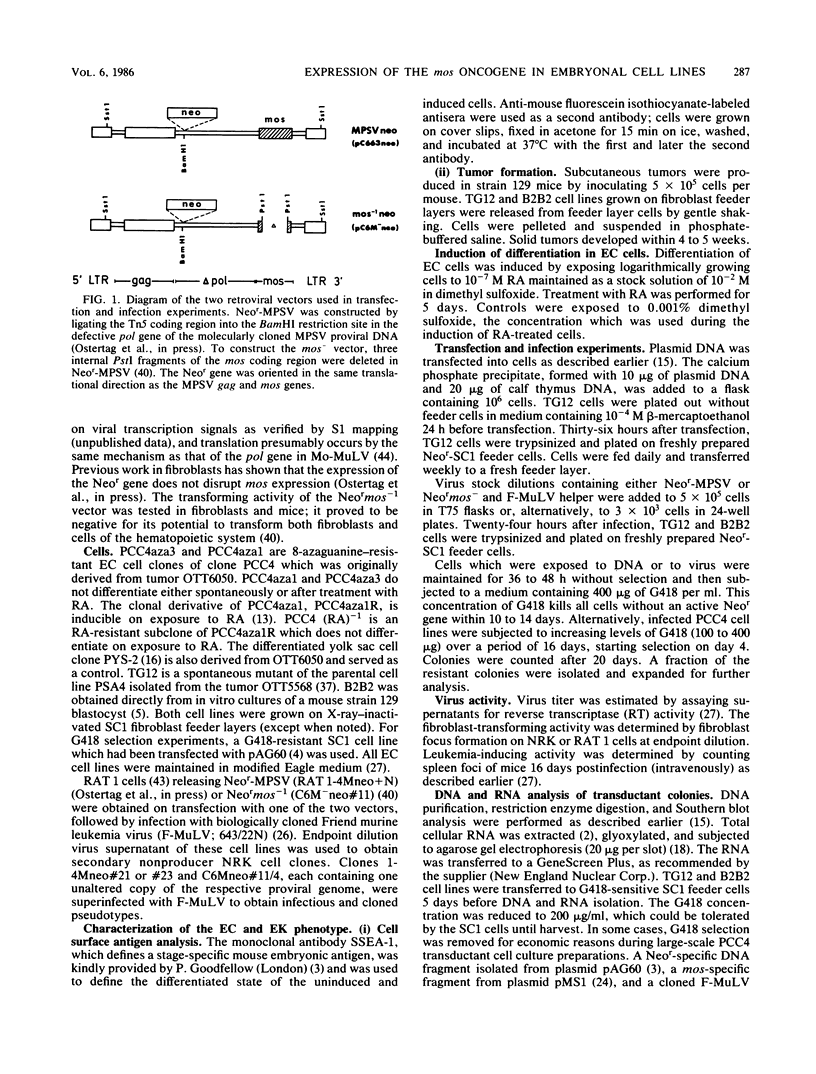
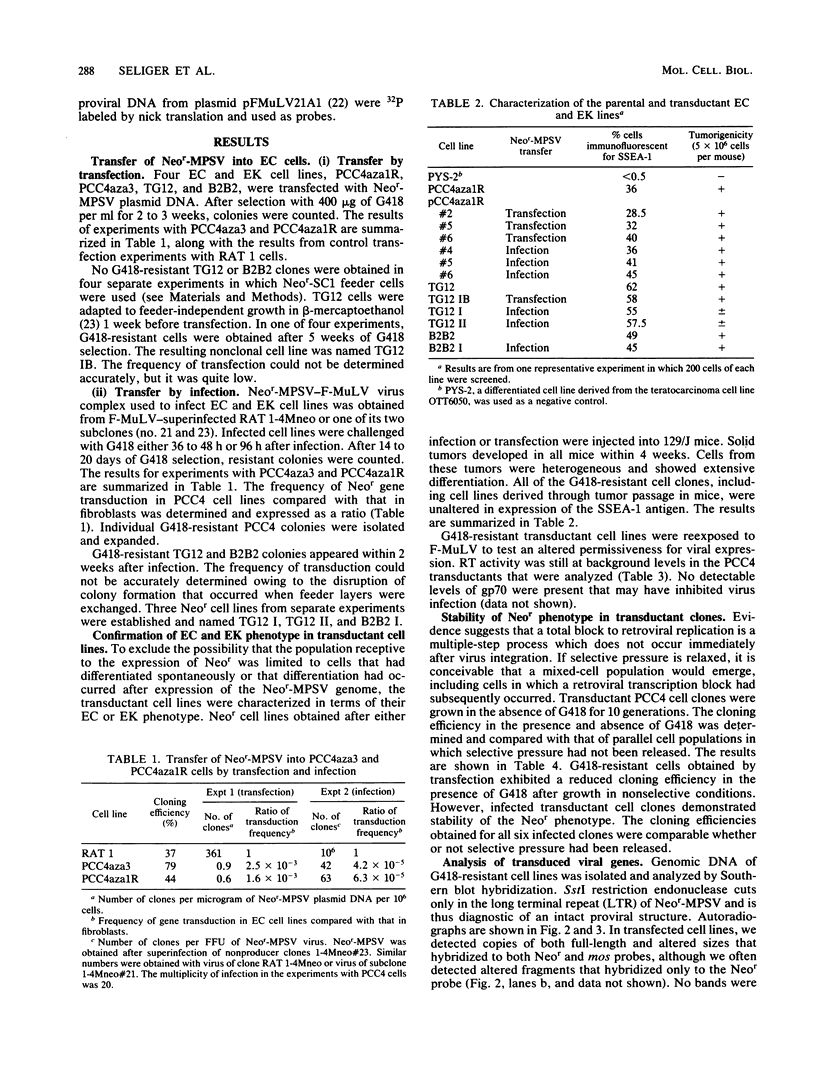
A derivative of the myeloproliferative sarcoma virus (Neor-MPSV) carrying the mos oncogene and dominant selection marker for neomycin resistance (Neor) was introduced into embryonal carcinoma and embryo-derived cell lines by transfection and infection using pseudotypes with Friend helper virus (Friend murine leukemia virus [F-MuLV]). Cells resistant to G418 (a neomycin analog) were cloned and expanded. Transductants retained an undifferentiated phenotype as judged by morphology, tumorigenicity, and cell-surface antigen analyses. Nucleic acid analysis of infectants revealed both Neor-MPSV and F-MuLV proviruses, although no virus was released. G418-resistant transductants remained nonpermissive for the expression of other proviruses and for subsequent superinfection. Northern analysis showed expression of full-length Neor-MPSV, as well as mos-specific subgenomic RNA. mos sequences were deleted from Neor-MPSV (Neor mos-1), and pseudotypes were used to infect embryonal carcinoma cells. No morphological differences were observed in either mos+ or mos- transductants as compared with parental cell lines. However, mos+ transductants showed an enhanced anchorage-independent growth compared with that of mos- transductants in agar cloning. PCC4 transductants were induced to differentiate with retinoic acid and superinfected with F-MuLV. Infection with viral supernatant in fibroblasts and in mice confirmed the rescue of biologically active Neor-MPSV.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asche W., Colletta G., Warnecke G., Nobis P., Pennie S., King R. M., Ostertag W. Lack of retrovirus gene expression in somatic cell hybrids of friend cells and teratocarcinoma cells with a teratocarcinoma phenotype. Mol Cell Biol. 1984 May;4(5):923–930. doi: 10.1128/mcb.4.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bradley A., Evans M., Kaufman M. H., Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984 May 17;309(5965):255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Kaufman M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981 Jul 9;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Frankel A. E., Fischinger P. J. Nucleotide sequences in mouse DNA and RNA specific for Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3705–3709. doi: 10.1073/pnas.73.10.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattoni S., Kirschmeier P., Weinstein I. B., Escobedo J., Dina D. Cellular Moloney murine sarcoma (c-mos) sequences are hypermethylated and transcriptionally silent in normal and transformed rodent cells. Mol Cell Biol. 1982 Jan;2(1):42–51. doi: 10.1128/mcb.2.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautsch J. W., Wilson M. C. Delayed de novo methylation in teratocarcinoma suggests additional tissue-specific mechanisms for controlling gene expression. Nature. 1983 Jan 6;301(5895):32–37. doi: 10.1038/301032a0. [DOI] [PubMed] [Google Scholar]
- Greiser-Wilke I., Ostertag W., Goldfarb P., Lang A., Furusawa M., Conscience J. F. Inducibility of spleen focus-forming virus by BrdUrd is controlled by the differentiated state of the cell. Proc Natl Acad Sci U S A. 1981 May;78(5):2995–2999. doi: 10.1073/pnas.78.5.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Jähner D., Nobis P., Simon I., Löhler J., Harbers K., Grotkopp D. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981 May;24(2):519–529. doi: 10.1016/0092-8674(81)90343-3. [DOI] [PubMed] [Google Scholar]
- Jakob H., Boon T., Gaillard J., Nicolas J., Jacob F. Tératocarcinome de la spuris: isolement, culture et propriétés de cellules a potentialités multiples. Ann Microbiol (Paris) 1973 Oct;124(3):269–282. [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E., Sherman M. I. Stimulation of differentiation of several murine embryonal carcinoma cell lines by retinoic acid. Exp Cell Res. 1979 Dec;124(2):381–391. doi: 10.1016/0014-4827(79)90213-1. [DOI] [PubMed] [Google Scholar]
- Katinka M., Vasseur M., Montreau N., Yaniv M., Blangy D. Polyoma DNA sequences involved in control of viral gene expression in murine embryonal carcinoma cells. Nature. 1981 Apr 23;290(5808):720–722. doi: 10.1038/290720a0. [DOI] [PubMed] [Google Scholar]
- Kollek R., Stocking C., Smadja-Joffe F., Ostertag W. Molecular cloning and characterization of a leukemia-inducing myeloproliferative sarcoma virus and two of its temperature-sensitive mutants. J Virol. 1984 Jun;50(3):717–724. doi: 10.1128/jvi.50.3.717-724.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman J. M., Speers W. C., Swartzendruber D. E., Pierce G. B. Neoplastic differentiation: characteristics of cell lines derived from a murine teratocarcinoma. J Cell Physiol. 1974 Aug;84(1):13–27. doi: 10.1002/jcp.1040840103. [DOI] [PubMed] [Google Scholar]
- Linney E., Davis B., Overhauser J., Chao E., Fan H. Non-function of a Moloney murine leukaemia virus regulatory sequence in F9 embryonal carcinoma cells. 1984 Mar 29-Apr 4Nature. 308(5958):470–472. doi: 10.1038/308470a0. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Tremblay J. M., Cline M. J., Verma I. M. Differential expression of cellular oncogenes during pre- and postnatal development of the mouse. Nature. 1982 Oct 14;299(5884):640–644. doi: 10.1038/299640a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Wagner E. F. Differentiation of F9 teratocarcinoma stem cells after transfer of c-fos proto-oncogenes. Nature. 1984 Oct 4;311(5985):438–442. doi: 10.1038/311438a0. [DOI] [PubMed] [Google Scholar]
- Niwa O., Yokota Y., Ishida H., Sugahara T. Independent mechanisms involved in suppression of the Moloney leukemia virus genome during differentiation of murine teratocarcinoma cells. Cell. 1983 Apr;32(4):1105–1113. doi: 10.1016/0092-8674(83)90294-5. [DOI] [PubMed] [Google Scholar]
- Oliff A. I., Hager G. L., Chang E. H., Scolnick E. M., Chan H. W., Lowy D. R. Transfection of molecularly cloned Friend murine leukemia virus DNA yields a highly leukemogenic helper-independent type C virus. J Virol. 1980 Jan;33(1):475–486. doi: 10.1128/jvi.33.1.475-486.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima R. Stimulation of the clonal growth and differentiation of feeder layer dependent mouse embryonal carcinoma cells by beta-mercaptoethanol. Differentiation. 1978;11(3):149–155. doi: 10.1111/j.1432-0436.1978.tb00978.x. [DOI] [PubMed] [Google Scholar]
- Oskarsson M., McClements W. L., Blair D. G., Maizel J. V., Vande Woude G. F. Properties of a normal mouse cell DNA sequence (sarc) homologous to the src sequence of Moloney sarcoma virus. Science. 1980 Mar 14;207(4436):1222–1224. doi: 10.1126/science.6243788. [DOI] [PubMed] [Google Scholar]
- Ostertag W., Freshney M., Vehmeyer K., Jasmin C., Rutter G. Action of temperature-sensitive mutants of myeloproliferative sarcoma virus suggests that fibroblast-transforming and hematopoietic transforming viral properties are related. J Virol. 1984 Jan;49(1):253–261. doi: 10.1128/jvi.49.1.253-261.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostertag W., Pragnell I. B. Differentiation and viral involvement in differentiation of transformed mouse and rat erythroid cells. Curr Top Microbiol Immunol. 1981;94-95:143–208. doi: 10.1007/978-3-642-68120-2_4. [DOI] [PubMed] [Google Scholar]
- Ostertag W., Vehmeyer K., Fagg B., Pragnell I. B., Paetz W., Le Bousse M. C., Smadja-Joffe F., Klein B., Jasmin C., Eisen H. Myeloproliferative virus, a cloned murine sarcoma virus with spleen focus-forming properties in adult mice. J Virol. 1980 Feb;33(2):573–582. doi: 10.1128/jvi.33.2.573-582.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Verma I. M., Hunter T. Detection of a transforming gene product in cells transformed by Moloney murine sarcoma virus. Cell. 1982 Jun;29(2):417–426. doi: 10.1016/0092-8674(82)90158-1. [DOI] [PubMed] [Google Scholar]
- Pellicer A., Wagner E. F., el-Kareh A., Dewey M. J., Reuser A. J., Silverstein S., Axel R., Mintz B. Introduction of a viral thymidine kinase gene and the human beta-globin gene into developmentally multipotential mouse teratocarcinoma cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2098–2102. doi: 10.1073/pnas.77.4.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propst F., Vande Woude G. F. Expression of c-mos proto-oncogene transcripts in mouse tissues. Nature. 1985 Jun 6;315(6019):516–518. doi: 10.1038/315516a0. [DOI] [PubMed] [Google Scholar]
- Rechavi G., Givol D., Canaani E. Activation of a cellular oncogene by DNA rearrangement: possible involvement of an IS-like element. Nature. 1982 Dec 16;300(5893):607–611. doi: 10.1038/300607a0. [DOI] [PubMed] [Google Scholar]
- Rothstein S. J., Jorgensen R. A., Postle K., Reznikoff W. S. The inverted repeats of Tn5 are functionally different. Cell. 1980 Mar;19(3):795–805. doi: 10.1016/s0092-8674(80)80055-9. [DOI] [PubMed] [Google Scholar]
- Slack J. R., Morgan R. H., Hooper M. L. Action potentials recorded intracellularly from differentiated mouse teratocarcinoma cells in culture. Exp Cell Res. 1977 Jul;107(2):457–459. doi: 10.1016/0014-4827(77)90372-x. [DOI] [PubMed] [Google Scholar]
- Sorge J., Cutting A. E., Erdman V. D., Gautsch J. W. Integration-specific retrovirus expression in embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6627–6631. doi: 10.1073/pnas.81.21.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speers W. C., Gautsch J. W., Dixon F. J. Silent infection of murine embryonal carcinoma cells by Moloney murine leukemia virus. Virology. 1980 Aug;105(1):241–244. doi: 10.1016/0042-6822(80)90171-3. [DOI] [PubMed] [Google Scholar]
- Stacey A., Arbuthnott C., Kollek R., Coggins L., Ostertag W. Comparison of myeloproliferative sarcoma virus with Moloney murine sarcoma virus variants by nucleotide sequencing and heteroduplex analysis. J Virol. 1984 Jun;50(3):725–732. doi: 10.1128/jvi.50.3.725-732.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L. C. The development of transplantable teratocarcinomas from intratesticular grafts of pre- and postimplantation mouse embryos. Dev Biol. 1970 Mar;21(3):364–382. doi: 10.1016/0012-1606(70)90130-2. [DOI] [PubMed] [Google Scholar]
- Stewart C. L. Formation of viable chimaeras by aggregation between teratocarcinomas and preimplantation mouse embryos. J Embryol Exp Morphol. 1982 Feb;67:167–179. [PubMed] [Google Scholar]
- Stewart C. L., Stuhlmann H., Jähner D., Jaenisch R. De novo methylation, expression, and infectivity of retroviral genomes introduced into embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4098–4102. doi: 10.1073/pnas.79.13.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocking C., Kollek R., Bergholz U., Ostertag W. Long terminal repeat sequences impart hematopoietic transformation properties to the myeloproliferative sarcoma virus. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5746–5750. doi: 10.1073/pnas.82.17.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketo M., Gilboa E., Sherman M. I. Isolation of embryonal carcinoma cell lines that express integrated recombinant genes flanked by the Moloney murine leukemia virus long terminal repeat. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2422–2426. doi: 10.1073/pnas.82.8.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teich N. M., Weiss R. A., Martin G. R., Lowy D. R. Virus infection of murine teratocarcinoma stem cell lines. Cell. 1977 Dec;12(4):973–982. doi: 10.1016/0092-8674(77)90162-3. [DOI] [PubMed] [Google Scholar]
- Topp W. C. Normal rat cell lines deficient in nuclear thymidine kinase. Virology. 1981 Aug;113(1):408–411. doi: 10.1016/0042-6822(81)90168-9. [DOI] [PubMed] [Google Scholar]