Abstract
Introduction
In December 2010, the World Health Organization recommended a single Xpert MTB/RIF assay as the initial diagnostic in people suspected of HIV-associated or drug resistant tuberculosis. Few data are available on the impact of this recommendation on patient outcomes. We describe the diagnostic follow-up, clinical characteristics and outcomes of a cohort of tuberculosis suspects screened using a single point-of-care Xpert.
Methods
Consecutive tuberculosis suspects at a primary care clinic in Johannesburg, South Africa were assessed for tuberculosis using point-of-care Xpert. Sputum smear microscopy and liquid culture were performed as reference standards. Xpert-negatives were evaluated clinically, and further assessed at the discretion of clinicians. Participants were followed for six months.
Results
From July-September 2011, 641 tuberculosis suspects were enrolled, of whom 69% were HIV-infected. Eight percent were positive by a single Xpert. Among 116 individuals diagnosed with TB, 66 (57%) were Xpert negative, of which 44 (67%) were empirical or radiological diagnoses and 22 (33%) were Xpert negative/culture-positive. The median time to tuberculosis treatment was 0 days (IQR: 0–0) for Xpert positives, 14 days (IQR: 5–35) for those diagnosed empirically, 14 days (IQR: 7–29) for radiological diagnoses, and 144 days (IQR: 28–180) for culture positives. Xpert negative tuberculosis cases were clinically similar to Xpert positives, including HIV status and CD4 count, and had similar treatment outcomes including mortality and time to antiretroviral treatment initiation.
Conclusions
In a high HIV-burden setting, a single Xpert identified less than half of those started on tuberculosis treatment, highlighting the complexity of TB diagnosis even in the Xpert era. Xpert at point-of-care resulted in same day treatment initiation in Xpert-positives, but had no impact on tuberculosis treatment outcomes or mortality.
Introduction
Access to rapid and accurate diagnosis of tuberculosis (TB) remains a barrier to TB control–globally only 65% of TB cases were diagnosed in 2011 [1]. The Xpert MTB/RIF assay (Xpert) represents a potential revolution in TB diagnosis, with good accuracy, rapid time to results and the possibility of decentralized implementation close to the point-of-care [2]–[4]. The World Health Organization has endorsed the use of Xpert as the initial diagnostic in individuals suspected of HIV-associated TB or MDR-TB, or as a follow-on test to sputum smear microscopy [5].
South Africa has committed to a national scale-up of Xpert, with the ultimate goal of screening all adult pulmonary TB suspects with a single Xpert performed at a centralized laboratories [6]. Implementing Xpert at the point-of-care has been demonstrated to be safe in terms of infection control [7], and has the potential for same day treatment initiation [8], though may be more expensive than placement in a central laboratory [9]. Moving the test away from the point-of-care could result in delays to results that decrease the potential patient-level impact of Xpert [10]. Few data are available on the impact of Xpert at point-of-care on time to treatment and patient outcomes. Furthermore, although Xpert has a higher sensitivity than smear microscopy [3], [11], [12], reliance on a single Xpert may result in a substantial number of missed TB cases. The few studies presenting diagnostic information on Xpert negative individuals suggest that smear microscopy and chest x-ray have limited utility in detecting culture positive, Xpert negative TB [13], [14].
Using operational data collected during the course of routine clinical care at a primary care clinic in South Africa, we sought to describe the diagnostic follow-up, clinical characteristics and outcomes of a cohort of TB suspects screened using a single point-of-care Xpert.
Methods
Study Design
Witkoppen Health and Welfare Centre (WHWC) is a high-volume primary health clinic primarily serving informal settlement communities in northern Johannesburg, South Africa. Since 2011, all patients presenting at the clinic were routinely tested for HIV and screened for the presence of TB symptoms. Consecutive TB suspects who consented were enrolled into this prospective cohort study. As part of the WHWC routine diagnostic process, all TB suspects also were assessed by fluorescent sputum smear microscopy for acid-fast bacilli and liquid culture for M. tuberculosis, performed at a centralized National Health Laboratory Service laboratory. In addition, Xpert was performed as a research procedure at point-of-care by a low-skilled health care worker. All results, including Xpert, were used for clinical decision making.
Patients with a TB diagnosis received TB treatment at WHWC, and TB/HIV co-infected individuals were eligible for antiretroviral treatment (ART), while among those without TB co-infection the ART treatment threshold was ≤350 cells/mm3. As part of routine care and independent of study participation, Xpert negative TB suspects were treated with a course of antibiotics if clinically indicated, and asked to return for follow-up after one week. At follow-up, Xpert negative TB suspects were evaluated clinically, and had additional diagnostic testing (chest xray and a second Xpert) at the discretion of the clinician. Chest x-rays were performed at a nearby clinic. Chest x-ray results were classified as positive when the radiologist’s report stated the x-ray was suggestive of TB.
Demographic and clinical characteristics as well as laboratory findings were collected through chart review. The clinical status of participating Xpert negative TB suspects was determined through chart review and/or telephone calls at two months from the initial Xpert test, and again at six months for those who remained symptomatic or could not be contacted at two months. Patients lost to follow-up were traced in the national death registry. Treatment outcomes at six months were determined for all those initiated on TB treatment.
Definitions
The TB suspect definition was stratified by HIV status. For HIV-infected or unknown status: cough, fever, night sweats, weight loss of any duration; for HIV-uninfected: cough or fever ≥ two weeks, or night sweats or weight loss of any duration.
The basis for the decision to initiate TB treatment was defined as the earliest positive diagnostic test (smear, culture, Xpert or xray), or an empiric diagnosis if treatment was started in absence of or prior to any positive diagnostic test.
TB suspects were classified as confirmed TB if smear microscopy, culture or Xpert were positive, independent of whether TB treatment was initiated. TB suspects were classified as possible TB if started on treatment in the absence of mycobacteriological confirmation (i.e. Xpert negative, smear negative or missing and culture negative, missing or contaminated). TB suspects were classified as not TB if culture, smear and Xpert were negative and TB treatment was not started, or if the clinical outcome was good in the absence of TB treatment among those with missing or contaminated culture results.
Time to treatment was defined as the time from the baseline visit, when the initial Xpert test was performed, until initiation of TB treatment, whether at WHWC or elsewhere.
Statistical Analysis
Standard descriptive statistics were used to characterize the cohort. Multinomial logistic regression was used to estimate odds ratios and their 95% confidence intervals (CI) for the association between baseline characteristics and the Xpert result among TB cases (both definite and possible), using those -classified as not TB as the referent population. Predictors with significance p<0.10 in the univariate analysis were included in the multivariate model. Poisson regression with robust error variance was used to compare characteristics among Xpert positive and negative TB cases, as well as among confirmed and possible TB cases [15]. Time to treatment was compared by basis of TB treatment initiation using Kaplan-Meier curves and the log-rank test. All statistical analyses were conducted using Stata 12 (StataCorp, College Station, TX).
Ethics Statement
This study was approved by institutional review boards at the University of North Carolina, Chapel Hill and the University of the Witwatersrand in Johannesburg, South Africa. All participants gave written consent for use of routine clinical data for research purposes.
Results
Baseline Cohort Characteristics
Between July 15 and September 22, 2011, 641 consecutive TB suspects were consented and enrolled into the study. The median age was 35 years (IQR: 29–44), 65% were female, and 35% were of non-South African nationality, with the majority (73%) from Zimbabwe (see Table 1). Sixty-nine percent were HIV-infected, of whom 45% were on ART at the baseline visit for a median of 21 months (IQR: 12–35). Among those not on ART, the majority (174/280, 62%) were eligible for treatment, with a CD4 count of ≤350 cells/mm3. The most common presenting TB symptom was cough (97%), followed by night sweats (34%).
Table 1. Characteristics of 641 TB suspects screened by Xpert MTB/RIF assay as the initial TB diagnostic, stratified by baseline Xpert result.
| TB Suspects | ||||
| Initial | Initial | All | ||
| Characteristic | Xpert positive(n = 50) | Xpert negative(n = 591) | p value | (n = 641) |
| Age, median (IQR) | 34 (29–39) | 36 (29–44) | 0.108 | 35 years (29–44) |
| Female gender (%) | 23(46) | 392 (66) | 0.004 | 415 (65) |
| Non-South African nationality (%) | 19 (38) | 260 (44) | 0.412 | 223 (35) |
| History of TB (%) | 5 (10) | 57 (10) | 0.935 | 62 (10) |
| Presenting TB symptom | ||||
| Cough (%) | 49 (98) | 569 (96) | 0.529 | 618 (96) |
| Loss of weight(%) | 30 (60) | 188 (32) | <0.001 | 218 (34) |
| Fever (%) | 5 (10) | 38 (6) | 0.333 | 43 (7) |
| Night sweats(%) | 33 (66) | 181 (31) | <0.001 | 214 (33) |
| HIV status | 0.026 | |||
| Positive | 43 (86) | 400 (68) | 443 (69) | |
| Negative | 6 (12) | 156 (26) | 162 (25) | |
| Unknown | 1 (2) | 35 (6) | 36 (6) | |
| Among HIV positive | ||||
| CD4 count, mean (95% CI) | 179 cells/mm3 (128–230) | 344 cells/mm3 (319–370) | <0.001 | 329 cells/mm3 (305–353) |
| CD4 count, median (IQR) | 148 cells/mm3 (66–249) | 288 cells/mm3 (151–487) | <0.001 | 276 cells/mm3 (138–458) |
| CD4 count category | 0.001 | |||
| CD4≤200 cells/mm3 (%) | 24 (56) | 126 (32) | 152 (34) | |
| CD4 201–350 cells/mm3 (%) | 10 (20) | 99 (25) | 109 (25) | |
| CD4 351–500 cells/mm3 (%) | 3 (6) | 71 (18) | 74 (17) | |
| CD4>500 cells/mm3 (%) | 2 (4) | 92 (23 | 94 (21) | |
| CD4 missing (%) | 4(9) | 10 (3) | 14 (3) | |
| On ART at first suspect visit (%) | 4 (9) | 198 (45) | <0.001 | 202 (45) |
| Time on ART, median (IQR) | 23 months | 21 months (11–35) | 0.606 | 21 months |
| (14–25) | (11–35) | (12–35) | ||
Abbreviations: TB, tuberculosis; IQR, interquartile range; CI, confidence interval; ART, antiretroviral therapy.
Diagnostic Follow-up and TB Diagnosis
The initial Xpert was positive in 8% (50/641) of TB suspects (Figure 1). Among these, 32% (16/50) had a positive smear and culture, 52% (26/50) were smear-negative/culture-positive, 4% (2/50) were culture-negative and 12% (6/50) had missing or unreported laboratory results. Among the 551 TB suspects with a valid culture result, the sensitivity of a single Xpert compared to culture was 66% (95% CI: 53–77%) and the specificity was 99% (95% CI: 98–100%).
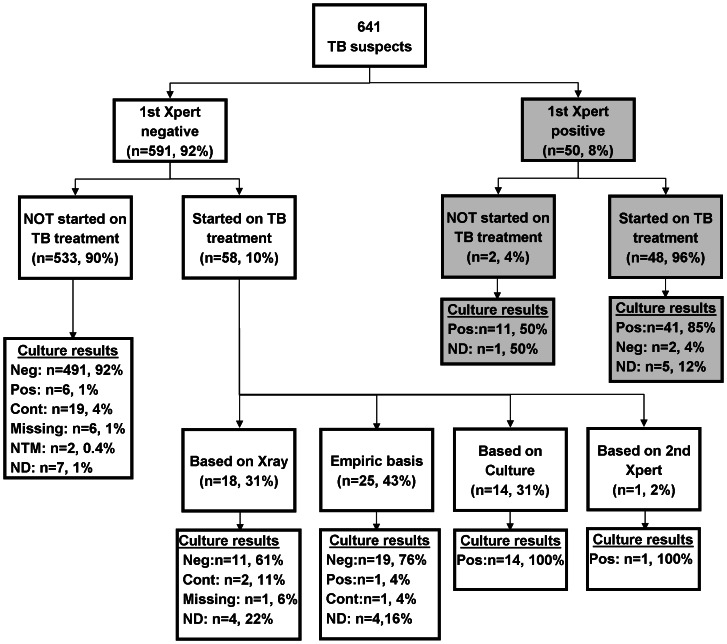
Figure 1. Flow chart of diagnostic assessment and basis of TB treatment initiation in 641 TB suspects presenting to a primary care clinic in Johannesburg, South Africa.
The basis of diagnosis was defined as the earliest positive diagnostic test (smear, culture, Xpert or x-ray), or an empiric diagnosis if treatment was started in absence of or prior to any positive diagnostic test. Abbreviation: TB, tuberculosis; Neg, negative; Pos, positive; Cont, contaminated; NTM, non-tuberculous mycobacteria; ND, not done.
The initial Xpert was negative in 92% of suspects (591/641). Among these, 72% were prescribed antibiotic treatment at the baseline visit, with 66% receiving amoxicillin, 17% erythromycin, 11% amoxicillin/clavulanate potassium, and 6% another antibiotic. Among Xpert negative TB suspects, smear microscopy and liquid culture were performed in the majority (98%) of patients, chest x-ray in 15% and a second Xpert in 8%. Culture was positive in 4% of Xpert-negatives (22/591), while smear microscopy was positive in only 1/591 (0.2%). Chest x-ray was performed in 84 and suggestive for TB in 26 (31%), and a second Xpert was requested in 49 and positive in 2. In addition, non-tuberculous mycobacteria grew in the sputum cultures of 2 (4%) Xpert negative TB suspects.
Overall, 11% (66/591) Xpert-negative TB suspects were classified as TB cases, of which 35% were confirmed cases (22 culture-positive, 1 smear-positive culture-negative and 65% possible TB cases (18 radiological diagnosis and 25 clinical diagnosis).
Factors Associated with TB Diagnosis
Among 641 TB suspects, the odds of receiving a TB diagnosis was higher if HIV-infected (aOR 7.22 [95% CI: 2.71–19.22] for Xpert-positives and aOR 18.96 [95% CI: 6.42–55.98] for Xpert-negatives), or ART-naïve (aOR 5.6 [95% CI: 1.78–17.24] for Xpert-positives and aOR 2.70 [95% CI: 1.43–5.55] for Xpert-negatives) and (Table 2). The presence of night sweats (aOR 2.61 [95%I 1.28–5.30] and loss of weight (aOR 2.29 [1.16–4.52] was associated with having a positive Xpert, while night sweats was the only significant presenting symptom associated with an Xpert-negative TB diagnosis (aOR 3.01 [95% CI: 1.67–5.41]). A higher CD4 count was associated with a lower odds of a TB diagnosis, but this was only significant among Xpert-negative TB suspects (aOR 0.14 to 0.37).
Table 2. Multinomial logistic models comparing baseline characteristics between 50 Xpert positive and 66 Xpert negative TB cases compared to those classified as not having TB.
| Univariate | Multivariate | ||||||||||
| Characteristic | No TB (REF) n = 525 | Xpert-positive TB diagnosis n = 50 | Xpert-negative TB diagnosis n = 66 | Xpert-positive TB diagnosis n = 50 | Xpert-negative TB diagnosis n = 66 | ||||||
| n (%) | n (%) | OR | 95% CI | n (%) | OR | 95% CI | aOR | 95% CI | aOR | 95% CI | |
| SA nationality | 291(55) | 31(62) | REF | 40(61) | REF | ||||||
| Non SA nationality | 234(44) | 19(38) | 0.76 | 0.42–1.39 | 26(39) | 0.81 | 0.48–1.36 | ||||
| No history of TB | 476(90) | 45(90) | REF | 58(88) | REF | ||||||
| History of TB | 49(9) | 5(10) | 1.08 | 0.412.84 | 8(12) | 1.34 | 0.60–2.97 | ||||
| Age ≤35 years | 263(50) | 31(62) | REF | 27(41) | REF | REF | REF | ||||
| Age >35 years | 262(49) | 19(38) | 0.62 | 0.34–1.11 | 39(59) | 1.45 | 0.86–2.44 | 0.61 | 0.30–1.24 | 1.65 | 0.92–2.94 |
| Female gender | 354(67) | 23(46) | REF | 38(58) | REF | REF | REF | ||||
| Male gender | 226(35) | 27(54) | 2.43 | 1.35–4.36 | 28(42) | 1.53 | 0.91–2.57 | 1.63 | 0.82–3.29 | 1.04 | 0.57–1.89 |
| HIV status | |||||||||||
| Negative | 152(29) | 6(12) | REF | 4(6) | REF | REF | REF | ||||
| Positive | 341(65) | 59(89) | 3.19 | 1.33–7.67 | 43(86) | 6.57 | 2.35–18.4 | 7.22 | 2.71–19.22 | 18.96 | 6.42–55.98 |
| Unknown | 32(6) | 1(2) | 0.79 | 0.092–6.80 | 3(5) | 3.56 | 0.76–16.70 | 0.59 | 0.067–5.26 | 0.137 | 0.68–15.97 |
| CD4 count | |||||||||||
| CD4<100 | 41(7) | 17(34) | REF | 25(38) | REF | REF | REF | ||||
| CD4 101–200 | 50(9) | 8(16) | 0.41 | 0.19–0.91 | 13(20) | 0.33 | 0.16–0.67 | 0.78 | 0.32–1.89 | 0.37 | 0.17–0.82 |
| CD4 201–350 | 88(16) | 10(20) | 0.17 | 0.048–0.57 | 12(18) | 0.18 | 0.068–0.49 | 0.39 | 0.11–1.48 | 0.29 | 0.10–0.82 |
| CD4>350 | 154(29) | 10(15) | 0.082 | 0.019–0.36 | 5(10) | 0.14 | 0.051–0.36 | 0.28 | 0.061–1.32 | 0.14 | 0.051–0.36 |
| No ART | 156(45) | 39(91) | REF | 47(80) | REF | REF | REF | ||||
| ART | 185(54) | 4(9) | 0.10 | 0.06–0.29 | 12(20) | 0.24 | 0.12–0.47 | 0.18 | 0.058–0.56 | 0.37 | 0.18–0.79 |
| TB Symptoms | |||||||||||
| No night sweats | 382(72) | 17(34) | REF | 28(42) | REF | REF | REF | ||||
| Night sweats | 143(27) | 33(66) | 5.19 | 2.80–9.60 | 38(58) | 3.63 | 2.15–6.13 | 2.61 | 1.28–5.30 | 3.01 | 1.67–5.41 |
| No loss of weight | 365(69) | 20(40) | REF | 38(58) | REF | REF | REF | ||||
| Loss of weight | 35(6) | 30(60) | 3.42 | 1.89–6.21 | 28(42) | 1.68 | 0.99–2.83 | 2.29 | 1.16–4.52 | 1.29 | 0.72–2.31 |
| Cough | 507(96) | 49(98) | REF | 62(94) | REF | ||||||
| No cough | 18(3) | 1(2) | 1.74 | 0.23–13.31 | 4(6) | 0.55 | 0.18–1.68 | ||||
| No fever | 493(93) | 45(90) | REF | 60(91) | REF | ||||||
| Fever | 32(6) | 5(10) | 1.71 | 0.64–4.61 | 6(9) | 1.54 | 0.62–3.84 | ||||
Abbreviations: TB, tuberculosis; OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval; ART, antiretroviral treatment; REF, reference category; SA, South African.
Results in bold are significant at the p<0.05 level.
Among the 116 TB cases, age was the only factor associated with a positive Xpert result, with those over 35 years being 40% less likely to have Xpert positive TB compared to Xpert negative TB (aRR 0.60 [95% CI: 0.36–0.98]) (Table 3). Compared to those with confirmed TB, possible TB s were more likely to be HIV-infected (98% vs 82%, p 0.028), but were similar on other clinical characteristics, including demographics, CD4 count, HAART use and TB symptoms in univariate and multivariate regression analysis (results not shown).
Table 3. Poisson regression models with robust error comparing characteristics among 50 Xpert positive and 66 negative TB diagnoses.
| Univariate | Multivariate | |||
| Characteristic | RR (95% CI) | p value | aRR (95% CI) | p value |
| SA nationality | REF | |||
| Non SA nationality | 1.03 (0.67–1.60) | 0.880 | ||
| No history of TB | REF | |||
| History of TB | 0.88 (0.43–1.82) | 0.730 | ||
| Age ≤35 years | REF | REF | ||
| Age >35 years | 0.61 (0.39–0.95) | 0.030 | 0.60 (0.36–0.98) | 0.042 |
| Female gender | REF | REF | ||
| Male gender | 1.30 (0.85–1.99) | 0.200 | 1.28 (0.81–2.02) | 0.290 |
| HIV status | ||||
| Negative | REF | REF | ||
| Positive | 0.70 (0.40–1.23) | 0.214 | 0.68 (0.39–1.17) | 0.164 |
| Unknown | 0.42 (0.07–2.47) | 0.335 | ||
| CD4 count | ||||
| CD4<200 | REF | REF | ||
| CD4≥200 | 1.06 (0.66–1.72) | 0.807 | 1.32 (0.49–3.51) | 0.583 |
| No ART | REF | |||
| ART | 0.57 (0.23–1.380 | 0.211 | ||
| TB Symptoms | ||||
| No loss of weight | REF | REF | ||
| Loss of weight | 1.50 (0.97–2.32) | 0.068 | 1.25 (0.79–2.01) | 0.337 |
| No night sweats | REF | |||
| Night sweats | 1.23 (0.78–1.93) | 0.369 | ||
| Cough | REF | |||
| No cough | 0.45 (0.08–2.67) | 0.381 | ||
| No fever | REF | |||
| Fever | 1.06 (0.53–2.11) | 0.867 | ||
Abbreviations: TB, tuberculosis; ART, antiretroviral treatment; RR, relative risk; aRR, adjusted relative risk; REF, reference.
Among culture-positive TB cases, individuals testing Xpert-negative had significantly longer time to culture positivity compared to Xpert-positives (median of 23 days [IQR: 18–32] versus 12 days [IQR: 10–16], p<0.001).
TB Treatment Initiation
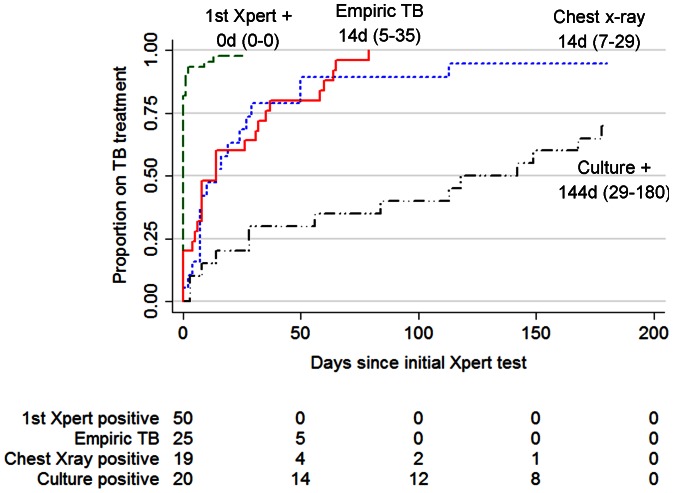
In total, 106 of the 116 TB caseswere started on TB treatment by six months following the initial Xpert assay. Almost all (48/50, 96%) Xpert positive patients started treatment, 2 were lost to follow-up., The proportion of Xpert-negative TB cases starting TB treatment was 70% (14/20) of culture positives, 95% (18/19) of individuals with suggestive chest x-ray, 100% (1/1) with positive 2nd Xpert, and 0% (0/1) with positive smear microscopy. In addition, 25 individuals started empiric TB treatment. The median time to TB treatment was 0 days (IQR: 0–0) for those positive on the initial Xpert, 14 days (IQR: 5–35) for empiric TB, 14 days (IQR: 7–29) for those starting treatment based on suggestive chest x-ray findings, and 144 days (IQR: 28–180) for culture positive, Xpert-negative patients (see Figure 2).
Figure 2. Time to TB treatment in 114 TB cases, by basis of TB treatment initiation.
Kaplan-Meier curves showing time to treatment stratified by basis of TB diagnosis, excluding those diagnosed based on 2nd Xpert or sputum smear microscopy (n = 2). The median time to treatment and IQR for each basis of TB treatment initiation are listed. Abbreviation: TB, tuberculosis.
Two and Six Month Follow-up
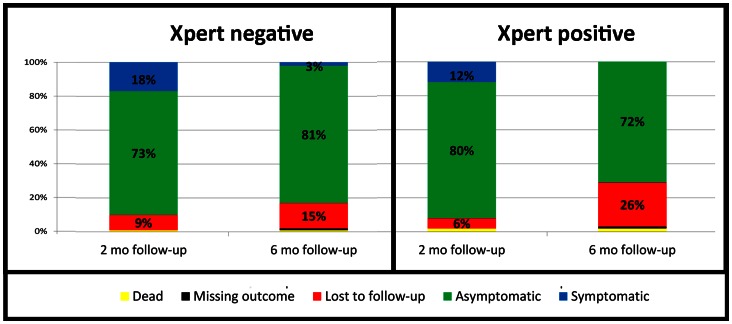
Among TB suspects with a negative initial Xpert, 18% had persistent TB symptoms at two months of follow-up, but only 3% remained symptomatic at six months (see Figure 3). The prevalence of persistent symptoms was similar between possible and confirmed TB cases, with 14% of possible and 18% of confirmed cases having symptoms at two months (p = 0.121), and 1% of possible and 0% of confirmed TB cases having TB symptoms at six months follow-up (p = 0.072). TB treatment outcomes between Xpert-positive and negative TB cases were not different (p 0.460). Among the 48 Xpert-positive cases started on treatment, 48% had a successful treatment outcome (six month treatment completion or cure), 25% transferred out, 23% defaulted, 2% were still on treatment and 2% died. Among the 58 Xpert-negative patients started on treatment, 64% completed or cured, 19% transferred, 12% defaulted, 3% still on treatment, and 2% died. Among TB suspects classified as not having TB, 1% died (3/535). TB treatment outcomes were also similar between confirmed and possible TB cases (p = 0.262).
Figure 3. Two and six-month outcomes of 591Xpert-negative TB suspects and 50 Xpert-positive TB suspects presenting to a primary care clinic in Johannesburg, South Africa.
Abbreviations: LTFU, lost to follow-up; TB, tuberculosis; mo, month; Rx, treatment.
During follow-up, a similar proportion of HIV co-infected Xpert positive individuals (55%) started ART as co-infected Xpert negatives (51%, p 0.130). The median time to starting ART was similar among Xpert positives, Xpert negative TB patients and those without TB (23 days [IQR: 16–56], 24 days [IQR: 19–54] and 22 days [IQR: 14–31] respectively).
Discussion
In this prospective cohort of TB suspects with high HIV prevalence screened by Xpert at point-of-care, 8% tested positive on initial Xpert and an additional 4% were bacteriologically confirmed with TB on culture, smear or second Xpert. Surprisingly less than half of those initiated on TB treatment had a positive Xpert result on initial screening, and 41% initiated treatment based on clinical or radiological findings alone.
Those diagnosed with TB were more likely to be HIV-infected, ART-naive, have a lower CD4 count and present with more symptoms, as could be expected in a setting with high HIV prevalence [16]. Xpert-negative, culture-positive TB cases had a lower sputum bacillary burden, with significantly longer time to culture positivity compared to Xpert-positives. Xpert-negative TB cases were similar to Xpert-positive cases on most clinical characteristics, including HIV status, CD4 count and ART use. This is in contrast to findings from Cape Town, where Xpert-negative TB cases had less advanced immunosuppression compared to Xpert-positive cases [13]. Differences in the study populations may partially explain the discrepancy. Our study population was more heterogeneous in terms of HIV infection, level of immunosuppression and ART experience, and thus more generalizable to the target population for Xpert testing according to the South African implementation strategy than the population studied by Lawn et al, which was restricted to ART-eligible HIV-infected persons regardless of TB symptoms.
Point-of-care Xpert did afford a clear advantage to patients in terms of time to TB treatment initiation. Almost all TB suspects testing Xpert-positive were started on TB treatment the same day as presenting with TB symptoms. This was two weeks faster than Xpert-negative suspects who started empirically or based on suggestive chest x-ray, and 20 weeks faster than those diagnosed by culture. Only 70% of those diagnosed based on culture were ever started on treatment, as some patients did not return to the clinic following the long delay for results, which is consistent with findings from other clinics in South Africa [17]. Furthermore, 12% of all culture results were either contaminated, not reported, or otherwise missing, highlighting the limited clinical value of culture. Despite this faster TB treatment initiation, Xpert-negatives had similar six-month TB treatment outcomes, including mortality, confirming findings by Lawn et al [13] and Yoon et al [18], and time to antiretroviral treatment initiation.
Xpert-negative TB cases who started treatment based on clinical or radiological criteria only were more likely to be HIV-infected, but were otherwise clinically similar to bacteriologically confirmed cases. This likely reflects a clinician’s decision to initiate TB treatment in the absence of a positive confirmatory diagnostic in HIV-infected individuals to avoid the high mortality of untreated TB in this population [19]. The finding that more than half of all patients starting TB treatment were Xpert-negative, and 41% started TB treatment based on radiological or clinical findings was surprising given the high sensitivity of Xpert in this population. These results are similar to a cohort of TB suspects screened by sputum microscopy, in which 44% of TB cases at an HIV clinic in South Africa started treatment based on a suggestive chest x-ray [20]. These findings emphasize the need for evidence-based assessment strategies for Xpert-negative TB suspects.
Our findings should be interpreted taking several limitations into account. Firstly, we studied a single primary care center with a clinical decision making process and access to diagnostic testing that may not be representative of all clinics in South Africa or other regions. Secondly, the observed time to treatment and proportion of patients started on treatment likely represents a “best case” scenario and may not be generalizable to settings where Xpert is performed at centralized laboratories. Thirdly, even though cultures were negative in almost all patients started on treatment based on clinical or radiological suspicion, we cannot exclude the presence of sputum culture-negative TB in these individuals, and that some patients started on treatment may not have had TB. Finally, our assessment of the impact of Xpert at point-of-care on treatment outcomes was limited by small sample size and relatively high (15%) loss to follow-up.
Conclusions
This operational research, the first to assess the operational impact of the 2010 recommendation for use of Xpert as the initial diagnostic highlights the diagnostic advantage of Xpert at point-of-care as this results in same day treatment initiation, the low mortality among Xpert-negative TB suspects, and the inadequacy of culture and x-ray in terms of speed and accuracy, respectively, for the assessment of Xpert-negative TB suspects. The prevalence of treatment without bacteriological confirmation reflects the complexity of TB diagnosis, even in the Xpert era. Future research should focus on optimizing assessment strategies of Xpert-negative TB suspects, accounting for setting specific factors such as HIV co-infection, laboratory capacity and cost concerns, to ensure the ultimate goal of improved patient outcomes through rapid and accurate diagnosis in all TB suspects.
Acknowledgments
Bridgette Moatlhodi, Veronica Modise, Annelize Theron, Nandipha Qangule, Sebolelo Phiri, Witkoppen Health and Welfare Centre patients, laboratory staff at NHLS Braamfontein.
Funding Statement
This work was supported by PEPFAR and National Institutes of Health grant UM1 AI069463, United States Agency for International Development in a grant to Right to Care 674-A-00-08-00007. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO (2011) Global Tuberculosis Control. Geneva.
- 2. Blakemore R, Nabeta P, Davidow AL, Vadwai V, Tahirli R, et al. (2011) A multisite assessment of the quantitative capabilities of the Xpert MTB/RIF assay. Am J Respir Crit Care Med 184: 1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, et al. (2010) Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363: 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang K, Lu W, Wang J, Zhang K, Jia S, et al. (2012) Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay: a meta-analysis. J Infect 64: 580–588. [DOI] [PubMed] [Google Scholar]
- 5.WHO (2011) Policy statement: automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. Geneva, Switzerland. [PubMed]
- 6. Meyer-Rath G, Schnippel K, Long L, MacLeod W, Sanne I, et al. (2012) The impact and cost of scaling up GeneXpert MTB/RIF in South Africa. PLoS One 7: e36966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banada PP, Sivasubramani SK, Blakemore R, Boehme C, Perkins MD, et al. (2010) Containment of bioaerosol infection risk by the Xpert MTB/RIF assay and its applicability to point-of-care settings. J Clin Microbiol 48: 3551–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clouse K, Page-Shipp L, Dansey H, Moatlhodi B, Scott L, et al. (2012) Implementation of Xpert MTB/RIF for routine point-of-care diagnosis of tuberculosis at the primary care level. S Afr Med J 102: 805–807. [DOI] [PubMed] [Google Scholar]
- 9. Schnippel K, Meyer-Rath G, Long L, MacLeod W, Sanne I, et al. (2012) Scaling up Xpert MTB/RIF technology: the costs of laboratory- vs. clinic-based roll-out in South Africa. Trop Med Int Health 17: 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawn SD, Kerkhoff AD, Wood R (2012) Location of Xpert(R) MTB/RIF in centralised laboratories in South Africa undermines potential impact. Int J Tuberc Lung Dis 16: 701; author reply 702. [DOI] [PubMed]
- 11. Theron G, Peter J, van Zyl-Smit R, Mishra H, Streicher E, et al. (2011) Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med 184: 132–140. [DOI] [PubMed] [Google Scholar]
- 12. Scott LE, McCarthy K, Gous N, Nduna M, Van Rie A, et al. (2011) Comparison of Xpert MTB/RIF with other nucleic acid technologies for diagnosing pulmonary tuberculosis in a high HIV prevalence setting: a prospective study. PLoS Med 8: e1001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lawn SD, Kerkhoff AD, Vogt M, Ghebrekristos Y, Whitelaw A, et al. (2012) Characteristics and early outcomes of patients with Xpert MTB/RIF-negative pulmonary tuberculosis diagnosed during screening before antiretroviral therapy. Clin Infect Dis 54: 1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Theron G, Pooran A, Peter J, van Zyl-Smit R, Kumar Mishra H, et al. (2012) Do adjunct tuberculosis tests, when combined with Xpert MTB/RIF, improve accuracy and the cost of diagnosis in a resource-poor setting? Eur Respir J 40: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zou G (2004) A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159: 702–706. [DOI] [PubMed] [Google Scholar]
- 16. Harries AD (1997) Tuberculosis in Africa: clinical presentation and management. Pharmacol Ther 73: 1–50. [DOI] [PubMed] [Google Scholar]
- 17. Lawn SD, Kerkhoff AD, Vogt M, Wood R (2012) Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. Lancet Infect Dis 12: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoon C, Cattamanchi A, Davis JL, Worodria W, den Boon S, et al. (2012) Impact of Xpert MTB/RIF testing on tuberculosis management and outcomes in hospitalized patients in Uganda. PLOS ONE 7: e48599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Straetemans M, Glaziou P, Bierrenbach AL, Sismanidis C, van der Werf MJ (2011) Assessing tuberculosis case fatality ratio: a meta-analysis. PLoS One 6: e20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kufa T, Mngomezulu V, Charalambous S, Hanifa Y, Fielding K, et al. (2012) Undiagnosed tuberculosis among HIV clinic attendees: association with antiretroviral therapy and implications for intensified case finding, isoniazid preventive therapy, and infection control. J Acquir Immune Defic Syndr 60: e22–28. [DOI] [PubMed] [Google Scholar]