Abstract
Background
Women are twice as likely to develop posttraumatic stress disorder (PTSD) than men. As shown in our previous work, the inability to suppress fear responses in safe conditions may be a biomarker for PTSD. Low estrogen in naturally cycling women is associated with deficits in fear extinction. On the basis of these findings, we have now examined the influence of estrogen levels on fear extinction in women with and without PTSD.
Methods
We measured fear-potentiated startle during fear conditioning and extinction in women. The study sample (N = 81) was recruited from an urban, highly traumatized civilian population at Grady Memorial Hospital in Atlanta, Georgia. We assayed serum estrogen levels and used a median split to divide the sample into high and low estradiol (E2) groups. Seventeen of 41 women (41.5%) in the low E2 group and 15 of 40 women (37.5%) met criteria for PTSD in the high E2 group.
Results
The results showed that all groups had equivalent levels of fear conditioning. However, we found significant interaction effects between high versus low E2 groups and PTSD diagnosis [F(1,71) = 4.55, p < .05] on extinction. Among women with low estrogen levels, fear-potentiated startle was higher during extinction in the PTSD group compared with traumatized control women [F(1,38) = 5.04, p < .05]. This effect was absent in the High E2 group.
Conclusion
This study suggests that low estrogen may be a vulnerability factor for development of PTSD in women with trauma histories. Research on the role of estrogen in fear regulation may provide insight into novel treatment strategies for PTSD.
Keywords: Anxiety disorders, estrogen, fear extinction, fear-potentiated startle, PTSD, trauma
Posttraumatic stress disorder (PTSD) is a debilitating psychiatric illness with neurobiological abnormalities that can develop after exposure to a life-threatening event. Women are twice as likely to develop PTSD as men (1–4), which may be related to psychosocial factors (gender differences in types of trauma exposure) or biological factors (differences in gonadal hormone influences). Studies have shown that neither the prevalence nor types of traumatic events nor the presence of comorbid psychiatric disorders significantly account for increased PTSD risk in women (2,3,5). Little is known, however, about the influence of female gonadal hormones, especially estrogen, on PTSD etiology. Some evidence points to a protective role of estrogen in anxiety regulation (6). Several studies have found that women are more likely to report symptoms of depression and anxiety during premenstrual, postpartum, and perimenopausal periods when estrogen levels are low (7–13). Studies using rodent models of anxiety have shown that females in the proestrus phase of their cycle (marked by high estrogen levels) show less fear- and anxiety-related behaviors relative to females in the metestrus or diestrus phases (lower estrogen levels) (14–17). Moreover, neuroimaging studies show greater activation of neural networks involved in fear excitation when women are scanned during the early follicular phase of their menstrual cycle (marked by low estrogen levels) relative to those scanned midcycle (high estrogen levels) (18,19). Hence, natural fluctuations of estrogen across the reproductive cycle may factor into the disproportionate incidence of PTSD in women. However few studies have looked at the influence of estrogen in laboratory-based models that explicitly probe for PTSD phenotypes.
There is growing evidence that impaired inhibition of conditioned fear is a biomarker of PTSD (20–23). Understanding the influence of estrogen on fear inhibition could shed light on mechanisms underlying sex differences in psychopathology. One laboratory model used for assessing fear inhibition is extinction. In Pavlovian fear extinction, a conditioned stimulus (CS) that was previously paired with an aversive stimulus (US) is repeatedly presented in the absence of the US, leading to a reduction of conditioned fear responding (22). Milad and colleagues (24) studied the influence of gonadal hormones on fear extinction in women and female rats. They found that low estrogen levels were associated with deficits in fear extinction recall, whereas high estrogen levels were associated with enhanced extinction recall (24–26). In addition, they showed that high estrogen levels corresponded to greater functional activation of brain regions previously implicated in fear extinction memory (i.e., ventromedial prefrontal cortex and hippocampus) (25).
These findings suggest that estrogen plays an important role in fear regulation. However, these effects in cycling females may differ from tonic levels of exogenous estrogen administered in ovariectomized females in which high estrogen has been observed to have a detrimental role on fear inhibition (27). Importantly, estrogen is only one of the hormones in the complex milieu that changes across the menstrual cycle; progesterone levels, as well as the ratio of estrogen to progesterone, may have strong influences on fear and anxiety. For example, progesterone has been implicated in neuroimaging studies using emotionally arousing images (28); additionally, its metabolite allopregnanolone has been associated with impaired fear extinction in rodent models (29). However, Milad and colleagues (26) investigated fear extinction with respect to both progesterone and estrogen levels and found that deficits in extinction recall seemed to be more highly associated with estrogen.
The present study examines the influence of estrogen on fear extinction in an inner-city population previously shown to have high rates of trauma exposure and PTSD incidence (30). Given the association between deficits in extinction and low estrogen levels (24,26), and PTSD (31,32), we hypothesized that there would be an interaction between these variables in that women with low estrogen and PTSD would show the greatest impairments in fear extinction.
Methods and Materials
Participants
The study included 81 women recruited from primary care clinics at Grady Memorial Hospital in Atlanta, Georgia, which serves a primarily African American, low socioeconomic, inner-city population (30,33). Women were excluded from participation for active psychosis or bipolar disorder, pregnancy, hearing impairment, and major medical illnesses as assessed by health and physical examinations conducted by study clinicians. Medication use was not an exclusion criterion for the study. Of the 81 participants, 9 were taking psychotropic medications: 4 were on antipsychotics (2 PTSD+, 2 PTSD−), 4 were taking benzodiazepines (2 PTSD+, 2 PTSD−), and 2 were taking selective serotonin reuptake inhibitors (both PTSD+). Women were screened for hearing impairment with an audiometer (Model GS1710; Grason-Stadler, Eden Prairie, Minnesota) and were required to detect tones at 30 dB [A] SPL at frequencies ranging from 250 to 4000 Hz. Before participation, all participants provided written informed consents that were approved by the Emory University internal review board and the Grady Memorial Hospital Research Oversight Committee.
Clinical Assessment
The following measures were used to index PTSD symptoms, childhood maltreatment and lifetime trauma history, respectively: Modified PTSD Symptom Scale (PSS) (34–36), Childhood Trauma Questionnaire (CTQ) (37,38), and the Traumatic Events Inventory (TEI) (36). These measures have all been used previously in our work with this population (39). The categorical definition of PTSD+ versus PTSD− was determined from responses to the PSS questionnaire based on DSM-IV A–E criteria (A, presence of trauma; B, presence of at least one reexperiencing symptom; C, presence of at least 3 avoidant/numbing symptoms; D, presence of at least 2 hyperarousal symptoms; E, occurrence for at least 1 month). Although the PSS measures self reported symptom severity, its use in diagnosing PTSD has been validated using the Clinician Administered PTSD Scale (40) in the Grady population (39).
Experimental Design
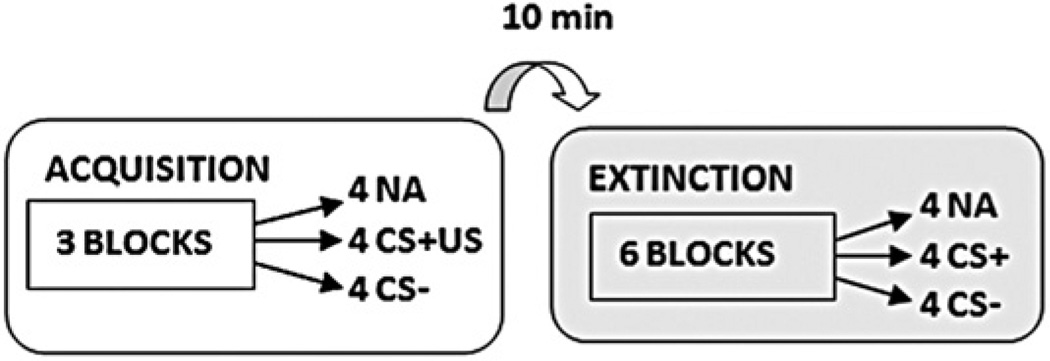
Figure 1 shows a diagram of the experimental session. The fear-potentiated startle protocol was based on our prior work (32) and consisted of two phases: fear acquisition and fear extinction. The fear acquisition phase began with a habituation phase in which the CSs were presented without any reinforcement; the conditioning phase consisted of three blocks with four trials of each type (a reinforced conditioned stimulus, CS+; a nonreinforced conditioned stimulus, CS−; and the noise probe alone, NA) for 12 trials per block and 36 total trials. Both CSs were colored shapes presented on a computer monitor for 6 sec. The US was a 250-msec air blast with an intensity of 140 psi directed at the larynx. The air blast was emitted by a compressed air tank attached to polyethylene tubing and controlled by a solenoid switch. This US has been used in several of our previous studies (23,32,41,42) and consistently produces robust fear-potentiated startle. In all phases, the intertrial intervals were randomized to be 9 to 22 sec in duration.
Figure 1.
Schematic illustration of the experimental paradigm. The previously reinforced conditioned stimulus (CS+) in the extinction session did not include the air blast (aversive stimulus [US]). CA−, nonreinforced conditioned stimulus; NA, noise probe alone.
Ten minutes after the conclusion of fear acquisition, participants underwent the fear extinction phase. During these 10 min, the participants engaged in a trauma-neutral task designed to assess attention. The extinction phase consisted of 6 blocks with four trials of each type (the previously reinforced CS+, CS−, and NA) for a total of 12 trials per block and 72 total trials (Figure 1). None of the CS presentations during extinction were reinforced with an air-blast US.
Startle Response Measurements
The startle response data were acquired using the electromyography (EMG) module of the BIOPAC MP150 for Windows (Biopac Systems, Goleta, California). The acquired data were filtered, rectified, and smoothed using the MindWare software suite (MindWare Technologies, Gahanna, Ohio) and exported for statistical analyses. The EMG signal was sampled at a frequency of 1kHz and filtered with low- and high-frequency cutoffs at 28 and 500 Hz, respectively. The maximum amplitude of the eye-blink muscle contraction 20 to 200 msec after presentation of the startle probe was used as a measure of the acoustic startle response.
As in our previous work (23,32,41,42), the eye-blink component of the acoustic startle response was measured by EMG recordings of the right orbicularis oculi muscle with two 5-mm Ag/AgCl electrodes filled with electrolyte gel. One electrode was positioned 1cm below the pupil of the right eye, and the other was placed 1 cm below the lateral canthus. Impedance levels were less than 6 kilo-Ohms for each participant. The startle probe was a 108-dB [A] SPL, 40-msec burst of broadband noise with near instantaneous rise time, delivered binaurally through headphones.
Estrogen Assays
Fasting whole blood specimens were obtained by venipuncture between 8 and 9 am by experienced nurses at the Clinical Interactions Network within the Atlanta Clinical and Translational Science Institute. Blood draws occurred the morning of the startle testing. To obtain serum for estradiol measurement, blood was kept at room temperature for 30 min to allow clotting and then centrifuged; the serum was transferred to a −80°C freezer for storage until analysis. Estradiol assays were completed by the Yerkes Biomarkers Core Laboratory at Emory University using a commercially available radioimmunoassay kit (product KE2D1; Siemens Healthcare Diagnostics, Deerfield, Illinois). Samples whose coefficient of variation (CV) between the replicates exceeded 20% were repeated and the values averaged. The interassay CV% = 11.3% at 176.13 pg/mL, and 14.56% at 1308.09 pg/mL. The intraassay CV% = 17.61% at 31.40 pg/mL. Individuals whose estradiol levels were too high for the assay detection (n = 7) were not included in any further analyses, resulting in a final sample of 81 women.
Data Analysis
The group variables in the analyses were the high and low estrogen groups derived from the median split of serum estradiol (E2) levels, and PTSD diagnosis (PTSD+, PTSD−). Demographic and clinical data such as age, PTSD symptoms, and childhood and adult trauma history were compared between the groups using a two-way analysis of variance (ANOVA).
Fear-potentiated startle was assessed by comparing average startle magnitude on the CS+ trials to the average startle magnitude to the NA trials using a mixed-model ANOVA with Trial Type and Block as within-subjects factors. Fear acquisition was measured using a difference score by subtracting startle magnitude to the NA trials from startle magnitude in the presence of a CS in each conditioning block. As in our previous work (43,44) late fear acquisition was defined as blocks 2 and 3 of acquisition, when discrimination learning was at maximum. Extinction was divided into three phases: early (blocks 1 and 2), mid (blocks 3 and 4), and late (blocks 5 and 6) extinction. Differential conditioning between CS+ and CS− was analyzed using a mixed-model ANOVA with Trial Type as the within-subjects factor and between-group factors of Diagnosis (PTSD+, PTSD−) and Estrogen (low E2, high E2) groups. Extinction was analyzed using a mixed-model ANOVA with Phase (early, mid, late) as the within-subjects factor and the same between-group factors above. Significant interactions were followed up by univariate ANOVAs. We also performed linear regression analyses to see whether PTSD and estrogen independently predicted the fear conditioning outcomes after controlling for age and trauma history. All statistical analyses were performed in SPSS 17.0 for Windows (SPSS, Chicago, Illinois), with alpha set at .05.
Results
Participant Characteristics
Of the 81 participants enrolled in the study, 32 women met PSS-based criteria for PTSD diagnosis (PTSD+), and 49 women did not (PTSD−). The participants ranged in age from 18 to 66 years old, and their self-identified race was African American (93.3%), Caucasian (4%), mixed (1.3%), or other (1.3%). We used a median split to divide women into low and high estradiol (E2) groups. The mean levels of estradiol were 8.00 pg/mL in the low E2 group and 92.50 pg/mL in the high E2 group. Seventeen of 41 women (41.5%) met criteria for PTSD in the low E2 group, and 15 of 40 women (37.5%) met criteria for PTSD in the high E2 group, χ2 = .13, not significant. Table 1 shows the clinical assessment across the PTSD and estrogen groups. As expected, the PTSD+ group had significantly higher PTSD symptoms, as well as more severe trauma exposure compared to the PTSD− group. The estrogen groups did not differ on degree of trauma exposure; however, the low E2 group had higher average PTSD symptoms than the high E2 group. Furthermore, the low E2 group was significantly older (mean = 47.4, SE = 1.9) than the High E2 group [(mean = 36.9, SE = 1.8), F(1,80) = 16.52, p < .001]. The diagnostic groups did not differ in age.
Table 1.
Outcome of Cinical Assessments Across Groups
| PTSD− | PTSD+ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low E2 | High E2 | Low E2 | High E2 | Effect (p Value) | |||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | PTSD | Estrogen | Interaction | |
| Childhood Trauma (CTQ) | 35.2 | 3.5 | 42.8 | 3.4 | 48.2 | 4.2 | 55.7 | 4.5 | .002 | .06 | .99 |
| Adult Trauma (TEI) | 2.2 | .5 | 2.8 | .5 | 3.9 | .6 | 4.44 | .6 | .003 | .33 | .99 |
| PTSD Symptoms (PSS) | 7.8 | 1.5 | 5.8 | 1.5 | 30.6 | 1.8 | 23.7 | 1.9 | <.0001 | .009 | .15 |
CTQ, Childhood Trauma Questionnaire; E2, estradiol; PSS, Modified PTSD Symptom Scale; PTSD, posttraumatic stress disorder; TEI, Traumatic Events Inventory.
Fear Acquisition
Participants displayed robust fear-potentiated startle to the CS+ compared with startle to the noise alone probe during conditioning [repeated-measures ANOVA, Block by Trial Type interaction, F(2,154) = 27.34, p < .001] with no main effects of PTSD diagnosis or estrogen level. CS+ versus NA Trial Type effects were strongest during Block 2 [F(1,77) = 25.58, p < .001] and Block 3 [F(1,77) = 22.67, p < .001] of acquisition. During late acquisition, defined as the second and third block of the acquisition phase, participants demonstrated a clear discrimination between the CS+ (mean = 39.46, SE = 7.59) and CS− (mean = 18.72, SE = 5.66), [repeated-measures ANOVA, main effect of Trial Type [F(1,77) = 16.20, p < .001)] but no significant main effects of diagnosis or estrogen (both Fs < 1.00) and no interaction effect [F,(1,77) = 2.58, p = .11]. All groups showed higher fear-potentiated startle during the CS+ compared to the CS− (data not shown).
Fear Extinction
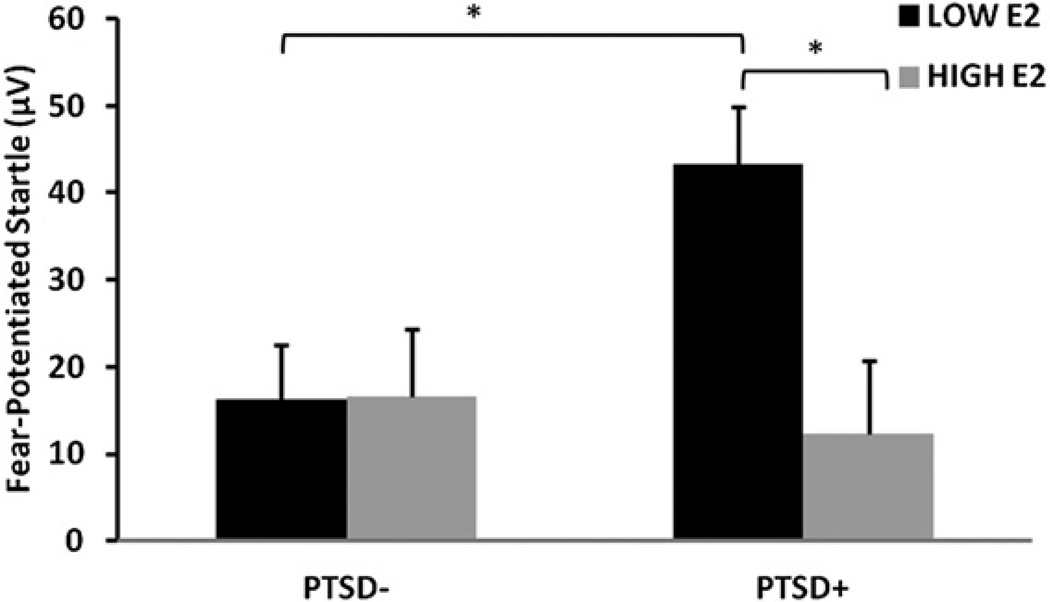
Participants displayed significant within-session extinction of fear-potentiated startle to the previously reinforced CS+ [repeated-measures ANOVA, main effect of Phase, F(2,142) = 27.42, p < .001] with a significant main effect of Estrogen group [F(1,71) = 4.41, p = .04] and a significant interaction effect of PTSD diagnosis and Estrogen group [F(1,71) = 4.55, p = .04]. Figure 2 shows fear-potentiate startle during extinction across the diagnostic and estrogen groups. We analyzed the effect of estrogen level within each Diagnostic group and found a significant effect of Phase in both groups, but the effect of estrogen was only significant in the PTSD+ group [F(1,27) = 4.56, p = .04] and not the PTSD− group [F(1,44) < 1.0]. To account for the difference in age and PTSD symptoms between the estrogen groups, we repeated the analysis with these variables as covariates. The effect of estrogen in the PTSD+ group remained significant after controlling for age and PTSD symptoms [F(1,24) = 5.44,p = .03] and in the PTSD− group was still not significant [F(1,37) = 1.21, p = .28]. Furthermore, because we did not exclude menopausal women from the study, we repeated the earlier analysis of estrogen effects by selecting only individuals younger than 50 and found the same results. In PTSD+ women under 50 years of age, the low E2 group had significantly higher levels of fear-potentiated startle during extinction compared to the high E2 group [F(1,14) = 5,71, p = .03].
Figure 2.
Mean ± SE fear-potentiated startle during extinction across diagnostic and estrogen groups. *p < .05. E2, estradiol; PTSD, posttraumatic stress disorder.
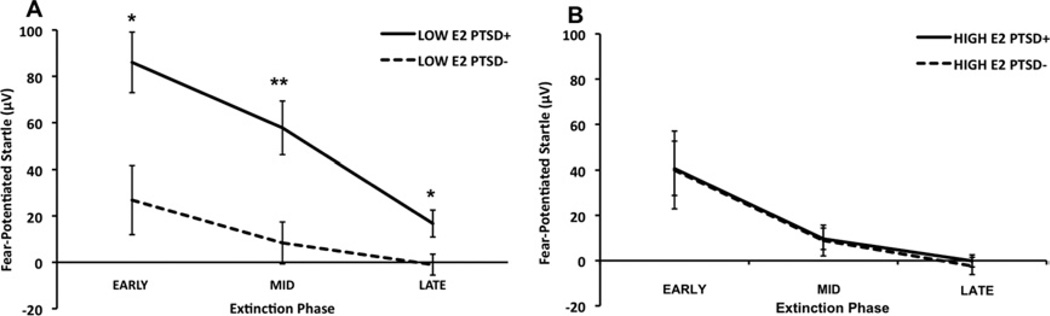
We also examined the effect of Diagnosis within low and high estrogen groups separately. Again, both groups demonstrated significant extinction across Phase; however, in the low E2 group, PTSD+ subjects had significantly higher fear-potentiated startle compared with PTSD− control women [F(1,38) = 5.04, p = .03]. This group difference was absent in the High E2 group [F(1,33) < 1.0]. Given that the diagnostic groups differed significantly in trauma exposure (Table 1), we repeated the analysis with CTQ and TEI scores as covariates. Figure 3A shows the differences between PTSD groups in the low E2 group, and Figure 3B shows the two diagnostic groups in the High E2 group. Again, all groups demonstrated significant effects of Phase (all groups, p < .001). Although there were still no differences within the high E2 group, the effect of PTSD in the low E2 group was strengthened [F(1,39) = 8.79, p = .005]. We followed up this significant effect with univariate analyses of each Phase of extinction and found that the greatest Diagnostic group differences within the low E2 group were during midextinction (blocks 3 and 4) [F(1,37) = 9.86, p = .003] but were also significant during early extinction (blocks 1 and 2) [F(1,37) = 5.21, p = .03] and late extinction (blocks 5 and 6) [F(1,37) = 5.07, p = .03; Figure 3A]. The Diagnostic groups within the high E2 group did not differ at any phase of extinction (Figure 3B).
Figure 3.
Mean ± SE fear-potentiated startle across extinction phases comparing diagnostic groups within each estrogen group. Data shown are covaried for trauma exposure. All groups demonstrated a significant linear effect of Phase, p < .001. (A) Low estradiol (E2) groups. (B) High E2 groups. *p < .05; **p < .01. PTSD, posttraumatic stress disorder.
To assess whether PTSD diagnosis and low estrogen levels independently accounted for deficits in midextinction, we performed a hierarchical stepwise regression analysis entering age in the first step, trauma history in the second step, PTSD diagnosis in the third step, and estrogen level in the final step (Table 2). The overall model with all four predictors accounted for 16% of the variance in midextinction, although neither age nor trauma history had significant contributions. A diagnosis of PTSD contributed significantly beyond age and trauma history [Fchange(1,61) = 6.62, p = .01]. After accounting for age, trauma, and PTSD, estrogen accounted for an additional 6% of the variance [Fchange(1,60) = 4.41, p = .04].
Table 2.
Outcome: Fear-Potentiated Startle During Midextinction
| Predictors | R2 | R2 Change | F Change | p |
|---|---|---|---|---|
| Age | .00 | .00 | .03 | .87 |
| Childhood and Adult Trauma | .00 | .00 | .06 | .94 |
| PTSD Diagnosis | .10 | .10 | 6.42 | .01a |
| Estrogen Level | .16 | .06 | 4.43 | .04b |
| Standardized Coefficients | β | p | ||
| Age | −1.02 | .31 | ||
| Childhood Trauma | −.40 | .69 | ||
| Adult Trauma | −.07 | .95 | ||
| PTSD Diagnosis | 2.34 | .02b | ||
| Estrogen Level | −2.10 | .04b | ||
PTSD, posttraumatic stress disorder.
p = .01.
p < .05.
Discussion
The results of the current study extend recent findings that demonstrate a significant effect of estrogen levels on one’s ability to inhibit learned fear (24–26). We now report that, among traumatized women with low estrogen levels, fear-potentiated startle was higher during extinction training in those with PTSD as compared to traumatized women without PTSD. In the high estrogen group, we found no effect of PTSD diagnosis. These data suggest that low levels of circulating systemic estrogen may be a vulnerability factor for the development of PTSD in women with a history of traumatic exposure. The fact that PTSD symptoms were higher in the low estrogen group compared with those in the high estrogen group further supports this hypothesis. It is important to note, however, that low estrogen alone is not sufficient to develop PTSD given that the proportion of women who met criteria for the disorder was not higher in the low estrogen group; however, low estrogen may be a contributing factor to a vulnerability profile that includes additional biological and psychological risk factors. Another possibility is that PTSD leads to low estrogen levels, rather than vice versa. Although our study cannot address this issue, studies in healthy cycling women (26), along with research on perimenopause and depression (13), suggest that low estrogen, due to changes in menstrual cycle or reproductive phase, may predate the onset of mental disorders. Our study suggests that impaired fear inhibition may provide a mechanism through which estrogen affects mental health outcomes. A longitudinal study testing extinction levels at different points of the cycle in the same individuals with and without PTSD would clarify the relationship between estrogen and PTSD.
Our findings complement previous reports by Milad and colleagues (24,26) who reported on the effect of estrogen levels (high vs. low) on the recall of extinction learning. In our study, which did not assess extinction recall, we observed differences between high and low estrogen groups during extinction training. It is important to note that the Milad group employed skin conductance measures, whereas the current study included fear-potentiated startle measures. Taken together, these two lines of research highlight the importance of further study on estrogen levels and menstrual phase on extinction learning as it potentially applies to clinical female populations with and without fear and anxiety symptoms.
The results of this study are consistent with our recent data showing impaired fear inhibition in women who were in the follicular phase (low estrogen) of their menstrual cycle (E.M. Glover et al., unpublished data). Our study is the first, however, to show an interaction of estrogen and PTSD-specific fear extinction deficits in a clinical sample. Thus, these findings have great clinical significance because they suggest that elevating estrogen levels in some patients may prove beneficial in rescuing extinction deficits observed in PTSD. Similar studies have found that estradiol administration significantly alleviated symptoms of depression in perimenopausal women (45,46).
How estrogen is linked to PTSD is not known, and few studies exist that explore this relationship in clinical populations (44,47). Several lines of evidence demonstrate sex and stress hormone interactions in women and female animals. However, the relationship between stress and gonadal hormones in modulating PTSD risk is not well understood. Studies have found sexually dimorphic effects of cortisol on fear conditioning and extinction (48), as well as sex and menstrual cycle effects on limbic neurocircuitry (19). A recent study conducted on the same traumatized population as the current study (i.e., Grady Trauma Project) found that a peptide in the stress response system, pituitary adenylate cyclase-activating polypeptide (PACAP), may be a biomarker for PTSD in women but not men (44). These studies underscore the complexity of the interactions between stress and sex-specific dimorphisms in neural and endocrine function and the need for further investigation of these interactions with regard to risk for anxiety disorders.
Dysregulated fear responses in PTSD have been demonstrated with neuroimaging studies showing heightened amygdala activation to fear-evoking stimuli (49–51), attention biases toward threatening faces and exaggerated fear-potentiated startle to danger cues (43), and deficient extinction of fear responses (31,32). Our study suggests that this increased “fear load” may, in part, result from hormonal status, in that low levels of estrogen may contribute to impaired fear inhibition. This argument is supported by the deficient fear extinction as well as increased symptoms of PTSD in the traumatized women with lower estrogen levels. The regression analysis indicates that low estrogen is predictive of impaired inhibition of fear even after accounting for PTSD diagnosis. Therefore, low estrogen may increase risk for PTSD by decreasing fear inhibition, which can serve as a biomarker of the disorder (52).
Given the higher prevalence of PTSD in women than men, it may seem contradictory that low, rather than high estrogen was associated with impaired fear extinction, which has been linked to PTSD (53). However, it is important to note that studies comparing fear extinction in men with either high or low estrogen women have found that men respond similarly to women with high levels of estrogen (26), indicating that testosterone may also affect fear inhibition either directly or through aromatization to estrogen. Given that progesterone and its metabolite allopregnanolone have been implicated in fear extinction in rats (29), as well as anxiety regulation (54) and emotion processing (28,55) in humans, further research is needed to elucidate the roles of progesterone and estrogen, or their ratios, in modulating fear inhibition in women. It is important to note that cycling hormones may have very different effects on behavior and emotion than continuous low levels, as seen during use of hormonal contraceptives.
A limitation of this study is that, on average, the low estrogen group was older than the high estrogen group in our clinical sample. This was because of the random recruitment approach in which we did not exclude participants based on hormonal status or menopause. To account for the difference in age between the estrogen groups, we repeated our statistical analysis with age as a covariate and found that the effects of estrogen in the PTSD+ group were even more robust and that the PTSD− group did not change. Furthermore, because we did not exclude menopausal women from the study, we repeated the analysis of estrogen effects by selecting only individuals younger than age 50 and replicating our original results. Another limitation of our study is that that the diagnostic groups differed significantly in trauma exposure (Table 1). One could argue that independent of PTSD diagnosis, lifetime trauma exposure might interact with low estrogen to contribute to extinction deficits. However, when we controlled for adult and childhood trauma exposure by using CTQ and TEI scores as covariates in our analysis, we found that although there were still no diagnostic differences within the high E2 group, the effect of PTSD in the low E2 group was strengthened. Our participants were recruited from the primary care patient pool at Grady Hospital in Atlanta, which serves mainly an African American, low socioeconomic status, highly traumatized population. This population is significantly more susceptible to trauma-related stress disorders and has been largely understudied in human clinical research. However, future research should replicate the current findings across a broad range of demographics.
These data suggest that independent of trauma exposure, individuals with PTSD may have an increased sensitivity to estrogen fluctuations. That this sensitivity to hormonal change is manifested through extinction deficits underscores the advantage of this paradigm in detecting biomarkers of PTSD. Considering the high prevalence of PTSD in women, further research is needed, especially in clinical populations, to understand the influence of estrogen on PTSD and to develop estrogen-sensitive treatments for its clinical management.
Acknowledgments
This research was supported by National Institute of Mental Health Grant Nos. MH071537 (principal investigator KJR), and MH070129 (principal investigator TJ), the Howard Hughes Medical Institute (KJR), and the Atlanta Clinical Translational Science Institute, the National Institutes of Health National Centers for Research Resources (Grant No. M01 RR00039), and the Burroughs Wellcome Fund. This work was funded in part by Institutional Research and Academic Career Development Awards Grant No. K12–GM000680 (EMG), National Alliance for Research on Schizophrenia and Depression (TJ and SDN), and Department of Defense/Congressionally Directed Medical Research Program(Award No. W81XWH-08-2-0170; principal investigator SDN). We thank Allen Graham, Angelo Brown, and the nursing staff at Grady Memorial Hospital for their assistance with participant recruitment and data collection.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Breslau N. The epidemiology of posttraumatic stress disorder: What is the extent of the problem? J Clin Psychiatry. 2001;62:16–22. [PubMed] [Google Scholar]
- 2.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 3.Olff M, Langeland W, Draijer N, Gersons BPR. Gender differences in posttraumatic stress disorder. Psychol Bull. 2007;133:183–204. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- 4.Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: A quantitative review of 25 years of research. Psychol Bull. 2006;132:959–992. doi: 10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- 5.Breslau N, Chilcoat HD, Kessler RC, Peterson EL, Lucia VC. Vulnerability to assaultive violence: Further specification of the sex difference in post-traumatic stress disorder. Psychol Med. 1999;29:813–821. doi: 10.1017/s0033291799008612. [DOI] [PubMed] [Google Scholar]
- 6.Solomon MB, Herman JP. Sex differences in psychopathology: Of gonads, adrenals and mental illness. Physiol Behav. 2009;97:250–258. doi: 10.1016/j.physbeh.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cloitre M, Yonkers KA, Pearlstein T, Altemus M, Davidson KW, Pigott TA, et al. Women and anxiety disorders: Implications for diagnosis and treatment. CNS Spectr. 2004;9:1–16. [PubMed] [Google Scholar]
- 8.Dean C, Kendell RE. The symptomatology of puerperal illnesses. Br J Psychiatry. 1981;139:128–133. doi: 10.1192/bjp.139.2.128. [DOI] [PubMed] [Google Scholar]
- 9.Douma SL, Husband C, O’Donnell ME, Barwin BN, Woodend AK. Estrogen-related mood disorders: Reproductive life cycle factors. Adv Nurs Sci. 2005;28:364–375. doi: 10.1097/00012272-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Pigott T. Anxiety disorders in women. Psychiatr Clin North Am. 2003;26:621–672. doi: 10.1016/s0193-953x(03)00040-6. [DOI] [PubMed] [Google Scholar]
- 11.Rapkin AJ, Mikacich JA, Moatakef-Imani B, Rasgon N. The clinical nature and formal diagnosis of premenstrual, postpartum, and perimenopausal affective disorders. Curr Psychiatry Rep. 2002;4:419–428. doi: 10.1007/s11920-002-0069-7. [DOI] [PubMed] [Google Scholar]
- 12.Yonkers KA. Anxiety symptoms and anxiety disorders: How are they related to premenstrual disorders? J Clin Psychiatry. 1997;58:62–67. [PubMed] [Google Scholar]
- 13.Schmidt PJ, Rubinow DR. Sex hormones and mood in the perimenopause. Ann N Y Acad Sci. 2009;1179:70–85. doi: 10.1111/j.1749-6632.2009.04982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 15.Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 2001;74:435–440. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 16.Mora S, Dussaubat N, Díaz-Véliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinol. 1996;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 17.Walf AA, Paris JJ, Frye CA. Chronic estradiol replacement to aged female rats reduces anxiety-like and depression-like behavior and enhances cognitive performance. Psychoneuroendocrinology. 2009;34:909–916. doi: 10.1016/j.psyneuen.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci. 2010;30:431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 23.Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, et al. Posttraumatic stress disorder may be associated with impaired fear inhibition: Relation to symptom severity. Psychiatry Res. 2009;167:151–160. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, et al. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry. 2011;70:920–927. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toufexis DJ, Myers KM, Bowser ME, Davis M. Estrogen disrupts the inhibition of fear in female rats, possibly through the antagonistic effects of estrogen receptor alpha (ERalpha) and ERbeta. J Neurosci. 2007;27:9729–9735. doi: 10.1523/JNEUROSCI.2529-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. Neuroimage. 2010;53:1286–1293. doi: 10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proc Natl Acad Sci. 2008;105:5567–5572. doi: 10.1073/pnas.0801853105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, et al. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31:505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, et al. Fear extinction in traumatized civilians with posttraumatic stress disorder: Relation to symptom severity. Biol Psychiatry. 2011;69:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, et al. Influence of child abuse on adult depression: Moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falsetti S, Resnick H, Resick P, Kilpatrick D. The Modified PTSD Symptom Scale: A brief self-report measure of posttraumatic stress disorder. Behav Ther. 1993;16:161–162. [Google Scholar]
- 35.Foa EB, Tolin DF. Comparison of the PTSD Symptom Scale—Interview Version with the Clinician Administered PTSD Scale. J Traum Stress. 2000;13:181–191. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz AC, Bradley RL, Sexton M, Sherry A, Ressler KJ. Posttraumatic stress disorder among african americans in an inner city mental health clinic. Psychiatr Serv. 2005;56:212–215. doi: 10.1176/appi.ps.56.2.212. [DOI] [PubMed] [Google Scholar]
- 37.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 38.Bernstein DP, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-Report Manual. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 39.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. J Traum Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 41.Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, et al. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norrholm SD, Vervliet B, Jovanovic T, Boshoven W, Myers KM, Davis M, et al. Timing of extinction relative to acquisition: A parametric analysis of fear extinction in humans. Behav Neurosci. 2008;122:1016–1030. doi: 10.1037/a0012604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ, et al. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol Med. 2012;42:533–543. doi: 10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, et al. Estrogen replacement in perimenopause-related depression: A preliminary report. Am J Obstet Gynecol. 2000;183:414–420. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- 46.de Novaes Soares C, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: A double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529–534. doi: 10.1001/archpsyc.58.6.529. [DOI] [PubMed] [Google Scholar]
- 47.Bremner D, Vermetten E, Kelley ME. Cortisol, dehydroepiandrosterone, and estradiol measured over 24 hours in women with childhood sexual abuse-related posttraumatic stress disorder. J Nerv Ment Dis. 2007;195:919–927. doi: 10.1097/NMD.0b013e3181594ca0. [DOI] [PubMed] [Google Scholar]
- 48.Zorawski M, Blanding NQ, Kuhn CM, LaBar KS. Effects of stress and sex on acquisition and consolidation of human fear conditioning. Learn Mem. 2006;13:441–450. doi: 10.1101/lm.189106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Ann N Y Acad Sci. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- 50.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research—past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Garfinkel SN, Liberzon I. Neurobiology of PTSD: A review of neuroimaging findings. Psychiatr Ann. 2009;39:370–381. [Google Scholar]
- 52.Jovanovic T, Norrholm SD. Neural mechanisms of impaired fear inhibition in posttraumatic stress disorder. Front Behav Neurosci. 2011;5:44. doi: 10.3389/fnbeh.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jovanovic T, Kazama A, Bachevalier J, Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62:695–704. doi: 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toufexis DJ, Myers KM, Davis M. The effect of gonadal hormones and gender on anxiety and emotional learning. Horm Behav. 2006;50:539–549. doi: 10.1016/j.yhbeh.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 55.Ferree NK, Kamat R, Cahill L. Influences of menstrual cycle position and sex hormone levels on spontaneous intrusive recollections following emotional stimuli. Conscious Cogn. 2011;20:1154–1162. doi: 10.1016/j.concog.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]