Abstract
The COMT Val158Met polymorphism may be a risk factor for nicotine addiction. This study examined the influence of the COMT Val158Met polymorphism on subjective, physiological, and cognitive effects of intravenous (IV) nicotine use in African American (AAs) (n=56) and European American (EAs) (n=68) smokers. Overnight abstinent smokers received saline followed by 0.5 and 1.0 mg/70 kg doses of nicotine, administered 30 minutes apart. Smokers with Val/Val genotype, compared to Met carriers, had greater negative subjective effects from IV nicotine and had more severe withdrawal severity following overnight abstinence from smoking. Women with Val/Val genotype reported greater difficulty concentrating and irritability than men with Val/Val or Met carrier genotypes. The Val/Val genotype was associated with better performance on the math task and in AA smokers it was associated with greater systolic blood pressure. These results support the rationale of pharmacologically inhibiting COMT to aid with smoking cessation among Val/Val genotype smokers.
Keywords: Nicotine, Genetics, Pharmacogenetic, Smoking, Cigarette, COMT
Introduction
Catechol-O-methyltransferase (COMT) is an enzyme that inactivates dopamine (DA), epinephrine, and norepinephrine as well as tightly regulating DA in the prefrontal cortical areas (1–3). The gene encoding COMT contains a well-studied single nucleotide polymorphism (SNP) that results in the presence of methionine (Met) or valine (Val) at codon 108 (in s-COMT) or codon 158 (in m-COMT) (4). The Val-coded allele is 3 to 4 times more active than the Met-coded allele, resulting in reduced DA levels in the synapse (5, 6). The COMT Val158Met variation has been widely researched for many phenotypes of psychiatric disorders including depression (7), psychosis (8), and drug addiction (9). Given the key role of DA in mediating drug reward, drug-seeking, and withdrawal states, studying the COMT Val158Met variation is especially important for addictive disorders, including nicotine addiction (10).
A recent meta-analysis concluded that the Val/Val genotype may be a risk factor for developing nicotine addiction (9). While some studies reported an association between the Val/Val genotype and poor response to smoking cessation treatments (11–13), other studies did not confirm these results, and some studies reported opposite findings (14, 15). Surprisingly, only a few studies have investigated the potential mechanism by which the COMT Val158Met polymorphism may modulate the risk for and treatment response to nicotine addiction. In a functional MRI study, abstinent smokers with the Val/Val genotype performed worse on the n-back test, which measures working memory (16). Furthermore, abstinent smokers with the Val/Val genotype had greater blood flow increases in brain areas associated with cigarette craving (17). These findings suggest that smoking cessation may be particularly difficult for smokers with the Val/Val genotype. However, systematic studies examining the COMT Val158Met polymorphism on withdrawal severity and nicotine responses are lacking. Such studies may provide better insight into the mechanisms of the observed COMT Val158Met effects on nicotine dependence.
The goal of this study was to determine the influence of the COMT Val158Met polymorphism on nicotine responses in smokers. The outcomes examined were those predicted to be likely modulated by the COMT enzyme, including measures of cognitive performance, withdrawal severity, subjective drug effects, and cardiovascular responses to nicotine (18–21). To assess the outcomes of interest, we used an IV nicotine administration procedure. In contrast to other slower nicotine delivery systems, IV nicotine administration produces rewarding effects in male and female smokers (22). Based on the known biological effects of the COMT Val158Met variant, we hypothesized that smokers who carry two copies of the Val allele would experience less rewarding effects from nicotine, perform worse on selected cognitive tasks, and experience more severe withdrawal symptoms compared with those who carry the Met allele.
Materials and methods
Subjects
We recruited 124 non-treatment-seeking cigarette smokers in and around New Haven, Connecticut through newspaper advertisements and flyers. All participants were between 18 and 50 years old and smoked between 10 and 25 cigarettes per day during the past year. The study sample included 100 smokers that were described in a previous study (23) as well as 24 additional smokers. The demographics are shown in Table 1.
Table 1.
Baseline measures for the study sample
| COMT genotype (rs4680) | Overall | Met (n=80) | Val/Val (n=44) | p* |
|---|---|---|---|---|
| Sex (male/female) | 90/34 | 59/21 | 31/13 | NS |
| Age, years | 37.4(8.5) | 37(8.7) | 39(8.1) | NS |
| Race (AA/EA) | 56/68 | 31/49 | 25/19 | 0.05 |
| Body Mass Index | 28.9(5.5) | 28.3(5.3) | 30.1(5.8) | NS |
| FTND | 5.5(2.1) | 5.2(2.2) | 6.1(2.0) | 0.02 |
| Cigarettes per day | 18.7(12.2) | 18.5(14.2) | 19.1(7.4) | NS |
| Years of smoking | 16.7(4.5) | 16.6(4.5) | 16.9(4.5) | NS |
| 3HC/Cotinine | 0.38(0.03) | 0.41(0.03) | 0.33(0.04) | NS |
| Nicotine (ng/ml) | 3.1(3.3) | 3.3(0.37) | 2.77(0.50) | NS |
| Heart rate | 67.5(10.4) | 68.0(10.9) | 66.5(9.4) | NS |
| Systolic blood pressure | 116.4(12.4) | 115(12.2) | 119(12.7) | NS |
| Diastolic Blood Pressure | 68.2(7.0) | 67.3(70) | 69.9(7.1) | 0.05 |
The participants were medically healthy and did not have current active medical problems (including hypertension) and were not on any current prescription medications. Potential participants were excluded if they were dependent on alcohol or any drugs other than nicotine, as determined by the Structured Clinical Interview for DSM-IV (24) and verified by urine drug screening. Written informed consent was obtained from each participant prior to study participation. The IV nicotine experimental sessions were conducted in the Biostudies Unit located at the West Haven campus of the VA Connecticut Healthcare System. The participants were compensated for their participation. This research protocol was approved by the Yale and VA Connecticut Healthcare System Human Subjects Subcommittees.
Procedure
Following an overnight abstinence from smoking, the participants arrived at the outpatient clinic at approximately 8 AM for the experimental session, which lasted about 3 hours. Abstinence from smoking was confirmed by measuring expired carbon monoxide (CO; <10 parts-per-million). The participants were instructed to continue their usual caffeine intake (to prevent caffeine withdrawal) and were asked to fast after midnight to minimize the nicotine-induced nausea that can be enhanced with food. Prior to the experimental sessions, a urine drug screen was conducted to rule out recent drug use and ensure that the inclusion criteria were met. An indwelling catheter was set in an antecubital vein, and the baseline measures were collected. The study used a single-blind design. Smokers received IV saline, followed by two increasing doses of IV nicotine, (0.5 and 1.0 mg/70 kg). An escalating dose schedule was selected to increase study safety. Subjects were first exposed to saline, followed by a low dose of nicotine, and finally a high dose of nicotine. This administration schedule was also used to avoid residual effects from the preceding nicotine dose to saline.
Nicotine Administration
Nicotine bitartrate was acquired from Interchem Corporation (Manchester, Connecticut). A research pharmacist at the VA CT Healthcare System prepared the nicotine samples. A total volume of 5 ml, containing either 0.5 mg or 1 mg/70 kg of nicotine, was injected intravenously over a 30-second interval via a catheter located in a forearm vein. The nicotine doses administered were within the range of nicotine delivered by smoking one cigarette. Our prior research demonstrated that these doses produced robust physiological and subjective responses in male and female smokers (22). The injections were given 30 minutes apart, which allowed subjective and cardiovascular responses to return to baseline levels (25–27). The genotyping was conducted after the experimental sessions were completed using a blinded method.
Dependent Measures
Outcome measures assessed biochemical, physiological, subjective, and cognitive domains. Biochemical measures included CO, plasma nicotine, cotinine, and 3-hydroxycotinine (3HC). The plasma nicotine concentrations and expired CO were used to confirm the overnight abstinence from smoking. Plasma cotinine concentrations were used as a measure of prior nicotine exposure (28). Expired CO, plasma nicotine, 3HC, and cotinine measurements were taken before the experimental session. Physiological measures included blood pressure (systolic and diastolic) and heart rate. These measures were taken during the medication treatment and in the experimental sessions at −5, 1, 2, 3, 5, 8, 10, and 15 min intervals following the saline or nicotine deliveries. Plasma cortisol measurements were taken at baseline, before each of the three injections, and at the end of the session. Plasma cortisol has been shown to be sensitive to nicotine administration and abstinence from nicotine (29–32). Studies also suggest that the COMT Val158Met polymorphism may influence cortisol release in response to stress or drugs of abuse (33, 34).
The subjective measures included the Drug Effects Questionnaire (DEQ), the Brief Questionnaire on Smoking Urges (BQSU), the Minnesota Nicotine Withdrawal Scale (MNWS) and the Positive and Negative Affect Schedule (PANAS). The DEQ was used to measure acute effects from IV nicotine and consisted of 10 items: drug strength, high, feel stimulated, good effects, bad effects, anxious, sedated, feel down, want more drug and like drug. Smokers rated each item on a visual analog (Likert) scale, from “not at all” to “extremely.” The DEQ was given at 1, 3, 5, 8, 10, and 15 minutes after the saline and nicotine administration. The BQSU is a 10-item scale originally developed by Tiffany and Drobes (35, 36). Smokers were asked how strongly they agreed or disagreed with items on a 7-point Likert scale. This scale has been found to be highly reliable in reflecting nicotine deprivation levels (37, 38). The MNWS measures withdrawal symptoms from tobacco and includes items to assess cigarette craving, irritability/anger, anxiety, difficulty concentrating, restlessness, increased appetite, depressed or sad mood, and insomnia (39, 40). The PANAS is a 20-item scale, which assesses both negative and positive affective states (41). The BQSU, MNWS and PANAS were administered before and after the session.
Cognitive testing was measured using the Mathematical Processing (MP) task, the Running Memory Continuous Performance Test (CPT) and the Stroop Test within the Automated Neuropsychological Assessment Metrics (ANAM) computer package, a computer-administered cognitive battery of performance tests developed by the Department of Defense (42). As the main cognitive outcome measure, we used the throughput score, which combines response speed, accuracy and consistency, and reflects cognitive efficiency (42). The MP, CPT, and Stroop assessments were chosen for their sensitivity to acute nicotinic administration (43–45).
The MP assessed basic computational skills. This task displays arithmetic problems involving three single-digit numbers and two operators (e.g., "5 − 2 + 3 ="). The participant presses buttons to indicate whether the answer to the problem is less than five or greater than five.
The CPT measured attention, concentration, and working memory. This task involves single characters shown on a computer monitor in rapid sequence. The participant presses designated buttons to indicate if the displayed character matches or does not match the preceding character.
The Stroop test assessed attention/concentration, shifting perceptual sets, and the suppression of a habitual response in favor of an atypical one (46). This task instructs the participant to say the color of the word displayed on a monitor, which may or may not differ from the actual word text. For example, a participant would push the designated blue button when observing the word written in blue that could spell red, blue or green. Cognitive assessments were conducted at the beginning and end of the session, about 2 hours apart.
Assays
Nicotine, cotinine and trans-3'-hydroxycotinine (3HC)/cotinine ratios were determined by tandem mass spectrometry (LC/MS/MS) employing stable isotope (deuterated) labeled internal standards as we have previously reported (47) and similar to that reported by others (48). Nicotine and cotinine were determined to compare baseline nicotine exposure between the three genotypes, and 3HC/cotinine ratios were determined to control for possible differences in the rate of nicotine clearance (48).
Genotyping
DNA was extracted from peripheral blood using a commercial kit (PureGene™; Gentra, Minneapolis, MN). We genotyped rs4680 using the TaqMan method and primers (supplied as pre-validated SNP Assays on Demand, Applied Biosystems). The rs4680 genotyping was performed using ABI PRISM 7900 Sequence Detection System (Applied Biosystems). All samples were genotyped in duplicate. An ancestry informative marker (AIM) panel of 90 SNPs chosen by our group for their ability to differentiate between major continental populations was genotyped using the Illumina BeadXpress Reader with VeraCode assays and the manufacturer’s protocol (Illumina, Inc., San Diego, CA). Genotypes were called using BeadStudio software (Illumina, Inc., San Diego, CA).
Data Analysis
Study outcomes were analyzed with a repeated-measures model using the Statistical Analysis System (SAS), version 9.2 (SAS Institute Inc., 2007). Because of the small number of smokers (n=22) with Met/Met genotype, we combined Met/Met group with Met/Val and compared this combined group with the Val/Val group, similar to previous studies on COMT and cigarette smoking (16, 49). For blood pressure, heart rate and the DEQ, multiple assessments were collected for each saline and nicotine dose. For these outcomes, the model included the COMT rs4680 genotype (Val/Val vs. Val/Met or Met/Met), nicotine dose (saline, 0.5 and 1mg/70 kg), sex (male vs. female), race (African-American vs. Caucasian), nicotine metabolite ratio, BMI, and interaction terms including genotype × dose, genotype × race, and genotype × sex. In previous studies, race, sex, BMI, and NMR have been shown to modulate nicotine’s pharmacological effects (23, 50). For the NWSC, BQSU, PANAS, and cognitive measures, the assessments were completed at the beginning and end of the session. For these outcomes, the model included time of measurement (pre- vs. post-session), rather than the dose. Significant main effects were followed by post hoc testing. Genotypes for AA and EA groups were consistent with Hardy Weinberg Equilibrium expectations [EA: χ2 (1) = 0.56, p = ns, AA: χ2 (1) = 0.70, p = ns]. Values of p ≤0.05 in two-tailed tests were considered statistically significant unless otherwise specified.
Subjects were classified as genetically AA or EA on the basis of the AIMs panel, using the program STRUCTURE v 2.3.2.1 (51), as described previously (52). Among the subjects included in the current study, 2 subjects reporting to be of AA descent clustered in the EA group, and 2 subjects reporting to be EA clustered in the AA group. Adjusting for the degree of admixture did not significantly change our results on the influence of COMT Val158Met variation on the study outcomes.
Results
Physiological
The systolic blood pressure, diastolic blood pressure, and heart rate responses were dose-dependent such that responses to 1.0 mg nicotine>0.5 mg nicotine> saline (all p values<0.0001) (Figure 1). Significant findings for heart rate, and systolic and diastolic blood pressure are summarized in Table 1 and Figure 1. The BMI was not significant for heart rate or blood pressure. The NMR values were positively correlated with the nicotine-induced heart rate and systolic blood pressure (p<0.01).
Figure 1.
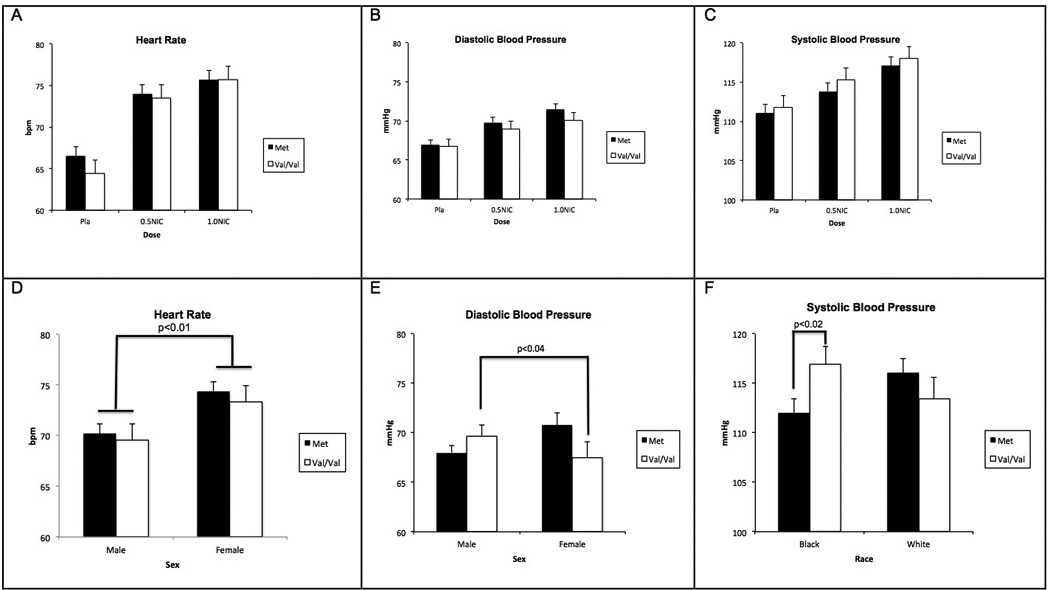
Mean±SEM of A) heart rate B) systolic and C) diastolic blood pressure as functions of COMT genotype and nicotine dose. Mean±SEM of D) heart rate E) diastolic blood pressure as functions of COMT genotype and sex and F) systolic blood pressure as functions of COMT genotype and race.
Subjective
For all the DEQ assessments, the rating for the 1.0 mg nicotine or 0.5 mg nicotine does were > than for saline (all p values<0.05). For the rating of “Stimulated,” “High,” “Feel Drug Strength,” the ratings for the 1.0 mg nicotine> 0.5 mg nicotine (p<0.05). The NMR values were positively correlated with the ratings of “Stimulated” “High,” and “Feel Anxious” (p<0.05). The results for the DEQ items are summarized in Table 1 and Figure 2.
Figure 2.
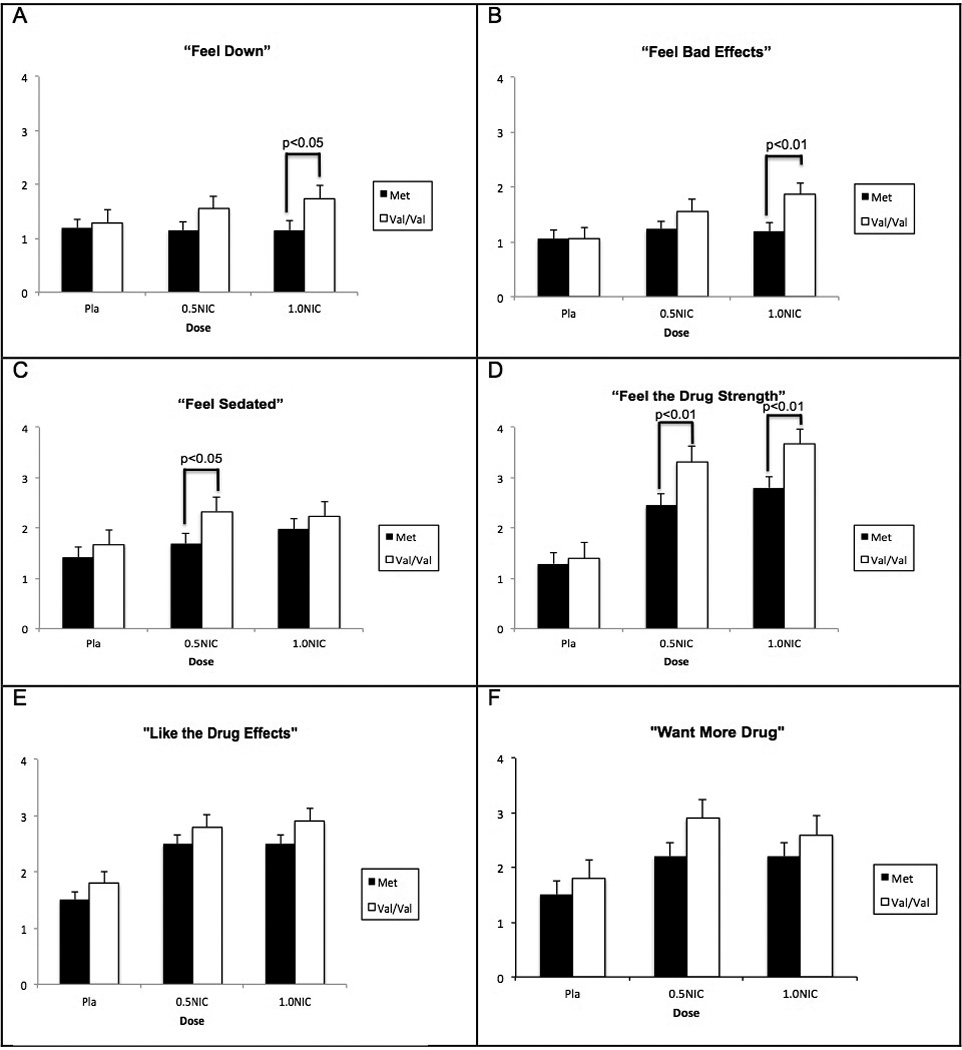
Mean±SEM of selected DEQ domains A) Feel Down B) Feel Bad C) Sedated D) Drug Strength E) Like the Drug, and F) Want More as functions of COMT genotype and nicotine dose.
The results for withdrawal severity, as measured by MNWS and BQSU, are summarized in Table 2 and Figure 3. The NMR values were positively correlated with the MNSW and BQSU factor 1 scores (p<0.05). For the PANAS, neither a main effect of genotype nor a genotype × session interaction was found to influence positive and negative affect states (p>0.05).
Table 2.
Vital and Drug Effects Questionnaire (DEQ) results as a function of COMT genotype and higher order interactions
| Measure | COMT | COMTXSex | COMTXRace | COMTXDose | Sex | Race |
|---|---|---|---|---|---|---|
| Heart rate | X | X | X | X | F=4.37, p=0.04 | X |
| F>M | ||||||
| Systolic BP | X | X | F=5.27, p=0.02 | X | X | X |
| AA Val>AA Met | ||||||
| Diastolic BP | X | F=4.41, p=0.04 | F=6.92, p=0.01 | X | X | X |
| F Val>M Val | AA Val>EA Val | |||||
| Anxious | F=4.82, p=0.03 | X | X | X | X | X |
| Val>Met | ||||||
| Feel Bad | X | X | X | F=10.58, p<0.0001 |
X | X |
| Val>Met 1.0NIC | ||||||
| Feel Down | X | X | X | F=7.29, p=0.0007 |
X | X |
| Val>Met 1.0NIC | ||||||
| Sedated | X | X | X | F=3.43, p=0.03 | X | F=4.64, p=0.03 |
| Val>Met 0.5NIC | B>W | |||||
| Drug Strength | X | X | X | F=7.76, p=0.0004 |
X | X |
| Val>Met 0.5NIC Val>Met 1.0NIC |
No significant effect (p<0.05).
Figure 3.
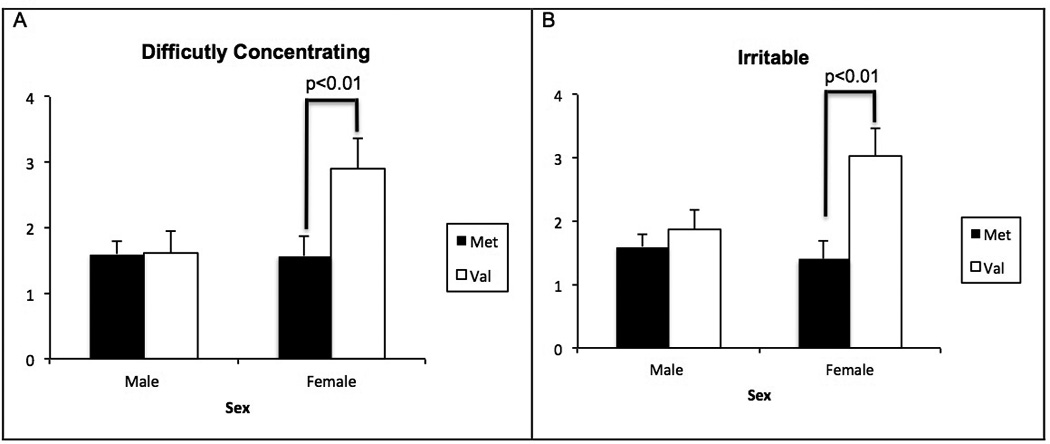
Mean±SEM of MNWS domains A) Difficulty Concentrating B) Irritable as functions of COMT genotype and sex.
Cognitive
As expected, the MP, CPT and Stroop Test performances at the end of the session were better than they were at baseline (p<0.0001). The results of the cognitive assessments are summarized in Table 3.
Table 3.
Subjective and cognitive results as a function of COMT genotype and higher order interactions
| Measure | Factor | COMT | COMTXSex | COMTXRace | COMTXTime | Sex | Race |
|---|---|---|---|---|---|---|---|
| MNWS | |||||||
| Total score | F=3.77, p=0.05 | X | X | X | X | X | |
| Val>Met | |||||||
| Crave cigarette | F=7.49,p=0.0072 | X | X | X | X | X | |
| Val>Met | |||||||
| Difficulty Concentrating | F=3.89,p=0.05 Val>Met |
F=3.87,p=0.05 | X | X | X | X | |
| F Val>M Val F Val>M Met |
|||||||
| Irritable | F=8.41,p=0.0045 | F=4.46,p=0.04 | X | X | X | X | |
| Val>Met | F Val>M Val | ||||||
| BQSU | F Val>M Met | ||||||
| Factor 1 | F=4.44,p=0.04 | X | X | X | X | X | |
| Val>Met | |||||||
| Factor 2 | F=6.12,p=0.01 | X | X | X | X | X | |
| Val>Met | |||||||
| Cognitive | |||||||
| Math Throughput Score | F=5.03,p=0.03 | X | X | X | X | X | |
| Val>Met | |||||||
| CPT | X | X | X | X | X | X | |
| Stroop | X | X | X | X | X | X |
No significant effect (p<0.05).
Cortisol
Plasma cortisol measurements did not show significant main effects for genotype or genotype × time interactions (p<0.05).
Discussion
We found that smokers with the Val/Val genotype, compared to the Met carriers, had greater negative subjective effects from IV nicotine and more severe withdrawal severity following overnight abstinence from smoking. Women with the Val/Val genotype reported greater difficulty concentrating and irritability than men with the Val/Val or Met allele containing genotypes. The Val/Val genotype was associated with better performance on the math task and in AA smokers, it was associated with greater systolic blood pressure readings. These findings are consistent with most of our hypotheses regarding COMT Val158Met polymorphism effects on nicotine responses.
In our study, smokers with the Val/Val genotype, compared with Met carriers, reported higher ratings of mostly negative drug effects, including “Feel Anxious,” “Feel Bad Effects,” “Feel Sedated,” “Feel Down” and “Feel the Drug Strength.” In contrast, no genotype effects were observed for the ratings of items that reflect nicotine’s reinforcing properties including “Good Drug Effects,” “Like the Drug Effects” or “Want More Drug.” This genotype effect was primarily observed in response to nicotine, and not to saline, administration, suggesting a pharmacogenetic effect of the Val/Val genotype. COMT effects were observed for both 0.5 and 1.0mg/70 kg of nicotine dose. Previous studies have shown that COMT Val158Met variation moderates responses to non-drug rewards or aversive stimuli (19, 20); however, the influence of the COMT Val158Met polymorphisms on the rewarding or aversive drug effects has not been reported. For example, healthy controls with the Val/Val genotype report a decreased ability to experience reward in the routine of daily life and report less happiness compared with those with the Met/Met genotype (20). In a recent functional fMRI study healthy controls with the Val/Val genotype had greater activation of amygdala in response to fearful/angry facial stimuli (53). Our findings extend these studies further by demonstrating that COMT Val158Met variation may also moderate subjective drug responses to nicotine.
Consistent with the DEQ findings, we found that smokers Val/Val genotype had greater withdrawal severity following an overnight abstinence, as measured by the MNWS and the BQSU. Further analysis of the individual MNWS items showed that smokers with the Val/Val genotype had greater craving, more difficulty concentrating and more irritability than Met carriers. To our knowledge, these findings are the first to demonstrate that the COMT Val158Met variation moderates withdrawal severity in smokers. In a previous study, smokers with the Val/Val genotype had greater blood flow to prefrontal cortical regions that are associated with cigarette craving upon abstinence from smoking (17). More severe withdrawal in smokers with the Val/Val genotype may have important treatment implications (see below).
The Val/Val genotype was associated with faster reaction time in the math test but did not influence the performance on the CPT and Stroop tasks. In contrast to our findings, a functional MRI study revealed that abstinent smokers with the Val/Val genotype performed worse on the n-back test, a test of working memory (16). The reason for these conflicting findings regarding the influence of Val/Val genotype on cognitive performance is not clear. In previous studies, the Val/Val genotype was associated with better or worse performance across many cognitive tasks. It has been argued that while individuals with the Val/Val genotype perform worse on tasks that require cognitive flexibility, they may perform better on tasks that require cognitive stability (54). The N-back is a relatively difficult task that requires sustained attention and working memory function, while the mathematical processing task is an easier task that assesses single-digit calculations (54). We did not observe a genotype effect on the Stroop or CPT tasks.
Understanding the underlying mechanisms that mediate the moderating effects of COMT Val158Met on subjective drug effects, withdrawal severity, and cognitive performance are an important topic for further research. The high COMT enzyme activity associated with Val allele may reduce tonic DA and increase phasic DA release in subcortical areas as well as reduce DA levels in prefrontal cortical areas (55). This reduced tonic DA release in smokers with the Val allele, may be further accentuated by smoking abstinence, which also reduces DA transmission in subcortical regions (56). As a result, the Val/Val genotype may be associated with more severe withdrawal symptoms. In contrast, the enhanced phasic DA release associated with the Val/Val genotype may contribute to greater responses to some of nicotine’s subjective effects. It is unclear why the COMT Val158Met variation was related to particularly the negative or aversive responses to nicotine. Both the aversive and rewarding effects of nicotine are likely be mediated by DA release in the nucleus accumbens, although the exact mechanisms remain to be determined.
We observed that AA, but not EA, cigarette smokers with the Val/Val genotype had significantly higher systolic and diastolic blood pressure readings throughout the session. In previous studies, the Val/Val genotype has been associated with higher blood pressure in mainly EA populations (57, 58). To our knowledge, the influence of the COMT Val158Met polymorphism on blood pressure regulation in cigarette smokers has not been examined. The underlying mechanism for the association of the Val/Val genotype with higher blood pressure in AA smokers is unclear. Previous studies have shown that AAs excrete less sodium and are more salt-sensitive than EAs (59–61). DA plays an important role in blood pressure regulation, primarily by influencing sodium excretion from the kidneys. Other mechanisms, such as the noradrenergic system, may also contribute to our findings, although the role of COMT Val158Met polymorphism on NE function has not been well described. Higher systolic and diastolic blood pressure in AA cigarette smokers with the Val/Val genotype may have noteworthy health and treatment implications, especially given the cardiovascular risks associated with smoking (62).
Our results also indicated a significant sex-by-COMT interaction for withdrawal severity. Women with the Val/Val genotype reported greater difficulty concentrating and irritability than men who were with Val/Val or Met carriers. These findings are consistent with the sexually dimorphic activity of the COMT enzyme that may be mediated by multiple mechanisms including the estrogen response element in COMT promoter, reduced COMT mRNA expression by estradiol as well as breakdown of catechol estrogens by the COMT enzyme (63). Similar to our findings, other studies have shown that the Val/Val genotype may have greater influence in women for endophenotypes related to negative affect (53, 64). The association of greater irritability, and difficulty concentrating with the Val/Val genotype in women may contribute to greater difficulty of women quitting smoking than men. As mentioned before, the Val/Val genotype may be a risk factor for developing nicotine addiction (9) and poor treatment response to smoking cessation treatments (11–13). Whether COMT variation contributes to the greater difficulty of women to quit smoking remain to be examined.
Our study also had several limitations. First, due to the small number of smokers with the Met/Met genotype, this group was combined with the Met/Val group. As a result, we could not test for the differences between the Met/Met and Met/Val groups. Similarly, due to small sample sizes of the race and sex subgroups, we could not conduct detailed analysis to explain race and sex interactions with the COMT Val158Met polymorphism. Second, the smokers were tested once following overnight abstinence from smoking. For optimum assessment of withdrawal or cognitive performance, multiple days of abstinence from smoking may be needed. Third, the IV nicotine responses may not be generalizable to cigarette smoking, given that tobacco addiction includes both nicotinic and non-nicotinic components (65). Fourth, we did not assess smokers during a smoking as usual condition, which would have allowed further delineation of the influence of COMT Val158Met polymorphism on the study outcomes. Lastly, we did not correct for multiple testing due to exploratory and hypothesis-generating nature of the study. Thus, the results should be interpreted cautiously, although we fell they warrant replication in larger studies.
With these caveats in mind, our results have several treatment implications. As suggested by previous studies, the association of the Val/Val genotype with greater withdrawal severity, worse cognitive performance and greater negative subjective effects suggests that smokers with this genotype, especially female smokers, may experience greater difficulty with smoking cessation. If greater activity of the COMT enzyme is associated with a poor treatment response for smoking cessation, then COMT inhibitors such as tolcapone and entacapone may improve outcomes for smoking cessation in smokers with the Val/Val genotype. Pharmacological COMT inhibition via increased synaptic DA levels improved working memory function and reduced marijuana craving (66–68). This treatment may be especially helpful for female smokers if it is provided immediately after the quit date, when the withdrawal severity is high and smokers are most likely to relapse.
Acknowledgements
Ann Marie Lacobelle, Greg Kay, Christa Robinson, Michelle Cucinelli, Catherine Aldi, Ellen Mitchell, Lance Barnes, and Stacy Minnix provided excellent technical assistance. This research was supported by the Veterans Administration Mental Illness Research, Education and Clinical Center (MIRECC) and NIH grants R03 DA027474, K12 DA00167 (AH); and R01 DA12690 and R01 DA12849.
Footnotes
Disclosure/conflict of Interest
MS serves as an expert witness on behalf of Pfizer in lawsuits related to varenicline.
References
- 1.Slifstein M, Kolachana B, Simpson EH, Tabares P, Cheng B, Duvall M, et al. COMT genotype predicts cortical-limbic D1 receptor availability measured with [11C]NNC112 and PET. Mol Psychiatry. 2008;13(8):821–827. doi: 10.1038/mp.2008.19. Epub 2008/03/05. [DOI] [PubMed] [Google Scholar]
- 2.Axelrod J, Tomchick R. Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem. 1958;233(3):702–705. Epub 1958/09/01. [PubMed] [Google Scholar]
- 3.Axelrod J. Methylation reactions in the formation and metabolism of catecholamines and other biogenic amines. Pharmacol Rev. 1966;18(1):95–113. Epub 1966/03/01. [PubMed] [Google Scholar]
- 4.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. Epub 1996/06/01. [DOI] [PubMed] [Google Scholar]
- 5.Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu Rev Pharmacol Toxicol. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. Epub 1999/05/20. [DOI] [PubMed] [Google Scholar]
- 6.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. Epub 2001/06/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Opmeer EM, Kortekaas R, Aleman A. Depression and the role of genes involved in dopamine metabolism and signalling. Prog Neurobiol. 2010;92(2):112–133. doi: 10.1016/j.pneurobio.2010.06.003. Epub 2010/06/19. [DOI] [PubMed] [Google Scholar]
- 8.Costas J, Sanjuan J, Ramos-Rios R, Paz E, Agra S, Ivorra JL, et al. Heterozygosity at catechol-O-methyltransferase Val158Met and schizophrenia: new data and meta-analysis. J Psychiatr Res. 2011;45(1):7–14. doi: 10.1016/j.jpsychires.2010.04.021. Epub 2010/05/22. [DOI] [PubMed] [Google Scholar]
- 9.Tammimaki AE, Mannisto PT. Are genetic variants of COMT associated with addiction? Pharmacogenet Genomics. 2010;20(12):717–741. doi: 10.1097/FPC.0b013e328340bdf2. Epub 2010/10/27. [DOI] [PubMed] [Google Scholar]
- 10.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. Epub 2006/06/17. [DOI] [PubMed] [Google Scholar]
- 11.Colilla S, Lerman C, Shields PG, Jepson C, Rukstalis M, Berlin J, et al. Association of catechol-O-methyltransferase with smoking cessation in two independent studies of women. Pharmacogenet Genomics. 2005;15(6):393–398. doi: 10.1097/01213011-200506000-00004. Epub 2005/05/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munafo MR, Johnstone EC, Guo B, Murphy MF, Aveyard P. Association of COMT Val108/158Met genotype with smoking cessation. Pharmacogenet Genomics. 2008;18(2):121–128. doi: 10.1097/FPC.0b013e3282f44daa. Epub 2008/01/15. [DOI] [PubMed] [Google Scholar]
- 13.Omidvar M, Stolk L, Uitterlinden AG, Hofman A, Van Duijn CM, Tiemeier H. The effect of catechol-O-methyltransferase Met/Val functional polymorphism on smoking cessation: retrospective and prospective analyses in a cohort study. Pharmacogenet Genomics. 2009;19(1):45–51. doi: 10.1097/fpc.0b013e328317f3f8. Epub 2009/01/24. [DOI] [PubMed] [Google Scholar]
- 14.David SP, Munafo MR. Genetic variation in the dopamine pathway and smoking cessation. Pharmacogenomics. 2008;9(9):1307–1321. doi: 10.2217/14622416.9.9.1307. Epub 2008/09/11. [DOI] [PubMed] [Google Scholar]
- 15.McKinney EF, Walton RT, Yudkin P, Fuller A, Haldar NA, Mant D, et al. Association between polymorphisms in dopamine metabolic enzymes and tobacco consumption in smokers. Pharmacogenetics. 2000;10(6):483–491. doi: 10.1097/00008571-200008000-00001. Epub 2000/09/07. [DOI] [PubMed] [Google Scholar]
- 16.Loughead J, Wileyto EP, Valdez JN, Sanborn P, Tang K, Strasser AA, et al. Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Mol Psychiatry. 2009;14(8):820–826. doi: 10.1038/mp.2008.132. Epub 2008/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Ray R, Faith M, Tang K, Wileyto EP, Detre JA, et al. Nicotine abstinence-induced cerebral blood flow changes by genotype. Neurosci Lett. 2008;438(3):275–280. doi: 10.1016/j.neulet.2008.04.084. Epub 2008/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egan MF, Hyde TM, Bonomo JB, Mattay VS, Bigelow LB, Goldberg TE, et al. Relative risk of neurological signs in siblings of patients with schizophrenia. Am J Psychiatry. 2001;158(11):1827–1834. doi: 10.1176/appi.ajp.158.11.1827. Epub 2001/11/03. [DOI] [PubMed] [Google Scholar]
- 19.Dreher JC, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proc Natl Acad Sci U S A. 2009;106(2):617–622. doi: 10.1073/pnas.0805517106. Epub 2008/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wichers M, Aguilera M, Kenis G, Krabbendam L, Myin-Germeys I, Jacobs N, et al. The catechol-O-methyl transferase Val158Met polymorphism and experience of reward in the flow of daily life. Neuropsychopharmacology. 2008;33(13):3030–3036. doi: 10.1038/sj.npp.1301520. Epub 2007/08/10. [DOI] [PubMed] [Google Scholar]
- 21.Htun NC, Miyaki K, Song Y, Ikeda S, Shimbo T, Muramatsu M. Association of the catechol-O-methyl transferase gene Val158Met polymorphism with blood pressure and prevalence of hypertension: interaction with dietary energy intake. Am J Hypertens. 2011;24(9):1022–1026. doi: 10.1038/ajh.2011.93. Epub 2011/07/22. [DOI] [PubMed] [Google Scholar]
- 22.Sofuoglu M, Yoo S, Hill KP, Mooney M. Self-administration of intravenous nicotine in male and female cigarette smokers. Neuropsychopharmacology. 2008;33(4):715–720. doi: 10.1038/sj.npp.1301460. Epub 2007/05/31. [DOI] [PubMed] [Google Scholar]
- 23.Sofuoglu M, Herman AI, Nadim H, Jatlow P. Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology. 2012;37(6):1509–1516. doi: 10.1038/npp.2011.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 25.Sofuoglu M, Mouratidis M, Yoo S, Culligan K, Kosten T. Effects of tiagabine in combination with intravenous nicotine in overnight abstinent smokers. Psychopharmacology (Berl) 2005;181(3):504–510. doi: 10.1007/s00213-005-0010-y. Epub 2005/06/29. [DOI] [PubMed] [Google Scholar]
- 26.Sofuoglu M, Poling J, Mouratidis M, Kosten T. Effects of topiramate in combination with intravenous nicotine in overnight abstinent smokers. Psychopharmacology (Berl) 2006;184(3–4):645–651. doi: 10.1007/s00213-005-0296-9. Epub 2006/01/25. [DOI] [PubMed] [Google Scholar]
- 27.Sofuoglu M, Waters AJ, Mooney M, O'Malley SS. Minocycline reduced craving for cigarettes but did not affect smoking or intravenous nicotine responses in humans. Pharmacol Biochem Behav. 2009;92(1):135–140. doi: 10.1016/j.pbb.2008.11.004. Epub 2008/12/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. Epub 2002/05/25. [DOI] [PubMed] [Google Scholar]
- 29.al'Absi M, Amunrud T, Wittmers LE. Psychophysiological effects of nicotine abstinence and behavioral challenges in habitual smokers. Pharmacol Biochem Behav. 2002;72(3):707–716. doi: 10.1016/s0091-3057(02)00739-6. Epub 2002/08/15. [DOI] [PubMed] [Google Scholar]
- 30.Mendelson JH, Sholar MB, Goletiani N, Siegel AJ, Mello NK. Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology. 2005;30(9):1751–1763. doi: 10.1038/sj.npp.1300753. Epub 2005/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newhouse PA, Sunderland T, Narang PK, Mellow AM, Fertig JB, Lawlor BA, et al. Neuroendocrine, physiologic, and behavioral responses following intravenous nicotine in nonsmoking healthy volunteers and in patients with Alzheimer's disease. Psychoneuroendocrinology. 1990;15(5–6):471–484. doi: 10.1016/0306-4530(90)90070-p. Epub 1990/01/01. [DOI] [PubMed] [Google Scholar]
- 32.Pickworth WB, Fant RV. Endocrine effects of nicotine administration, tobacco and other drug withdrawal in humans. Psychoneuroendocrinology. 1998;23(2):131–141. doi: 10.1016/s0306-4530(97)00075-9. Epub 1998/06/11. [DOI] [PubMed] [Google Scholar]
- 33.Alexander N, Osinsky R, Mueller E, Schmitz A, Guenthert S, Kuepper Y, et al. Genetic variants within the dopaminergic system interact to modulate endocrine stress reactivity and recovery. Behav Brain Res. 2011;216(1):53–58. doi: 10.1016/j.bbr.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Wolff K, Tsapakis EM, Pariante CM, Kerwin RW, Forsling ML, Aitchison KJ. Pharmacogenetic studies of change in cortisol on ecstasy (MDMA) consumption. Journal of psychopharmacology (Oxford, England) 2012;26(3):419–428. doi: 10.1177/0269881111415737. [DOI] [PubMed] [Google Scholar]
- 35.Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British journal of addiction. 1991;86(11):1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. Epub 1991/11/01. [DOI] [PubMed] [Google Scholar]
- 36.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2001;3(1):7–16. doi: 10.1080/14622200020032051. Epub 2001/03/22. [DOI] [PubMed] [Google Scholar]
- 37.Bell SL, Taylor RC, Singleton EG, Henningfield JE, Heishman SJ. Smoking after nicotine deprivation enhances cognitive performance and decreases tobacco craving in drug abusers. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 1999;1(1):45–52. doi: 10.1080/14622299050011141. Epub 2000/11/10. [DOI] [PubMed] [Google Scholar]
- 38.Morgan MJ, Davies GM, Willner P. The Questionnaire of Smoking Urges is sensitive to abstinence and exposure to smoking-related cues. Behavioural pharmacology. 1999;10(6–7):619–626. doi: 10.1097/00008877-199911000-00008. Epub 2000/04/26. [DOI] [PubMed] [Google Scholar]
- 39.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 40.Hughes JR, Hatsukami DK. Effects of three doses of transdermal nicotine on post-cessation eating, hunger and weight. J Subst Abuse. 1997;9:151–159. doi: 10.1016/s0899-3289(97)90013-4. [DOI] [PubMed] [Google Scholar]
- 41.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. Epub 1988/06/01. [DOI] [PubMed] [Google Scholar]
- 42.Reeves D, Kane R, Winter K. Automated neuropsychological assessment metrics (ANAM v3.11a/96) user’s manual: Clinical and neurotoxicology subset. San Deigo: National Cognitive Foundation; 1996. NCRF-SR-96-01. [Google Scholar]
- 43.Snyder FR, Henningfield JE. Effects of nicotine administration following 12 h of tobacco deprivation: assessment on computerized performance tasks. Psychopharmacology (Berl) 1989;97(1):17–22. doi: 10.1007/BF00443406. Epub 1989/01/01. [DOI] [PubMed] [Google Scholar]
- 44.Mancuso G, Warburton DM, Melen M, Sherwood N, Tirelli E. Selective effects of nicotine on attentional processes. Psychopharmacology (Berl) 1999;146(2):199–204. doi: 10.1007/s002130051107. Epub 1999/10/20. [DOI] [PubMed] [Google Scholar]
- 45.Mancuso G, Andres P, Ansseau M, Tirelli E. Effects of nicotine administered via a transdermal delivery system on vigilance: a repeated measure study. Psychopharmacology (Berl) 1999;142(1):18–23. doi: 10.1007/s002130050857. Epub 1999/04/02. [DOI] [PubMed] [Google Scholar]
- 46.Spreen O, Strauss E. In: A compendium of neuropsychological tests. Spreen O, Strauss E, editors. New York, NY: Oxford University Press; 1991. [Google Scholar]
- 47.Sofuoglu M, Herman AI, Nadim H, Jatlow P. Rapid Nicotine Clearance is Associated with Greater Reward and Heart Rate Increases from Intravenous Nicotine. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2011.336. Epub 2012/02/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dempsey D, Tutka P, Jacob P, 3rd, Allen F, Schoedel K, Tyndale RF, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clinical pharmacology and therapeutics. 2004;76(1):64–72. doi: 10.1016/j.clpt.2004.02.011. Epub 2004/07/02. [DOI] [PubMed] [Google Scholar]
- 49.Guo S, Chen da F, Zhou DF, Sun HQ, Wu GY, Haile CN, et al. Association of functional catechol O-methyl transferase (COMT) Val108Met polymorphism with smoking severity and age of smoking initiation in Chinese male smokers. Psychopharmacology (Berl) 2007;190(4):449–456. doi: 10.1007/s00213-006-0628-4. Epub 2007/01/09. [DOI] [PubMed] [Google Scholar]
- 50.Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handbook of experimental pharmacology. 2009;(192):29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–959. doi: 10.1093/genetics/155.2.945. Epub 2000/06/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang BZ, Zhao H, Kranzler HR, Gelernter J. Characterization of a likelihood based method and effects of markers informativeness in evaluation of admixture and population group assignment. BMC Genet. 2005;6:50. doi: 10.1186/1471-2156-6-50. Epub 2005/10/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Domschke K, Baune BT, Havlik L, Stuhrmann A, Suslow T, Kugel H, et al. Catechol-O-methyltransferase gene variation: impact on amygdala response to aversive stimuli. Neuroimage. 2012;60(4):2222–2229. doi: 10.1016/j.neuroimage.2012.02.039. Epub 2012/03/06. [DOI] [PubMed] [Google Scholar]
- 54.Witte AV, Floel A. Effects of COMT polymorphisms on brain function and behavior in health and disease. Brain research bulletin. 2012;88(5):418–428. doi: 10.1016/j.brainresbull.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 55.Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29(11):1943–1961. doi: 10.1038/sj.npp.1300542. Epub 2004/08/12. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Dong Y, Doyon WM, Dani JA. Withdrawal from chronic nicotine exposure alters dopamine signaling dynamics in the nucleus accumbens. Biol Psychiatry. 2012;71(3):184–191. doi: 10.1016/j.biopsych.2011.07.024. Epub 2011/08/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stewart SH, Oroszi G, Randall PK, Anton RF. COMT genotype influences the effect of alcohol on blood pressure: results from the COMBINE study. American journal of hypertension. 2009;22(1):87–91. doi: 10.1038/ajh.2008.321. Epub 2008/11/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hagen K, Pettersen E, Stovner LJ, Skorpen F, Holmen J, Zwart JA. High systolic blood pressure is associated with Val/Val genotype in the catechol-o-methyltransferase gene. The Nord-Trondelag Health Study (HUNT) American journal of hypertension. 2007;20(1):21–26. doi: 10.1016/j.amjhyper.2006.05.023. Epub 2007/01/03. [DOI] [PubMed] [Google Scholar]
- 59.Grim CE, Luft FC, Miller JZ, Meneely GR, Battarbee HD, Hames CG, et al. Racial differences in blood pressure in Evans County, Georgia: relationship to sodium and potassium intake and plasma renin activity. Journal of chronic diseases. 1980;33(2):87–94. doi: 10.1016/0021-9681(80)90032-6. Epub 1980/01/01. [DOI] [PubMed] [Google Scholar]
- 60.Chrysant SG, Danisa K, Kem DC, Dillard BL, Smith WJ, Frohlich ED. Racial differences in pressure, volume and renin interrelationships in essential hypertension. Hypertension. 1979;1(2):136–141. doi: 10.1161/01.hyp.1.2.136. Epub 1979/03/01. [DOI] [PubMed] [Google Scholar]
- 61.Luft FC, Grim CE, Higgins JT, Jr, Weinberger MH. Differences in response to sodium administration in normotensive white and black subjects. The Journal of laboratory and clinical medicine. 1977;90(3):555–562. Epub 1977/09/01. [PubMed] [Google Scholar]
- 62.Armani C, Landini L, Jr, Leone A. Molecular and biochemical changes of the cardiovascular system due to smoking exposure. Curr Pharm Des. 2009;15(10):1038–1053. doi: 10.2174/138161209787846973. Epub 2009/04/10. [DOI] [PubMed] [Google Scholar]
- 63.Tunbridge EM, Harrison PJ. Importance of the COMT gene for sex differences in brain function and predisposition to psychiatric disorders. Current topics in behavioral neurosciences. 2011;8:119–140. doi: 10.1007/7854_2010_97. Epub 2011/07/20. [DOI] [PubMed] [Google Scholar]
- 64.Olsson CA, Anney RJ, Lotfi-Miri M, Byrnes GB, Williamson R, Patton GC. Association between the COMT Val158Met polymorphism and propensity to anxiety in an Australian population-based longitudinal study of adolescent health. Psychiatr Genet. 2005;15(2):109–115. doi: 10.1097/00041444-200506000-00007. Epub 2005/05/19. [DOI] [PubMed] [Google Scholar]
- 65.Sofuoglu M, Lesage MG. The reinforcement threshold for nicotine as a target for tobacco control. Drug and alcohol dependence. 2012;125(1–2):1–7. doi: 10.1016/j.drugalcdep.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, et al. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology. 2007;32(5):1011–1020. doi: 10.1038/sj.npp.1301227. Epub 2006/10/26. [DOI] [PubMed] [Google Scholar]
- 67.Roussos P, Giakoumaki SG, Bitsios P. Tolcapone effects on gating, working memory, and mood interact with the synonymous catechol-O-methyltransferase rs4818c/g polymorphism. Biological psychiatry. 2009;66(11):997–1004. doi: 10.1016/j.biopsych.2009.07.008. Epub 2009/08/25. [DOI] [PubMed] [Google Scholar]
- 68.Shafa R. COMT-Inhibitors may be a Promising Tool in Treatment of Marijuana Addiction Trials. A J Addict. 2009;18(322) [Google Scholar]


