Abstract
Chronic pain in children is associated with significant negative impact on social, emotional, and school functioning. Previous studies on the impact of pain on children's functioning have primarily used mixed samples of pain conditions or single pain conditions (e.g., headache, abdominal pain) with relatively small sample sizes. As a result, the similarities and differences in the impact of pain in sub-groups of children with chronic pain have not been closely examined.
Objectives
To compare pain characteristics, quality of life, and emotional functioning among youth with pediatric chronic migraine (CM) and juvenile fibromyalgia (JFM).
Methods
We combined data obtained during screening of patients for two relatively large intervention studies of youth (ages 10-18) with CM (N = 153) and JFM (N = 151). Measures of pain intensity, quality of life (Pediatric Quality of Life; PedsQL™, child and parent-proxy), depressive symptoms (Children's Depression Inventory; CDI), and anxiety symptoms (Adolescent Symptom Inventory-4 - Anxiety subscale) were completed by youth and their parent. A multivariate analysis of co-variance (MANCOVA) controlling for effects of age and gender was performed to examine differences in quality of life and emotional functioning between the CM and JFM groups.
Results
Youth with JFM had significantly higher anxiety and depressive symptoms, and lower quality of life in all domains. Among children with CM, overall functioning was higher but school functioning was a specific area of concern.
Discussion
Results indicate important differences in sub-groups of pediatric pain patients and point to the need for more intensive multidisciplinary intervention for JFM patients.
Keywords: Pediatric pain, chronic migraine, pediatric headache, juvenile fibromyalgia, quality of life
Chronic pain in children and adolescents is a significant public health problem with over 25% of school-age children reporting recurrent or chronic pain.1 The impact of chronic pain on social and emotional functioning has been studied in various pediatric pain conditions2-10. Results from these studies suggest lower overall functioning in children and adolescents with chronic pain conditions when compared to their healthy counterparts but less is known about how children with the various chronic pain syndromes differ from one another.
Most of the aforementioned research studies examining the impact of pediatric pain have focused on either combined samples of various pain conditions or on single conditions in isolation such as abdominal pain, fibromyalgia, or headache. Research on combined pain conditions is limited by relatively small sample sizes for each pain subtype. Similarly, functioning examined in a single pain condition may not generalize to other pain syndromes; therefore, little is known about the potential similarities and differences between various pediatric chronic pain conditions in terms of social and emotional functioning. Results from a recent study suggested that children and adolescents with recurrent headache may have lower levels of disability and depressive symptoms compared to those with widespread musculoskeletal pain. 11 However, a limitation of the study was that classification of pain subtypes was designated based on primary pain location only and not based on clear medical diagnoses based on specific diagnostic criteria.
Despite the relative paucity of research on sub-groups of pediatric pain conditions, clinicians anecdotally report distinct differences in patients with various chronic pain syndromes such as greater impairment in those with widespread musculoskeletal pain compared to other pain conditions. It is plausible that chronic pain may have differential impact depending on underlying pathophysiology, specific pain characteristics such as pain location, frequency, and severity, or presence of comorbid somatic symptoms or mood difficulties. The reasons why different pain conditions may result in greater or lesser impact on children's daily lives is as yet poorly understood, yet this topic has important implications for treatment planning. As an initial step, more empirical work is needed to identify areas of commonalities and differences between pain conditions in children, even as science into the underlying biological mechanisms of chronic pain and associated symptoms advances our understanding of specific pain conditions.
Juvenile Fibromyalgia (JFM) and pediatric Chronic Migraine (CM) are two chronic pain syndromes that have several common elements including persistent or recurrent pain that occurs daily or almost daily. Research on adults has shown that fibromyalgia and migraine headaches share some overlapping features 12-14 including similar neurobiological changes associated pain hypersensitivity 15,16. However, adult fibromyalgia patients experience poorer quality of life (QOL) compared to those with migraine headaches 14. Similar to adult fibromyalgia, JFM in children is characterized by constant widespread pain, fatigue, and sleep disturbance, along with multiple associated somatic symptoms. JFM patients also appear to have substantially higher rates of anxiety and mood disturbances 17 than children with CM 18 but there are no studies comparing QOL differences in the two conditions . Treatment regimens for both chronic pain conditions typically consists of medication management and often recommendations for non-drug interventions (e.g., cognitive-behavioral therapy, lifestyle changes) to promote self-management and coping. A greater understanding of the impact of these pain subtypes would help inform more tailored approaches specific to their needs.
As part of the screening procedure for two ongoing clinical research studies we obtained extensive information about pain characteristics, quality of life, and emotional functioning for over 300 patients who met diagnostic criteria for pediatric CM or JFM. The objective of the current study was to compare pain characteristics, quality of life and emotional functioning (depressive and anxiety symptoms) between patients with CM and those with JFM from the combined samples from these relatively large pediatric studies. Based on prior findings, we expected the CM and JFM groups to be similar in terms of pain intensity. However, we hypothesized that quality of life and emotional functioning would be significantly poorer in children and adolescents diagnosed with JFM as compared to those with CM.11
Materials and Methods
Participants
Participants in the study were pediatric pain patients (ages 10 to 18 years) diagnosed with JFM or CM and recruited from subspecialty clinics (four pediatric rheumatology clinics in the Midwestern United States and a single pediatric Headache Center at a children's hospital for the JFM and CM studies, respectively). Participants with JFM were diagnosed by a pediatric rheumatologist based on the 1985 Yunus and Masi criteria19 for JFM classification (generalized musculoskeletal pain in at least 3 sites for at least 3 months; 5 or more painful tender points upon palpation; associated symptoms such as sleep disturbance, fatigue, headaches, irritable bowel syndrome). Participants with JFM were excluded if they were diagnosed with another underlying chronic pediatric disease, such as juvenile arthritis or lupus. Participants with CM were diagnosed by a pediatric neurologist board certified in headache medicine using the International Classification of Headache Disorders – 2nd Edition (ICHD-II) definition of migraine (modified for pediatric use)20 including unilateral or bilateral headaches of 1-72 hours duration, nausea and/or vomiting, and 2 of 5 associated symptoms (photophobia, phonophobia, difficulty thinking, or lightheadedness). At least 15 headaches per month were required for a classification of CM and this was confirmed by a prospective 4-week daily headache diary completed by participants.21 Patients with CM diagnosed with medication overuse or other primary pain syndromes (including JFM) were excluded from the CM group. Participants with known developmental delays were not included in either sample.
Procedure
Participants were identified and introduced to the study by their study physician. If interested in learning more about the study, a research coordinator contacted families to give them detailed information and request their participation. Eligible participants and parents provided written informed consent prior to the initiation of any study procedures. Institutional Review Board approvals were obtained from all participating study sites.
Measures
Demographic Information
Families were asked to complete forms detailing background and demographic information including race, ethnicity, age, gender, and socioeconomic status.
Pain Characteristics
Participants completed daily pain diaries and returned diaries at their study visit. Based on the two separate study protocols for JFM and CM, participants with JFM completed 0-10 centimeter daily Visual Analog Scales (VAS) to assess pain intensity over one week, and participants with CM completed daily 0-10 Numeric Pain Rating Scales for four weeks (28 days). The longer duration of diary completion for patients with CM was required to confirm headache frequency of ≥ 15 headaches per month, in addition to assessing average pain intensity. For the purposes of this study, average pain intensity was defined as average pain over seven days (for the CM group, this was the average pain intensity of headaches they experienced in the last 7 days of their 4–week diary). The VAS and numeric pain ratings are reliable, valid instruments used to measure pain in both adult and pediatric populations 22 and provide comparable information 23,24. For patients with CM, headache frequency and average duration of pain was also calculated from diaries.
Quality of Life
Pediatric Quality of Life Inventory (PedsQL™) – Generic Core Scales
The Pediatric Quality of Life Inventory- Generic Version (PedsQL™) is an instrument commonly used to assess overall quality of life in children and adolescents with chronic health conditions, such as cancer, diabetes, rheumatoid arthritis, and headache. 6 Each item has four response options - never, sometimes, often, and always. Self-report and parent-proxy versions of the PedsQL™ were administered. Participants were asked to rate their own (or their child's) quality of life in the 4 domains of physical, social, emotional and school functioning over the last month. The PedsQL™ is reverse-scored with scores ranging from 0-100, with higher scores indicative of better functioning. 25 Scores on the PedsQL™ scales range from about 78-91 (for child and parent-proxy report) among healthy children 26.
Emotional Adjustment and Depression
Children's Depression Inventory (CDI)
The CDI is a 27-item self-report measure that assesses cognitive, behavioral, and affective symptoms of depression in children and adolescents. 27 Each question has three response choices related to the individual's feelings during the past two weeks. Total scores range from 0-54 and normative scores based on age and gender are available. CDI total raw scores > 10 indicate at least mild depression.
Adolescent Symptom Inventory-4 (ASI-4) – Generalized Anxiety Sub-scale
The Adolescent Symptom Inventory-4 is an instrument used to measure caregiver ratings of a child's emotional functioning. The Generalized Anxiety Sub-scale of the ASI-4 consists of eight questions which ask parents to rate how often their child displays a symptom (never, sometimes, often, or always). Items are then scored either 0 or 1 (0-never and sometimes; 1-often and always) to get a symptom criterion score. Scores of greater than or equal to five on the Generalized Anxiety Sub-scale of the ASI is an indication that the participant exhibits significant symptoms consistent with Generalized Anxiety.28
Statistical Analysis
First, descriptive data were computed for all demographic variables and measures of pain, quality of life, anxiety and depression. Pearson correlations between measures of pain, quality of life and emotional functioning were calculated for each group independently to examine relationships between these variables within each illness group. Due to the multiple correlations, we used a more conservative level of significance (p < .01) to interpret correlations. A one-way multivariate analysis of co-variance (MANCOVA) with age and gender as co-variates, was conducted to test the primary aim, i.e., to determine if there were significant differences between the JFM and CM groups based on parent and self-report quality of life and emotional functioning (i.e., anxiety and depressive symptoms). A Bonferroni correction (.05 × 10 comparisons = .005) was used to interpret results of the individual comparisons between groups in the MANCOVA.
In exploratory analyses, we undertook a closer examination of the link between elevated distress and longer pain duration on QOL by assessing whether subgroups of CM patients with higher levels of depressive symptoms and those with constant/nearly constant pain (i.e., those that presented most similarly to the JFM group) had QOL scores that were as low as the JFM group. First, the proportion of CM patients versus JFM patients who showed at least mild elevations in depressive symptoms (CDI score >10, Kovacs et al.) was calculated and QOL scores for this “elevated distress” subgroup of CM patients were computed, and 2) the proportion of CM patients who had constant (24 hours per day) or nearly constant (>10 hours per day) pain was calculated and QOL scores for this “high pain duration” subgroup of CM patients were computed. Although formal statistical analyses were not conducted due to power issues (relatively small number of CM patients in the elevated distress and constant daily pain groups) descriptive data on QOL was compared to the JFM group and the overall CM sample.
Results
Demographic Information
A total of 310 individuals were eligible and of these, 304 were included (JFM=151, CM=153) with a mean age of 15.07 and 14.54 years, respectively. One JFM and 5 CM subjects were excluded from the final sample due to missing data. The majority of the participants were female (JFM: 90.1%; CM: 81.7%) and over 80% of the combined sample was Caucasian. The median income for the sample was over $50,000. Detailed demographic information is provided in Table 1. There were no significant differences in race or income level between the two groups (p's > .05); however, participants in the JFM group were significantly older than the CM group (p = .015) and the JFM group had a greater proportion of females (p = .036).
Table 1.
Demographics of study participants by group
| JFM (N=151) | CM (N=153) | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | |
| Age | 15.07 | 1.82 | 14.54 | 1.94 | 0.015 |
| Number | % | Number | % | ||
| Gender | |||||
| Male | 15 | 9.9 | 28 | 18.3 | 0.036 |
| Female | 136 | 90.1 | 125 | 81.7 | |
| Race/Ethnicity | |||||
| White | 130 | 86.1 | 130 | 85 | |
| Black/African American | 10 | 6.6 | 12 | 7.8 | |
| Asian | 1 | 0.7 | 1 | 0.7 | |
| American Indian/ Alaskan Native | 2 | 1.3 | 1 | 0.7 | 0.663 |
| Hispanic/Latino | 5 | 3.3 | 6 | 3.9 | |
| Biracial | 3 | 2.0 | ---- | ---- | |
| Missing | ---- | ---- | 3 | 2 | |
| Family Income | Number | % | Number | % | |
| $0-$9,999 | 7 | 4.6 | 7 | 4.6 | |
| $10,000-$19,999 | 9 | 6.0 | 6 | 3.9 | |
| $20,000-$29,999 | 8 | 5.3 | 12 | 7.8 | |
| $30,000-$39,999 | 20 | 13.2 | 9 | 5.9 | 0.838 |
| $40,000-$49,999 | 11 | 7.3 | 19 | 12.4 | |
| $50,000 and over | 92 | 60.9 | 85 | 55.6 | |
| Missing | 4 | 2.6 | 15 | 9.8 | |
Pain Characteristics
Average pain intensity reported by youth with CM was 5.48 (SD = 1.88) and for those with JFM was 5.36 (SD = 1.79) and this difference was not statistically significant (t = 0.566, p = .572). The average number of migraines per week within the CM group was 5.7 (SD = 1.44) indicating that headaches occurred almost daily, and the average headache duration was 9.94 hours (SD = 8.73).
Correlations between parent and child QOL reports and measures of emotional functioning
Bivariate correlations between measures were conducted separately for each group (Tables 2 and 3). Results indicated that parent-proxy and child reports of quality of life were significantly associated with one another (Pearson r's ranging from 0.42 – 0.69; p < .01) and parent-reported anxiety and self-reported depression were positively correlated for both JFM and CM groups (r = 0.42 and 0.42; p< .01 respectively). These results suggest moderate to high agreement between parent and child report on the various domains of functioning. Anxiety and depressive symptoms were significantly negatively correlated with all quality of life domains for both groups.
Table 2.
Correlations between Quality of Life domains, emotional functioning and pain intensity in the JFM Group (N = 151)
| PPF | PEF | PSF | PSCF | CPF | CEF | CSF | CSCF | CDI | ASI | |
|---|---|---|---|---|---|---|---|---|---|---|
| PedsQL™ (Range: 0-100) | ||||||||||
| Parent Report | ||||||||||
| Physical Functioning (PPF) | ||||||||||
| Emotional Functioning (PEF) | 0.45** | |||||||||
| Social Functioning (PSF) | 0.51** | 0.48** | ||||||||
| School Functioning (PSCF) | 0.48** | 0.42** | 0.48** | |||||||
| Child Report | ||||||||||
| Physical Functioning (CPF) | 0.67** | 0.34** | 0.33** | 0.31** | ||||||
| Emotional Functioning (CEF) | 0.30** | 0.64** | 0.34** | 0.17* | 0.38** | |||||
| Social Functioning (CSF) | 0.36** | 0.36** | 0.42** | 0.19* | 0.50** | 0.41** | ||||
| School Functioning (CSCF) | 0.38** | 0.36** | 0.30** | 0.67** | 0.45** | 0.35** | 0.31** | |||
| Child Depression Inventory (CDI) (Range: 1-27) | -0.26** | -0.53** | -0.30** | -0.34** | -0.39** | -0.67** | -0.48** | -0.46** | ||
| Adolescent Symptom Inventory (ASI) (Range: 0-8) | ||||||||||
| Anxiety Symptoms | -0.32** | -0.61** | -0.30** | -0.48** | -0.29** | -0.33** | -0.27** | -0.30** | 0.42** | |
| Visual Analogue Scale (VAS) (Range: 0-10) | -0.35** | -0.19 | -0.18 | -0.17 | -0.42** | -0.19 | -0.16 | -0.11 | 0.29** | 0.11 |
Correlation significant at the 0.01 level (2-tailed)
Table 3.
Correlations between Quality of Life domains, emotional functioning and pain intensity in the CM Group (N = 153)
| PPF | PEF | PSF | PSCF | CPF | CEF | CSF | CSCF | CDI | ASI | |
|---|---|---|---|---|---|---|---|---|---|---|
| PedsQL™ (Range: 0-100) | ||||||||||
| Parent Report | ||||||||||
| Physical Functioning (PPF) | ||||||||||
| Emotional Functioning (PEF) | 0.48** | |||||||||
| Social Functioning (PSF) | 0.49** | 0.58** | ||||||||
| School Functioning (PSCF) | 0.53** | 0.61** | 0.58** | |||||||
| Child Report | ||||||||||
| Physical Functioning (CPF) | 0.54** | 0.26** | 0.32** | 0.37** | ||||||
| Emotional Functioning (CEF) | 0.28** | 0.50** | 0.33** | 0.29 | 0.47** | |||||
| Social Functioning (CSF) | 0.27** | 0.38** | 0.52** | 0.29 | 0.48** | 0.57** | ||||
| School Functioning (CSCF) | 0.46** | 0.48** | 0.43** | 0.69** | 0.54** | 0.48** | 0.37** | |||
| Child Depression Inventory (CDI) (Range: 1-27) | -0.28** | -0.51** | -0.38** | -0.46** | -0.39** | -0.67** | -0.43** | -0.63** | ||
| Adolescent Symptom Inventory (ASI) (Range: 0-8) | ||||||||||
| Anxiety Symptoms | -0.42** | -0.54** | -0.28** | -0.41** | -0.32** | -0.35** | -0.23** | -0.36** | 0.42** | |
| Numeric Pain Scale (NPS) (Range: 0-10) | -0.05 | -0.12 | -0.09 | -0.11 | -0.10 | -0.19 | -0.17 | -0.28** | 0.21 | 0.04 |
Correlation significant at the 0.01 level (2-tailed)
Quality of Life in JFM and CM
Mean scores of both JFM and CM groups were lower than those reported for healthy children 26 with the exception of social functioning which was not impaired in the CM group. There were however, significant differences between the 2 pain samples in this study. The omnibus MANCOVA showed significant differences between the two groups on the combined dependent variables, F (11, 284) =19.56, p < 0.001 (Table 4) after controlling for age and gender effects. When the individual dependent measures were considered separately, children and adolescents with CM evidenced significantly higher functioning across all domains of the PedsQL™ (emotional, social, physical, and school) using a Bonferroni adjusted alpha level of .005, compared to participants with JFM, particularly in physical and emotional functioning. Youth with CM also had significantly lower scores on both the ASI and CDI (indicating lower distress) compared to participants with JFM (Table 4).
Table 4.
Differences between youth with JFM and CM on Quality of Life and emotional functioning (after adjusting for age and gender)
| JFM (N=151) | CM (N=153) | |||||
|---|---|---|---|---|---|---|
| PedsQL™ (Range: 0-100) | Mean† | SE | Mean† | SE | F | Sig. |
| Parent Report | ||||||
| Physical Functioning | 47.32 | 1.60 | 73.45 | 1.57 | 133.82 | 0.000 |
| Emotional Functioning | 57.82 | 1.64 | 68.81 | 1.61 | 22.56 | 0.000 |
| Social Functioning | 70.87 | 1.59 | 86.15 | 1.57 | 45.93 | 0.000 |
| School Functioning | 54.06 | 1.89 | 63.54 | 1.86 | 12.69 | 0.000 |
| Child Report | ||||||
| Physical Functioning | 46.47 | 1.51 | 71.50 | 1.49 | 137.85 | 0.000 |
| Emotional Functioning | 54.62 | 1.56 | 70.23 | 1.53 | 50.28 | 0.000 |
| Social Functioning | 71.26 | 1.43 | 86.72 | 1.40 | 59.06 | 0.000 |
| School Functioning | 46.17 | 1.64 | 60.37 | 1.62 | 37.54 | 0.000 |
| Child Depression Inventory (Range: 1-27) | 12.92 | 0.55 | 7.98 | 0.54 | 40.36 | 0.000 |
| (Child Report) | ||||||
| Adolescent Symptom Inventory (Range: 0-8) | ||||||
| Anxiety Symptoms (Parent Report) | 2.46 | 0.15 | 1.03 | 0.15 | 45.18 | 0.000 |
| Pain Intensity (Range: 0-10) | ||||||
| (Child Report) | 5.32 | 0.15 | 5.53 | 0.15 | 0.93 | 0.335 |
*Omnibus Results for MANCOVA, Hotellings Trace: F (11, 284) = 19.56, p ≤ .001
Marginal Means
In exploratory analysis, it was found that 28.8% of the CM group fell in the “elevated distress” group (CDI score > 10) compared to 60.3% of the JFM sample. Descriptive data on child-reported QOL domains for this “elevated distress” CM subgroup showed that they had lower emotional (Mean = 55.11; SD = 17.83), social (Mean = 79.88; SD =18.35) and school functioning (Mean = 42.27; SD = 16.05) than the overall CM group and these scores were more similar to the JFM group. When duration of pain was considered, the subgroup of CM patients with “high pain duration” (constant/nearly constant pain; 36.6% of the CM sample) had similar QOL scores to the overall CM group (scores ranging from 58.48-84.82) which were much higher than the JFM group - indicating that headaches of longer duration did not appear to be associated with lowered QOL.
Discussion
The findings of this study highlight the utility of studying the similarities and differences between pediatric pain sub-groups in order to get a more in depth understanding of the impact of these pain conditions on quality of life. CM and FM are similar in that they are both characterized by persistent, daily or almost daily pain. Studies in adults have shown that these have some overlapping features including underlying neurobiological changes associated with pain hypersensitivity 15,16. However, the impact of migraines and fibromyalgia on QOL and psychosocial function, particularly in youth, is poorly understood. The marked differences between the CM and JFM groups found in this study highlight how patients with different pain subtypes may present to pediatric care centers with varying psychosocial profiles. As the results suggest, the negative impact on QOL is much less severe for youth with CM than for those with JFM. Despite similar levels of pain intensity in both groups, youth with JFM had markedly poorer physical, emotional, social and school functioning (per parent and child report) than youth with CM. Even the subgroup of CM patients who had constant or nearly constant pain had higher QOL than JFM patients, suggesting that pain characteristics (intensity or duration) do not directly influence QOL. On the other hand, psychological distress appeared to demonstrate a stronger connection with QOL across groups. In general, the JFM group reported markedly greater problems with anxiety and depressive symptoms than CM patients along with poorer QOL in all domains. When a subgroup of the more distressed CM patients were compared with JFM patients on QOL, it was observed that their scores were more similar to JFM patients, supporting a link between emotional functioning and quality of life.
It should be noted that while patients with CM appear to have a higher level of functioning in most domains, their functioning at school was reported as being lower than healthy children. Therefore the impact of headaches on school performance may be an area of specific concern for CM patients. JFM patients also reported very poor school functioning, but this was in the context of poor emotional and physical function as well which may have slightly different implications from a treatment perspective. Although the underlying reasons for the marked differences in the impact of CM and JFM on QOL are not well understood, the findings provide some guidance into potential avenues for intervention.
Specifically, the results of this study suggest that the clinical care for patients with CM and JFM can be designed to be more appropriate to their specific needs. Offering training in relatively basic pain coping strategies (e.g., relaxation, distraction and pacing techniques) and recommending healthy lifestyle changes (such as more attention to hydration, nutrition and physical activity) may be helpful for most youth suffering from CM. For difficulties at school, more support and resources might be provided to help them with academic concerns. These strategies may prove insufficient for the management of young patients with JFM who are likely to have more complex needs. Patients with JFM are more likely to experience symptoms of elevated depression and anxiety and they tend to have significantly poorer quality of life across multiple domains. Hence, greater attention should be given to the comprehensive assessment of youth with JFM followed by a multidisciplinary treatment plan that is sufficiently intensive to address the physical limitations and psychosocial difficulties experienced by youth with JFM that cut across the domains of emotional, social and school functioning. In particular, most JFM patients could benefit from a complete course of cognitive-behavioral therapy for pain coping skills training 30, and for those who have clinically significant depressive symptoms, consideration of antidepressant medication may be necessary as well. JFM patients have also been found to be very sedentary 31, likely leading to a cycle of inactivity and deconditioning contributing to further pain and impairment. For these patients, increasing physical activity and exercise participation should form an essential component of treatment. Early studies of exercise interventions for JFM patients have been promising 32 although careful attention to designing exercise programs keeping in mind JFM patients’ exercise intolerance is needed to maximize their engagement in treatment 33.
This study had several strengths including the relatively large pediatric sample with similar demographic characteristics, with clear diagnoses, and recruitment from similar (tertiary care) treatment settings. However, some limitations of this study include 1) recruitment from tertiary care settings somewhat limits generalizability of the findings to all health care settings and 2) not being able to examine underlying reasons for the substantial differences in QOL observed between JFM and CM. Further studies might examine the role of emotional distress and the role of associated comorbidities which are much more salient in JFM. Finally, the focus of this study was limited to two of the many pain conditions of childhood. Nevertheless, we believe that the study represents a step towards a more sophisticated understanding of the characteristics and the needs of pediatric pain subpopulations which have thus far been viewed as a more or less homogenous group from a research perspective. While it is true that many pediatric pain patients respond well to similar types of treatment approaches, such as cognitive-behavioral therapy,34 there may be ways to apply resources in a more targeted or tailored fashion, and vary the intensity (dose) and comprehensiveness of services based on the particular needs of each of the subgroups. It is hoped that future research with sufficient samples of different pediatric subpopulations will further expand on key differences between pediatric pain sub-populations and examine whether current treatment approaches can be improved to incorporate a more fine-grained knowledge of each medical condition.
Figure 1.
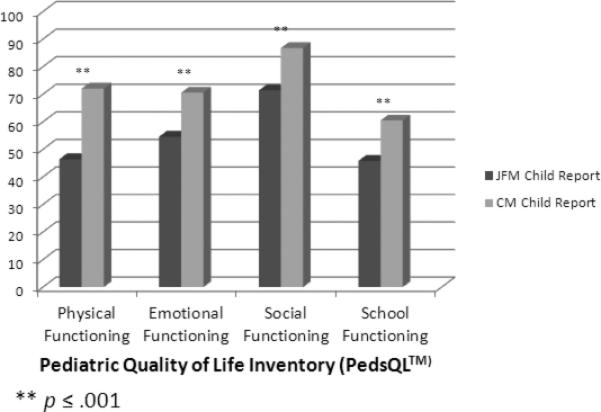
Quality of life in youth with JFM or CM
Acknowledgments
We would like to thank the Headache Center team at Cincinnati Children's Hospital for their assistance with the Chronic Migraine study and the multi-site Juvenile Fibromyalgia Study team for their assistance with the planning and data collection for the JFM clinical intervention study. JFM study team members include – Drs. Anne Lynch-Jordan, Daniel Lovell (Cincinnati Children's Hospital), Dr. Lesley Arnold (University of Cincinnati), Dr. Kenneth Schikler (Kosair Children's Hospital, University of Louisville), Dr. Murray Passo (Medical University of South Carolina), Dr. T. Brent Graham (Vanderbilt University School of Medicine), Dr. Philip Hashkes (Shaare Zedek Medical Center, Jerusalem), Drs. Steven Spalding, Margaret Richards and Gerard Banez (Cleveland Clinic Lerner School of Medicine), and the research coordinators at each site.
Source of Financial Support: National Institute of Arthritis and Musculoskeletal and Skin Diseases - NIAMS R01AR050028 (S.Kashikar-Zuck); National Institute of Neurological Disorders and Stroke – NINDS R01NS050536 (PI: S. Powers)
Footnotes
Conflict of Interest: None of the authors have a financial or other conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perquin CW, Hazebroek-Kampschreur AA, Hunfeld JA, et al. Pain in children and adolescents: a common experience. Pain. 2000 Jul;87(1):51–58. doi: 10.1016/S0304-3959(00)00269-4. [DOI] [PubMed] [Google Scholar]
- 2.Kashikar-Zuck S, Lynch AM, Graham TB, Swain NF, Mullen SM, Noll RB. Social functioning and peer relationships of adolescents with juvenile fibromyalgia syndrome. Arthritis and Rheumatism. 2007 Apr 15;57(3):474–480. doi: 10.1002/art.22615. [DOI] [PubMed] [Google Scholar]
- 3.Kashikar-Zuck S, Johnston M, Ting TV, et al. Relationship between school absenteeism and depressive symptoms among adolescents with juvenile fibromyalgia. J Pediatr Psychol. 2010 Oct;35(9):996–1004. doi: 10.1093/jpepsy/jsq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker LS, Smith CA, Garber J, Claar RL. Appraisal and coping with daily stressors by pediatric patients with chronic abdominal pain. J Pediatr Psychol. 2007 Mar;32(2):206–216. doi: 10.1093/jpepsy/jsj124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker LS, Greene JW. Children with recurrent abdominal pain and their parents: More somatic complaints, anxiety, and depression than other patient families. Journal of Pediatric Psychology. 1989;14(2):231–243. doi: 10.1093/jpepsy/14.2.231. [DOI] [PubMed] [Google Scholar]
- 6.Powers SW, Patton SR, Hommel KA, Hershey AD. Quality of life in childhood migraines: clinical impact and comparison to other chronic illnesses. Pediatrics. 2003 Jul;112(1 Pt 1):e1–5. doi: 10.1542/peds.112.1.e1. [DOI] [PubMed] [Google Scholar]
- 7.Merlijn VP, Hunfeld JA, van der Wouden JC, Hazebroek-Kampschreur AA, Passchier J, Koes BW. Factors related to the quality of life in adolescents with chronic pain. Clinical Journal of Pain. 2006;22(3):306–315. doi: 10.1097/01.ajp.0000177509.93523.68. [DOI] [PubMed] [Google Scholar]
- 8.Gold JI, Mahrer NE, Yee J, Palermo TM. Pain, Fatigue, and Health-related Quality of Life in Children and Adolescents With Chronic Pain. The Clinical Journal of Pain. 2009;25(5):407–412. doi: 10.1097/AJP.0b013e318192bfb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logan DE, Simons LE, Kaczynski KJ. School Functioning in Adolescents With Chronic Pain: The Role of Depressive Symptoms in School Impairment. Journal of Pediatric Psychology. 2009;34(8):882–892. doi: 10.1093/jpepsy/jsn143. [DOI] [PubMed] [Google Scholar]
- 10.Logan DE, Simons LE, Stein MJ, Chastain L. School Impairment in Adolescents With Chronic Pain. The Journal of Pain. 2008;9(5):407–416. doi: 10.1016/j.jpain.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Kashikar-Zuck S, Flowers SR, Claar RL, et al. Clinical utility and validity of the Functional Disability Inventory among a multicenter sample of youth with chronic pain. Pain. 2011 Jul;152(7):1600–1607. doi: 10.1016/j.pain.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Tommaso M. Prevalence, clinical features and potential therapies for fibromyalgia in primary headaches. Expert review of neurotherapeutics. 2012 Mar;12(3):287–295. doi: 10.1586/ern.11.190. quiz 296. [DOI] [PubMed] [Google Scholar]
- 13.de Tommaso M, Federici A, Serpino C, et al. Clinical features of headache patients with fibromyalgia comorbidity. The journal of headache and pain. 2011 Dec;12(6):629–638. doi: 10.1007/s10194-011-0377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ifergane G, Buskila D, Simiseshvely N, Zeev K, Cohen H. Prevalence of fibromyalgia syndrome in migraine patients. Cephalalgia. 2006 Apr;26(4):451–456. doi: 10.1111/j.1468-2982.2005.01060.x. [DOI] [PubMed] [Google Scholar]
- 15.Nicolodi M, Volpe AR, Sicuteri F. Fibromyalgia and headache. Failure of serotonergic analgesia and N-methyl-D-aspartate-mediated neuronal plasticity: their common clues. Cephalalgia. 1998 Feb;18(Suppl 21):41–44. doi: 10.1177/0333102498018s2111. [DOI] [PubMed] [Google Scholar]
- 16.Sarchielli P, Mancini ML, Floridi A, et al. Increased levels of neurotrophins are not specific for chronic migraine: evidence from primary fibromyalgia syndrome. J Pain. 2007 Sep;8(9):737–745. doi: 10.1016/j.jpain.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Kashikar-Zuck S, Parkins IS, Graham TB, et al. Anxiety, mood, and behavioral disorders among pediatric patients with juvenile fibromyalgia syndrome. Clinical Journal of Pain. 2008 Sep;24(7):620–626. doi: 10.1097/AJP.0b013e31816d7d23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slater SK, Kashikar-Zuck SM, Allen JR, et al. Psychiatric comorbidity in pediatric chronic daily headache. Cephalalgia. 2012 Sep 18; doi: 10.1177/0333102412460776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yunus MB, Masi AT. Juvenile primary fibromyalgia syndrome. A clinical study of thirty-three patients and matched normal controls. Arthritis Rheum. 1985 Feb;28(2):138–145. doi: 10.1002/art.1780280205. [DOI] [PubMed] [Google Scholar]
- 20.Hershey AD, Winner P, Kabbouche MA, et al. Use of the ICHD-II Criteria in the Diagnosis of Pediatric Migraine. Headache: The Journal of Head and Face Pain. 2005;45(10):1288–1297. doi: 10.1111/j.1526-4610.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- 21.Hershey AD, Powers SW, Vockell AL, LeCates S, Kabbouche MA, Maynard MK. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001 Dec 11;57(11):2034–2039. doi: 10.1212/wnl.57.11.2034. [DOI] [PubMed] [Google Scholar]
- 22.McGrath PJ, Walco GA, Turk DC, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain. 2008 Sep;9(9):771–783. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Breivik EK, Bjornsson GA, Skovlund E. A comparison of pain rating scales by sampling from clinical trial data. Clin J Pain. 2000 Mar;16(1):22–28. doi: 10.1097/00002508-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011 Jun;41(6):1073–1093. doi: 10.1016/j.jpainsymman.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Varni J, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a Pedatric Population Health Measure: Feasibility, Reliability, and Validity. Ambulatory Pediatrics. 2003;6:329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001 Aug;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Kovas M. Children's Depression Inventory. Mental Health Systems; North Tonowanda, NY: 1992. [Google Scholar]
- 28.Gadow KD, Sprafkin J. Adolescent Symptom Inventory - 4: Norms Manual. Checkmate Plus; Stony Brook: 1998. [Google Scholar]
- 29.Kovacs M. Children's Depression Inventory. Available from Multi-Health systems, Inc.; 908 Niagara Falls Blvd., North Tonawanda, N.Y.: 1992. pp. 14120–2060. [Google Scholar]
- 30.Kashikar-Zuck S, Ting TV, Arnold LM, et al. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia: A multisite, single-blind, randomized, controlled clinical trial. Arthritis Rheum. 2012 Jan;64(1):297–305. doi: 10.1002/art.30644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashikar-Zuck S, Flowers SR, Verkamp E, et al. Actigraphy-based physical activity monitoring in adolescents with juvenile primary fibromyalgia syndrome. J Pain. 2010 Sep;11(9):885–893. doi: 10.1016/j.jpain.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephens S, Feldman BM, Bradley N, et al. Feasibility and effectiveness of an aerobic exercise program in children with fibromyalgia: results of a randomized controlled pilot trial. Arthritis Rheum. 2008 Oct 15;59(10):1399–1406. doi: 10.1002/art.24115. [DOI] [PubMed] [Google Scholar]
- 33.Kashikar-Zuck S, Myer G, Ting TV. Can behavioral treatments be enhanced by integrative neuromuscular training in the treatment of juvenile fibromyalgia? Pain Management. 2012;2(1):9–12. doi: 10.2217/pmt.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palermo TM, Eccleston C, Lewandowski AS, Williams ACdC, Morley S. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: An updated meta-analytic review. Pain. 2010;148(3):387–397. doi: 10.1016/j.pain.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


