Abstract
Problem
IL-22 has important functions at mucosal surfaces, including the induction of antimicrobial peptides and maintenance of epithelium. However IL-22 has not been investigated in the genital tract during TV infection.
Methods of Study
Women who visited an STD clinic and women from a cohort with frequent Trichomoniasis were studied. IL-22, IL-17 and antimicrobial peptides were measured in cervicovaginal lavage by ELISA.
Results
In women visiting the STD clinic, those without STDs (n=10) had a median IL-22 of 0 pg/ml while women with infections (n=30) had 27 pg/ml (p=0.04). In the cohort, women with Trichomoniasis (n=19) had significantly higher IL-22 than women with no infections (n=21, 74 versus 0 pg/ml, p=0.0001). IL-17 was also significantly increased in Trichomoniasis and there was a correlation between IL-22 and IL-17 (p=0.001).
Conclusion
IL-22 is increased in STDs generally and in Trichomoniasis specifically suggesting an antimicrobial response of the mucosa and an epithelial repair process induced by the STDs.
Keywords: STDs, Trichomoniasis, Genital Epithelium, Antimicrobial peptides, Cervicovaginal lavage
Introduction
IL-22 is a cytokine produced by innate and adaptive immune cells1. The IL-22 receptor is expressed on epithelial cells2 and IL-22 appears to promote epithelial homeostasis and barrier function. Many studies of IL-22 function have been carried out to identify its role in mucosal immunity of the gut. For example, during infection with Citrobacter rodentium, mice lacking IL-22 have increased intestinal epithelium damage and bacterial invasion. At least some of the effects of IL-22 in this model are due to IL-22 inducing production of anti-microbial peptides3. IL-22 is also involved in tissue repair and mucous secretion4. Although it has been suggested that IL-22 may function at all mucosal epithelial surfaces, there have been no studies to elucidate the role IL-22 plays in the lower female genital tract mucosa.
The female genital tract epithelium plays an important role in resistance to pathogens. It serves as a physical barrier to pathogen invasion and it has a layer of mucous that helps to trap pathogens5,6. Certain antimicrobial peptides are also produced by the lower genital tract epithelium7,8.
Some sexually transmitted pathogens cause damage to the epithelium. For example, in Chlamydia trachomatis infection, epithelial cells become infected and are targeted by adaptive and innate immune responses. These immune responses can lead to epithelium damage, fibrosis and scarring, especially in chronic infections or during re-infections9. Trichomonas vaginalis (TV) infection, while asymptomatic in many women, has been linked to severe health issues such as pre-term birth, low birth weight infants, increased risk of HIV acquisition and increased HIV shedding 10-12. TV is known to adhere to epithelial cells and secrete proteases to degrade the extracellular matrix 13-15. Some infected women present with inflammation of the vagina or cervix both, and includes hemorrhaging or reddening of the cervix, also known as strawberry cervix 16,17.
Since some STDs, including TV and Chlamydia, can cause damage to the epithelium, we hypothesized that IL-22 would be produced in response to these infections in order to maintain epithelial barrier integrity or induce antimicrobial peptide defenses. To investigate this hypothesis, IL-22 levels were measured in genital mucosal fluid from women visiting an STD clinic and from a cohort of women at high risk for HIV infection that had a high rate of TV infection.
Materials and Methods
STD Clinic Subjects
This group of subjects has been previously described18. Briefly, the subjects were recruited at the Ruth M. Rothstein CORE Center STD Screening Clinic of Cook County Stroger Hospital, Chicago, IL. Symptomatic women (n=40) between the ages of 18 and 54, presenting for an STD evaluation were approached for study participation and written informed consent was obtained. The largest proportion of subjects were between 18 and 24 years old (40%), while 33% were between 25 and 34, 18% were between 35 and 44 and 8% were between 45 and 54. The subjects were 90% African American, 7% Latino, and 3% Caucasian. The Institutional review boards of Rush University Medical Center and Cook County Stroger Hospital approved all studies. The control and case subjects were not age matched for this study. Samples were collected prior to treatment. Standard of care genital exams were performed, 3 swabs were taken for diagnosis before 10 mL of CVL was obtained by irrigating the cervix with saline. Urine was also obtained for a pregnancy test, none of the subjects were pregnant. The tests performed were BD Probetect ET (BD Franklin Lakes, NJ) for Chlamydia and GC, rapid plasma regain test (Arlington Scientific, Utah) for syphilis, enzyme immunoassay (Biorad Laboratories, Hercules CA) for HIV, wet mount examination and ELISA for p65 (HyTest, Turku, Finland) for TV. Amsel criteria (wet mount for clue cells, vaginal pH, whiff test) and Nugent gram stain were used to diagnose BV and a KOH preparation that was examined microscopically was used to diagnose Candida. A clinical exam was used to determine the presence of Pelvic Inflammatory Disease and/or Warts. The menstrual cycle of the participants at the time of collection was unknown. Visually there was no blood contamination and the Abacard test for prostate specific antigen (Abacus Diagnostics, West Hills, CA) was used to assess semen. There was no information on contraceptive methods used by the subjects.
Collection of samples from high-risk cohort
CVL samples were also collected from women who had visited an STD clinic and who are classified as having a high risk of HIV infection. The high-risk HIV seronegative group was enrolled at the University of Illinois at Chicago. They were women aged 18-45 years old who met at least 2 of the following criteria: crack use in the last 6 months; exchange of sex for money, drugs, or shelter in the last 6 months; at least 5 sexual partners in the last 6 months; history of an STD in the last year, and/or sexual relations with a known HIV positive man. Samples were collected prior to treatment. Swabs were collected for testing and CVL samples were collected by irrigation of the cervix with 10 mL of saline. An Aptima test (Hologic Gen-Probe Inc, San Diego, USA) was performed to diagnose Chlamydia and GC, BV was diagnosed using the Amsel criteria, slides were examined for Candida, and the OSOM/Genzyme Rapid test was used for Trichomonas. Subjects were also examined for blood and samples were not collected from patients with gross blood on examination. A pregnancy test was performed and a PSA test was also done to look for semen contamination. All CVL samples were stored at −80° C.
ELISA
ELISA kits for IL-22, IL-17, IL-6, IL-10, IP-10, sTNFR1, CXCL5 and MMP3 were obtained from R&D systems (Minneapolis MN). The manufacturer’s listed lower limits of detection are as follows; IL-22: 5.8 pg/mL, IL-17: 15 pg/mL, IL-6: 0.7 pg/mL, IL-10: 3.9 pg/mL, sTNFRI: 1.2 pg/ml, IP-10: 4.4 pg/ml, CXCL5: 15 pg/ml, MMP3: 9 pg/ml. The HBD2 ELISA was from Phoenix Pharmaceuticals (Burlingame CA) and the LL-37 ELISA was from Hycult Biotech (Plymouth Meeting PA) (detection limits for these were 7.8 pg/ml and 0.1 ng/ml repectively). Total protein in CVL samples was measured by Pierce BCA assay (Thermo Scientific, Rockford, IL). BioTek Synergy HT plate reader (BioTek Instruments, Inc, Winooski, VT) was used to read the protein assay and ELISA plates after development
Statistical Analysis
All statistical analysis on the data was performed using the Instat statistical software package (Graphpad Softward). Correlation between IL-22 and the other mediators was determined using the Spearman test. The Mann Whitney unpaired test was used to compare the medians of two groups. A “p” value of 0.05 or lower was considered significant. Samples with cytokine levels that were below the detection limit were assigned a 0 pg/ml value when performing statistical analysis. This resulted in the mean and median for IL-17 and IL-10 falling below the detection limit.
Results
Since some STDs are known to damage the genital epithelium, we measured IL-22 in genital secretions of women who had visited an STD clinic. Out of 40 subjects that were studied, 10 had no detectable infection or condition; 5 had only bacterial vaginosis, 4 had only TV, 4 had only Chlamydia, 4 had only gonorrhea, 8 had other infections and the 5 others had multiple infections. Only 7 out of 40 subjects were positive for PSA indicating the presence of semen in our test samples. Of these 7 none of them were TV positive and one of them had no STD or BV, 2 had BV, 2 had gonorrhea, one had both BV and gonorrhea and the other had cervicitis. IL-22 levels were significantly higher in women with STDs (median 27 pg/ml) compared to the no-STD group (median 0 pg/ml) (Table 1). Although the number of women with only TV or Chlamydia was small, levels of IL-22 were significantly higher in those two groups (Table 1).
Table I.
IL-22 levels in genital secretions from women who visited STD clinic
| Category | N | IL-22 Mean | IL-22 Median (Range) | P*** |
|---|---|---|---|---|
| No STD* | 10 | 6.5 | 0 (0 - 65) | |
| All STD** | 30 | 54 | 27 (0 - 250) | 0.04 |
| BV only | 5 | 31 | 0 (0 - 153) | |
| Chlamydia only | 4 | 103 | 82 (0 - 249) | 0.05 |
| Gonorrhea only | 4 | 54 | 47 (0 - 123) | |
| Trichomonas only | 4 | 108 | 112 (0 - 208) | 0.05 |
| Multiple STDs | 5 | 40 | 53 (0 - 93) |
No STDs, No Candidiasis nor BV
Includes women with BV, Candidiasis, individual STDs and multiple STDs
Mann Whitney Test Compared to women with No STD (2 tailed)
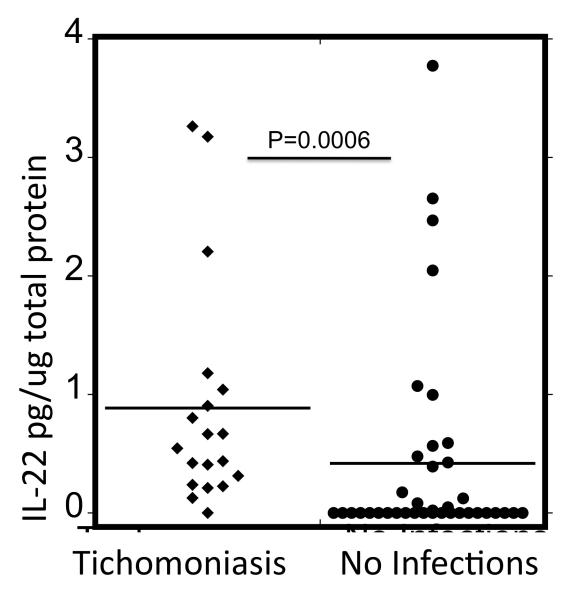
Since the level of IL-22 in samples from women with TV was higher than women without TV, we next evaluated IL-22 levels in CVL from a cohort of women at a high risk of HIV infection that had a high prevalence of TV. Of these 57 women, 19 of them were positive for TV infection only (no other STDs, bacterial vaginosis or Candida), while 38 of them tested negative for all infections as well as bacterial vaginosis and Candida. Levels of IL-22 in the TV positive women were significantly higher (median of 74 pg/ml) than in the women with no infections (median of 0 pg/ml, Table 2, p=0.0001 Mann Whitney Test). Levels of IL-22 in the two groups were also compared after adjusting for total protein levels in CVL. Total average protein levels for the TV negative and positive samples were 210 μg/mL, (median 50.57 μg/mL) and 188 μg/mL, (median= 135.75 μg/mL) respectively. IL-22 was significantly higher in the TV positive group when either unadjusted (Table 2) or adjusted for total protein (Fig.1). It is also interesting to note that there was not a significant difference in the level of IL-22 between the High Risk cohort control group and the STD clinic control group (median of 0 for both groups, p=0.117).
Table II.
Levels of Cytokines in TV positive and STD negative Subjects
| TV+ Subjects (n=19) |
STD- Subjects |
|||||
|---|---|---|---|---|---|---|
| Cytokine | Mean | Median (Range) | n | Mean | Median (Range) | P** |
|
|
|
|||||
| IL-22 | 72.6 | 74 (0-123) | 38 | 22.4 | 0 (0-110) | 0.0001 |
| IL-17 | 8.6 | 9.9 (0-25) | 38 | 1.62 | 0 (0-110) | 0.002 |
| IL-6 | 2.4 | 1.49 (0-7.58) | 38 | 4.98 | 0.8 (0-110) | 0.11 |
| IL-10 | 0.13 | 0.08 (0-0.46) | 38 | 0.14 | 0.02 (0-) | 0.55 |
| IP-10 | 23.7 | 15 (0-0.46) | 38 | 25.8 | 0 (0-361) | 0.44 |
| sTNFR1 | 159 | 137.6 (15-446) | 38 | 101.3 | 58 (0-606) | 0.015 |
| MMP3 | 85 | 24 (0-479) | 21 | 30 | 20 (0-188) | 0.48 |
| LL-37 | 4267 | 2222 (348-10422) | 21 | 1863 | 828 (32-7683) | 0.02 |
| CXCL-5 | 153 | 40(0-1664) | 21 | 159 | 18 (0-2100) | 0.53 |
| hβD-2 | 2565 | 2453 (1395-4131) | 21 | 2817 | 3074 (644- 4116) |
0.40 |
Mann Whitney Test (2 tailed)
All values are in pg/ml
Fig 1.
IL-22 Levels in Vaginal Secretions of HIV high-risk cohort women when adjusted for total protein. A Mann Whitney test was performed(2 tailed, non parametric), the lines represent the median values.
Since Th17 cells are one of the cell types known to secrete IL-22, we also tested the samples from the high-risk cohort for IL-17. IL-17 was significantly higher in the TV positive group (9.9 pg/ml) compared to the uninfected group (median 0 pg/ml, p=0.002, Table 2). This difference was also significant (p-0.004) when adjusted for protein levels (not shown). There was also a highly significant correlation between the IL-22 and IL-17 levels (Table 3).
Table III.
Correlation of IL-22 with other cytokines in High Risk women
| All Subjects |
|||
|---|---|---|---|
| Cytokine | N | Spearman r | P* |
| IL-17 | 65 | 0.39 | 0.001 |
| IL-6 | 66 | −0.06 | 0.63 |
| IL-10 | 66 | 0.10 | 0.42 |
| IP-10 | 66 | 0.09 | 0.46 |
| sTNFR1 | 66 | 0.17 | 0.18 |
| MMP3 | 40 | −0.12 | 0.47 |
| CXCL-5 | 40 | −0.09 | 0.57 |
| hβD-2 | 40 | −0.24 | 0.14 |
| LL-37 | 40 | 0.29 | 0.07 |
Spearman test (2 tailed)
IL-22 has been reported to induce certain antimicrobial peptides such as human ß2 defensin (HBD-2)19 which prompted us to determine whether levels of HBD-2 or the antimicrobial peptide LL-37 correlated with IL-22 levels in the high-risk cohort. Levels of HBD-2 were not increased in TV positive women compared to the women with no infections, and HBD-2 levels were not significantly correlated with IL-22 levels. However, elevated levels of LL-37 were detected in the TV positive subjects (p=0.02, Table 2), and there was a trend towards correlation (p=0.07) of LL-37 with IL-22 (Table 3).
Although studies have reported that IL-22 induces the production of some chemokines such as CXCL520 and matrix metalloproteinases like MMP321,22, we did not find any significant difference between the TV positive and TV negative groups of CXCL5 or MMP-3 (Table 2). There was also no significant correlation of these with IL-22 levels.
A significant difference in the levels of sTNFR1 was observed between the TV positive subjects and the TV negative subjects (p=0.015, Table 2), but there was no correlation between sTNFR1 and IL-22 levels. We also did not find any significant correlation between IL-22 and IL-6, IL-10, and IP-10.
Discussion
This study shows that IL-22 can be detected in lower female genital tract secretions of some women, and that IL-22 levels are increased during STDs in general and specifically in TV infection. To our knowledge, this is only the second study to show that IL-22 is expressed in the lower genital tract of women, but it is the first to show that it is elevated in TV infection. A previous study by Jha et al.23, showed that IL-22 is increased in acute Chlamydia trachomatis infection. That study showed levels of IL-22 that were higher than those detected in our study, even when comparing levels found in women in the control groups. Possible differences that could explain this difference include 1) The Jha study used 5 ml of diluent to collect CVL while our study used 10 ml; 2). Gram stain was used to diagnose Trichomonas in the Jha study which is likely less sensitive than the molecular methods used in our study; and 3) the ELISAs were from different manufacturers. Another study showed that T-lymphocytes that are capable of producing IL-22 are present in cervical tissue24. However, in that study, in vivo production of IL-22 was not assessed but T cells were isolated from the cervix and stimulated in vitro to induce IL-22 secretion.
We hypothesized that IL-22 would be expressed in the secretions from women with TV since TV as well as some other STDs, can cause epithelial damage13,15 and a number of studies show a protective role for IL-22 and its ability to increase epithelial barrier function. For example, IL-22 levels are elevated and IL-22 plays a protective role during murine Citrobacter rodentium3 infections that results in extensive damage to the intestinal epithelium. IL-22 is induced during lung epithelium damage and helps protect airway epithelial cells from apoptosis25. IL-22 has also been shown to be important for wound healing in an acute colitis model26 and protect against epithelial damage in a model of inflammatory bowel disease 27. A recent study shows IL-22 is also involved in containment of commensal bacteria28.
In this study, we measured both IL-17 and IL-22 in the high-risk cohort since both of these cytokines can be induced by similar stimuli and both can be made by T helper cell subsets. We observed that IL-17 was increased in secretions from women with TV infection and that IL-17 levels significantly correlated with IL-22. McKinnon et al24 showed that not only are T cells capable of producing IL-22 present in the genital tract of women, but that T cells capable of producing IL-17 are also found there. About 20% of the IL-17-producing T cells co-expressed IL-22 in that study, suggesting the possibility that Th17 cells could have produced some of the IL-22 present in women with STDs that we observed in the current study. However, innate lymphoid cells (ILCs) cannot be ruled out as the main producers of IL-22 in the genital tract. In the gut epithelium, ILCs are the major producers of IL-221,29. ILCs are of hematopoietic origin and include subsets of NK cells and Lymphoid Tissue Inducer (LTi) cells. ILCs also play major roles in lymphoid tissue formation as well as tissue remodeling29. In the STD clinic subjects, prostate specific antigen (PSA) was detected in 6 out of the 40 samples. One of the control subjects was PSA positive but none of TV positive subjects tested positive for PSA. The absence of PSA in the TV samples suggests that the higher levels of IL-22 seen in the TV positive women are not due to semen contamination.
We observed that human LL-37 (cathelicidin) was significantly increased in women who were TV positive compared to TV negative women. There was also a trend toward a correlation between the LL-37 and IL-22 levels, suggesting that the IL-22 might be inducing the elevated levels of LL-37. However, even though IL-22 is known to induce antimicrobial peptides such as beta defensins, Kanda et al.30 found that LL-37 at high concentrations induced the production of IL-22 by T-cells30. Peric et al demonstrated induction of LL-37 by IL-1731. Taken together, these studies along with our data suggest an alternative possibility that IL-17 produced during TV infection induced LL-37, which in turn induced expression of IL-22.
We observed that the levels of sTNFR1 were significantly higher in TV positive than in TV negative subjects. There are no reports that link IL-22 or TV with sTNFR1, but it has been observed that TV infection induces TNF-α production32,33. Studies also show that sTNFR1 is a strong antagonist of TNF-α34.It can bind to the pro-inflammatory cytokine and neutralize its effects35 or it can cause apoptosis of activated monocytes that produce TNFα at the site of infection36. The presence of elevated levels of sTNFR1 in the TV positive subjects could indicate a host response to neutralize inflammation present due to the presence of TV infection.
Surprisingly, we did not see any significant difference between hBD2 levels in TV-positive and -negative women, and further, hBD2 levels did not correlate with IL-22 levels. This was unexpected, since several studies have shown an induction of hBD2 by IL-22 as well as IL-22 inducing hBD2 cooperatively with IL-172,37. Since most of those studies focused on keratinocytes, it is possible the lack of correlation of IL-22 and hBD2 could be because lower genital tract epithelial cells respond differently to IL-22 than keratinocytes or that inhibitors of hBD2 induction are present in the lower female genital tract. It is also possible that IL-22 levels were too low to have an impact.
We also did not detect any significant difference in CXCL5 or MMP3 levels between TV-positive and -negative women. Several studies showed that MMP3 was strongly induced in human keratinocytes by IL-2221,22. IL-22 also induced increased CXCL5 production which in turn induced strong neutrophil chemotactic activity20. It is possible that other cytokines present in the genital tract are more potent inducers of MMP3 and CXCL5 and mask any effect of IL-22.
In summary, we found that IL-22 was increased in women with STDs in general and infection with Trichomonas specifically. Limitations of this study included a relatively small number of subjects, an absence of possibly relevent information on the subjects such as method of contraception and menstrual cycle of the subjects. However, based on previous studies, these results suggest a host response to TV infection and STDs that involves epithelial repair and secretion of anti-microbial peptides. Further dissection of these responses could help in understanding what factors are important for epithelial homeostasis in the lower female genital tract in response to STDs.
Acknowledgements
This work was supported by NIH grant P01 AI082971
REFERENCES
- 1.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 2.Wolk K, et al. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nature medicine. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 4.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annual review of immunology. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 5.Roberts L, Liebenberg L, Barnabas S, Passmore JA. Vaginal microbicides to prevent human immunodeficiency virus infection in women: Perspectives on the female genital tract, sexual maturity and mucosal inflammation. Best practice & research. Clinical obstetrics & gynaecology. 2012 doi: 10.1016/j.bpobgyn.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Quayle AJ. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. Journal of reproductive immunology. 2002;57:61–79. doi: 10.1016/s0165-0378(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 7.Hirbod T, Broliden K. Mucosal immune responses in the genital tract of HIV-1-exposed uninfected women. J Intern Med. 2007;262:44–58. doi: 10.1111/j.1365-2796.2007.01822.x. [DOI] [PubMed] [Google Scholar]
- 8.Frohm Nilsson M, et al. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infection and immunity. 1999;67:2561–2566. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darville T, Hiltke TJ. Pathogenesis of genital tract disease due to Chlamydia trachomatis. The Journal of infectious diseases. 2010;201(Suppl 2):S114–125. doi: 10.1086/652397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutton M, et al. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001-2004. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;45:1319–1326. doi: 10.1086/522532. [DOI] [PubMed] [Google Scholar]
- 11.Fichorova RN. Impact of T. vaginalis infection on innate immune responses and reproductive outcome. Journal of reproductive immunology. 2009;83:185–189. doi: 10.1016/j.jri.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kissinger P, et al. Trichomonas vaginalis treatment reduces vaginal HIV-1 shedding. Sex Transm Dis. 2009;36:11–16. doi: 10.1097/OLQ.0b013e318186decf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwebke JR, Burgess D. Trichomoniasis. Clin Microbiol Rev. 2004;17:794–803. doi: 10.1128/CMR.17.4.794-803.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav M, Dubey ML, Gupta I, Bhatti G, Malla N. Cysteine proteinase 30 in clinical isolates of T. vaginalis from symptomatic and asymptomatic infected women. Exp Parasitol. 2007;116:399–406. doi: 10.1016/j.exppara.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Lehker MW, Sweeney D. Trichomonad invasion of the mucous layer requires adhesins, mucinases, and motility. Sex Transm Infect. 1999;75:231–238. doi: 10.1136/sti.75.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehker MW, Alderete JF. Biology of trichomonosis. Curr Opin Infect Dis. 2000;13:37–45. doi: 10.1097/00001432-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Mirmonsef P, Krass L, Landay A, Spear GT. The Role of Bacterial Vaginosis and Trichomonas in HIV Transmission Across The Female Genital Tract. Curr HIV Res. 2012 doi: 10.2174/157016212800618165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spear GT, et al. Multiplex immunoassay of lower genital tract mucosal fluid from women attending an urban STD clinic shows broadly increased IL1ss and lactoferrin. PloS one. 2011;6:e19560. doi: 10.1371/journal.pone.0019560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubino SJ, Geddes K, Girardin SE. Innate IL-17 and IL-22 responses to enteric bacterial pathogens. Trends Immunol. 2012;33:112–118. doi: 10.1016/j.it.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Guilloteau K, et al. Skin Inflammation Induced by the Synergistic Action of IL-17A, IL-22, Oncostatin M, IL-1{alpha}, and TNF-{alpha} Recapitulates Some Features of Psoriasis. J Immunol. 2010 doi: 10.4049/jimmunol.0902464. [DOI] [PubMed] [Google Scholar]
- 21.Wolk K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 22.Boniface K, et al. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 23.Jha R, et al. Spontaneous secretion of interleukin-17 and -22 by human cervical cells in Chlamydia trachomatis infection. Microbes and infection / Institut Pasteur. 2011;13:167–178. doi: 10.1016/j.micinf.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 24.McKinnon LR, et al. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol. 2011;187:6032–6042. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- 25.Sonnenberg GF, et al. Pathological versus protective functions of IL-22 inairway inflammation are regulated by IL-17A. J Exp Med. 2010;207:1293–1305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickert G, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zenewicz LA, et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonnenberg GF, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 30.Kanda N, Hau CS, Tada Y, Sato S, Watanabe S. Decreased serum LL-37 and vitamin D3 levels in atopic dermatitis: relationship between IL-31 and oncostatin M. Allergy. 2012;67:804–812. doi: 10.1111/j.1398-9995.2012.02824.x. [DOI] [PubMed] [Google Scholar]
- 31.Peric M, et al. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J Immunol. 2008;181:8504–8512. doi: 10.4049/jimmunol.181.12.8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zariffard MR, et al. Trichomonas vaginalis infection activates cells through toll-like receptor 4. Clin Immunol. 2004;111:103–107. doi: 10.1016/j.clim.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Han IH, et al. Proinflammatory cytokine and nitric oxide production by human macrophages stimulated with Trichomonas vaginalis. Korean J Parasitol. 2009;47:205–212. doi: 10.3347/kjp.2009.47.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selinsky CL, Boroughs KL, Halsey WA, Jr., Howell MD. Multifaceted inhibition of anti-tumour immune mechanisms by soluble tumour necrosis factor receptor type I. Immunology. 1998;94:88–93. doi: 10.1046/j.1365-2567.1998.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallach D, et al. Soluble and cell surface receptors for tumor necrosis factor. Agents Actions Suppl. 1991;35:51–57. [PubMed] [Google Scholar]
- 36.Waetzig GH, et al. Soluble tumor necrosis factor (TNF) receptor-1 induces apoptosis via reverse TNF signaling and autocrine transforming growth factor-beta1. FASEB J. 2005;19:91–93. doi: 10.1096/fj.04-2073fje. [DOI] [PubMed] [Google Scholar]
- 37.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]