Abstract
Changes in memory performance are one of the hallmark symptoms of mild cognitive impairment and are affected by healthy aging as well. Pattern separation, which refers to the process of orthogonalizing overlapping inputs into distinct memory representations, may be a sensitive marker of these memory changes. Here, we describe a paradigm, the Behavioral Pattern Separation Task – Object Version (BPS-O task), which reveals age-related changes in pattern separation performance. Specifically, we report an age-related decline in pattern separation in healthy adults, ranging from ages 20–89. When we classify those individuals ages 60 and older into two groups, Aged Unimpaired (AU) and Aged Impaired (AI) based on their delayed word recall performance, we observe impairments in pattern separation performance in the Impaired group, but no overall impairment in recognition performance. In contrast, those individuals diagnosed with mild cognitive impairment demonstrate worse performance than age-matched controls in both pattern separation and recognition memory performance. Therefore, the BPS-O task provides a sensitive measure for observing changes in memory performance across the lifespan and may be useful for the early detection of memory impairments that may provide an early signal of later development to mild cognitive impairment.
Keywords: healthy aging, pattern separation, dentate gyrus (DG), MCI
Introduction
In recent years there has been growing evidence from both humans (Bakker, Kirwan, Miller, & Stark, 2008; Lacy, Yassa, Stark, Muftuler, & Stark, 2011) and rodents (Leutgeb, Leutgeb, Moser, & Moser, 2007; for review, see Yassa, & Stark, 2011) that a process known as pattern separation relies critically on the dentate gyrus (DG) of the hippocampus. Pattern separation refers to the orthogonalization of similar inputs into distinct, non-overlapping representations such that new memories can be stored rapidly without inducing large amounts of interference (McClelland, McNaughton, & O’Reilly, 1995; Norman, & O’Reilly, 2003). This rapid storage of arbitrary information is a critical component of episodic memory. Evidence from aging in humans (Yassa, Mattfeld, Stark, & Stark, 2011; Yassa, … Stark, 2010c; Stark, Yassa, & Stark, 2010) and rodents (Wilson, … Tanila, 2004; Wilson, Ikonen, Gallagher, Eichenbaum, & Tanila, 2005; Wilson, Gallagher, Eichenbaum, & Tanila, 2006) has suggested that paradigms designed to tax pattern separation can be used to investigate dentate gyrus function. For example, in humans, BOLD fMRI activity consistent with pattern separation has been observed in the DG and CA3 subfields of the hippocampus (Bakker, … Stark, 2008; Lacy, … Stark, 2011). This subfield-level activity changes in at least two different ways with age in a manner that predicts behavioral deficits in pattern separation. First, elevated BOLD fMRI response during performance of a pattern separation task has been noted in older adults (Yassa, … Stark, 2010c) and individuals with amnestic mild cognitive impairment (aMCI; Yassa, … Stark, 2010b). In both cases, increased activity is correlated with worse performance on the task, suggesting that it is a marker of network dysfunction rather than a compensatory response. This finding is consistent with electrophysiological recording in aged rodents with pattern separation impairments which show elevated firing rates in CA3 neurons (see Wilson, … Tanila, 2006 for review).
Both computational accounts and cross-species empirical studies of CA3 function have ascribed a process known as pattern completion to the recurrent collateral connections in this region which form an auto-associative network engaged during retrieval (Yassa, & Stark, 2011 for review). Pattern completion is the ability to retrieve a pre-existing representation when given a partial or degraded cue. This is a computational bias that opposes pattern separation and predisposes the hippocampal network to retrieval of prior memories rather than encoding new ones (Norman, & O’Reilly, 2003; Treves, & Rolls, 1992). The elevated firing rates in aged CA3 neurons as well as the increased BOLD fMRI signal in the same region may contribute to the behavioral propensity towards pattern completion as the expense of pattern separation, a feature that is typically observed in aging adults with memory impairment.
Moreover, a parametric investigation of pattern separation across varying levels of item similarity reveals a “representational rigidity” in the DG/CA3 BOLD fMRI signal in which a larger degree of dissimilarity is required for the network to properly encode the stimulus as a new item instead of treating it as a repetition. The extent of this rigidity is correlated with the integrity of the perforant path input to the DG/CA3 from the entorhinal cortex, measured using high-resolution diffusion tensor imaging, and is also correlated with functional connectivity between the entorhinal cortex and DG/CA3 measured using high-resolution fMRI (Yassa, … Stark, 2011). Critically, this rigidity is also the hallmark of CA3 neurons in aged rats with memory impairment (Wilson, … Tanila, 2005).
Thus, cross-species neural investigations, including electrophysiology in rats and brain imaging in humans, provide the impetus for using tasks designed to tax pattern separation to investigate dentate gyrus and CA3 function. These Behavioral Pattern Separation (BPS) tasks were developed in order to characterize the behavioral outcomes of neural pattern separation. While they are only inferential measures of pattern separation, the evidence above suggests that they are strongly tied to the neurobiology of this process, and can be used as a proxy to assess neurobiological changes in the course of aging and brain disease. For any such task to be useful in reflecting the changes that may be disproportionately associated with dentate gyrus function, it would need to demonstrate reliability across different groups of participants and across different labs administering the task. In this paper, we detail the history and reliability of such a task in humans and demonstrate its sensitivity at detecting age-related memory loss.
We have designed and established the reliability of a behavioral pattern separation task using pictures of everyday objects, coined the Behavioral Pattern Separation Task – Object Version (BPS-O). In this paper, we will review the existing data that demonstrate the utility of this task to assess pattern separation behavior and also present new data detailing a gradual change with age, as predicted by models of aging that stress changes occurring in the dentate gyrus. We also report an important dissociation in pattern separation performance and recognition in those individuals with mild cognitive impairment (MCI). Finally, we provide the information necessary to disseminate this task as a stand-alone application for use by other research groups, making it easy to use and apply to other populations.
The BPS-O Task (see Figure 1) consists of two phases. In the first phase, participants engage in an indoor/outdoor judgment for pictures of everyday objects. Immediately following this encoding task, participants are given instructions regarding a surprise recognition memory test in which they must identify each item as “Old”, “Similar”, or “New”. One-third of the images in the test phase are exact repetitions of images presented in the study phase (targets); one-third of the images are new images not previously seen (foils); and one-third of the images are perceptually similar to those seen during the study phase, but not identical (lures). We are particularly interested in the responses to these lure trials and the rates at which participants correctly identify these as “Similar”, avoiding the propensity to identify these as “Old”. Identifying these lure trials as “Old” (i.e., overgeneralization) is likely driven by pattern completion processes. In contrast, discriminating these lure trials from the similar study item requires a distinct representation of the objects – a hallmark of pattern separation. Typically, we assess pattern separation performance by calculating the ratio of “Similar” responses given to the lure items minus the “Similar” responses given to the foils to account for any bias the participant has in using the “Similar” response overall. We term this ratio the BPS score. Thus, if a participant has poor pattern separation performance, their BPS score will be low because they have made fewer “Similar” responses to lure trials (i.e., typically making more “Old” responses).
Figure 1.
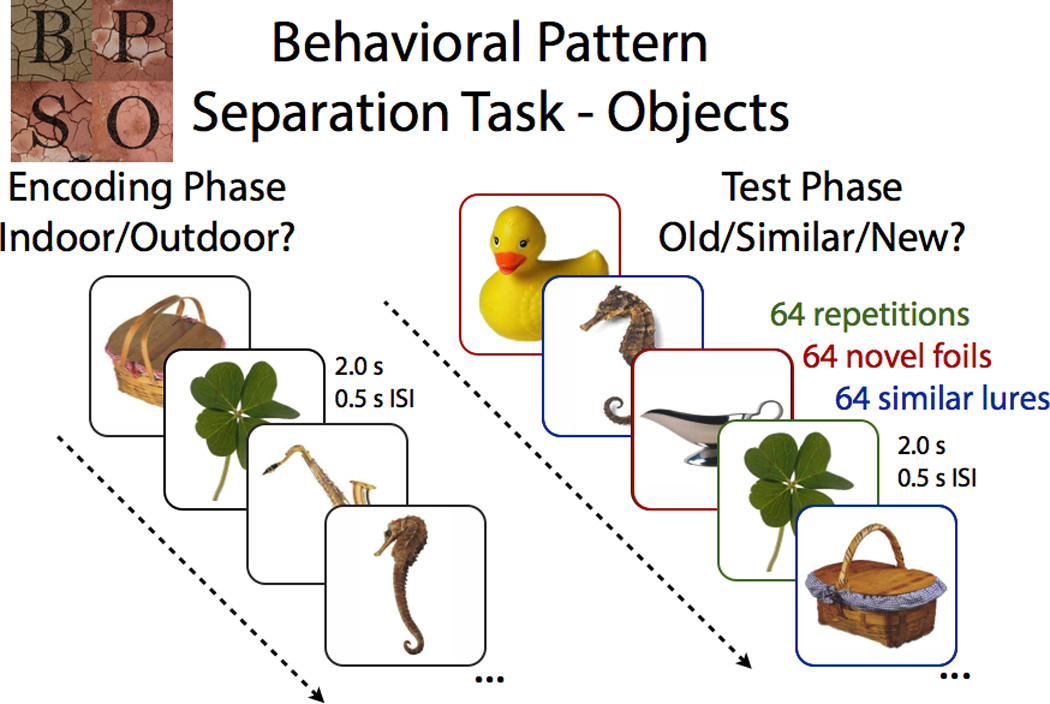
Participants encoded a series of pictures, followed by a surprise recognition test, which contained exact repetitions (identical to those in the encoding task), novel foils (completely new items), and similar lures (related, but not identical, to those in the encoding task).
Using high-resolution fMRI, we have demonstrated activity in the CA3/DG regions of the hippocampus that are consistent with strong pattern separation using the incidental encoding version of the BPS-O task (Bakker, … Stark, 2008; Lacy, … Stark, 2011) and an explicit recognition version of the task (Kirwan, Jones, Miller, & Stark, 2007). These studies also reported activity in the CA1 field of the hippocampus consistent with pattern completion processes. Likewise, using a match/mismatch task, Kumaran and Maguire (2007) reported hippocampal activity when novel items overlap significantly with stored representations, which was confined to CA1 when measured with high-resolution fMRI (Chen, Olsen, Preston, Glover, & Wagner, 2011). While high-resolution imaging studies are not able to distinguish between activity for CA3 and dentate gyrus independently, models of pattern separation processes are attributed to the dentate gyrus.
Several studies have previously demonstrated that older adults have a significantly lower BPS scores relative to young adults (Toner, Pirogovsky, Kirwan, & Gilbert, 2009; Yassa, … Stark, 2011). These data emphasize the utility of this task for assessing changes in memory performance in healthy aging. Similarly, Yassa, Stark et al. (2010c) reported lower BPS score for individuals with MCI compared with healthy aging individuals. Amnestic MCI is a diagnostic entity that is often a preclinical phase of Alzheimer’s disease and is characterized by an impairment in memory functioning (Peterson, … Kokmen, 1999). In addition to this behavioral disparity between MCI and healthy age-matched controls, MCI patients exhibited an elevated BOLD fMRI response in the CA3/DG subfields of the hippocampus. Activity in these same regions has been correlated with the BPS-O score in both older and younger adults as well (Lacy, … Stark, 2011; Yassa, … Stark, 2011).
In both the rodent and human studies, the effect of aging on hippocampal function and memory performance has typically been assessed by using two endpoints: younger adults (20–30 years old) and older adults (60–80 years old). While there are clear differences between these two groups, this approach is not able to address the time-course of these changes. Understanding how performance changes across the lifespan and how the time-course is related to the time-course of other behavioral or neurobiological changes will give us a far better understanding of the mechanisms upon which the task draws. In addition, a wealth of evidence from a well-established rodent model of aging suggests that there are individual differences in neurocognitive aging that argue for dividing the aging group into aged impaired and unimpaired groups. (Gallagher, … Wilson, 2006). Recent evidence from our research group further suggests that this approach is also fruitful in humans (Stark, … Stark, 2010). Are these individual differences in memory performance present earlier in the lifespan or only identifiable later in life? How does memory performance differ in those individuals with mild cognitive impairment? In particular, how does behavioral pattern separation change as a function of age and in the context of cognitive impairment?
Materials and Methods
To address these questions, 98 healthy, cognitively intact adults, ranging in age from 20–89 years of age, performed the BPS-O. We divided participants into 4 groups: 20–39 years of age (N = 26, mean age = 26.9, 10 Males), 40–59 years of age (N = 20, mean age = 50.8, 8 Males), 60–75 years of age (N = 29, mean age = 67.5, 10 Males), and 75–89 years of age (N = 18, mean age = 78.1, 8 Males). Participants were tested to insure normal performance on measures of general cognition: Mini Mental State Exam (Crum, Anthony, Bassett, & Folstein, 1993); memory: Rey Auditory Verbal Learning Test (Rey, 1941) and Wechsler Memory Scale Logical Memory (Wechsler, 1997b); executive functioning: Trails A and B (Tombaugh, 2004), Verbal Fluency (Tombaugh, Kozak, & Rees, 1999) and Letter Number Sequencing (Wechsler, 1997a); working memory: Digit Span (Wechsler, 1997a); and general intelligence: Wechsler Adult Intelligence Score III (Wechsler, 1997a). Mean score, standard deviation, and z-score compared to age-matched norms are presented in Table 1. All cognitively intact adults scored within 1.5 standard deviations of the mean of their age group for all neuropsychological measures.
Table 1.
| Neuropsychological Test | 20–39 | 40–59 | 60–74 | 75–89 | MCI |
|---|---|---|---|---|---|
| MMSE | 29.4 (0.8, 1.9) | 29.3 (0.8, .19) | 28.8 (1.2, .09) | 28.7 (1.2, 1.1) | 27.2 (2.2, −.63) |
| RAVLT Total | 55.5 (8.4, .05) | 55.4 (8.4, .72) | 50.8 (9.2, .96) | 50.9 (8.5, 1.8) | 30.5 (5.3, −1.3) |
| RAVLT Immediate | 12.2 (2.0, .32) | 12.1 (3.0, .70) | 10.8 (2.7, .52) | 10.9 (2.6, .95) | 3.4 (2.8, −1.7) |
| RAVLT Delay | 12.2 (1.2, .38) | 11.8 (2.9, .60) | 10.4 (3.4, .53) | 10.1 (2.5, 1.3) | 3.3 (2.8, −1.7) |
| WMS LM Immediate | 51.1 (8.3, .97) | 43.3 (6.8, .39) | 48.1 (8.7, 1.3) | 46.8 (10.5, 1.4) | 33.1 (10.0, .03) |
| WMS LM Delayed | 34.0 (5.5, 1.14) | 28.7 (6.9, .91) | 30.0 (7.0, 1.4) | 29.2 (7.5, 1.7) | 16.6 (8.8, −.08) |
| Digit Span | 18.4 (3.6, .22) | 19.0 (3.8, .56) | 17.1 (3.1, .38) | 18.6 (5.1, .94) | 16.4 (4.2, .30) |
| L-N Sequencing | 10.9 (2.0, 0) | 11.0 (2.3, .43) | 10.0 (1.8, 0) | 9.6 (2.5, .69) | 7.2 (2.0, −.30) |
| Trails A | 18.8 (4.8, .62) | 22.1 (5.6, .80) | 25.2 (5.7, 1.1) | 30.1 (8.1, 1.1) | 36.2 (13.6, .17) |
| Trails B | 43.6 (10.8, .49) | 51.1 (16.8, .65) | 64.1 (19.5, .24) | 68.3 (13.3, 1.0) | 105 (27, −1.9) |
| Verbal Fluency | 45.0 (8.2, .03) | 44.2 (9.6, −.04) | 44.0 (12.3, .17) | 49.3 (11.1, 1.1) | 34.3 (9.2, −.44) |
| Category Fluency | 22.9 (4.1, .18) | 22.4 (3.9, .10) | 21.7 (4.4, .83) | 19.6 (4.8, .76) | 12.8 (4.9, −1.1) |
| WAIS III IQ | 113.9 (8.9, .92) | 118.4 (9.2, 1.2) | 118.5 (9.3, 1.2) | 117.9 (9.9, 1.2) | 107 (10.8, .49) |
Scores on standardized neuropsychological testing: mean score (standard deviation, z-score from standardized mean). Scores in bold are greater than one standard deviation from age-normed means. Mini Mental State Examination (MMSE), Rey Auditory Verbal Learning Test (RAVLT), Wechsler Memory Scale Logical Memory (WMS LM), Letter-Number Sequencing (L-N), Wechsler Adult Intelligence Scale 3rd Edition (WAIS III) Intelligence Quotient (IQ).
In addition, 11 individuals with amnestic mild cognitive impairment (MCI) also performed the BPS-O and neuropsychological testing (mean age = 74.4, 6 Males). All individuals were diagnosed with mild cognitive impairment with memory deficits by the UCI Alzheimer’s Disease Research Center. The Clinical Dementia Rating (CDR) scale (Hughes, Berg, Danziger, Coben, & Martin, 1982) was used to assess function in daily life and to assign a CDR rating reflecting overall functional capacity. All MCI patients had CDR scores of 0.5. Diagnosis of MCI was based on the criteria proposed by Peterson (Petersen, … Kokmen, 1999), which include a memory complaint (corroborated by an informant), impaired memory function on testing (e.g. 1.5 standard deviations below norm), otherwise preserved cognitive functioning (e.g. within 1 standard deviation of norm), no decline in basic activities of daily living, and no dementia. Final amnestic MCI diagnoses were reached by clinical consensus conferences within the ADRC. Our exclusion criteria included major neurological or psychiatric disorders, head trauma with loss of consciousness, and history of drug abuse or dependency.
The BPS-O (Figure 1) consisted of a series of 192 color photographs of everyday objects on a white background. In the first phase, participants engaged in an indoor/outdoor judgment for each picture via a button press (128 items total, 2s each, 0.5s ISI). Immediately following the encoding task, participants were given instructions regarding a surprise recognition memory test in which they identified each item as “Old”, “Similar”, or “New” via button press (192 items total – 64 repeated items, 64 lure items, and 64 foil items; 2s each, 0.5s ISI). One-third of the images in the test phase were exact repetitions of images presented in the study phase (targets); one-third of the images were new images not previously seen (foils); and one-third of the images were similar to the those seen during the study phase, but not identical (lures). Behavioral pattern separation performance (BPS score) was calculated as the difference between the rate of “Similar” responses given to the lure items minus “Similar” responses given to the foils (to correct for response biases).
Results
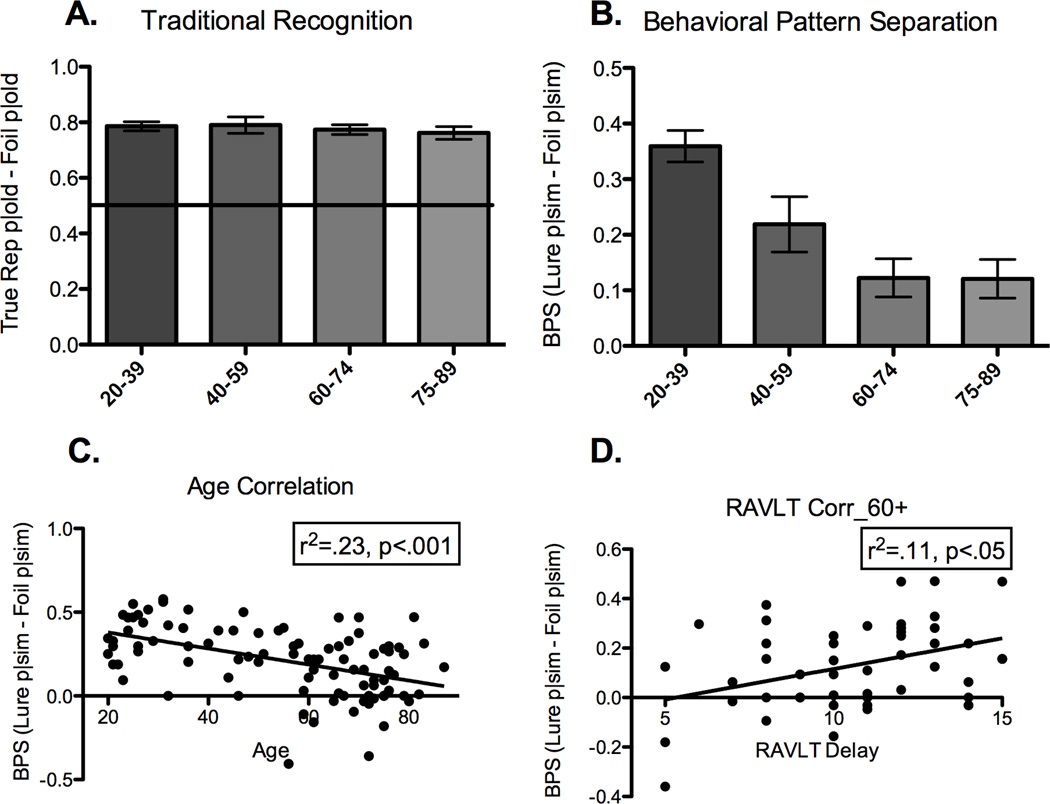
Response proportions for each stimulus and response type are presented in Table 2. In addition to assessing behavioral pattern separation, the BPS-O task provides a measure of recognition memory performance as well. Traditional recognition memory performance for healthy individuals was operationalized as the percent of targets endorsed as “Old” minus the percent of foils endorsed as “Old” (Hits minus false alarms; Figure 2A). There were no differences among the healthy groups when recognition memory scores were entered into a one-way ANOVA (p=.81). This traditional measure of recognition memory does not particularly tax pattern separation, nor does it differentiate among age groups. Therefore, we calculated the BPS score (Figure 2B) to provide a more sensitive measure of memory performance that may be able to evaluate age-related changes. A one-way ANOVA revealed a significant effect of age group (F(3,92) = 10.61, p<.0001) with a prominent linear trend (r2 = .20, p<.0001). These data indicate a gradual transition in pattern separation performance for healthy individuals across the lifespan. In support of this hypothesis, we found a negative correlation between age and BPS score (r2 = .23, p<.001; Figure 2C). We chose to bin the ages in such a way that the 60+ participants could be divided into two groups with sufficient power to detect any differences in performance. Binning them by decade (20s, 30s, 40s, etc.) did not alter the pattern of the data in any way. While pattern separation performance appears to plateau at age 60 and not continue to decline, the variability in this age group can be at least partially accounted for by other cognitive factors (see below).
Table 2.
| Targets | Lures | Foils | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age Group | Old | Similar | New | Old | Similar | New | Old | Similar | New |
| 20–39 | 81.2% (8.8) |
11.7% (6.2) |
4.1% (3.8) |
40.3% (14.5) |
47.6% (15.4) |
8.3% (8.0) |
2.6% (2.8) |
11.6% (6.4) |
82.0% (8.4) |
| 40–59 | 81.6% (12.8) |
10.4% (6.6) |
4.3% (4.5) |
48.2% (16.0) |
39.2% (16.0) |
8.1% (7.2) |
2.9% (3.7) |
15.7% (11.7) |
76.1% (15.4) |
| 60–74 | 82.2% (9.1) |
7.6% (5.3) |
5.3% (3.9) |
54.6% (17.2) |
23.6% (14.9) |
12.3% (11.1) |
4.8% (4.9) |
12.3% (11.1) |
73.4% (18.8) |
| 75–89 | 80.0% (10.6) |
7.0% (6.9) |
9.2% (7.6) |
59.4% (14.8) |
22.5% (18.6) |
13.6% (9.9) |
6.6% (6.5) |
10.9% (11.7) |
76.6% (14.0) |
| MCI | 75.9% (21.2) |
8.7% (12.4) |
16% (12.3) |
64.9% (18.5) |
12.5% (11.6) |
22.7% (13.8) |
15.9% (12.9) |
8.7% (10.8) |
75.4% (14.0) |
Percent endorsed for each group and each stimulus and response type (standard deviations below). Note the shift in old and similar responses to lures across the aging spectrum, extending to the MCI group.
Figure 2.
There is no effect of age on recognition performance (old responses to true repetitions – old responses to foils) (2A). In contrast, there is a linear decrease across age groups for pattern separation performance (similar responses to lures – similar responses to foils) (2B). This linear decrease can also be characterized by a negative correlation between pattern separation score and age (2C). For older individuals (ages 60–89), there is a positive relationship between pattern separation performance and delayed word recall on the RAVLT (2D).
As a follow-up set of analyses, we ran one-way ANOVAs on each response type×condition separately (i.e., rates of calling repetitions “old”, calling them “similar”, etc.). The only analyses that showed significant effects of age were on the rates of calling lure items “old” (F(3,92) = 5.6, p<.01) and on calling lure items “similar” (F(3,92 = 12.1, p<.01). No other age-related differences were found, even without correcting for Type I errors (all p’s>.18). The rate of calling lure items “similar” is used to calculate the BPS score (along with the base rate of calling foil items “similar”). The main effect of age here is indicative of a decrease in pattern separation behavior and the increase in calling these items “old” is an almost necessary mirror response. Older participants do not believe these items to be new, but rather are more likely to mistakenly believe them to be “old”.
We have previously reported several correlations between measures of pattern separation or the integrity of the CA3/DG network in aged individuals and the RAVLT delayed word list recall score. For older individuals, the BPS-O correlates with the RAVLT (Stark, … Stark, 2010). Furthermore, the integrity of the perforant path (the input to the CA3 and DG from the entorhinal cortex) correlates with the RAVLT (Yassa, Muftuler, & Stark, 2010b) and a measure of the BOLD functional signal linked to pattern separation correlates with all three of these (Yassa, … Stark, 2011). By stressing rapid learning and, in particular, learning that must be resistant to interference (the “List B” presented for study after the target list), the RAVLT has the hallmarks of a task that taxes pattern separation (Norman, & O’Reilly, 2003; Yassa, & Stark, 2011).
To further explore the relationship between the BPS score and the RAVLT, we restricted our analysis to those individuals ages 60 and above and evaluated the relationship between pattern separation performance and the RAVLT delayed recall. Consistent with our previous findings, we found a positive correlation, such that better BPS sores are associated with higher RAVLT scores (r2 = .11, p<.05; Figure 2D). In fact, we observed a correlation between pattern separation in the BPS-O task and age across all ages (r2= .22, p<.001), as well as between the BPS-O task and RAVLT delay for all ages (r2 = .11, p<.01). There is also a correlation between RAVLT delay and age (r2= .14, p<.001), however it only explains about half as much variance as the correlation between BPS-O performance and age. BPS-O performance is also correlated with several other neuropsychological measures. Here, we found positive correlations between the BPS-O task and other measures of memory performance for those individuals older than 60 years old, including the RAVLT total score (the sum of the 5 encoding trials), the RAVLT immediate recall, and the Logical Memory paragraph immediate and delayed recall (all p’s<.05). These correlations are not surprising since all of these measures use memory tasks that ostensibly tap into pattern separation. By providing a more direct measure of pattern separation ability and by simultaneously assessing traditional recognition memory ability, the BPS-O task may be a more sensitive assay for detecting age-related changes in memory.
We sought to explore these individual differences further and extend our previous findings by dividing the 60+ age group into two groups based on their RAVLT delayed word list recall scores, akin to what has been done in the rodent literature using the water maze (Wilson, … Tanila, 2006 for review). Participants were ranked by RAVLT score and split into thirds. The top third recalled 12–15 of the 15 words on the delayed RAVLT test and constituted the “Aged-Unimpaired (AU)” group (N=19). The bottom third recalled 5–8 words on the RAVLT test and constituted the Impaired group (N=12). While we have labeled this group as “Aged-Impaired (AI)”, it is important to note that these individuals are not clinically impaired as these scores fall within the normal range for this age group. However, we have used this metric previously (Stark, … Stark, 2010) and it is consistent with the rodent aging model (e.g., Gallagher, … Wilson, 2006) that identifies a subset of healthy aging as similar to the young (Unimpaired) and a subset with significantly worse memory performance (Impaired).
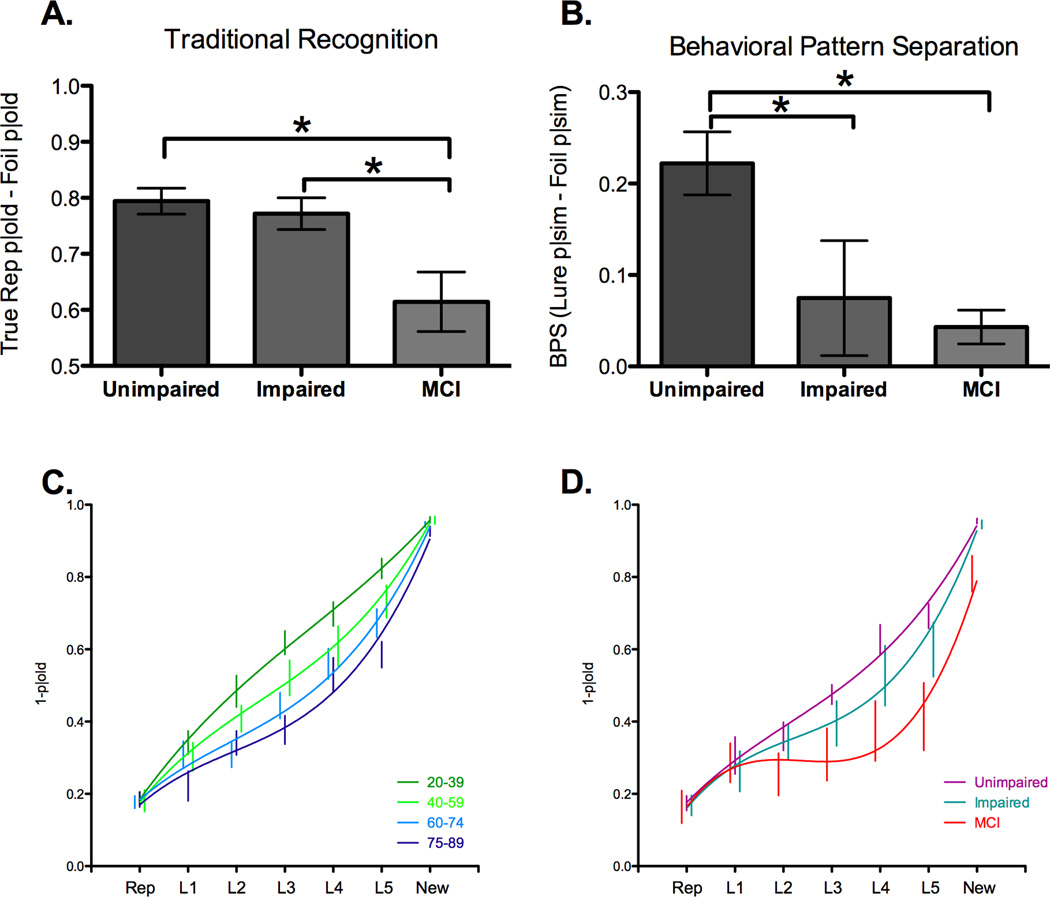
We then compared these two groups to the MCI group to determine how traditional recognition and pattern separation are affected by amnestic MCI. Consistent with their memory-impairment diagnosis, a one-way ANOVA on traditional recognition memory scores detected significant differences among the three groups, (F(2, 40) = 5.88, p<.01). Paired t-tests revealed that the MCI group (61%) was impaired relative to the Unimpaired group (79%; t(29) = 3.08, p<.01) and the Impaired group (77%; t(22) = 2.24, p<.05; Figure 3A). In contrast, there was no evidence for a recognition memory difference between the Impaired and Unimpaired groups (p=.61). A one-way ANOVA confirmed differences among the three groups in their BPS scores, our assay of pattern separation (F(2,40) = 5.64, p<.05; Figure 3B). Paired t-tests revealed impaired pattern separation performance for the MCI group (4%) compared to the Unimpaired group (22%; t(29) = 3.75, p<.001). In contrast to their recognition performance, the Impaired group (7%) performed significantly worse than the Unimpaired group on pattern separation (t(29) = 2.23, p<.05), with no difference between the MCI and Impaired groups (p=.71). Thus, while the MCI group was impaired on both traditional measures of recognition and pattern separation performance, the Impaired group is selectively impaired on this measure of pattern separation.
Figure 3.
We split the healthy older individuals into two groups: the aged unimpaired group has RAVLT scores of 12–15 and the impaired group has scores of 5–8. While there is no difference in recognition performance between the two healthy aged groups, the MCI group has a significant decrease in recognition memory (3A). In contrast, pattern separation performance is impaired for both the aged impaired and MCI groups compared to the unimpaired group (3B). Ranking the lure bins by degree of similarity (L1 – most similarity; L5 – least similarity) revealed a main effect of age group (3C) and a group effect for the unimpaired, impaired, and MCI groups (3D).
The BPS-O not only gives a basic measure of pattern separation performance as described above, but it also measures the behavioral response to varying degrees of change in the input (varying amounts of dissimilarity). In previous development of the task, we rank-ordered the lures by level of mnemonic similarity to the targets by assessing the probability of producing false alarms to the lures (calling them “old”) for each lure item using a large, independent population of young participants (Yassa, … Stark, 2010c). In that study, the stimuli were divided into 5 lures bins, with the more mnemonically similar lures in lure bin 1 (L1) and the least mnemonically similar lure items in lure bin 5 (L5). The same assignment of lure stimuli to lure bins was used here. To evaluate the data from this range of ages, we calculated a pattern separation metric, 1 minus the percent of “old” responses, for each condition (repetitions, lure bins 1–5, and new items). This metric allows us to evaluate the tuning curve of these responses to emphasize pattern separation instead of pattern completion.
The behavioral tuning curves as a function of age are shown in Figure 3C for healthy individuals. Note, that the normative procedure used will virtually ensure a linear trend in younger individuals (as a different group of younger individuals was used to derive the x-axis). Here, we can see a clear shift in the tuning curve as a function of age, with greater changes in the input (higher lure bin numbers) needed to produce a change in the output (successfully inhibiting the propensity to erroneously call the lures “old”). Performance on repetition and new trials was matched across age, but performance on the lures varied systematically across the aging spectrum. To create a simple measure that would be sensitive to different shapes of the curve while weighing each point consistently, we began by regressing out the average linear response (thus turning each graph in Figure 3C into horizontal lines or U-shaped curves). With this component removed, we calculated a simple area under the curve (AUC) across all seven conditions for each participant. A one-way ANOVA on the AUC revealed an effect of age group (F(3,88) = 6.80, p<.01) that followed a linear trend (r2 = .17, p<.001). This pattern was repeated and amplified when we examined the MCI participants relative to the 60+ individuals separated into Impaired and Unimpaired groups (Figure 3D) where there was a clear difference across groups (F(2,40) = 3.83, p<.05). As we moved from the Unimpaired to the Impaired and then MCI, there was a clear linear decline in the AUC (r2 = .16, p<.01).
In an effort to limit differences in strategy that might be employed by the younger and older groups, we designed both the study and test phases to be speeded (2 seconds to view the picture and make a response). However, the older age groups did have an increased number of missed trials due to this speeded response window. A one-way ANOVA revealed a significant effect of age group (F(3,92) = 3.4, p<.05), with the average number of trials missed increasing with age (20–39, 6.5%; 40–59, 8.4%; 60–74, 15.4%; 75–89, 13.3%). In addition, the MCI group had significantly more missed trials than every other age group (37.2%, all p’s<.05). Given this increase in the number of missed trials, it is possible that the results for the MCI group could show an artificially reduced BPS score. If they did not respond in time for “similar” responses to lure items differentially, we might induce such an effect. An investigation of the number of trials missed for each condition for the MCI group revealed a comparable number of missed trials for lure and new conditions (an average of 12 and 14 respectively; paired t(9) = .82, p=.43). Likewise, there was only a small (and unreliable) increase in reaction time for the similar responses to the lure items relative to the similar responses to new items (147 ms, p=.33). Therefore, while the MCI group missed more trials than the healthy aging groups, we do not have reason to believe that this would have substantially affected the BPS metric.
Discussion
Pattern separation is often thought to be a vital component of episodic memory and other complex, multi-dimensional forms of memory that rely upon the hippocampus (Norman, & O’Reilly, 2003; Yassa, & Stark, 2011). Previous research has identified age-related changes in pattern separation performance for individuals aged 60 and older (Toner, … Gilbert, 2009; Yassa, … Stark, 2010c; Yassa, … Stark, 2011). The data presented here expand these results by demonstrating that pattern separation performance linearly declines with increasing age. In contrast, our traditional measure of recognition memory did not change across the aging spectrum. When we divided older participants (ages 60–89) into those who are Impaired and Unimpaired based on how their delayed word-list recall compares to young adults, an interesting pattern emerged. Although AI adults are clinically normal and do not present with memory complaints, their pattern separation performance was impaired relative to young adults and AU adults. However, recognition performance was intact in both AU and AI individuals. MCI individuals on the other hand were impaired on both pattern separation and recognition, likely owing to the more substantial neurological and cognitive deficits associated with MCI.
Traditional recognition memory remained consistent across the aging spectrum in this paradigm. Additionally, no differences were observed in the 60+ AU and AI groups. This finding is in contrast to recall measures of memory, such as word recall, story recall, and paired-associate learning of words, all of which exhibit a linear decrease across age (Salthouse, 2009). It is quite likely that, like many tests of recognition memory, old/new recognition scores from the BPS-O task relies more on gist and familiarity than processes such as recollection, and thus may be spared with aging. Consistent with this account, prior literature has demonstrated that aging is associated with a shift toward gist and familiarity-based memory and a loss of encoding details and recollection-based memory (e.g., Ferguson, Hashtroudi, & Johnson, 1992; Koutstaal, Schacter, Galluccio, & Stofer, 1999). Likewise, amnesic patients with hippocampal damage also demonstrate this dissociation, with intact traditional recognition performance, but an impairment in pattern separation behavior as indexed by the BPS (Kirwan, … Stark, 2012; see Holdstock, … Norman, 2002 for similar findings using a related task).
So what makes the BPS score special or different from other measures of memory performance in aging? First, the BPS-O task provides us with two simultaneous measures of memory (which we believe tap into different underlying memory processes): a recognition memory measure and a pattern separation measure. As our data have indicated, the pattern of behavior across these measures differs for those who have been categorized as impaired or unimpaired, and different too from those who have been diagnosed as MCI. Second, the BPS-O task is specifically designed to tax pattern separation. Thus, the BPS score captures more of the age-related variance than other neuropsychological measures and has a tighter correlation than the RAVLT with the perforant path (Yassa, … Stark, 2011). While these points argue for the BPS being a more sensitive measure to changes associated with age, the goal is not to predict age per se, but changes in underlying neural function. Future research will be necessary to determine if it better correlates with the rate of decline or future diagnosis of MCI and other neural measures of age-related change, like perforant path integrity and BOLD fMRI signal.
Models of hippocampal aging have identified several changes occurring within the hippocampal circuit that could contribute to the differences in pattern separation performance of older adults.Wilson et al. (2006) proposed a model of hippocampal aging that emphasized reduced connectivity from the entorhinal cortex to the hippocampus via the perforant path, possibly due to synapse loss (Geinisman, deToledo-Morrell, Morrell, Persina, & Rossi, 1992). Consistent with this rodent data, Yassa, Muftuler et al. (2010b) reported reduced perforant path integrity in older adults compared to younger adults using ultrahigh-resolution microstructural diffusion tensor imaging (DTI). Likewise, this perforant path diffusion signal correlates with pattern separation performance on the BPS-O for aging individuals (Yassa, & Stark, 2011).Wilson et al. (2006) also argue that reduced inhibitory drive in the CA3 region may lead to rigidity in place cell firing patterns resulting in an increased propensity for pattern completion in the network. Yassa et al. (2011) documented evidence for similar representational rigidity in the CA3 and DG subfields in humans using high-resolution BOLD fMRI. Although the mechanism for this representational rigidity cannot be determined from these data, the degree of rigidity correlated with pattern separation performance on the BPS-O task. Thus, the BPS-O has demonstrated strong links to two different neurobiological features of the aging hippocampal network: (1) representational rigidity in the DG/CA3, and (2) perforant path integrity. Coupled with its strong correlations with an orthogonal test of hippocampal memory, the RAVLT, this demonstrates a powerful brainbehavior relationship that can be used to better understand how this network changes with age and disease.
In addition to documenting pattern separation impairments with age, we also found that pattern separation performance is impaired in amnestic MCI. However, unlike healthy aging, this change was also coupled with impaired recognition memory performance for targets. It is clear that the nature of the memory deficit in MCI is much more complex than in healthy aging, and it is quite likely that some of these changes in memory performance may be the result of non-hippocampal deficits (e.g., possibly changes in parahippocampal cortices as well as the frontal lobes). The sensitivity of this task may be more critical for identifying risk in what we have characterized as our Aged Impaired group. This group of individuals falls within the normal range on neuropsychological tests of memory, yet they demonstrate reliably lower pattern separation performance on the BPS-O. They do not demonstrate the recognition memory impairments found in the MCI group, leading to a clear dissociation. Follow-up studies will be critical to determine whether those same participants are more likely to develop cognitive impairment. Early detection of these individuals is paramount for many therapeutic approaches to be effective, emphasizing the importance of identifying them as soon as possible.
The BPS-O has strong potential for application to clinical populations and as an outcome measure in intervention trials. A recent clinical trial in amnestic MCI (Bakker, … Gallagher, 2012) used the BPS-O as an outcome measure and documented improvement in pattern separation performance with two-week exposure to a low-dose antiepileptic medication (Levetiracetam). These findings highlight the usefulness of using the BPS-O as a sensitive measure for detecting behavioral changes for investigational drugs aimed at improving memory.
Elsewhere, we have conducted a number of control experiments to ascertain that the task preferentially taxes mnemonic pattern separation processes and not other cognitive capacities. Across different stimulus sets, institutions, variations on the task instructions, response type (old/new vs. old/similar/new), and format (study-test vs. continuous), the BPS-O has remained remarkably consistent. For example, we established that participants have the ability to perceptually distinguish even highly similar lures from their related targets using a working memory version of the task (Lacy, … Stark, 2011) and that this is unaffected by age (Yassa, … Stark, 2010c). If older participants were merely naming the objects during encoding instead of encoding the details, then we would expect more “old” responses and fewer “similar” responses in the working memory version of the task, but that is not the case. While it is possible that there are different strategies applied during encoding, these age-related changes cannot be simply due to group differences in vision, working memory, attention or motivation, or strategy use. In addition, participant confidence did not alter pattern separation behavior, with similar confidence ratings for “old” and “similar” responses, indicating that “old” responses to lures do not merely reflect reduced confidence in memory for those targets (Lacy, … Stark, 2011).
In summary, both human and animal studies have emphasized the role of the dentate gyrus of the hippocampus in pattern separation (Yassa, & Stark, 2011) – a computational component of episodic memory. Changes in the dentate gyrus have been implicated in studies of aging in humans (Yassa, … Stark, 2011) and rodents (Wilson, … Tanila, 2006). The BPS-O has proven to be sensitive to changes in pattern separation behavior across the aging spectrum, suggesting a gradual change in dentate network function. Changes in this network have also been documented with MCI (Yassa, … Stark, 2010b), which may be detectable at an earlier stage based on performance in this task. Furthermore, pattern separation performance on the BPSO has been linked to systemic noradrenergic activation (Segal, Stark, Kattan, Stark, & Yassa, 2012), which could be due to the noradrenergic projections from the perforant path to the DG. Taken together, these findings emphasize the utility of this task for measuring pattern separation behavior, possibly as a proxy for the integrity of the DG and may be a useful task for assessing changes in other disorders associated with disruptions of the dentate gyrus, such as Schizophrenia, depression, and sleep disorders. We have developed a stand-alone version of the BPS-O for free download at http://darwin.bio.uci.edu/~cestark/bpso/bpso.html to be used by other research groups interested in examining pattern separation.
Highlights.
Behavioral pattern separation declines in human aging.
MCI individuals exhibit impairments in both recognition memory and behavioral pattern separation.
Aged Impaired adults demonstrate pattern separation deficits but normal recognition.
Acknowledgements
This research was supported in part by a grant from the National Institutes on Aging R03 AG032015 and R01 AG034613, and an Alzheimer’s Disease Research Center Project Award AG016573-11. We thank Samantha Rutledge for assistance in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakker A, Kirwan CB, Miller MI, Stark CEL. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74(3):467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends in Cognitive Sciences. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Hashtroudi S, Johnson MK. Age differences in using source-relevant cues. Psychology and Aging. 1992;7(3):443. doi: 10.1037//0882-7974.7.3.443. Retrieved from http://memlab0.eng.yale.edu/PDFs/1992_Ferguson_Hashtroudi_Johnson_PsychAging.pdf. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Colantuoni C, Eichenbaum H, Haberman RP, Rapp PR, Tanila H, Wilson IA. Individual differences in neurocognitive aging of the medial temporal lobe. Age (Dordr) 2006;28(3):221–233. doi: 10.1007/s11357-006-9017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus Hippocampus. 1992;2(4):437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt= Citation&list_uids=7104545. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Schacter DL, Galluccio L, Stofer KA. Reducing gistbased false recognition in older adults: encoding and retrieval manipulations. Psychol Aging. 1999;14(2):220–237. doi: 10.1037//0882-7974.14.2.220. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt= Citation&list_uids=10403710. [DOI] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Mem. 2011;18(1):15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science (New York, N.Y.) Science. 2007;315(5814):961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Mattfeld AM, Stark CEL. Striatal and medial temporal lobe functional interactions during visuomotor associative learning. Cerebral Cortex. 2010;21(3):647–658. doi: 10.1093/cercor/bhq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura EM, Reber PJ. A review of medial temporal lobe and caudate contributions to visual category learning. Neurosci Biobehav Rev. 2008;32(2):279–291. doi: 10.1016/j.neubiorev.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychological review Psychol.Rev. 2003;110(4):611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65(1):65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology Arch.Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41(3):245–251. doi: 10.1016/s0028-3932(02)00157-4. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt= Citation&list_uids=12457750. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends in cognitive sciences Trends Cogn.Sci. 2006;10(2):59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Rey A. L'examen psychologique dans les cas d'encephalopathie traumatique. Arch Psychol. 1941;28:286–340. [Google Scholar]
- Rugg MD, Vilberg KL, Mattson JT, Yu SS, Johnson JD, Suzuki M. Item memory, context memory and the hippocampus: fMRI evidence. Neuropsychologia. 2012 doi: 10.1016/j.neuropsychologia.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Decomposing age correlations on neuropsychological and cognitive variables. J Int Neuropsychol Soc. 2009;15(5):650–661. doi: 10.1017/S1355617709990385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal SK, Stark SM, Kattan D, Stark CE, Yassa MA. Norepinephrine-mediated emotional arousal facilitates subsequent pattern separation. Neurobiol Learn Mem. 2012;97(4):465–469. doi: 10.1016/j.nlm.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Stark CEL. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learning and Memory. 2010;17(6):284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14(2):167–177. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14590600. [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learning & memory (Cold Spring Harbor, N.Y.) Learn.Mem. 2009;16(5):338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Instelligence Scale (WAIS-III): Administration and scoring manual. San Antonio, TX: The Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale - Third Edition (WMS-III) San Antonio, TX: The Psychological Corporation; 1997b. [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends in neurosciences Trends Neurosci. 2006;29(12):662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Ageassociated alterations of hippocampal place cells are subregion specific. The Journal of neuroscience : the official journal of the Society for Neuroscience J.Neurosci. 2005;25(29):6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gureviciene I, McMahan RW, Gallagher M, Eichenbaum H, Tanila H. Cognitive aging and the hippocampus: how old rats represent new environments. The Journal of neuroscience : the official journal of the Society for Neuroscience J.Neurosci. 2004;24(15):3870–3878. doi: 10.1523/JNEUROSCI.5205-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Muftuler LT, Stark CEL. Ultrahigh-resolution microstructural diffusion tensor imaging (msDTI) reveals perforant path degradation in aged humans in vivo. Proc Natl Acad Sci U S A. 2010b;107(28):12687–12691. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CEL. High-resolution functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. NeuroImage. 2010b;51:1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus, E-pub ahead of print: 2010. 2010c doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CE. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci U S A. 2011;108(21):8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34(10):515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Aly M, Wang WC, Koen JD. Recollection and familiarity: examining controversial assumptions and new directions. Hippocampus. 2010;20(11):1178–1194. doi: 10.1002/hipo.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]