Abstract
Purpose
To describe the computed tomographic (CT) appearances and clinical consequences of tumor fistulization as a complication of targeted therapy for cancer.
Methods
The committee on human research approved this Health Insurance Portability and Accountability Act–compliant study and waived written informed consent. Based on the records of the senior author and our multidisciplinary Tumor Boards, we retrospectively identified 4 patients (1 man and 3 women with a mean age of 55.25 years; range, 47 to 64 years) who developed tumor fistulization while being treated with targeted therapy consisting of sunitinib (n = 2); bevacizumab (n = 1); and XL184, an investigational c-Met inhibitor (n = 1). All available clinical, imaging, and histopathological records were reviewed, with particular emphasis on treatment administered, CT findings, and clinical course.
Results
All 4 patients developed fistulae from large metastatic deposits in the abdomen (mean size before treatment, 10.55 cm; range, 7.4–13.4 cm) to the gastrointestinal tract, and one patient also developed fistulae from a lung metastasis of undetermined size to the bronchial tree. All fistulae manifested as the appearance of air within a pre-existing tumor mass. At the time of fistula detection, disease at other sites in the 4 patients showed signs of regression (n = 1), progression (n = 2), or stability (n = 1). Currently, one patient is alive without evidence of disease, and the 3 other patients are deceased.
Conclusions
Targeted therapy can be associated with tumor fistulization to the gastrointestinal tract or tracheobronchial tree; familiarity with the CT findings should facilitate the diagnosis of this complication, which seems to be of variable and patient-specific prognostic significance.
Keywords: VEGF inhibitor, fistula, bevacizumab, sunitinib, angiogenesis inhibitor
Targeted therapy has substantially improved outcomes for many cancers. Notable success stories include tyrosine kinase inhibitors (imatinib and sunitinib) for treatment of gastrointestinal stromal tumors and antibodies to vascular endothelial growth factor (VEGF) receptor (bevacizumab) or epidermal growth factor receptor (cetuximab) in metastatic colorectal cancer.1 These agents may have specific effects, such as the rapid reduction of increased 18F-fluorodeoxyglucose uptake in gastrointestinal stromal tumors treated with imatinib,2 or result in specific complications, such as the development of pneumatosis in patients receiving bevacizumab.3 These changes are often first seen at imaging performed for assessment of tumor response. As such, it is important for radiologists to be aware of the spectrum of complications that may develop during therapy with these novel anticancer antibodies. We describe 4 recent cases of tumor fistulization developing in patients receiving targeted therapy, which was first recognized on surveillance computed tomography (CT). The purpose of this study was to report these 4 cases to describe the CT appearances and clinical consequences of tumor fistulization as a complication of targeted therapy.
MATERIALS AND METHODS
Patients
This was a retrospective single-institution study approved by our Committee on Human Research (approval number 10-01837) and compliant with the requirements of the Health Insurance Portability and Accountability Act. Patient consent was not required. Over a 16-month period, based on the records of the senior author (F.V.C) and our regular multidisciplinary Urologic Oncology and Gastrointestinal tumor boards, we retrospectively identified 4 patients who developed tumor fistulization seen on CT while receiving cancer immunotherapy. Tumor fistulization was defined as the development of a new communication between a tumor mass and another organ such as the gastrointestinal tract or bronchial tree, as manifested by direct visualization or indirect signs such as the otherwise unexplained passage of air or oral contrast into a tumor mass. All available clinical, imaging, and histopathological records were reviewed by the principal investigator (H.C.), with particular emphasis on treatment regimens and clinical outcome.
CT Technique
All patients were scanned with a 64–row multidetector CT (LightSpeed, General Electric, Milwaukee, Wis) using a 1.25-mm slice thickness. The peak tube voltage was set at 120 kilovolt (peak), and the current (mA) was automatically adjusted to maintain a noise index of 12. All scans were obtained in the portal venous phase of enhancement (70-second scan delay) after the power injection of 150-mL intravenous iohexol (Omnipaque 350, Nycomed Amersham, Princeton, NJ) at a rate of 3 to 5 mL/s. Where appropriate, to better delineate anatomic findings, multiplanar reformats were constructed.
RESULTS
The clinical and imaging characteristics of the 4 patients are summarized in Table 1 and illustrated in Figures 1 to 4. The primary sites of malignancy were colonic adenocarcinoma, lower extremity cutaneous melanoma, gastric gastrointestinal stromal tumor, and renal cell carcinoma. All 4 patients developed a fistula between a tumor deposit in the abdomen and the gastrointestinal tract while being treated with sunitinib (n = 2), bevacizumab (n = 1), or XL184, an investigational c-Met inhibitor (n = 1). The patient with metastatic melanoma also developed a second fistula between a pulmonary metastasis and the tracheobronchial tree.
TABLE 1.
Clinical and Imaging Characteristics of the 4 Patients in the Study Group
| Patient Number | Age and Sex | Initial Disease Status | Treatment Administered | CT Findings After Treatment | Outcome |
|---|---|---|---|---|---|
| 1 | 47 F | 11-cm pelvic sidewall recurrence of colonic adenocarcinoma | 16 cycles of FOLFIRI (olinic acid, 5-f luorouracil, irinotecan) and bevacizumab | Marked shrinkage of mass with new intralesional loculated air and fistulous track to colon | Radical resection with takedown of fistula, intraoperative radiation, and adjuvant chemotherapy. Alive with no evidence of disease 5 mos later |
| 2 | 50 F | Stage IV melanoma with pulmonary, hepatic, splenic, and peritoneal metastases, including 6.1-cm pelvic mass | 2 cycles of XL184 | Direct visualization of fistula from pelvic mass to small bowel with new associated small bowel obstruction. Simultaneous development of a fistula between a large pulmonary metastasis and the tracheobronchial tree Shrinkage of other metastases. Attempted laparascopic diversion of ileostomy unsuccessful because of disease extent | Treatment converted to palliative care. Died 1 month later |
| 3 | 60 F | Large gastric gastrointestinal stromal tumor with hepatic and peritoneal metastases, including a 7-cm upper abdominal implant | Progressed on imatinib 400 mg 2 times a day. Changed to sunitinib 37.5 mg qD. 2 cycles of sunitinib 37.5 mg qD. | New air foci in upper abdominal implant extending to the transverse colon. Shrinkage of other metastases | Treatment converted to palliative care. Died 5 mos later |
| 4 | 61 M | Large locally recurrent renal cell carcinoma in right nephrectomy bed with pulmonary metastases | 3 cycles of sunitinib 50 mg daily (4 weeks on, 2 weeks off) | New fistula between tumor and bowel with passage of oral contrast into mass. Stability of other metastases | Died 1 month later |
qD indicates four times a day.
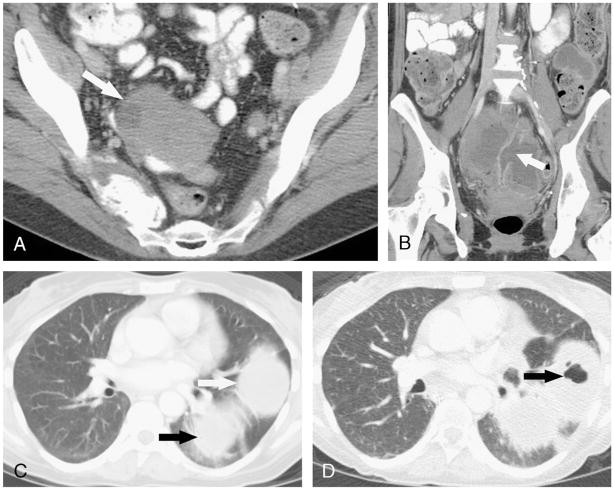
FIGURE 1.
A, Axial contrast-enhanced CT image in a 47-year-old woman showing a large pelvic sidewall recurrence (asterisk) of colonic adenocarcinoma. B, Sagittal reformatted contrast-enhanced CT image after treatment with 16 cycles of FOLFIRI (folinic acid, 5-fluorouracil, and irinotecan) and bevacizumab shows a fistula (arrow) between the shrunken tumor mass and the colon (C).
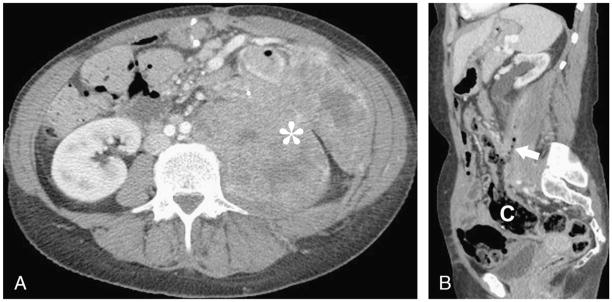
FIGURE 4.
A, Axial contrast-enhanced CT image in a 61-year-old man with a large locally recurrent renal cell carcinoma in right nephrectomy bed. B, Axial contrast-enhanced CT image after treatment with 3 cycles of 50 mg sunitinib daily (4 weeks on, 2 weeks off the drug) shows a new fistula between tumor and bowel, as demonstrated by the passage of air and oral contrast (arrow) into the mass. C, Coronal reformatted contrast-enhanced CT image demonstrates several contrast-filled bowel loops (arrows) in near the contrast within the tumor mass, suggesting this was the site of fistulous communication, although the underlying fistula track could not be directly visualized.
DISCUSSION
This small case series suggests that tumor fistulization may be an underrecognized complication of targeted therapy. More sweeping conclusions regarding the mechanism or clinical importance of this complication are difficult to establish in a study of only 4 patients, but we would tentatively suggest 2 inferences. First, whereas the development of a tumor fistula to the gastrointestinal tract or tracheobronchial tree could simply reflect tumor necrosis as a treatment response “un-masking” previously unapparent connection to or invasion of these structures, we suspect that this is only part of the pathophysiological process and that impaired tissue healing due to antiangiogenesis contributes to the double whammy leading to fistula formation. Inhibition of angiogenesis, often through targeting of VEGF and its associated ligands and receptors, is intended to limit or reverse the growth of susceptible tumors by regulating the chemical factors related to angiogenesis.4 These systemic treatments also inhibit angiogenesis in healthy tissues and accordingly can cause impaired wound healing, wound dehiscence, gastrointestinal perforation, and decreased capillary density of the intestinal villi.5 Our supposition that this mechanism contributed to tumor fistulization in our study patients is supported by the commonality of antiangiogenic activity between the 3 agents implicated in this report. Bevacizumab is a monoclonal antibody targeting VEGF.5 Sunitinib is an oral small-molecule tyrosine kinase inhibitor that interacts with several angiogenesis receptors including platelet-derived growth factor receptors and the VEGF receptors. It is currently approved for use in patients with imatinib-resistant or intolerant gastrointestinal stromal tumor and advanced renal cell carcinoma.6,7 XL184 is an experimental drug that inhibits Vascular Endothelial Growth Factor Receptor 2, Met hepatocyte growth factor receptor, and kinases. It is currently in a phase 1 dose escalation study for safety and pharmacokinetics in patients with advanced malignancies.8
Second, the development of a tumor fistula does not seem to have a clear prognostic consequence. Although the fistula itself might be regarded as a morbid complication, it could equally be regarded as a marker of disease response. Although 3 of our patients died of their disease, the fistula only seems to have contributed to the cause of death in one case, and one patient is alive without evidence of disease, although the fistula persists. As such, the clinical consequence seems variable and should be assessed on a case-by-case basis.
Tumor fistulization has not been previously reported as a complication of targeted therapy with antiangiogenic agents in a general sense, although fistula formation (gastrointestinal, enterocutaneous, tracheoesophageal, bronchopleural, biliary, vaginal, renal, and bladder) and gastrointestinal perforation are described complications of treatment with bevacizumab. In the pivotal study regarding the effectiveness of bevacizumab as an adjuvant to FOLFIRI ( folinic acid, f luorouracil, and irinotecan), 6 patients receiving bevacizumab developed gastrointestinal perforation (1.5%) compared to zero in the FOLFIRI group. Of these 6, one died, 2 recovered and discontinued therapy, and 3 recovered and continued therapy.9 A pooled analysis of the effect of the drug in older patients reported gastrointestinal perforation in 14 patients (<1%).10 Gastrointestinal perforation has also been reported with sunitinib. The phase 1 study of sunitinib involving 28 patients noted that at higher doses (≥75 mg/d), tumor response included decreased tumor vascularization and central necrosis that eventually resulted in organ perforation.11 However, neither the study that established the role of sunitinib as second-line therapy for gastrointestinal stromal tumor involving 207 patients nor the study establishing sunitinib as an effective treatment of renal cell carcinoma involving 375 patients reported any instances of gastrointestinal perforation or fistula.7,8 The relationship between the experimental drug XL184 and gastrointestinal perforation or fistulization is not yet known. As of this article’s publication, the phase 1 trial of XL184 is ongoing and is expected to conclude in August of 2011.9
Our study has several limitations. First, this is a small retrospective case series with potential sample selection bias. Second, cases were not collected systematically, and the absolute or relative frequency of tumor fistulization related to bevacizumab, sunitinib, or XL184 cannot be assessed. Third, no histopathological specimens were obtained for any of the tumor fistulas in the study, so the potential mechanism of fistulization remains speculative. Fourth, whereas we believe the association of tumor fistulization with novel targeted therapy agents is more than coincidental, the evidence remains circumstantial.
In conclusion, targeted therapy can be associated with tumor fistulization to the gastrointestinal tract or tracheobronchial tree; familiarity with the CT findings should facilitate the diagnosis of this complication, which seems to be of variable and patient-specific prognostic significance.
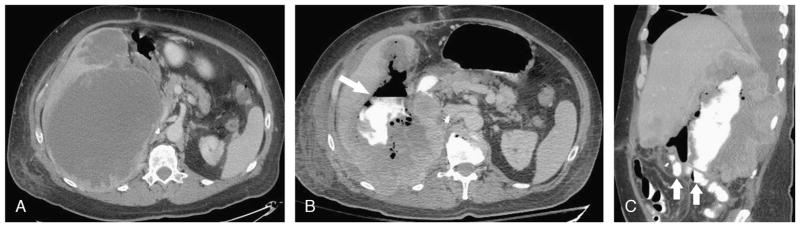
FIGURE 2.
A, Axial contrast-enhanced CT image in a 50-year-old woman with metastatic melanoma showing a pelvic tumor deposit (arrow). B, Coronal reformatted contrast-enhanced CT image after treatment with 2 cycles of XL184 shows a wide-mouth fistula (arrow) between a loop of small bowel in the pelvis and the tumor mass, which is now largely cystic and fluid-filled. C, Axial contrast-enhanced CT image obtained at the same time as A shows 2 pulmonary metastases (arrows) in the left lower lobe. D, Axial contrast-enhanced CT image obtained at the same time as B shows new air foci (arrow) in the anterior metastasis, consistent with development of a fistula between the tumor and the tracheobronchial tree.
FIGURE 3.
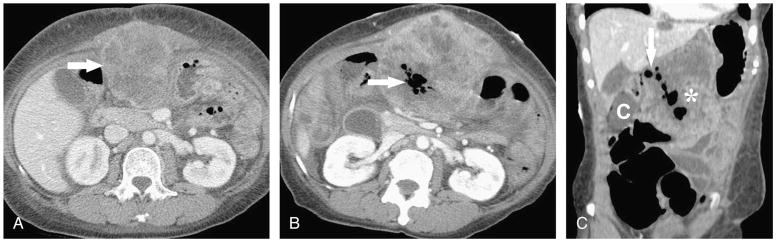
A, Axial contrast-enhanced CT image in a 60-year-old woman with gastric gastrointestinal stromal tumor shows an upper abdominal tumor deposit (arrow). B, Axial contrast-enhanced CT image after treatment with 2 weeks of 25 mg sunitinib and 9 weeks of 37.5 mg sunitinib qD shows new air foci (arrow) in upper abdominal implant, which extended to the transverse colon on other images (not shown) consistent with tumor fistulization. C, Coronal reformatted contrast-enhanced CT image demonstrates gas bubbles within a fistulous track (arrow) connecting the colon (C) to the tumor mass (asterisk).
Acknowledgments
JT was supported by NIBIB T32 Training Grant 2T32EB001631-06.
References
- 1.Bayraktar UD, Bayraktar S, Rocha-Lima CM. Molecular basis and management of gastrointestinal stromal tumors. World J Gastroenterol. 2010;16:2726–2734. doi: 10.3748/wjg.v16.i22.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van den Abbeele AD for the GIST Collaborative PET Study Group, Dana-Farber Cancer Institute, Boston, Massachusetts; OHSU, Portland, Oregon; Helsinki University Central Hospital, Turku University Central Hospital, Finland; Novartis Oncology. F18-FDG-PET provides early evidence of biological response to STI571 in patients with malignant gastrointestinal stromal tumors (GIST) Proc Am Soc Clin Oncol. 2001;20:362a. [Google Scholar]
- 3.Asmis TR, Chung KY, Teitcher JB, et al. Pneumatosis intestinalis: a variant of bevacizumab related perforation possibly associated with chemotherapy related GI toxicity. Invest New Drugs. 2008;26:95–96. doi: 10.1007/s10637-007-9094-z. [DOI] [PubMed] [Google Scholar]
- 4.Kou CJ. Overview of angiogenesis inhibitors. In: Goldberg RM, editor. UpToDate. Waltham, MA: UpToDate; 2010. [Google Scholar]
- 5.Han ES, Monk BJ. What is the risk of bowel perforation associated with bevacizumab therapy in ovarian cancer. Gynecol Oncol. 2007;105:3–6. doi: 10.1016/j.ygyno.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 6.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Hutson TE, Tomczak P. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 8.Exelixis Inc. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [Accessed June 13, 2010]. Study of XL184 in Adults With Advanced Malignancies. [cited 2010 June 13]. Available at: http://clinicaltrials.gov/ct2/show/NCT00215605 NLM Identifier: NCT00215605. [Google Scholar]
- 9.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 10.Cassidy J, Saltz LB, Giantonio BJ. Effect of bevacizumab in older patients with metastatic colorectal cancer: pooled analysis of four randomized studies. J Cancer Res Clin Oncol. 2010;136:737–743. doi: 10.1007/s00432-009-0712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faivre S, Delbaldo C, Vera K. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]