Abstract
Gonadal steroids affect a variety of brain processes. Cognitive consequences of hormonal changes associated with menopause are of scientific interest and of public heath relevance. Natural menopause is a normal physiological process that can be directly studied only through observational research. Similarly, surgical menopause in humans is rarely directly amenable to experimental research. Causality with respect to cognitive outcomes is therefore difficult to infer. Cross-sectional and longitudinal findings from the Melbourne Women’s Midlife Health Project, the Study of Women’s health Across the Nation, and other midlife cohorts suggest that cognitive consequences of the natural menopausal transition are probably small, at least during midlife and at least for episodic memory, a key cognitive domain for which data are the most robust. Midlife episodic memory performance is similar shortly after natural menopause compared to shortly before, and serum estradiol concentration in midlife is unassociated episodic memory performance. Effects of natural menopause on other cognitive domains, cognitive consequences of surgical menopause, and late-life cognitive consequences of midlife hormonal exposures are less well understood and merit continued study.
Keywords: Cognition, Dementia, Estrogen, Memory, Menopause, Testosterone
It is convenient to consider midlife as the period of a woman’s life that begins with the menopausal transition and extends through menopause to age 65 years, an arbitrary chronological milestone regarded as the threshold of older adult life. Menopause itself is a normal physiological process reflecting age-associated depletion of the primordial ovarian follicles. As a consequence, the ovary can no longer produce estrogens and progesterone. Natural menopause occurs at a median age of about 51 years [1]. It is dated according to the final menstrual period, which is in turn defined by 12 months of amenorrhea [2]. The mean serum concentration of 17β-estradiol, the primary ovarian estrogen, begins to decline about two years before the final menstrual period and reaches a permanent nadir about two years after the final menstrual period [3]. This transition is characterized by menstrual cycle irregularity and hormonal instability [4,5]. Levels of testosterone, the principal androgen, decline gradually during a woman’s reproductive years but are largely unchanged by natural menopause [6]. Androgen precursors are produced by the ovarian stroma. For this reason, endocrine effects of surgical menopause differ from those of natural menopause. In addition to the precipitous nature of the hormonal loss, bilateral oophorectomy also leads to reductions in testosterone concentrations [6].
An interest in the relation between gonadal aging and brain aging is far from new. Perhaps not surprisingly, initial concerns were for older men. Early in the 20th century, “monkey gland” testicular grafts were in vogue for male rejuvenation [7]. It is only during the past half century that estrogens for women gained favor, spurred in part by Robert Wilson’s Feminine Forever published in 1966 [8], touting ovarian hormones as a means to “prolonged well-being and extended youth” (p. 206). Despite temporary setbacks, estrogen prescriptions in the US and other countries soared over the next 35 years, with a sustained decline only after principal findings from the Women’s Health Initiative were first reported in 2002 [9]. Although Wilson mentions loss of memory in his paternalistic paean to estrogen and femininity [8] and cognitive effects of estrogen therapy had been studied even earlier [10], current interests in the role of estrogen in cognition and other aspects of human brain aging stem largely from preliminary research in the late 1980s and early 1990s related to estrogen and Alzheimer’s disease treatment [11–13] and risk [14–16].
The brain is a target of gonadal hormones, with receptors for estrogen, progesterone, and testosterone. Menopause-associated hormonal effects on aging brain function are potentially relevant to various neurological and psychiatric disorders. Particularly important concerns are cognitive decline associated with normal aging and more severe loss of cognitive ability attributed to pathological alterations of Alzheimer’s disease. The extent to which menopausal loss of ovarian hormones influences these cognitive outcomes remains an area of controversy. The following sections examine cognitive aging from a midlife perspective, considering cognitive changes associated with natural menopause, the relation between serum hormones and midlife cognition, and the relation between estrogen-containing hormone therapy and midlife cognition. The focus is on a form of memory (episodic memory) vulnerable to cognitive aging, as studied in cohorts of midlife women. Findings from the Melbourne Women’s Midlife Health Project, where some of our own work has taken place, are highlighted first and then discussed in relation to results from other cohorts and from pertinent clinical trials.
Cognitive aging: the central role of episodic memory
Cognition refers to mental processes by which knowledge is acquired, stored and used. These include skills concerned with attention and concentration, learning and memory, language, complex perceptual and motor skills, and judgment, planning and reasoning. Of these domains, a particular type of memory — episodic memory (also referred to as declarative memory [17]) — is the most relevant both to cognitive aging and to dementia. Episodic memory refers to the ability to learn some new bit of information and then to recall this information in a conscious manner after a period of time. Episodic memory is tested, for example, by a person’s ability to learn and recall details from a narrative story or recall items from a list of words. Verbal episodic memory refers to episodic memory for material that can be encoded through words or retrieved through verbal strategies; it is a domain in which women tend to outperform men [18]. Episodic memory depends on the integrity of medial temporal structures that include hippocampus, subiculum, entorhinal cortex, and parahippocampal gyrus [17].
Cognitive aging often entails noticeable decline in episodic memory [19]. More pronounced episodic memory impairment is also an early symptom of Alzheimer’s disease [20], the most common cause of dementia [21]. In old age, an episodic memory deficit beyond that expected on the basis of usual cognitive aging is associated with a high likelihood of clinically apparent Alzheimer’s disease over an ensuing several year period [22]. In this setting, the initial episodic memory deficit could simply represent an early manifestation of underlying Alzheimer pathology. Alternatively, poor performance on episodic memory tasks could reflect increased Alzheimer vulnerability related to low cognitive reserve (for example, below average innate ability or limited educational opportunities [23]) or related to such factors as depression, prior concussive head injury, or ischemic vascular disease. Any of these might modify the clinical expression of Alzheimer pathology and hence reduce the threshold for the emergence of Alzheimer symptoms. Pathological changes of Alzheimer’s disease affect the entorhinal cortex and the CA1 region of the hippocampus early in the disease course [24]; metabolic decline in these regions as imaged by positron emission tomography precedes cognitive symptoms [25,26].
In the laboratory, estrogens affect the hippocampus and hippocampal function. Some effects are mediated by alpha and beta estrogen receptors expressed in hippocampal pyramidal neurons [27]. Effects on basal forebrain cholinergic neurons, which express estrogen receptor alpha [28] and project to the hippocampus, play a role as well [29]. Together with progesterone, estradiol acts to increase numbers of dendritic spines, sites of excitatory synapses, on CA1 hippocampal pyramidal neurons [30]. Long-term potentiation is a physiological process believed critical to episodic memory formation. In hippocampal slice preparations, estradiol enhances long-term potentiation of these CA1 neurons [31]. Higher levels of progesterone, in contrast, reduce long-term potentiation amplitude [32]. Within the dentate gyrus of the hippocampus, estradiol — but not progesterone — appears to promote survival of new granule cell neurons [33].
Other estrogen effects may have an impact on processes contributing to the pathology of Alzheimer’s disease. Biochemical hallmarks of Alzheimer’s disease include accumulation in the brain of β-amyloid and a hyperphosphorylated form of tau protein. Estradiol reduces brain amyloid burden in some animal models [34] but not others [35]. In transgenic models of Alzheimer’s disease, estrogen depletion generally increases levels of β-amyloid [36–39] (but see [40]), and in primary brain cultures estradiol reduces tau phosphorylation [41]. Mitochondrial effects that diminish brain oxidative stress would also be predicted to lower Alzheimer risk [42]. Net effects of estrogens on the vascular endothelium and on processes involved in inflammation, atherosclerosis, thrombosis, thrombolysis, and fibrinolysis could increase or decrease dementia risk (reviewed in [43]). Some effects may differ depending on the type of estrogen or route of administration [44–46].
Estrogens and the menopausal transition
During a woman’s reproductive years, cognitive performance is reported to fluctuate during the course of the menstrual cycle. Performance differences, which tend to be small, may reflect cyclical fluctuations in the ovarian production of estradiol and progesterone. Findings are far from consistent, and implications are uncertain. In some reports, menstrual cycle phases when estradiol circulates at higher levels are positively associated with verbal episodic memory [47], but no memory effects are observed in other studies [48,49]. Intriguingly, hippocampal volume as measured by magnetic resonance imaging is reported to be larger (and globus pallidus and putamen volume to be smaller) during the late follicular phase of the menstrual cycle compared to menstrual phase [47]. For women of reproductive age, short-term pharmacological suppression of ovarian hormone production with a gonadotropin-releasing hormone analog impairs verbal episodic memory, and memory performance is restored when estrogens are added back [50].
Memory complaints are common during midlife [51,52], a time when ovarian production of estradiol is declining [3]. Because of the close association between episodic memory deficits and Alzheimer’s disease, these symptoms might seem troubling. However, a dire conclusion seems premature, since a clear relation between midlife memory complaints and objective performance on episodic memory tasks is not well established [53].
It is worth pointing out that one can study the relation between the natural menopausal transition and memory scientifically, but one cannot study this relation experimentally [54]. Menopause is not an experimental intervention that can be randomly assigned to one group of women and withheld from another. Inferences regarding natural menopause and cognition are of necessity based on observational studies, where the strength of evidence can be less than ideal, and causality is difficult to infer. These include cross-sectional and longitudinal studies of midlife women in different reproductive stages and studies examining the association between serum concentrations of gonadal hormones and cognition. Clinical trials of ovarian hormones after menopause provide an additional perspective on the cognitive consequences of menopause, but the experimental treatment model differs from menopause per se. In a similar manner, it is likely that cognitive outcomes of surgical menopause will be studied experimentally only in nonhuman animal models. It is difficult to envision an ethical scenario wherein some healthy women are assigned to bilateral oophorectomy and others to a nonsurgical (or shame surgical) control condition. Such a consideration could arise only in highly unusual circumstances (e.g., a trial of prophylactic oophorectomy involving women with mutations in a BRCA tumor suppressor gene).
Cognition in the Melbourne Women’s Midlife Health Project
The Melbourne Women’s Midlife Health Project was established through random digit telephone dialing in 1991 by Dennerstein and colleagues [55]. Eligible women were born in Australia, aged 45 to 55 years and residing in Melbourne, Australia. Respondents who had menstruated during the three preceding months, were not using oral contraceptives or hormone therapy, and had a uterus and at least one ovary were invited to participate in the longitudinal cohort. Four hundred thirty-eight women (response rate of 56 percent) agreed.
Reproductive stage and midlife cognition
Episodic memory was first assessed during year 8 and involved 326 women (mean age 57 years, range 52 to 63 years) who had not undergone surgical menopause [56] (Table 1). Testing was limited to verbal episodic memory based on three immediate recall trials and one delayed recall trial from a list of 10 unrelated words. At this time, 50 cohort members were in the menopausal transition (27 early and 23 late), 151 were in the early postmenopause (within five years of the final menstrual period), and 49 were in the late postmenopause (at least five years from the final menstrual period). The reproductive stage could not be determined for 49 women using hormone therapy. Among the 250 women not using hormone therapy, there was no relation between reproductive stage and verbal episodic memory (immediate recall or delayed recall) [56] (Fig. 1A). Moreover, for 139 postmenopausal midlife women who had never used hormone therapy, memory scores were unrelated to the interval between test date and date of the final menstrual period. If estrogens are important for maintaining episodic memory abilities, one would have expected performance differences based on reproductive stage and a performance decline following the last menstrual period. These cross-sectional findings thus imply that menopausal loss of ovarian hormones has no substantial effect on episodic memory ability during this midlife period.
Table 1.
Relation between midlife reproductive stage and cognitive abilities in midlife cohorts
| Midlife cohort, publication year | Number of women | Episodic memory | Other cognitive domains | Reference |
|---|---|---|---|---|
| Melbourne, Australia, 2003 | 326 | NS | — | [56] |
| SWAN, United States, 2003 | 803 | — | NS | [61] |
| Kinmen, Taiwan, 2006 | 495 | NS | Most NSa | [64] |
| United Kingdom, 2006 | 997 | NS | Slower visual searchb | [65] |
| Umea, Sweden, 2007 | 242 | NS | NS | [66] |
| SWAN, United States, 2007 c | 1657 | NS | NS | [62] |
| SWAN, United States, 2009d | 2362 | NSe | NSe | [63] |
| Umea, Sweden, 2010f | 193 | NS | Variableg | [67] |
Reduced verbal fluency for women in the menopausal transition compared to premenopause; no differences on digit span forward, digit span backward, or the Trail Making Test, Parts A and B.
Slower letter cancellation in postmenopausal women.
Includes women in [61].
No significant effect or nonsignificant trend for cognitive differences (story recall, Symbol Digit Modalities Test, Digit Span Backward) based on reproductive stage at time of baseline testing or for comparisons between postmenopause and premenopause. Nonsignificant trend (0.05 < p < 0.1) for reduced practice effect (lower rate of improvement) for story recall across annual visits in menopausal transition compared to premenopause; nonsignificant trend for reduced practice effect for the Symbol Digit Modalities Test for women in the late menopausal transition compared to premenopause.
Includes women in [66].
After menopause, reduced practice effects for phonemic fluency and Block Design compared to before menopause. No practice effect differences for vocabulary.
NS = nonsignificant probability p > 0.05. SWAN = Study of Women’s health Across the Nation
Figure 1. Reproductive stage, serum estradiol, and hormone therapy use: effects on verbal episodic memory.
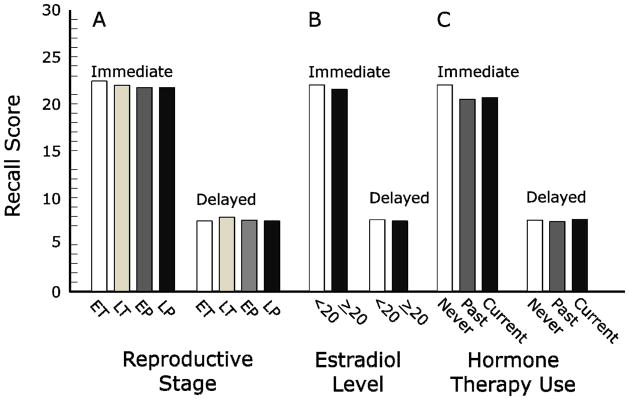
In the Melbourne Women’s Midlife Health Project, there were no significant between-group differences on any comparison for immediate or delayed recall on a word-list learning task (see [56]). A. Comparisons based on reproductive stage for 250 midlife women not using hormone therapy. B. Comparisons based on serum total estradiol for 231 midlife women not using hormone therapy, dichotomized as <20 pmol/L and ≥ 20 pmol/L. Results were similar when levels were analyzed continuously and for analyses based on the free estradiol index. C. Comparisons based on use of hormone therapy for 299 midlife women in the late menopausal transition or postmenopause. EP: early postmenopause; ET: early menopausal transition; LP: late postmenopause; LT: late menopausal transition.
Hormone levels and midlife cognition
Serum for estradiol concentrations was obtained from Melbourne cohort participants at the time of episodic memory testing and was assayed by double-antibody radioimmunoassay. Among women not using hormone therapy, there were no significant associations between the two word list recall scores and the total estradiol concentration (Fig. 1B) or between test scores and the free estradiol index (an index based on the ratio of estradiol to sex hormone binding globulin) [56]. These results reinforce inferences regarding episodic memory based on analyses of reproductive stage.
Three years later, Melbourne cohort members were assessed with a comprehensive neuropsychological battery [57]. By this time, most cohort members were postmenopausal but still middle-aged. New analyses focused on the relation between hormone concentrations and cognition for the 148 women who had undergone natural menopause and were not using hormone therapy. The mean age when the full battery was first administered was 60 years, and all but six women were below the age of 65. Because of the large number of neuropsychological variables, principal component analysis was undertaken to reduce the likelihood of false positive associations. The four identified cognitive factors represented verbal episodic memory, nonverbal episodic memory, semantic memory, and executive–visuospatial function. Blood for hormone assays was obtained on the morning of neuropsychological testing. Free (nonprotein bound) levels of estradiol and testosterone were calculated from validated algorithms based on total levels and the concentration of sex hormone binding globulin. Because we were interested in the relation between estradiol and testosterone, we also assessed the ratio of free testosterone to free estradiol.
There were significant associations or near-significant trends between some hormone measures and two of the four cognitive factors, viz., verbal episodic memory and semantic memory. The concentration of free estradiol was unrelated to the verbal episodic memory factor [57], confirming observations three years earlier in this cohort [56]. This factor was composed of immediate and delayed recall scores from two list learning tasks (an unrelated word list — the same task administered during year 8 — and a list containing words related in meaning to other words in the list—the California Verbal Learning Test). Free estradiol concentrations, however, were positively associated with semantic memory (p = 0.02). This factor was derived from tests of naming (Boston Naming Test) and category fluency (number of animal names generated in 60 seconds). Post-hoc analyses indicated that free estradiol levels were significantly associated with the naming scores (p = 0.002) but not with category fluency. The ratio of free testosterone to free estradiol showed a negative trend with verbal episodic memory (p = 0.06) and a significant negative association with semantic memory (p = 0.007) [57].
In summary, serum estradiol concentrations in midlife women were not associated with episodic memory scores, but higher levels of free estradiol were related to better semantic memory (naming) skills. This finding could indicate that naming skills — unlike verbal episodic memory — are influenced by menopausal status. A higher concentration of testosterone relative to estradiol was associated with worse performance in both of these cognitive domains, raising new questions regarding gonadal steroids and cognition. An important caveat of these and similar analyses is that hormone levels at one point in time provide an imprecise estimate of long-term, cumulative hormone exposures.
Hormone therapy and midlife cognition
Effects of hormone therapy were examined for 299 Melbourne women in the late menopausal transition or postmenopause [56]. Word-list learning did not vary significantly among never users, past users, or current users (Fig. 1C). For the 76 current users of hormone therapy, exploratory analyses indicated that episodic memory was better for women who initiated treatment before the final menstrual period than after this event. The mean difference was 2.0 words on the immediate recall trials (p = 0.03) and 0.7 words on the delayed recall trial (p = 0.1).
Effects of hormone use were also examined in relation to episodic memory performance five years later among the 145 women who had undergone natural menopause by the time of the final assessment [58]. Primary analyses compared never-users to ever-users based on the sum of all four recall trials. There was no significant difference between hormone therapy groups (mean declines of 0.8 words and 0.6 words in the two respective groups). Findings were similar when hormone therapy status was analyzed as never-use, past use, or current use, and also when immediate and delayed recall trials were examined separately.
A decline in episodic memory is a risk factor or an early marker for the development of Alzheimer’s disease [59]. Other analyses therefore focused on women who declined the most over the five year interval [58]. For women in the lowest 16th percentile (at least one standard deviation below the mean memory change), the average decline was 7.8 words, compared to an average improvement of 0.7 words for other women. The proportion of hormone therapy use was similar in the two groups, however, 17.4% of the large-decliners and 14.5% of other women. The odds of a large decline in episodic memory was therefore not significantly associated with hormone therapy use. These observational results suggest no substantial effect of midlife hormone therapy on episodic memory and no substantial impact on memory performance five years later. Small effects could have been missed by these analyses. As a further cautionary note, hormone users in general tend to be healthier than nonusers [60], and results could be biased by residual confounding.
Reproductive stage and cognition in other midlife cohorts
The relation between menopausal status and cognition has been examined in other midlife cohorts. The largest is the Study of Women’s health Across the Nation, or SWAN. This ethnically diverse voluntary cohort involves sites in seven United States cities. Initial cognitive analyses were confined to the Chicago site (Table 1) [61]. Data for Digit Span Backward and the Symbol Digit Modalities Test were available for women followed for two years, most of whom were premenopausal or in the early menopausal transition at the time of baseline testing. These tasks tap into aspects of executive functions but do not assess episodic memory or semantic memory. About 800 women contributed scores on at least one occasion. Mean scores improved from one year to the next (practice effect), but there was no significant incremental change as women progressed from one reproductive stage to another [61]. Cross-sectional analyses reported from all the SWAN sites included the East Boston Memory Test as well as Digit Span Backward and the Symbol Digit Modalities Test. The former assesses verbal episodic memory through the immediate recall and 10-minute delayed recall of a brief (36-word, three sentence) story. There were no cognitive differences based on reproductive stage [62] (Table 1).
A more complicated SWAN analysis again included women from all sites [63] (Table 1). At cognitive baseline (study year 4, mean participant age of 50 years), most women were in the menopausal transition. Thirty-one percent were already postmenopausal and only eight percent of women were still premenopausal. Women were then followed for four more years. For SWAN participants not using hormone therapy, cognitive scores did not differ by reproductive stage, and test performances generally improved from one year to the next in all menopausal groups (practice effect).
Despite absence of cognitive differences based on reproductive stage, there were subtle findings related to the rate of test score improvement, which was modeled within reproductive stages. Differences were observed when the menopausal transition was compared to premenopause [63]. During the early menopausal transition, there was a nonsignificant trend (p < 0.1) for the rate of improvement to be less on delayed recall of the East Boston Memory Test story; during the late menopausal transition, there was a similar nonsignificant trend for delayed story recall and also for the Symbol Digit Modalities Test score. Rates of change did not differ when the postmenopause was compared to premenopause, and other comparisons based on reproductive stage were neither significant nor showed a trend toward significance [63].
Another midlife cohort is from the Kinmen islands, a rural administrative unit of the Republic of China (Taiwan). Here, nearly 500 premenopausal women were cognitively tested at the beginning of the study and again 18 months later, by which time 23 percent of women had entered the menopausal transition [64]. Comparisons to women who remained premenopausal indicated no differences on two tests of episodic memory (the Rey Auditory Verbal Learning Test and a nonverbal continuous recognition task) or on other tasks (the Trail Making Test and the forward and backward digit span). Category fluency (animal naming), however, improved less among women who had entered the menopausal transition (p < 0.001) (Table 1).
In a nationally representative British birth cohort, all women were assessed at the identical age of 53 years [65]. Some women were still premenopausal, some were in the menopausal transition, while the majority were postmenopausal. Cross-sectional findings suggested a weak effect of the natural menopausal transition on cognitive function (Table 1). There were no trends across reproductive stage for verbal episodic memory (word list recall), but there was a significant trend toward slower search speed (letter cancellation task) for postmenopausal women (p for trend 0.04). Interestingly, better childhood cognitive ability was associated with later age of natural menopause, implying that analyses in other midlife cohorts should consider the possibility of confounding by pre-existing cognitive ability.
Finally, in the population-based Betula project, Swedish women aged 45, 50, or 55 years old were given a neuropsychological battery that included a composite measure of verbal episodic memory, a test of nonverbal episodic memory (face recognition), vocabulary (synonym selection), phonemic fluency (generating words beginning with a specified letter), and Block Design [66]. There was no effect of reproductive stage (premenopausal, menopausal transition, postmenopause) on any cognitive test score (Table 1).
Follow-up analyses within the same Swedish cohort, however, suggested a nuanced effect of reproductive stage on some cognitive measures [67]. Women in the second report were between ages 40 and 65 years who contributed data during at least one of three test sessions five years apart. Modeling compared linear trends before menopause (premenopause plus menopausal transition) and after menopause. For phonemic fluency and Block Design scores (but not for vocabulary and a somewhat different episodic memory composite score), practice effects between test sessions separated by five years were reduced after menopause compared to before menopause (Table 1). After menopause, there was an interaction between body mass index (dichotomized as normal versus overweight or obese) and rates of change on two cognitive tests: Block Design and the episodic memory composite score. Before menopause, overweight or obese women performed worse than normal weight women on both tasks. After menopause, the slope of the practice effect differed such that performance trajectories for overweight or obese women exceeded (improved more than or declined less than) that of normal weight women.
Findings from these midlife cohorts thus reinforce the general conclusion that natural menopause does not have a substantial effect on episodic memory abilities measured during midlife [61,64–66]. Some SWAN results [63] leave open the possibility of a small, nuanced effect on episodic memory manifest through changes in the practice effect during the menopausal transition (but not after menopause). Other cognitive domains are not as well studied, but some findings (e.g., practice effect changes after menopause noted in the Betula project [67]) do raise concern of modest effects of menopause beyond those attributable to age alone (Table 1).
Hormone levels and cognition in other midlife cohorts
SWAN investigators examined the cross-sectional relation between total estradiol concentrations and performance on story recall (episodic memory) and other SWAN tasks [62]. Analyses involved 1657 women (mean age 50 years) at the time of their first cognitive assessment (the fourth annual follow-up visit). Hormone therapy users and women who were surgically menopausal were excluded. There were no significant associations between serum estradiol and performance on the SWAN cognitive tests.
Consistent with findings on episodic memory from the Melbourne Women’s Midlife Health Project [56,57] and from SWAN [62], total estradiol levels in the Betula project were not related to episodic memory scores or to scores on other tests [66]. However, the absence of association between estradiol and vocabulary, a measure of semantic memory, differs from the positive association in the Melbourne cohort [57]. Reasons for the discrepancy are unclear, although semantic memory tasks differed in the two studies. In other Betula analyses that included elderly as well as middle-age women, free testosterone was negatively associated with both vocabulary score and the composite score for verbal episodic memory [68]. These results are similar to adverse associations described for the Melbourne cohort which, however, were based on the ratio between free testosterone and free estradiol rather than on free testosterone in isolation [57].
Hormone therapy and cognition during midlife
Hormone therapy and cognition in other midlife cohorts
In the British birth cohort, investigators reported no evidence for an effect of hormone therapy on cognitive measures [65]. In SWAN, a woman’s use of hormone therapy before the final menstrual period was associated with significantly better recall scores and better Symbol Digit Modalities Test scores [63]. In the smaller Melbourne cohort, hormone initiation prior to the final menstrual period was also associated with better recall [56]. Current hormone therapy use in SWAN, however, was associated with reduced year-to-year practice effects on these same tasks. The SWAN findings might be interpreted in several ways. First, as pointed out by study authors, results are consistent with the critical window, or timing, hypothesis, which suggests a better cognitive outcome when hormone therapy is initiated and used at a younger age or in closer temporal proximity to the menopause compared to later initiation and use [29,69,70]. Higher initial test scores on the part of hormone users could also reflect a healthy-user bias, whereby better educated women who lead healthier life styles are more apt to be prescribed hormone therapy; performance differences could be related to these other factors rather than hormone therapy per se [71]. A reduced practice effect might also be a test artifact, since women with higher scores have less opportunity to improve, especially on tests like the East Boston Memory Test with a low test ceiling. If the practice effect is indeed reduced among hormone users and the reduction is sustained over the long term, test scores would be expected eventually to fall below those of women not using hormones, as predicted, for example, by the critical window hypothesis.
Hormone therapy and cognition in randomized clinical trials
The link between midlife cognition and hormone therapy use is better addressed through clinical trials than through observational analyses described above [56,63,65]. In postmenopausal women well beyond midlife, the answer already seems clear: hormone initiation does not improve episodic memory and probably does not improve other cognitive skills in this older age group. An important source for this conclusion is the Women’s Health Initiative Study of Cognitive Aging (WHISCA), an ancillary study of the Women’s Health Initiative [72,73]. For women with a uterus, conjugated estrogens combined with medroxyprogesterone acetate slightly enhanced mean nonverbal episodic memory (Benton Visual Retention Test) and slightly reduced mean verbal episodic memory (California Verbal Learning Test). For women without a uterus, conjugated estrogens had no discernible effect on episodic memory. Findings in other large trials of older postmenopausal women are congruent [54].
For midlife women, however, clinical trial evidence on cognitive effects of hormone therapy is still sparse. Most trials in this age range have been small and of short duration [54]. The largest midlife hormone therapy trial randomized 180 naturally-menopausal women aged 45–55 years, where active treatment was with oral conjugated estrogens combined with medroxyprogesterone acetate [74]. After four months, there were no significant differences between treatment groups for tests of episodic memory (California Verbal Learning Test, paragraph recall, Benton Visual Retention Test) or for other neuropsychological test outcomes. In another trial, naturally menopausal women aged 50 to 65 years were assigned for four weeks to oral estradiol, testosterone undecanoate, or placebo (about 67 women per treatment group). There were no between-group differences for episodic memory (word list immediate recall) or other tasks [75]. These findings from relatively small clinical trials in midlife women comport with results from larger trials in older women [54].
The possibility remains that cognitive outcomes of menopause could differ when ovarian function is induced abruptly by oophorectomy, irradiation, or cancer chemotherapy [54,76]. A younger age of surgical menopause is associated with increased risk of later cognitive impairment or dementia [77]. Although surgical menopause per se in one midlife cohort was not linked to memory decrements [65], limited short-term clinical trial evidence suggests that estradiol may indeed improve verbal episodic memory when started immediately after bilateral oophorectomy [78,79]. For testosterone, evidence after surgical menopause is both limited and conflicting, with one report suggesting improvement compared to placebo in verbal episodic memory (story recall) [78] and another report indicating impairment on a similar task when estradiol plus testosterone was compared to estradiol alone [80].
More convincing evidence regarding cognitive effects of exogenous estrogens during midlife awaits completion of two large randomized clinical trials. These are ELITE (Early versus Late Intervention Trial with Estradiol; ClinicalTrials.gov identifier NCT00114517) and KEEPS (Kronos Early Estrogen Prevention Study) (ClinicalTrials.gov identifier NCT00623311). Over 600 women are enrolled in ELITE, recruited within two strata defined by menopause: women within six years of menopause and women 10 or more years beyond menopause. Over 700 women, all within three years of natural menopause, have been enrolled in KEEPS. Participants in both clinical trials have undergone extensive neuropsychological testing.
Future perspective
Cognitive consequences of the natural menopausal transition are beginning to be revealed. As reviewed above, observational findings are generally heartening with respect to objective midlife outcomes. For the domain that has been studied most carefully, viz. verbal episodic memory, there is no evidence that abilities are meaningfully compromised by the natural menopausal transition. Midlife episodic memory performance is the same shortly after menopause as before [56,62,63,65], and low estradiol levels in midlife are not associated with reductions in episodic memory test scores [56,57,62,66]. Whether semantic memory is affected [57] or whether executive functions are compromised [81] will require additional research. Effects of estrogen-containing hormone therapy are still uncertain, with hints that hormone use prior to the final menstrual period may improve episodic memory but that hormone use after menopause may not [56,63]. Results from the ELITE and KEEPS trials should eventually provide clear, and perhaps definitive, answers regarding near-term (several year) consequences of midlife hormone therapy on episodic memory and other cognitive outcomes. The answers will be of immense practical and theoretical importance. These large trials, however, do not address the narrower, yet still important, question of hormone therapy use during the short window preceding the final menstrual period.
It is more difficult to unravel long-term cognitive consequences of menopause or long-term consequences of midlife hormone therapy. Late-life initiation of hormone therapy does not improve memory [54] or reduce dementia risk [82,83]. Observational evidence, however, which may be biased, suggests that hormone therapy used during a critical midlife window may reduce Alzheimer’s disease risk years later [71]. For younger women in the Multi-Institutional Research in Alzheimer’s Genetic Epidemiology (MIRAGE) case-control study — but specifically not for older women in this study — there was a significant association between hormone therapy and Alzheimer risk reduction [84], and in the Cache County cohort prior hormone therapy use — but specifically not current use — was associated with reduced incidence of Alzheimer’s disease [85].
Studies such as these provide a foundation for the critical window, or timing, hypothesis [69,70,86]. The theory is better developed with respect to cardiovascular effects of estrogens, where clinical consequences may vary according to the health of vascular endothelium [87–89]. Speculatively, in a roughly analogous fashion, initiation and use of hormone therapy during a temporal window close to the time of menopause might reduce risk of late-life Alzheimer’s disease, despite evidence that initiation and use in the late postmenopause increases risk. Practically speaking, the critical window hypothesis as applied to dementia risk will be difficult to address through experimental research requiring outcome assessments one to two decades later. Scheduled follow-up in ELITE and KEEPS, for example, is too short, and the sample sizes are probably too small for clinical dementia outcomes. Long-term follow-up of Women’s Health Initiative participants who were in their 50s at the time of clinical trial randomization may eventually provide some answers. Other opportunity for new insight will come from careful follow-up of large midlife cohorts, from research into an understanding of basic mechanisms by which gonadal hormones can affect cognition and neurological function, and from clinical trials using appropriate biomarkers as surrogate outcomes. The issue remains important, because hormone therapy remains the best treatment for moderate or severe vasomotor symptoms of menopause [90,91], and many women continue to be exposed to midlife hormone therapy [92].
How other gonadal steroids influence midlife cognition is poorly addressed by extant data. Menopause entails the loss of ovarian progesterone as well as estradiol. Progesterone modifies some brain effects of estradiol [30,32,33], and it may have independent effects on cognition [93,94]. Testosterone, too, deserves closer scrutiny after natural menopause when levels are maintained (but are increased relative to estradiol) [57,68] and after surgical menopause, when levels fall abruptly [78,80]. Cognitive consequences of testosterone therapy, sometimes recommended for example for female hypoactive sexual desire disorder, are largely unknown.
Menopause is also associated with changes in levels of other reproductive hormones. Concentrations of follicle stimulating hormone (FSH) and luteinizing hormone (LH), gonadotropins secreted by the anterior pituitary gland, are elevated during the menopausal transition and during the postmenopause. The relation of FSH and LH to human cognitive function has been examined in only a few studies. There was no association between FSH levels and performance by midlife women on SWAN cognitive tasks [62]. LH receptors are expressed in hippocampus and other brain regions [95], and it has been argued that LH elevation is linked to Alzheimer’s pathogenesis [96]. Higher LH levels have been associated with poorer memory in older men [97] and poorer global cognition in older women [98], but possible associations in midlife women are not yet reported.
A final perspective pertains to the promise of personalized medicine and treatment benefit or toxicity predicted on the basis of genotype. Genetic or epigenetic variability affects age at menopause [99], cognitive aging [100], and Alzheimer’s disease risk [101]. Just as the presence and severity of midlife vasomotor symptoms varies widely among women, it is almost certain that women differ greatly in their risk for cognitive aging and dementia. Similar differences presumably exist for susceptibility to cognitive risks and benefits of different hormone therapy formulations. Most goals of personalized medicine are yet to be realized [102]. Differential vulnerability, resistance, and resiliency will depend on a number of factors related to health, lifestyle, and genetics, many of which remain unidentified. From perspectives provided by cohorts of midlife women, evidence thus far suggests that for most women hormonal consequences of the natural menopausal transition are not major determinants of cognitive aging. Cognitive consequences of surgical menopause and late-life cognitive consequences of endogenous and exogenous midlife hormonal exposures remain inadequately addressed.
Executive summary.
Role of episodic memory
Episodic memory refers to the conscious recollection of new information at some time after the information was first encountered.
Episodic memory performance is often compromised by cognitive aging.
Larger declines in episodic memory are linked to increased risk of Alzheimer’s disease.
Episodic memory, usually assessed with verbal tasks, is tested in most studies of cognitive aging, and consequently more is know about this cognitive domain than about other aspects of cognition.
In laboratory models, estrogens appear to act in ways that would enhance episodic memory and reduce some pathological hallmarks of Alzheimer’s disease.
Estrogens and the menopausal transition
Memory complaints are common during midlife.
Research in cohorts of midlife women provides insight into the cognitive consequences of menopause.
Cognition in the Melbourne Women’s Midlife Health Project
Cross-sectional findings indicate that midlife verbal episodic memory is unaffected by reproductive stage.
Midlife episodic memory is unrelated to serum concentrations of estradiol.
In postmenopausal midlife women, free estradiol levels are positively associated with semantic memory.
The ratio of testosterone to estradiol shows a significant negative association with semantic memory and a negative trend with verbal episodic memory performance.
In cross-sectional and longitudinal analyses, hormone therapy use is not significantly associated with midlife episodic memory performance.
Reproductive stage and cognition in other midlife cohorts
Reproductive stage was not significantly associated with episodic memory scores in other cohorts (SWAN, Taiwan, United Kingdom, and the Betula Project in Sweden); SWAN findings suggest subtle trends in episodic memory practice effects during the menopausal transition compared to premenopause but not during the postmenopause compared to premenopause.
Findings for other cognitive domains other than episodic memory are limited.
Hormone levels and cognition in other midlife cohorts
Estradiol levels are unrelated to episodic memory scores in other cohorts (SWAN and Sweden).
In a Swedish cohort that included elderly and midlife women, the testosterone level was negatively associated with measures of semantic memory and episodic memory.
Hormone therapy and cognition during midlife
Observational findings after natural menopause indicate no significant relation between estrogen-containing hormone therapy and episodic memory; clinical trial data are congruent but still sparse.
More definitive answers on midlife cognitive effects of hormone therapy are anticipated from large ongoing clinical trials (ELITE and KEEPS).
Future perspective
Cognitive consequences of endogenous and exogenous hormones are increasingly well understood. However, important questions remain.
Does hormone therapy used during the menopausal transition lead to cognitive outcomes different from those of midlife hormone therapy begun after the final menstrual period?
What are the cognitive consequences of surgical menopause? Do midlife cognitive consequences of estrogen therapy after surgical menopause differ from those of hormone therapy after natural menopause?
What are the late-life consequences for cognitive aging and dementia risk of midlife hormone therapy initiation and use?
What cognitive roles are played by midlife changes in the gonadotropins or in other gonadal steroids?
Footnotes
Financial & competing interests disclosure
Work in the Melbourne Women’s Midlife Health Project was supported in part by Alzheimer’s Association grant IIRG-01-2684. ELITE is supported by National Institutes of Health grant R01 AG024154. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in, or financial conflict with, the subject matter or materials discussed in the manuscript. This disclosure includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Bibliography
Papers of special note have been highlighted as
• of interest
•• of considerable interest
- 1.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 2.Soules MR, Sherman S, Parrott E, et al. Executive summary: stages of reproductive aging workshop (STRAW) Fertil Steril. 2001;76:874–878. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 3.Guthrie JR, Dennerstein L, Taffe JR, Lehert P, Burger HG. The menopausal transition: a 9-year prospective population-based study The Melbourne Women’s Midlife Health Project. Climacteric. 2004;7:375–389. doi: 10.1080/13697130400012163. [DOI] [PubMed] [Google Scholar]
- 4.Burger HG, Hale GE, Robertson DM, Dennerstein L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women’s Midlife Health Project. Hum Reprod Update. 2007;13:559–565. doi: 10.1093/humupd/dmm020. [DOI] [PubMed] [Google Scholar]
- 5.Prior JC. Ovarian aging and the perimenopausal transition: the paradox of endogenous ovarian hyperstimulation. Endocrine. 2005;26:297–300. doi: 10.1385/ENDO:26:3:297. [DOI] [PubMed] [Google Scholar]
- 6.Davison S, Bell R, Donath S, Montalto J, Davis SR. Androgen levels in adult females: changes with age, menopause and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847–3853. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton D. The Monkey Gland Affair. Chatto & Windus; London: 1986. [Google Scholar]
- 8.Wilson RA. Feminine Forever. M Evans and Company; New York: 1966. [Google Scholar]
- 9.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 10.Caldwell BM. An evaluation of psychological effects of sex hormone administration in aged women II Results of therapy after eighteen months. J Gerontol. 1954;9:168–174. doi: 10.1093/geronj/9.2.168. [DOI] [PubMed] [Google Scholar]
- 11.Fillit H, Weinreb H, Cholst I, et al. Observations in a preliminary open trial of estradiol therapy for senile dementia–Alzheimer’s type. Psychoneuroendocrinology. 1986;11:337–345. doi: 10.1016/0306-4530(86)90019-3. [DOI] [PubMed] [Google Scholar]
- 12.Honjo H, Ogino Y, Naitoh K, et al. In vivo effects by estrone sulfate on the central nervous system — senile dementia (Alzheimer’s type) J Steroid Biochem. 1989;34:521–525. doi: 10.1016/0022-4731(89)90137-4. [DOI] [PubMed] [Google Scholar]
- 13.Ohkura T, Isse K, Akazawa K, et al. Evaluation of estrogen treatment in female patients with dementia of the Alzheimer type. Endocr J. 1994;41:361–371. doi: 10.1507/endocrj.41.361. [DOI] [PubMed] [Google Scholar]
- 14.Birge SJ. The role of estrogen deficiency in the aging central nervous system. In: Lobo RA, editor. Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. Raven Press; New York: 1994. pp. 153–157. [Google Scholar]
- 15.Henderson VW, Paganini-Hill A, Emanuel CK, Dunn ME, Buckwalter JG. Estrogen replacement therapy in older women: comparisons between Alzheimer’s disease cases and nondemented control subjects. Arch Neurol. 1994;51:896–900. doi: 10.1001/archneur.1994.00540210068014. [DOI] [PubMed] [Google Scholar]
- 16.Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer’s disease in women. Am J Epidemiol. 1994;140:256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- 17.Squire LR. Memory and brain systems: 1969–2009. J Neurosci. 2009;29:12711–12716. doi: 10.1523/JNEUROSCI.3575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16:248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- 19.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welsh K, Butters N, Hughes J, Mohs R, Heyman A. Detection of abnormal memory decline in mild cases of Alzheimer’s disease using CERAD neuropsychological measures. Arch Neurol. 1991;48:278–281. doi: 10.1001/archneur.1991.00530150046016. [DOI] [PubMed] [Google Scholar]
- 21.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59:1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 22.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 23.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20 (suppl 3):S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- 24.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 25.Mosconi L, Mistur R, Switalski R, et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2009;36:811–822. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.González M, Cabrera-Socorro A, Pérez-García CG, et al. Distribution patterns of estrogen receptor alpha and beta in the human cortex and hippocampus during development and adulthood. J Comp Neurol. 2007;503:790–802. doi: 10.1002/cne.21419. [DOI] [PubMed] [Google Scholar]
- 28.Shughrue PJ, Scrimo PJ, Merchenthaler I. Estrogen binding and estrogen receptor characterization (ERα and ERβ) in the cholinergic neurons of the rat basal forebrain. Neuroscience. 2000;96:41–49. doi: 10.1016/s0306-4522(99)00520-5. [DOI] [PubMed] [Google Scholar]
- 29.Gibbs RB, Mauk R, Nelson D, Johnson DA. Donepezil treatment restores the ability of estradiol to enhance cognitive performance in aged rats: evidence for the cholinergic basis of the critical period hypothesis. Horm Behav. 2009;56:73–83. doi: 10.1016/j.yhbeh.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 31.Foy MR, Henderson VW, Berger TW, Thompson RF. Estrogen and neural plasticity. Curr Dir Psychol Sci. 2000;9:148–152. [Google Scholar]
- 32.Foy MR, Akopian G, Thompson RF. Progesterone regulation of synaptic transmission and plasticity in rodent hippocampus. Learn Mem. 2008;15:820–822. doi: 10.1101/lm.1124708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol. 2005;481:252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- 34.Petanceska SS, Nagy G, Frail D, Gandy S. Ovariectomy and 17β-estradiol modulate the levels of Alzheimer’s amyloid β peptides in brain. Neurology. 2000;54:2212–2217. doi: 10.1212/wnl.54.12.2212. [DOI] [PubMed] [Google Scholar]
- 35.Barron AM, Cake M, Verdile G, Martins RN. Ovariectomy and 17beta-estradiol replacement do not alter beta-amyloid levels in sheep brain. Endocrinology. 2009;150:3228–3236. doi: 10.1210/en.2008-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng H, Xu H, Uljon SN, et al. Modulation of Aβ peptides by estrogen in mouse models. J Neurochem. 2002;80:191–196. doi: 10.1046/j.0022-3042.2001.00690.x. [DOI] [PubMed] [Google Scholar]
- 37.Yue X, Lu M, Lancaster T, et al. Brain estrogen deficiency accelerates A(beta) plaque formation in an Alzheimer’s disease animal model. Proc Natl Acad Sci U S A. 2005;102:19198–19203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carroll JC, Rosario ER, Chang L, et al. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amtul Z, Wang L, Westaway D, Rozmahel RF. Neuroprotective mechanism conferred by 17beta-estradiol on the biochemical basis of Alzheimer’s disease. Neuroscience. 2010;169:781–786. doi: 10.1016/j.neuroscience.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 40.Golub MS, Germann SL, Mercer M, et al. Behavioral consequences of ovarian atrophy and estrogen replacement in the APPswe mouse. Neurobiol Aging. 2008;29:1512–1523. doi: 10.1016/j.neurobiolaging.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alvarez-De-La-Rosa M, Silva I, Nilsen J, et al. Estradiol prevents neural tau hyperphosphorylation characteristic of Alzheimer’s disease. Ann N Y Acad Sci. 2005;1052:210–224. doi: 10.1196/annals.1347.016. [DOI] [PubMed] [Google Scholar]
- 42.Henderson VW, Brinton RD. Menopause and mitochondria: windows into estrogen effects on Alzheimer’s disease risk and therapy. Prog Brain Res. 2010;182:77–96. doi: 10.1016/S0079-6123(10)82003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henderson VW. Hormone therapy and Alzheimer’s disease: benefit or harm? Expert Opinion on Pharmacotherapy. 2004;5:389–406. doi: 10.1517/14656566.5.2.389. [DOI] [PubMed] [Google Scholar]
- 44.Zhao L, Brinton RD. Select estrogens within the complex formulation of conjugated equine estrogens (Premarin) are protective against neurodegenerative insults: implications for a composition of estrogen therapy to promote neuronal function and prevent Alzheimer’s disease. BMC Neuroscience. 2006;13:24. doi: 10.1186/1471-2202-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chetkowski RJ, Meldrum DR, Steingold KA, et al. Biologic effects of transdermal estradiol. N Engl J Med. 1986;314:1615–1620. doi: 10.1056/NEJM198606193142505. [DOI] [PubMed] [Google Scholar]
- 46.Vongpatanasin W, Tuncel M, Wang Z, et al. Differential effects of oral versus transdermal estrogen replacement therapy on C-reactive protein in postmenopausal women. J Am Coll Cardiol. 2003;41:1358–1363. doi: 10.1016/s0735-1097(03)00156-6. [DOI] [PubMed] [Google Scholar]
- 47.Protopopescu X, Butler T, Pan H, et al. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008;18:985–988. doi: 10.1002/hipo.20468. [DOI] [PubMed] [Google Scholar]
- 48.Hatta T, Nagaya K. Menstrual cycle phase effects on memory and Stroop task performance. Arch Sex Behav. 2009;38:821–827. doi: 10.1007/s10508-008-9445-7. [DOI] [PubMed] [Google Scholar]
- 49.Mordecai K, Rubin L, Maki P. Effects of menstrual cycle phase and oral contraceptive use on verbal memory. Horm Behav. 2008;54:286–293. doi: 10.1016/j.yhbeh.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. J Clin Endocrinol Metab. 1996;81:2545–2549. doi: 10.1210/jcem.81.7.8675575. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell ES, Woods NF. Midlife women’s attributions about perceived memory changes: observations from the Seattle Midlife Women’s Health Study. J Womens Health Gend Based Med. 2001;10:351–362. doi: 10.1089/152460901750269670. [DOI] [PubMed] [Google Scholar]
- 52.Xu J, Bartoces M, Neale AV, et al. Natural history of menopause symptoms in primary care patients: a MetroNet study. J Am Board Fam Pract. 2005;18:374–382. doi: 10.3122/jabfm.18.5.374. [DOI] [PubMed] [Google Scholar]
- 53.Weber M, Mapstone M. Memory complaints and memory performance in the menopausal transition. Menopause. 2009;16:694–700. doi: 10.1097/gme.0b013e318196a0c9. [DOI] [PubMed] [Google Scholar]
- 54.Henderson VW, Sherwin BB. Surgical versus natural menopause: cognitive issues. Menopause. 2007;14:572–579. doi: 10.1097/gme.0b013e31803df49c. [DOI] [PubMed] [Google Scholar]
- 55.Dennerstein L, Smith AMA, Morse CA, et al. Menopausal symptoms in Australian women. Med J Aust. 1993;159:232–236. doi: 10.5694/j.1326-5377.1993.tb137821.x. [DOI] [PubMed] [Google Scholar]
- 56•.Henderson VW, Dudley EC, Guthrie JR, Burger HG, Dennerstein L. Estrogen exposures and memory at midlife: a population-based study of women. Neurology. 2003;60:1369–1371. doi: 10.1212/01.wnl.0000059413.75888.be. First midlife cohort to report on associations between episodic memory performance and reproductive stage and estradiol levels. [DOI] [PubMed] [Google Scholar]
- 57.Ryan J, Stanczky FZ, Dennerstein L, et al. Hormone levels and cognitive function in postmenopausal midlife women. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.07.014. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 58.Clark MS, Dennerstein L, Guthrie JR, Topol BB, Henderson VW. Hormone therapy use in midlife does not influence episodic memory: prospective analyses after natural menopause [abstract] Neurology. 2006;66(suppl 2):A351. [Google Scholar]
- 59.Elias MF, Beiser A, Wolf PA, et al. The preclinical phase of Alzheimer’s disease: a 22-year prospective study of the Framingham cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 60.Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prior to use of estrogen replacement therapy, are users healthier than nonusers? Am J Epidemiol. 1996;143:971–978. doi: 10.1093/oxfordjournals.aje.a008678. [DOI] [PubMed] [Google Scholar]
- 61.Meyer PM, Powell LH, Wilson RS, et al. A population-based longitudinal study of cognitive functioning in the menopausal transition. Neurology. 2003;61:801–806. doi: 10.1212/01.wnl.0000079051.91602.e2. [DOI] [PubMed] [Google Scholar]
- 62•.Luetters C, Huang MH, Seeman T, et al. Menopause transition stage and endogenous estradiol and follicle-stimulating hormone levels are not related to cognitive performance: cross-sectional results from the study of women’s health across the nation (SWAN) J Womens Health. 2007;16:331–344. doi: 10.1089/jwh.2006.0057. Associations in SWAN between cognitive test scores, reproductive stage, and serum estradiol. [DOI] [PubMed] [Google Scholar]
- 63••.Greendale GA, Huang M-H, Wight RG, et al. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology. 2009;72:1850–1857. doi: 10.1212/WNL.0b013e3181a71193. Longitudinal analysis in the large, multiethnic SWAN cohort examining changes in practice effect as a function of reproductive stage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64•.Fuh J-L, Wang S-J, Lee S-J, Lu S-R, Juang K-D. A longitudinal study of cognition change during early menopausal transition in a rural community. Maturitas. 2006;53:447–453. Longitudinal analysis of cognitive change between premenopause and the menopausal transition. [Google Scholar]
- 65•.Kok HS, Kuh D, Cooper R, et al. Cognitive function across the life course and the menopausal transition in a British birth cohort. Menopause. 2006;13:19–27. doi: 10.1097/01.gme.0000196592.36711.a0. Cognitive analyses in a midlife British birth cohort. [DOI] [PubMed] [Google Scholar]
- 66•.Herlitz A, Thilers P, Habib R. Endogenous estrogen is not associated with cognitive performance before, during, or after menopause. Menopause. 2007;14:425–431. doi: 10.1097/01.gme.0000247019.86748.e3. Associations between cognitive performance and reproductive stage and estradiol levels in a Swedish cohort. [DOI] [PubMed] [Google Scholar]
- 67.Thilers PP, Macdonald SW, Nilsson LG, Herlitz A. Accelerated postmenopausal cognitive decline is restricted to women with normal BMI: Longitudinal evidence from the Betula project. Psychoneuroendocrinology. 2010;35:516–524. doi: 10.1016/j.psyneuen.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 68.Thilers PP, Macdonald SW, Herlitz A. The association between endogenous free testosterone and cognitive performance: a population-based study in 35 to 90 year-old men and women. Psychoneuroendocrinology. 2006;31:565–576. doi: 10.1016/j.psyneuen.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 69.Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: a critical time. JAMA. 2002;288:2170–2172. doi: 10.1001/jama.288.17.2170. [DOI] [PubMed] [Google Scholar]
- 70.Maki PM. A systematic review of clinical trials of hormone therapy on cognitive function: effects of age at initiation and progestin use. Ann N Y Acad Sci. 2005;1052:182–197. doi: 10.1196/annals.1347.012. [DOI] [PubMed] [Google Scholar]
- 71.Henderson VW. Estrogen-containing hormone therapy and Alzheimer’s disease risk: understanding discrepant inferences from observational and experimental research. Neuroscience. 2006;138:1031–1039. doi: 10.1016/j.neuroscience.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 72.Resnick SM, Maki PM, Rapp SR, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91:1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 73.Resnick SM, Espeland MA, An Y, et al. Effects of conjugated equine estrogens on cognition and affect in postmenopausal women with prior hysterectomy. J Clin Endocrinol Metab. 2009;94:4152–4161. doi: 10.1210/jc.2009-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74••.Maki PM, Gast MJ, Vieweg A, Burriss SW, Yaffe K. Hormone therapy in menopausal women with cognitive complaints: a randomized, double-blind trial. Neurology. 2007;69:1322–1330. doi: 10.1212/01.wnl.0000277275.42504.93. Largest clinical trial examining cognitive effects of midlife hormone therapy. [DOI] [PubMed] [Google Scholar]
- 75.Kocoska-Maras L, Zethraeus N, Rådestad AF, et al. A randomized trial of the effect of testosterone and estrogen on verbal fluency, verbal memory, and spatial ability in healthy postmenopausal women. Fertil Steril. 2010 doi: 10.1016/j.fertnstert.2010.05.062. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 76.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen, and cognitive aging: the timing hypothesis. Neurodegener Dis. 2010;7:163–166. doi: 10.1159/000289229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rocca WA, Bower JH, Ahlskog JE, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 78.Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- 79.Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- 80.Möller MC, Bartfai AB, Rådestad AF. Effects of testosterone and estrogen replacement on memory function. Menopause. 2010;17:983–989. doi: 10.1097/gme.0b013e3181dc2e40. [DOI] [PubMed] [Google Scholar]
- 81.Joffe H, Hall JE, Gruber S, et al. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13:411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- 82.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study (WHIMS) JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 83.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 84.Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA. Postmenopausal hormone therapy and Alzheimer’s disease risk: interaction with age. J Neurol Neurosurg Psychiatry. 2005;76:103–105. doi: 10.1136/jnnp.2003.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zandi PP, Carlson MC, Plassman BL, et al. Hormone replacement therapy and incidence of Alzheimer’s disease on older women: the Cache County study. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 86.Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 87.Umetani M, Domoto H, Gormley AK, et al. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 88.Clarkson TB. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause. 2007;14:373–384. doi: 10.1097/GME.0b013e31803c764d. [DOI] [PubMed] [Google Scholar]
- 89.Sherwood A, Bower JK, McFetridge-Durdle J, et al. Age moderates the short-term effects of transdermal 17beta-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler Thromb Vasc Biol. 2007;27:1782–1787. doi: 10.1161/ATVBAHA.107.145383. [DOI] [PubMed] [Google Scholar]
- 90.North American Menopause Society: Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17:242–255. doi: 10.1097/gme.0b013e3181d0f6b9. [DOI] [PubMed] [Google Scholar]
- 91.Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95 (suppl 1):s1–s66. doi: 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buist DS, Newton KM, Miglioretti DL, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol. 2004;104:1042–1050. doi: 10.1097/01.AOG.0000143826.38439.af. [DOI] [PubMed] [Google Scholar]
- 93.Paganini-Hill A, Henderson VW. The effects of hormone replace therapy, lipoprotein cholesterol levels, and other factors on a clock drawing task in older women. J Am Geriatr Soc. 1996;44:818–822. doi: 10.1111/j.1532-5415.1996.tb03740.x. [DOI] [PubMed] [Google Scholar]
- 94.van Wingen G, van Broekhoven F, Verkes RJ, et al. How progesterone impairs memory for biologically salient stimuli in healthy young women. J Neurosci. 2007;27:11416–11423. doi: 10.1523/JNEUROSCI.1715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lei ZM, Rao CV. Neural actions of luteinizing hormone and human chorionic gonadotropin. Semin Reprod Med. 2001;19:103–109. doi: 10.1055/s-2001-13917. [DOI] [PubMed] [Google Scholar]
- 96.Webber KM, Perry G, Smith MA, Casadesus G. The contribution of luteinizing hormone to Alzheimer disease pathogenesis. Clinical Medicine and Research. 2007;5:177–183. doi: 10.3121/cmr.2007.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hyde Z, Flicker L, Almeida OP, et al. Higher luteinizing hormone is associated with poor memory recall: the health in men study. Journal of Alzheimer’s Disease. 2010;19:943–951. doi: 10.3233/JAD-2010-1342. [DOI] [PubMed] [Google Scholar]
- 98.Rodrigues MA, Verdile G, Foster JK, et al. Gonadotropins and cognition in older women. Journal of Alzheimer’s Disease. 2008;13:267–274. doi: 10.3233/jad-2008-13304. [DOI] [PubMed] [Google Scholar]
- 99.Mitchell ES, Farin FM, Stapleton PL, et al. Association of estrogen-related polymorphisms with age at menarche, age at final menstrual period, and stages of the menopausal transition. Menopause. 2008;15:105–111. doi: 10.1097/gme.0b013e31804d2406. [DOI] [PubMed] [Google Scholar]
- 100.Reichenberg A, Mill J, MacCabe JH. Epigenetics, genomic mutations and cognitive function. Cogn Neuropsychiatry. 2009;14:377–390. doi: 10.1080/13546800902978417. [DOI] [PubMed] [Google Scholar]
- 101.Seshadri S, Fitzpatrick AL, Ikram MA, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303:1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Holmes MV, Shah T, Vickery C, et al. Fulfilling the promise of personalized medicine? Systematic review and field synopsis of pharmacogenetic studies. PloS One. 2009;2:e7960. doi: 10.1371/journal.pone.0007960. [DOI] [PMC free article] [PubMed] [Google Scholar]


