Abstract
The eponymous term nucleus of Edinger-Westphal (EW) has come to be used to describe two juxtaposed and somewhat intermingled cell groups of the midbrain that differ dramatically in their connectivity and neurochemistry. On one hand, the classically defined EW is the part of the oculomotor complex that is the source of the parasympathetic preganglionic motoneuron input to the ciliary ganglion (CG), through which it controls pupil constriction and lens accommodation. On the other hand, EW is applied to a population of centrally projecting neurons involved in sympathetic, consumptive and stress-related functions. This terminology problem arose because the name EW has historically been applied to the most prominent cell collection above or between the somatic oculomotor nuclei (III), an assumption based on the known location of the preganglionic motoneurons in monkeys. However, in many mammals, the nucleus designated as EW is not made up of cholinergic, preganglionic motoneurons supplying the CG, and instead contains neurons using peptides, such as urocortin 1, with diverse central projections. As a result, the literature has become increasingly confusing. To resolve this problem, we suggest that the term EW be supplemented with terminology based on connectivity. Specifically, we recommend that: 1. The cholinergic, preganglionic neurons supplying the CG be termed the Edinger-Westphal preganglionic (EWpg) population, and 2. The centrally projecting, peptidergic neurons be termed the Edinger-Westphal centrally projecting (EWcp) population. The history of this nomenclature problem and the rationale for our solutions are discussed in this review.
The central problem: One term, two structures
In the case of the Edinger-Westphal (EW) nucleus, a circumstance has arisen in which two fundamentally different cell groups, both with important functions, have come to be called by the same name. This has occurred because the cholinergic, preganglionic neurons projecting to the ciliary ganglion (CG), to which the term was originally applied as a discrete cell group dorsal to the oculomotor nucleus (III), show unexpected variability in their location across different species. Since a population of centrally projecting, peptidergic neurons occupies a similar location, above III, they have come to share the same name, especially in those non-primate, mammalian species in which the CG preganglionic neurons are diffusely distributed, and the centrally projecting cells form a discrete group. Clearly, these two neuron populations need to be identified by separate names in order to alleviate the confusion caused in the literature by the assignment of the same term to both.
One suggestion that has been put forth is to call the centrally projecting cell group, the subgriseal paramedian midbrain neuronal stream (Cavani et al., 2003). This name was applied because in some species this peptidergic cell group extends rostrally, well beyond the confines of the cytoarchitectonically defined EW (e.g., cats; May et al., 2008a). Thus, these neurons can form a stream that extends to the mesodiencephalic junction. However, due to its length, this term has not obtained broad acceptance. It has also been suggested that the centrally projecting cells are reminiscent of those lying in the caudally adjacent dorsal raphe (May et al., 2008a), and so should be designated as a subdivision of the raphe system. However, this suggestion was rejected because it would engender a different set of confusions.
Another proposed solution is to adopt the terms preganglionic EW and non-preganglionic EW nuclei to define the two populations (Weitemier et al., 2005a; Ryabinin et al., 2005; Gaszner et al., 2007). This clearly represents an improvement in the clarity of the terminology, but it still does not adequately describe these sets of neurons, because the populations as a whole often ignore EW’s cytoarchitectural boundaries. For example, the diffuse distribution of preganglionic neurons in rodents and cats makes the term nucleus a misleading identifier for this population. Even in monkeys, the preganglionic neuron column extends into the anteromedian nucleus (AM), and so these cells are not confined to a single nucleus. The term non-preganglionic EW is also not sufficiently precise, as other non-preganglionic populations are also present in this area, such as the cholinergic S- and C-group motoneurons that project to and innervate extraocular muscle fibers (Büttner-Ennever and Akert, 1981; Büttner-Ennever et al., 2001). These actually overlap with the peptidergic neuron distribution in monkeys (Horn et al., 2008).
In an attempt to remedy these failings, it was recently proposed to identify the urocortin 1 (Ucn1) - positive neurons, which make up the largest segment of the centrally projecting peptidergic cells, as the perioculomotor Ucn 1-containing population, abbreviated as pIIIU (Horn et al., 2008; May et al., 2008a). The preganglionic population supplying CG were then referred to as the perioculomotor preganglionic population (pIIIPG). These authors argued that the term EW should not be retained, because it was associated with a history of inconsistent usage. They further argued that the use of eponyms has largely been discarded in the latter part of the 20th century, except in the case of a few pre-eminent anatomists (e.g. Cajal). The strength of these perioculomotor (pIII) terms is that while they indicate the general location of the neuronal populations, in accordance with neuroanatomical conventions, they are appropriate regardless of the precise location of the cells in a specific species, because they all lie near the oculomotor nucleus. Furthermore, since no cytoarchitectonically defined structure is specified using this term, any lack of coincidence with nuclear boundaries is not a problem. On the other hand, in many cases the location of these two populations cannot be readily defined in conventional brain atlases. Moreover, the Ucn1 neurons overlap other neurochemically-defined populations subserving similar functions, and it would be desirable to group them with the Ucn1 neurons by some common term. Finally, the wide use of the term EW by individuals outside those doing research in this area would tend to make adoption of an entirely new terminology more difficult.
Due to their variable location, the preganglionic neurons supplying CG that reside near the oculomotor nucleus cannot be given a single cytoarchitectonically based name that will suffice for all vertebrate species. Nonetheless, there is a need to have a term by which to refer to this functionally important set of neurons. After long and careful consideration of the merits of different approaches, we have developed a new terminology that we believe includes the most desirable aspects of the above solutions for the “EW problem”. We suggest that these two populations be specified based on their projection target. The neurons projecting to the ciliary ganglion would be designated the Edinger-Westphal preganglionic (EWpg) population and the peptidergic neurons with central projections would be designated as the Edinger-Westphal centrally projecting (EWcp) population. It should also be noted that this nomenclature eliminates the need to designate an AM. This is a positive change as the constituents and borders of this nucleus have varied in the hands of different investigators. As is true of many brain regions, this region is neurochemically diverse, with one of its constituent populations being Ucn1 cells. One could specify these subpopulations by their transmitter, e.g., urocortinergic neurons of EWcp (uEWcp). There is an important proviso to this terminology solution. Atlases for a given species must define the location of EWpg and EWcp cell groups based on connectivity data for that species, and must account for the fact that only in monkeys and birds does EWpg lie in a cytoarchitectonically distinct nucleus.
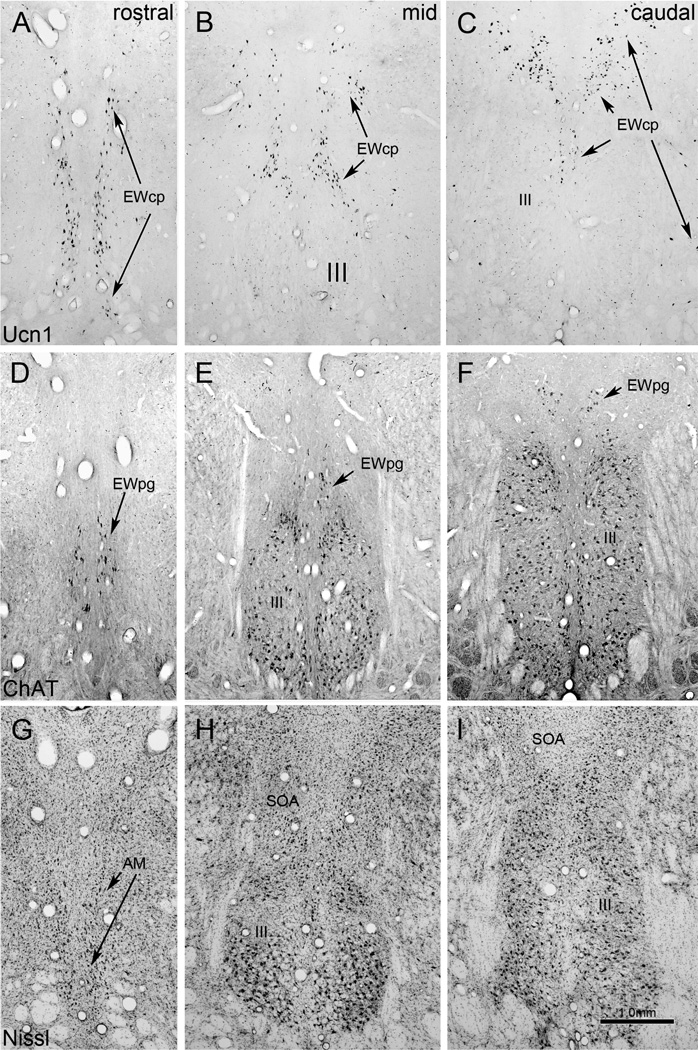
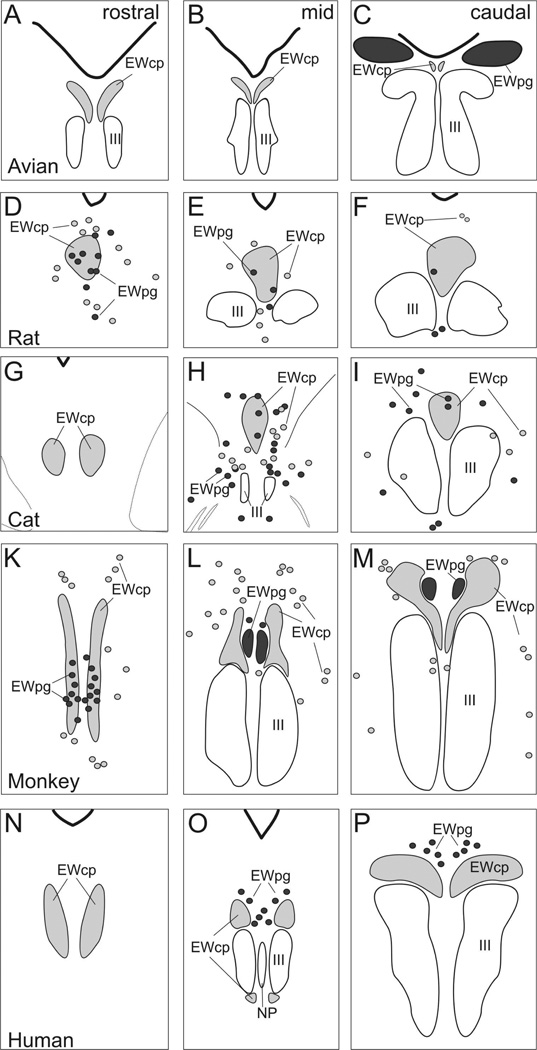
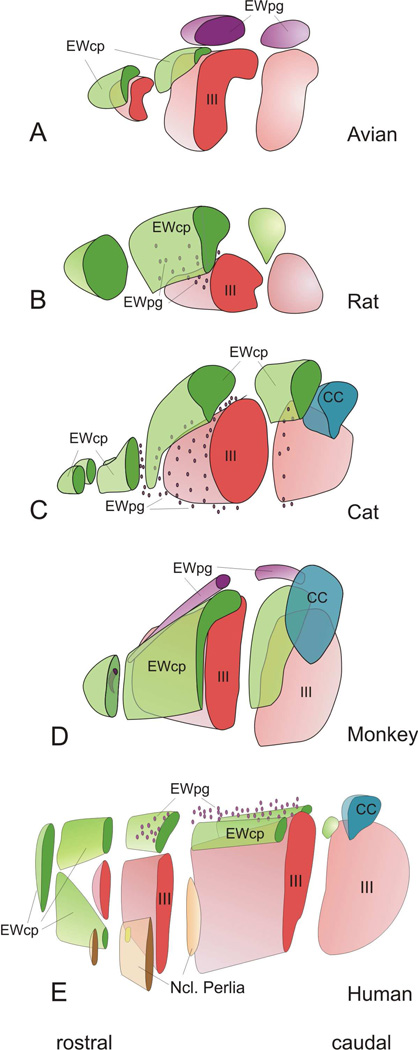
We have provided a series of figures illustrating the adoption of this new nomenclature for commonly used species (Figs. 1–8). Figures 1,6&7 show the arrangement in Macaca fascicularis monkeys. Ucn1-positive neurons in EWcp extend rostral to III as a narrow, dorso-ventrally oriented group, in and around AM. At more caudal levels, they mainly lie immediately dorsal and medial to III. In contrast, the ChAT-positive cells of EWpg form a discrete rostro-caudally oriented column, beginning rostral to III in AM, before sitting dorsal to III and EWcp in the nucleus historically termed EW. The main population of EWcp neurons lies between EWpg and III, and makes up the bulk of AM. The distribution of Ucn1 cells in EWcp of humans (Figs. 2,6&7) is actually quite similar to that of monkeys, although the cells cluster in two groups. However, in this case, it is this Ucn1-rich nucleus that makes up the bulk of what has historically been termed EW (Olszewski and Baxter, 1982). The EWpg population sits as a rostro-caudally oriented column dorsal to EWcp, but is more diffusely organized. In the pigeon (Figs. 3,6&7), the Ucn1-positive cells of EWcp are again located dorsomedial to III, and extend rostral to it. The ChAT-positive motoneurons in EWpg are also located in a discrete nucleus that has historically been termed EW, but it is found dorsolateral to III. In the cat (Figs. 4,6&7), the Ucn1-positive cells in EWcp begin caudally as a single midline nucleus dorsal to III that was historically termed EW. This extends rostral to III as AM, and splits into two columns that lie just off the midline at the rostral pole of EWcp. Finally, in the rat (Figs. 5,6&7), the Ucn1-positive neurons in EWcp have the familiar dorsomedial position immediately adjacent to III that extends rostral to III. They occupy the nucleus historically termed EW. Many ChAT-positive cells of EWpg are found rostral to III, while others are scattered above and between III. A three-dimensional (3-D) reconstruction of these populations in five species is provided in figure 7. It is clear that the location of the centrally projecting cells in EWcp is actually quite consistent across species. The core of this group is always located immediately dorsal and medial to III. In contrast, the preganglionic neurons in EWpg are much more variable. They occupy a discrete nucleus only in monkeys and birds. In contrast, in cats, rats and humans, these motoneurons are more diffusely organized (dots in Fig. 7). They also vary in location, with the preganglionic neurons all lying dorsal to III in humans, but being more widely distributed in the cat and rat.
Figure 1.
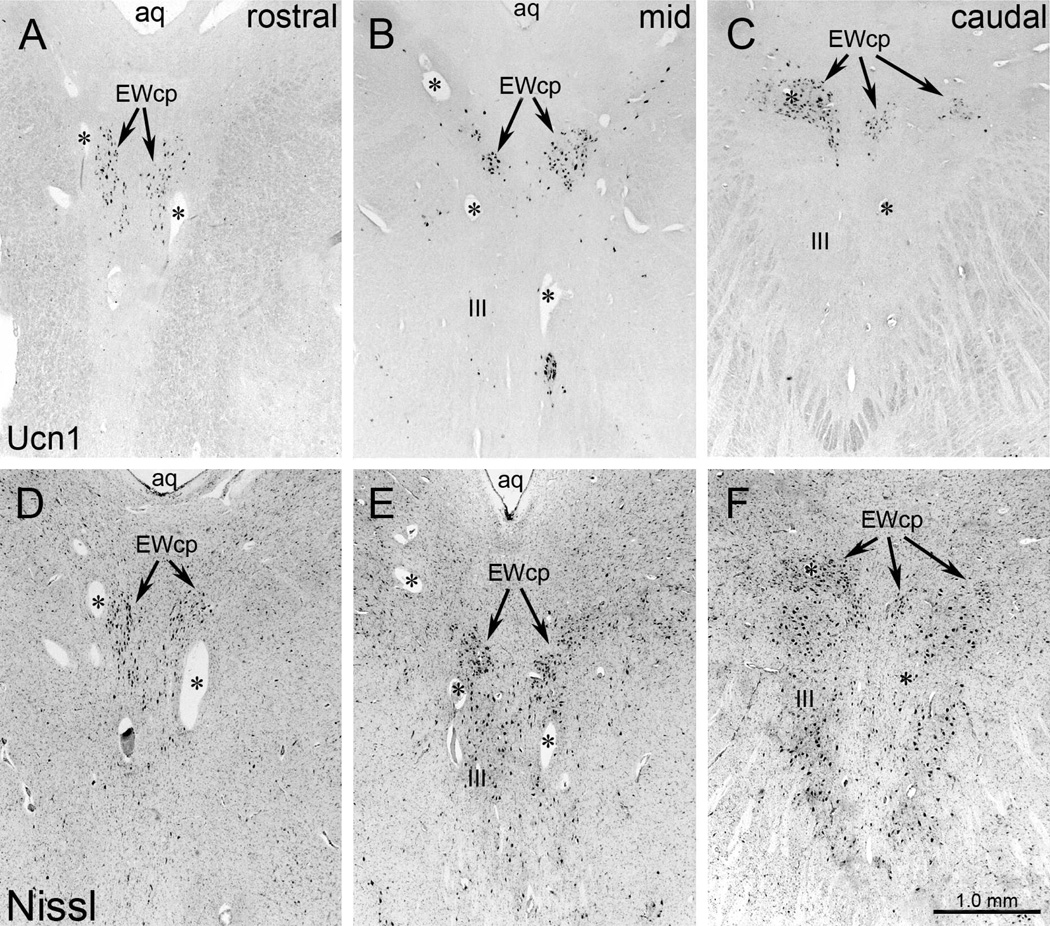
Digital photomicrographs of the macaque monkey perioculomotor area (pIII). Sections were stained using antibody to urocortin 1 (Ucn1) (A–C), antibody to choline acetyl transferase (ChAT) (D–F) and with cresyl violet (Nissl) (G–I), and are shown in a rostral to caudal sequence. The mid section is not midway between the others, but is instead located near the front of the oculomotor nucleus (III) because the interactions of the different populations are particularly complex at this point. Rostral to III, the Ucn1 cells are oriented dorsoventrally, with many of them occupying the anteromedian nucleus (AM). Note that EWpg motoneurons form a discrete nucleus in the monkey that is dorsal to somatic motoneurons in III (i.e, the former EW). The EWcpneurons do not form an obvious nucleus, but instead extend from between the two nuclei of III into the supraoculomotor area (SOA). A few are scattered amongst the myelinated bundles lateral to III. Scale bar: 1mm.
Figure 8.
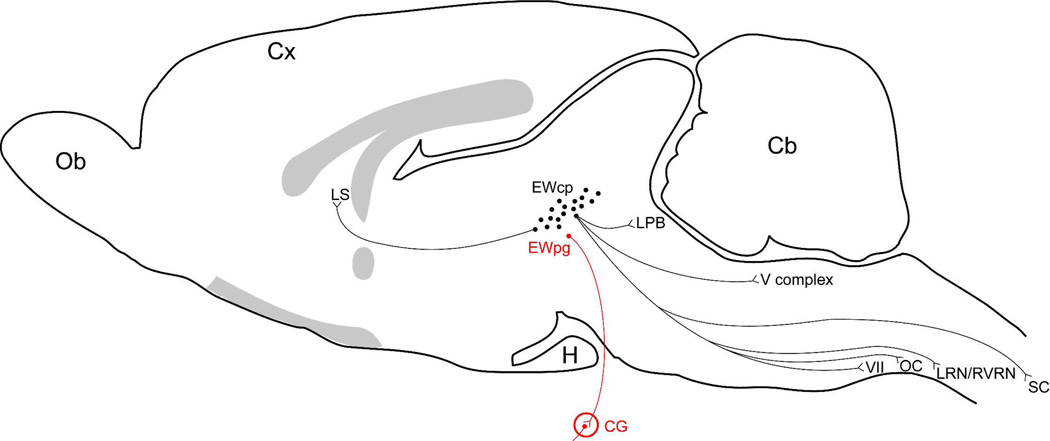
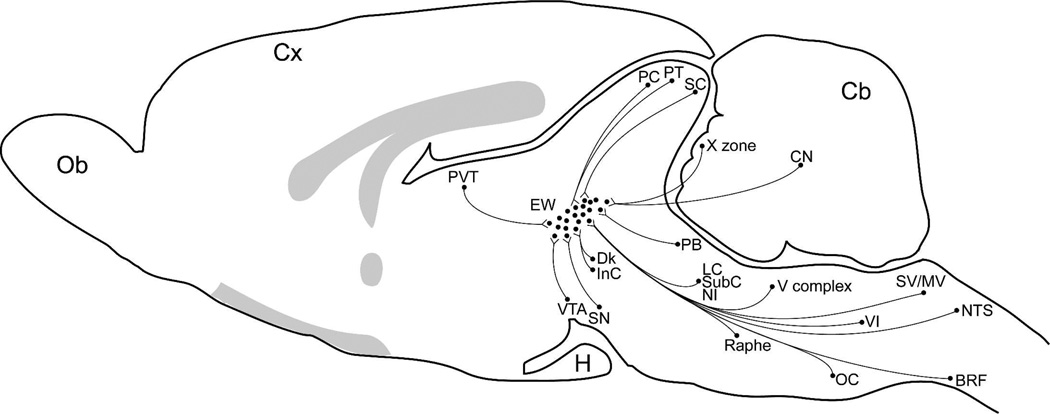
Schematic diagram of a midsagittal section through the rat brain to summarize the known afferents to the classical EW. Species differences in the location of Ewcp and EWpg were not considered. Abbreviations: BRF, bulbar reticular formation; Cb, cerebellum; CN, cerebellar nuclei; Cx, cerebral cortex; Dk, Darkeschewitsch nucleus; H, hypophysis; InC, nucleus interstitial of Cajal; LC, locus coeruleus; LPB, lateral parabrachial nucleus; MV, medial vestbibular nucleus; NI, nucleus incertus; Ob, Olfactory bulb; OC, olivary complex; PB, parabrachial nuclei; PC, nucleus of the posterior commissure; PTm, medial pretectal nucleus; Raphe, raphe nuclei; SC, superior colliculus; SubC, subcoeruleus; SV, superior vestibular nucleus; V complex, trigeminal nuclei complex; VTA, ventral tegmental area; VI, abducens nucleus; X zone, the X zone of the cerebellar cortex. The shaded gray areas represent the corpus callosum, fornix, anterior commissure and the optical chiasm and tract.
Figure 6.
Line drawings showing the organization of EWpg and EWcp in several selected species: avian, rat, cat, monkey and human. Representative rostral (left column), middle (middle column) and caudal (right column) sections are shown. The EWcp is indicated by light gray shading and EWpg is indicated by dark gray shading. Scattered cells located outside the nuclear boundaries are indicated by appropriately shaded circles.
Figure 7.
Three dimensional (3-D) representations of human, macaque, cat, rat and pigeon oculomotor complex, to illustrate the 3-D organization of EWpg and EWcp. The 3-D models are cut at selected points to illustrate how frontal sections through this level would look. In cases where the population is scattered, and so not contained in a discrete nucleus, dots are used.
Figure 2.
Photomicrographs of transverse sections through pIII in human immunostained for Ucn1 (A–C). Note that at caudal levels the Ucn1-positive neurons of EWcp form two separate cell groups dorsal to III, which merge at more rostral levels to form a large compact group. This is delineated in Nissl-stained sections as well (D–F). Portions of this population were formerly termed EW. At mid levels, sometimes an additional small ventral part of the Ucn1-positive cell group is apparent, which merges with the dorsal group rostraly to III (B). For clarity, corresponding blood vessels are indicated by asterisks in the matching picture pairs. Scale bar: 1 mm.
Figure 3.
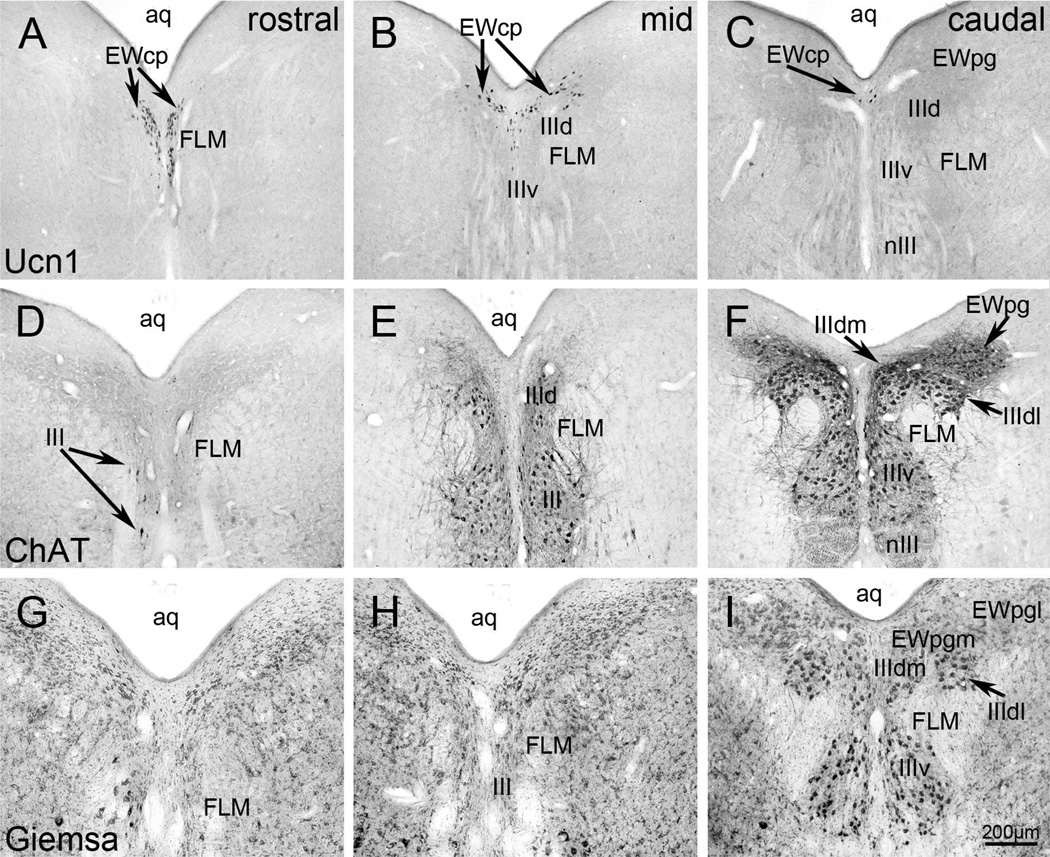
Digital images of oculomotor (III) region in the pigeon brain. In the upper row (A–C), Ucn1-positive neurons form the two medial columns of the EWcp which is located below the central aqueduct (aq). More caudally, the major group of the Ucn1-labelled neurons moves dorsally to form a winglike structure above III. D–F present ChAT-positive neurons that constitute the subdivisions of the III and EWpg. The latter is located dorsolateral to the former. G–I present sections stained with Giemsa, showing the relationship of III and EWpg. The latter is composed of medial (EWpgm) and the lateral (EWpgl) subdivisions. This is the nucleus formerly termed EW. Other abbreviations: FLM: fasciculus longitudinalis medialis; nIII: oculomotor nerve. Scale bar: 200µm.
Figure 4.
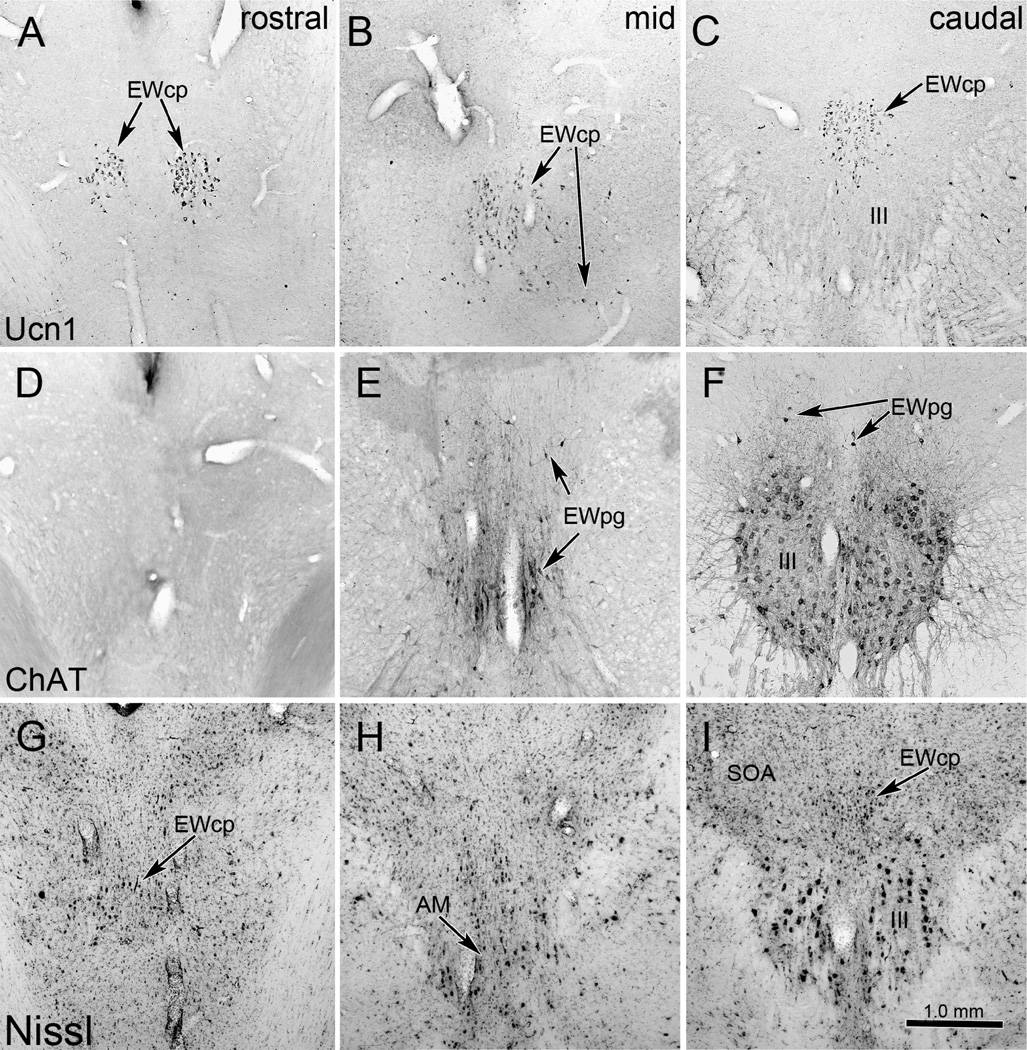
Photomicrographs of the feline oculomotor (III) region. Note that the ChAT-positive motoneurons of EWpg do not form a discrete nucleus in the cat (D–F), and are instead scattered dorsal, rostral and ventral to somatic motoneurons in III. The Ucn1-positive EWcp neurons do form a discrete nucleus dorsal to III that extends as a paired column at the most rostral levels (AC). As shown in the Nissl sections (G–I), this nucleus was formerly termed EW. However a few neurons are scattered in the supraoculomotor area (SOA) and amongst the myelinated bundles lateral to III. Scale bar: 1mm.
Figure 5.
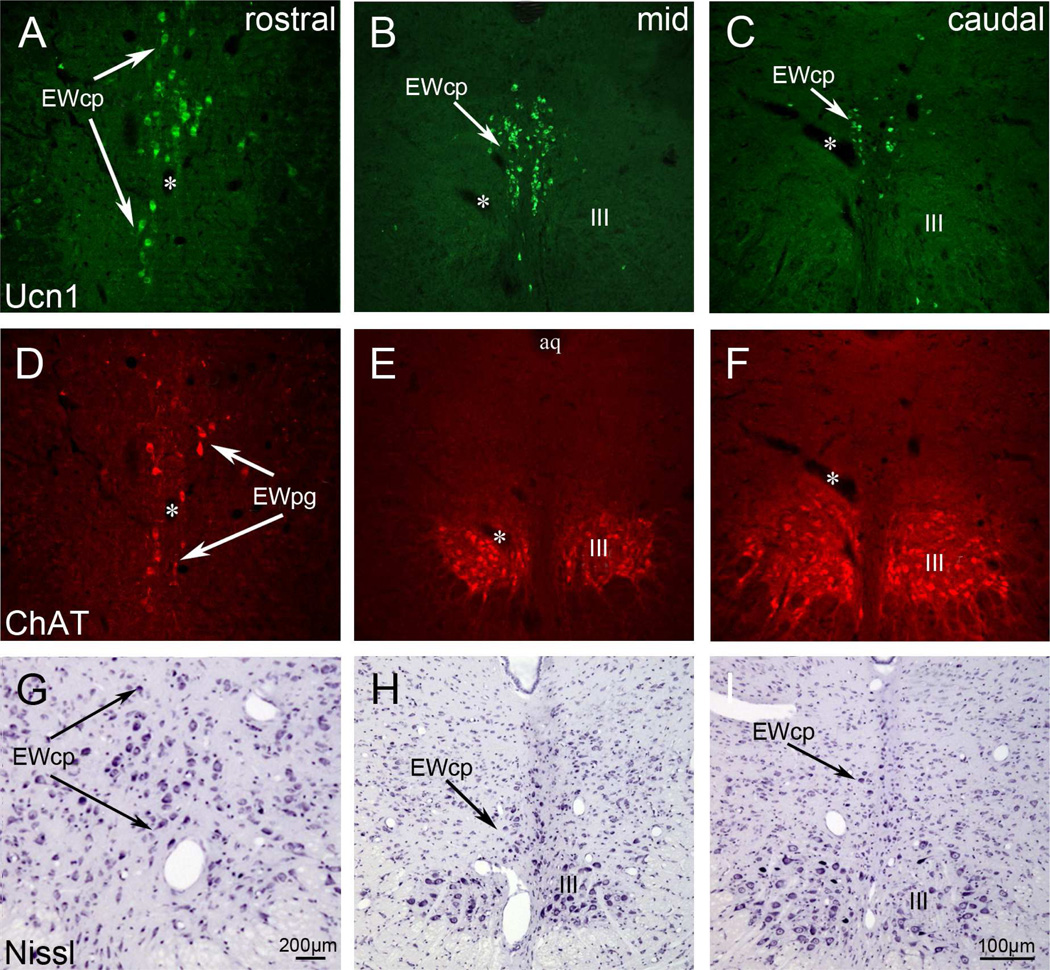
Photographs of transverse sections through the oculomotor (III) region in rat, with combined immunofluorescence for Ucn1 (green, A–C) and ChAT (red, D–F). At caudal and mid levels, the Ucn1-positive neurons of EWcp form a rather compact nucleus visible in the Nissl sections (G–I). This nucleus was formerly called EW, with its neurons more scattered at rostral levels. ChAT-positive neurons within III represent extraocular motoneurons, and scattered ChAT-positive neurons dorsal to III may represent preganglionic neurons (EWpg). These overlap with EWcp rostral to III (A,D.G). For clarity, corresponding blood vessels are labelled by asterisks in the picture pairs. Scale bar:200 µm. Other abbreviations: aq; cerebral aqueduct.
In summary, we seek to draw attention to the fact that two functionally different populations of neurons have come to be called EW. This has yielded a confusing literature for researchers in the field and outside it. For example, considerable research activity has been undertaken in an attempt to find pupillary reflex deficits in humans with Alzheimer’s disease based on the reports of degeneration in EW (Scinto et al., 1999). This was fruitless, since the population of cells that was degenerating was actually part of the EWcp, not EWpg. We believe that the best solution to this terminological problem is to introduce separate, connectivity-related terms for these two cell groups. We are not naive to the inherent resistance to any change in conventional nomenclature, but believe that over time, the proposed new terms will supplant the old, due to their scientific accuracy, and usefulness. The rest of this article will be directed at providing a more detailed understanding of how the term EW came to represent two different sets of neurons, and explaining the important functions EWpg and EWcp subserve. In order to remain faithful to the historical usage of EW in the papers we summarize, we will use the term EW throughout the following review.
Historical perspective
As reviewed by Warwick (1954), the study of the anatomy of the orbital contents can be traced back to Fallopius (1600) who described a postganglionic plexiform junction between the third and the fifth nerves in the orbit. The structure of the oculomotor complex and its role in controlling specific extraocular muscles has been known since the work of Stilling (1846) and von Gudden (1881). The role of the specialized smooth muscles controlling the papillary constrictor and ciliary muscles, and their innervation by the ciliary ganglion (CG) was established in the 1800s (for review, Loewenfeld, 1993). The short ciliary nerves and their origin in the third nerve were first described in 1664 by Willis. In 1701, Schacher described the short ciliary nerves collectively as a ganglion, and a connection with the trigeminal nerve was first described in humans by Eustachius in 1714 (see also Meckel, 1748). Later, Winslow (1732) and Haller (1743) recognized the actual structure of the short ciliary nerves and their ganglion, as well as the motor and sensory root, in humans. In 1815 and 1842, respectively, Muck, and then Longet cut the short ciliary nerves and observed pupillary dilation. In the 19th century, electrical impulses were used to stimulate the short ciliary nerves, thereby producing pupillary constriction (Mayo, 1823; Hall, 1846; Bernard, 1852; Budge, 1855).
The central source of input to the CG was identified in the latter half of the 19th century. In 1857, a Russian anatomist (Jacubowich, 1857) described a group of cells in the periaqueductal gray above III, extending from its rostral pole nearly to its caudal pole. However, the discovery of the cells of origin of the third nerve parasympathetic outflow to the CG is credited to Edinger and Westphal due to papers each published based on human material (Edinger, 1885; Westphal, 1887). Ludwig Edinger described small neurons associated with the upper and lower medial edges of the rostral oculomotor nucleus. Carl Friedrich Otto Westphal noted the prior evidence for pupillomotor control from the oculomotor area (Hensen and Volckers, 1868), and then described a clinical case from which he inferred that it was actually a small-celled neuronal group above III, which was involved in accommodation and pupil constriction during near vision. In this case, eye movement was lost, but verge-related pupillary constriction and lens accommodation were maintained after lesions that involved III, but not the area above it. In 1888, Spitzka described 3 similar human cases, and independently ascribed the losses to involvement of a collection of cells he noted dorsal to III.
Perlia (1893) described a specific EW subdivision in humans that consisted of two parallel lines of cells located immediately dorsomedial to III, and also described a group of cells, located anterior to the rostral pole of III, which he called the anteromedian nucleus (AM). The term AM was subsequently used for a medial cell group merging with EW cells at rostral levels, and therefore considered an extension of EW by many authors (Perlia, 1893; Brouwer, 1918; le Gros Clark, 1926; Adler, 1933), but others included regions within AM that do not contain preganglionic motoneurons (Figs. 3, 6&7; Benjamin, 1939). In addition, Perlia also described the presence of a circumscribed midline cell group between III at rostral levels. Originally this almond-shaped nucleus was termed the “central nucleus” (Perlia, 1893) or “median nucleus” (Bernheimer, 1897). Later it became referred to as the “nucleus of Perlia” (NP) (Olszewski and Baxter, 1982). NP may be unique to humans, as a systematic study of 95 monkey brains revealed only 9% with a clear midline group, which might correspond to the NP (Warwick, 1955), and it was not observed in other species (le Gros Clark, 1926). While a role in various aspects of the near triad has been proposed for NP (Knies, 1891; Majano, 1903; Brouwer, 1918; Adler, 1933; Leathart, 1941), it is likely that NP is simply a medial part of III, which has become separated by descending fiber bundles
Although there was early disagreement (von Bechterew, 1887; Zeri, 1895; Juliusberger and Kaplan, 1899; Bach, 1906), studies by a number of authors confirmed the involvement of EW in third nerve autonomic regulation, based on clinical – pathological correlations and experimental studies (Bernheimer, 1897; 1901; Marina, 1899; Lenz, 1928, 1929; Ranson and Magoun, 1933). Thus, it became an accepted fact that the EW was a preganglionic parasympathetic part of the oculomotor complex involved in key autonomic functions of the eye: pupillary constriction and lens accommodation.
Structural perspective
EW in non-human primates
In support of this autonomic role for EW, early degeneration studies in macaque monkeys described the preganglionic neurons innervating CG as forming a narrow cellular column dorsal to III (Warwick, 1954). Warwick studied the effects of a variety of CG-related lesions in monkeys and cats. All lesions which affected the short ciliary nerves resulted in chromatolysis in 97% of the CG cells. However, only 3% of the CG cells presented chromatolysis after iridectomy, suggesting the pupillary component of postganglionic motoneurons is small. Following ciliary ganglionectomy or transection of the third cranial nerve in monkeys, Warwick observed a retrograde, ipsilateral reaction in most of the cells of EW, as well as many in AM. Therefore, he concluded that both EW and AM are sources of the parasympathetic component of the oculomotor complex.
Contemporary retrograde tracer studies in Old and New World monkeys have supported Warwick’s observations, and demonstrated that the preganglionic parasympathetic neurons, supplying the ipsilateral CG, do in fact lie in rostrocaudally oriented columns on either side of the midline, dorsal to III (Figs. 1,6&7; Akert et al., 1980; Burde and Loewy, 1980; Jaeger and Benevento, 1980; Clarke et al., 1985). Rostrally, each column of preganglionic neurons extends slightly beyond III, where it occupies a portion of AM (Figs. 1,6&7; Akert et al., 1980; May et al., 1992). It has been argued that there is no justification for distinguishing the AM from EW, and that both could be considered parts of EW (e.g., Gamlin, 2000). Indeed, based on the retrograde labelling of preganglionic EW neurons, it is clear that the anteriorly located, preganglionic neurons within AM are anatomically continuous with preganglionic neurons within EW (Akert et al., 1980). On the other hand, the majority of cells within the cytoarchitectonic confines of AM in primates are not preganglionic neurons (Horn et al., 2008; May et al., 2008a). Other investigators have suggested that preganglionic neurons also lie in NP (Figs. 1,6&7; Burde and Loewy, 1980; Ishikawa et al., 1990). This suggestion was based on a few, scattered retrogradely labelled neurons located on the midline between III following injections of retrograde tracers into CG. However, these cases often also exhibited labelling of the somatic motoneurons in III, so these midline cells in monkeys may represent somatic motoneurons, more specifically those of the S-group that supply the multiply-innervated muscle fibers of the inferior oblique and superior rectus muscles (Warwick, 1955; Büttner-Ennever et al., 2001; Wasicky et al., 2004).
Subsequent retrograde labeling results showed that the arrangement in green monkeys, red monkeys, and the prosimian galago generally is the same as demonstrated in macaques (Sun and May, 1993; May et al., 2008b). Some differences are present: the preganglionic neurons of the red monkey and galago are organized as a single, midline nucleus, instead of paired columns.
Burde and Loewy (1980) injected retrograde tracers into the CG of monkeys and reported labeled cells in EW, AM and NP. The latter may represent the S-group (see above). In a more detailed analysis of this pattern of retrograde labeling, Burde (1988) and Burde and Williams (1989) reported that the nuclei projecting to the CG could be divided into lateral and medial visceral columns, with the latter subdivided into dorsal and ventral subgroups. However, more recent studies (May et al., 2008b) have failed to find these subdivisions, supporting the initial description of Akert et al. (1980). Burde and colleagues also used intraocular injections of WGA-HRP to test for the existence of a direct parasympathetic pathway to the ciliary muscle in monkeys, and found label in a few central cells (Parelman et al., 1984; Burde, 1988). However, there is evidence WGA or WGA-HRP can be transported trans-synaptically following an injection into a peripheral target (Porter et al., 1985; Erichsen and May, 2002). It is also possible that the tracer spread to accessory ganglia (Kuchiiwa et al., 1989; 1994; Gamlin, 2000).
EW in non-primate mammalian species
These primate studies established the concept of EW as a preganglionic neuron group lying above III. Initially, as defined by cytoarchitecture, a broad territory with several subdivisions came to be called EW in primates (Brouwer, 1918). Following this approach, Le Gros Clark (1926) and others (Zweig, 1921; Crosby and Woodburne, 1943) then identified an EW above III in a series of mammals based solely on cytoarchitecture and location. Some differences were observed. The cytoarchitectonically defined EW of the cat formed a single midline nucleus dorsal to III. That of the mouse was not only a single structure, it actually lay on the midline between the two sides of III. Subsequently, any distinct cell group occupying this area was assumed to be EW, and was believed to project to CG.
However, from early on, problems with this concept arose. For example, the claim by Bernheimer (1897) of retrograde degeneration in the presumptive EW of the rabbit after damaging the oculomotor nerve was not substantiated by the subsequent studies of van Biervliet (1899) or Levinsohn (1904). Similarly, removal of the ciliary ganglia of cats revealed minimal changes in their EWs (Crouch, 1936; Hogg, 1966). This study by Crouch was one of the very first attempts to understand the relationship between EW and the CG. The results of three types of lesions were examined in cats: removal of the CG; removal of the eyeball; and sectioning of the third cranial nerve. The author reached the following conclusions: a) extirpation of the CG does not cause sufficient chromatolysis in EW to indicate that fibers project from the nucleus to the ganglion; b) both enucleation of the orbital contents and cutting the third nerve results in chromatolysis of many EW cells, indicating that EW fibers arrive at the orbit via the third cranial nerve. In light of current evidence, one wonders whether this degeneration was trans-synaptic. Szentágothai (1942) reported that only one-fifth of EW neurons degenerate after CG removal in cat. These were the larger of the cells located rostral and dorsomedial to III. He hypothesized that the smaller EW neurons might have some “vegetative functions, like neurons in the hypothalamus”.
The advent of neuronal tracing techniques emphasized this problem, by demonstrating important differences between cats and monkeys in the source of parasympathetic outflow (Figs. 6&7; Loewy et al., 1978; Sugimoto et al., 1978; Toyoshima et al., 1980). The feline EW is a cytoarchitectonically distinct midline nucleus located immediately dorsal to III. However, as emphasized by Loewy and Saper (1978) and demonstrated by several other investigators (Saper et al, 1976; Sugimoto et al., 1977; Loewy et al., 1978; Toyoshima et al., 1980), most of the labeled preganglionic neurons were not found within the confines of this nucleus. This result was quantified by Erichsen and May (2002), who showed that EW contains less than 5% of the preganglionic population. The rest are scattered in the supraoculomotor area, along the midline between III, in and around AM, and among the exiting rootlets of the third nerve.
As more mammalian species have been investigated, it has become evident that the location of the preganglionic neuron population differs appreciably from species to species, and it is rarely synonymous with the cytoarchitectural borders of EW. In rabbits, the preganglionic neurons are largely found among the exiting third nerve rootlets (Johnson and Purves, 1981). Rodents also have a cytoarchitecturally designated EW (Figs. 5,6&7). It is a small, paired nucleus located on either side of the midline, between III in mice (Franklin and Paxinos, 1997), and it has a slightly more dorsal location in rats (Paxinos and Watson, 1998). The preganglionic neuron arrangement in rodents is less well understood because the small size of the CG makes it a difficult injection target. Inoculations of the anterior chamber with pseudorabies virus, a transneuronal retrograde marker, have been employed in rodents to overcome this difficulty (mouse: Vann and Atherton, 1991; rat: Smeraski et al., 2004; Kozicz, 2007). These studies suggest that the preganglionic motoneurons are mainly located rostral and dorsal to III, and are not confined within the cytoarchitectonically defined EW. In fact, a small injection of an anterograde tracer into the area rostral to the rat III results in terminal labeling within the CG (Klooster et al., 1993).
EW in birds and reptiles
Like that of monkeys, the EW observed in birds has come to be recognized as a distinct nucleus dorsal to III (Figs. 2,6&7; Narayanan and Narayanan, 1976; Lyman and Mugnaini, 1980; Reiner et al., 1991; Toledo et al., 2002). However, the avian EW is located dorsolateral to III, and is surrounded by a cell free and glial fiber rich zone. Furthermore, the avian EW only contains preganglionic neurons. Thus, the avian EW is even more circumscribed and distinct than that of primates (Reiner et al., 1991). As in primates, its neurons innervate the CG, which controls the pupil and lens (Marwitt et al., 1971; Pilar and Tuttle, 1982). However, the avian CG also clearly innervates and controls choroidal blood vessels (Pilar and Tuttle, 1982; Fitzgerald et al., 1996). In reptiles as well, the EW is a circumscribed nucleus dorsolateral to the somatomotor neurons of the oculomotor nucleus that innervates the ciliary ganglion (Barbas-Henry and Lohman, 1988; Medina et al., 1993; Powers and Reiner 1993). Thus, a cytoarchitectonically distinct EW projecting to the ciliary ganglion is likely to have been the ancestral condition for the bird-reptile radiation from stem amniotes.
Taken together, the studies in mammals, birds and reptiles provide strong evidence that the cytoarchitectonically-defined EW in monkeys, birds and reptiles is equivalent to EWpg in the proposed nomenclature, as it contains motor neurons supplying autonomic fibers to the III nerve. By contrast the evidence for an involvement of the cytoarchitectonically defined EW in oculomotor function in other mammalian species was weak, if present.
Central projections of EW
Connectional studies in the 70s and 80s provided additional insight into the nature of cytoarchitectonically-defined EW in mammals. The first study revealing that some component of what has been considered EW, in fact, had central projections was that of Saper and colleagues in 1976. HRP injections into the spinal cord of rats, cats and monkeys revealed a significant number of labeled cells in EW. This finding was confirmed by Loewy and colleagues in 1978. In a subsequent study, Loewy and Saper (1978) used [3H]-labeled amino acids as an anterograde tracer in order to define the central targets of EW in cats. Projections were found to the dorsal accessory, parabrachial, subtrigeminal, spinal trigeminal, gracile and medial cuneate nuclei, as well as to the spinal cord. As a retrograde labeling control, the authors injected HRP into the dorsal column nuclei, spinal trigeminal nucleus, inferior olivary nucleus, or spinal cord, and in each case found retrograde label in EW. They concluded that the conventional view of EW as a source of preganglionic outflow to CG needed to be reconsidered for the cat. Klooster et al. (1993) used Phaseolus vulgaris leucoagglutinin (PhAL) to map the efferents of the rat EW, and saw a similar pattern. The central descending pathway terminated in the inferior olivary nucleus, the parabrachial nuclei, the facial nucleus, the trigeminal nuclear complex, the rostroventrolateral reticular nucleus (C1 adrenergic neurons).
The cerebellum was added to the list of EW and AM targets in the cat by Chung et al. (1987). A similar projection was observed in the rat (Roste and Dietrichs, 1988). In the context of our argument, it is noteworthy that these authors suggested that this projection might be related to controlling extraocular, as opposed to intraocular, muscles. In contrast, monkeys show cerebellar projections from AM, but not EW (May et al., 1992; Sekiya et al., 1984).
Jansen et al. (1993) used trans-synaptic transport of pseudorabies virus to study the regions of the central nervous system that provide higher-order input to the rat stellate ganglion. Surprisingly, EW was among the nuclei in which the authors detected infection with the virus. This was interpreted as evidence for a direct connection between a parasympathetic motor nucleus and a sympathetic one. Such a possibility had been raised by the fact that Loewy et al. (1973) localized ascending inhibitory (pupillodilator) fibers from the spinal cord projecting to EW by use of electrical stimulation. In retrospect, it must be noted that the rat EW turned out not to be the location of preganglionic parasympathetic neurons.
It is noteworthy that in birds and reptiles, neurons in the vicinity of the circumscribed cell group projecting to the ciliary ganglion (i.e. EW) project to the spinal cord (ten Donkelaar et al., 1980; Cabot et al., 1982; Wolters et al., 1986). Due to the circumscribed nature of the avian and reptilian EW, it was clear that the dorsal midbrain cells projecting to the spinal cord do not belong to EW, and must be comparable to the EW neurons in mammals with central projections.
Thus, the presence of central projections is a defining characteristic of the EWcp population, even as the presence of peripheral projections defines EWpg (Fig. 8).
Inputs to EW
The light reflex input to EWpg arises from area pretectalis in birds and the olivary pretectal area in mammals. A clear ocular role for EW in primates and birds was confirmed by demonstrating input from pupillary control neurons of the pretectum (Pierson and Carpenter, 1974; Steiger and Büttner-Ennever, 1979; Gamlin et al., 1984). This pretectal area in mammals primarily contains wide-field luminance units (Pong and Fuchs, 2000; Clarke et al., 2003). Stimulation of this pretectal area constricts the pupil, and lesions of this area cause a loss of the pupillary light reflex (Reiner et al., 1983; Gamlin et al., 1984). There are species-specific variations in the laterality of the pretectal projection to EW. For example, birds display an entirely crossed pathway (Gamlin et al., 1984; Gamlin and Reiner, 1991), while primates show both ipsilateral and crossed projections (see Loewenfeld, 1993), although there is conflicting evidence for the ipsilateral projection (Steiger and Büttner-Ennever, 1978).
Inputs from the olivary pretectal nucleus have been shown to end directly on a subset of CG preganglionic EW neurons in primates and birds (Gamlin et al., 1984; May et al., 2008b). In birds, the pretectal input is selective for the caudolateral part of EW (Reiner et al., 1983; Gamlin et al., 1984). However, the precise targets of the pretectal projection within the primate EW have been in dispute. In monkeys, both conventional anterograde tracers placed in the pretectum and transneuronal anterograde tracers placed in the vitreous have been interpreted as suggesting the pupillary component lies in an EW subdivision termed the lateral visceral column (Benevento et al., 1977; Baleydier et al., 1990; Büttner-Ennever et al., 1996; Kourouyan and Horton, 1997). Others have shown, however, that this pretectal target region does not contain preganglionic motoneurons (Gamlin, 2000; Horn et al., 2008; May et al., 2008a). Instead, there is evidence that pretectal terminals form close associations with CG-projecting neurons in the ventromedial portion of the middle third of EW, suggesting this specific part of EW mediates pupillary control in monkeys (Clarke et al., 2003; May et al., 2008b).
In the monkey, injections of HRP that included both EW and III only labeled the olivary pretectal nuclei, and nuclei related to the oculomotor system (Steiger and Büttner-Ennever, 1979). In contrast, in a study of EW afferents in cats (Breen et al., 1983), not only were retrogradely labeled neurons identified in the olivary pretectal nuclei, they were also found in many other central nuclei that are not known to be related to the oculomotor system, including: the ventral tegmental area, the pars reticulata of the substantia nigra, the parabrachial nuclei, the locus coeruleus and subcoeruleus area, the nucleus incertus, the raphe nuclei and the nucleus of the solitary tract. In fact, projections from the olivary pretectal nucleus to EW were not seen in cats by anterograde means (Berman, 1977). This may be due to the fact, that more rostral portions of the cat preganglionic population in and around AM are related to pupillary control, while more caudal populations control lens accommodation (Erichsen and May, 2002).
The sources of lens accommodation signals to EW neurons are even less well understood (Gamlin, 1999). A midbrain near response region in the vicinity of III has been demonstrated based on recordings in macaques (Mays and Porter, 1984; Mays and Gamlin, 1995). In addition, cells whose activity is related to vergence and lens accommodation have been recorded in parietal and frontal cortex, the cerebellar nuclei and nucleus reticularis tegmenti pontis (Hiraoka and Shimamura, 1989; Gamlin and Yoon, 2000). However, there is little known about how these signals access the accommodative preganglionic neurons in EW (Gamlin, 1999; May et al., 1992).
In rats, medial cerebellar and vestibular nuclei injections produced labeled terminals in EW, as well as in many oculomotor-related areas of the brainstem (Buisseret-Delmas et al., 1998). Utilizing anterograde tracers, Balaban (2003) described topographically organized projections from the vestibular nuclei to the ipsilateral EW and AM of rabbits. Based on the assumption that EW contained preganglionic neurons, they concluded that this projection could facilitate lens accommodation, pupillary constriction and regulation of intraocular blood flow in response to vestibular self-motion signals, even though the vestibular projections were found to target ChAT-negative (and weakly immunoreactive) neurons in EW. However, as we have noted, the preganglionic neurons in the rabbit primarily lie beneath III, not in EW.
In summary, in those mammals where EWpg corresponds to the cytoarchitecturally-defined EW, the inputs related to pupillary control have been clearly delineated, but lens control-related inputs are yet to be defined. A much more diverse group of inputs supplies the cytoarchitecturally-defined EW when it is in fact part of the EWcp population. This dichotomy is illustrated in Figure 9, which emphasizes the need for more detailed investigation of the sources of input in light of the distinction between EWpg and EWcp.
Figure 9.
Schematic diagram of a midsagittal section through the rat brain to summarize the known sources of inputs supplying the EWpg and EWcp (together EW). Possible species differences are not indicated. Abbreviations: Cb, cerebellum; CG, ciliary ganglion; Cx, cerebral cortex; H, hypophysis; IO, inferior olivary nucleus; LPB, lateral parabrachial nucleus; LRN, lateral reticular nucleus; LS, lateral septal nucleus; MeON, medial olivary nucleus; MON, main olivary nucleus; Ob, Olfactory bulb; RVRN, rostroventral reticular nucleus; V complex, trigeminal complex; VII, facial nucleus. The shaded gray areas represent the corpus callosum, fornix, anterior commissure and the optical chiasm and tract.
Neurochemical evidence for inconsistencies in the role of EW
Since parasympathetic preganglionic neurons are cholinergic, ChAT immunostaining has recently been used in non-human primates to define the CG preganglionic neuron distribution (Figs. 1, 6&7; Horn et al., 2008; May et al., 2008a). Like retrograde labeling, this approach also reveals a pair of unitary cell columns dorsal to the III, indicating that the primate EW is not subdivided into several discrete visceral columns (Warwick, 1954; Burde, 1988). The preganglionic neurons in the bird and reptiles are also cholinergic and confined to EW (Fig. 3, 6&7; Medina et al., 1993; Powers et al., 1993; Cavani et al., 2003). However in cats, ChAT staining supports the contention that the cytoarchitecturally-defined EW and the source of the input to CG lie in different locations. Presumably this projection to CG originates from ChAT-positive neurons scattered between, dorsal, ventral and anterior to the somatic motoneurons of III (Figs. 4,6&7; Strassman et al., 1987; May et al., 2008a). The rodent EW also contains few ChAT-positive neurons (Figs. 5,6&7; Weitemier et al., 2005). In humans, ChAT staining indicates that preganglionic neurons are located dorsal to III in an indistinct column (Figs. 2,6&7; Ryabinin et al., 2005; Horn et al., 2008). However, in contradistinction to other primates, human ChAT positive motoneurons are not located in the cytoarchitecturally-defined EW of Olszewski and Baxter (1982), and so probably do not lie within the small-celled nucleus originally observed by Edinger (1885) and Perlia (1893). Taken together, the studies on projections and ChAT staining show that a cytoarchitecturally defined EW is the source of the cholinergic projection to CG in non-human primates, birds and reptiles, but is not in many other species. To further address these contradictions, combined connectional and immunohistochemical studies have been undertaken.
Neurochemical studies showed that not only does EW of many species project centrally, these central projections are not cholinergic (Hökfelt et al., 1976). Strassman et al. (1987) performed double-labeling experiments in cats, combining HRP injections into the CG with Nuclear Yellow injections into the spinal cord or cerebellum. The retrograde labeling was further combined with immunohistochemistry for ChAT. Neurons projecting to the CG expressed strong ChAT-immunoreactivity, but were primarily located outside EW and AM. Neurons around and within EW and AM that projected to the cerebellum and the spinal cord were not cholinergic. In addition to confirming that EW and AM project to the cervical and lumbar levels of the spinal cord in cats, Phipps et al. (1983) combined the tracer techniques with immunohistochemistry to prove that many of the relevant neurons are substance P-like immunoreactive. In a follow-up study, Maciewicz et al. (1984) demonstrated that some cat EW neurons projecting to the spinal cord and/or trigeminal complex are also cholecystokinin-immunoreactive. Finally, Otake (2005) studied the source of afferents of the paraventricular nucleus of the thalamus in rat by combining retrograde labeling with immunohistochemistry for cholecystokinin and substance P. Neurons projecting to paraventricular thalamus that contained both neuropeptides were distributed throughout EW, although principally at the rostral levels (see also Innis and Aghajanian, 1986).
Renewed attention was paid to these early studies showing the existence of non-oculomotor, peptidergic and centrally projecting components to the classically described EW in rodents, following the discovery of two new neuropeptides, viz. Ucn1 (Vaughan et al., 1995) and cocaine-and amphetamine-regulated transcript (CART; Koylu et al., 1998), in the late 1990s. When the brain distributions of these neuropeptides were subsequently mapped in the rat (Douglass and Daoud, 1996; Koylu et al., 1998; Kozicz et al., 1998; Bittencourt et al., 1999), most of the immunohistochemically defined neurons were reported to lie in EW. Previously, Chung et al. (1987) had used a combination of a retrograde tracer (HRP) injection into the cervical spinal cord and immunohistochemistry for corticotropin-releasing factor (CRF) in cats to demonstrate double-labeled cells throughout EW and AM. However, it was later proven that those cells were in fact immunoreactive to Ucn1 and not to CRF (Bittencourt et al., 1999). Bittencourt and colleagues (1999) placed retrograde fluorescent tracers in the upper thoracic spinal cord and the lateral septal nucleus of rats, because Ucn1 terminals had been identified in both regions (Vaughan et al., 1995). They found sets of Ucn1-positive double-labeled cells in EW from both injections. Thus, the rat EW gives rise to ascending and descending projections, and the ascending and descending projections arise from different Ucn1-positive populations. Ascending projections were also found to target the rat ventral tegmental area and substantia nigra (Yamamoto et al., 1998). In birds, Ucn1-positive neurons were found in the cell group near EWpg that was known to project to the spinal cord (Cavani et al., 2003).
Since the EWcp contains the vast majority of Ucn1-positive cells in the CNS, it is presumably the source of the widespread urocortinergic fiber projection (rat: Bittencourt, 1999; mouse: Weitemier et al., 2005; monkey: Vasconcelos et al., 2003). Further evidence for this comes from Bachtell et al. (2004), who performed electrolytic lesions of the cytoarchitectonically-defined EW in the mouse, and observed a significant reduction of Ucn1-positive fibers in the lateral septum and the dorsal raphe. This pattern is widespread, considering that neuropeptides, including those already mentioned and recent additions, i.e.: neuropeptide B and nesfatin, have been observed in EW of other species (frog: Kozicz et al., 2002; Lazar et al., 2004; mouse: Bachtell et al., 2002a&b; Tanaka et al., 2003; rat: Dun et al., 2005; Foo et al., 2008; Xu et al., 2009; vole: Lim et al., 2006).
The results summarized above were surprising in that they contradicted “textbook” views that only ascribed ocular functions to EW. Today, it is clear that the neurons with central projections are not the same as those that project to the CG. Nonetheless, because these EWcp cells occupy the nucleus previously identified as EW in some species, while ChAT-positive neurons of the EWpg occupy EW in other species, considerable confusion was generated in the literature. The question as to why EWpg is a distinct compact nucleus in some species but not others is likely to have an evolutionary basis. The ubiquity of this trait in birds and reptiles suggests it evolved early in this lineage as a corrollary of the emergence of relatively large eyes and keen visual ability (Ott, 2006). The general absence of this feature may be a reflection of the apparent nocturnal bottleneck and reduced visual reliance early in mammalian evolution (Heesy and Hall, 2010), with compactness separately emerging in primates in association with large frontal eyes and keen vision.
Functional perspective
Cholinergic projections controlling the lens and pupil
Anatomical results suggesting that the monkey EW contains cholinergic preganglionic neurons controlling the lens and pupil are supported by numerous physiological investigations, some of which were noted above. Additionally, records from units recorded in the macaque monkey EW demonstrate that their firing rates are correlated with the activity of the ciliary and sphincter pupillae muscles (Gamlin et al., 1994; Gamlin, 2000). Furthermore, EW mediates lens accommodation and pupillary constriction in primates, birds and reptiles (Reiner et al., 1983; Crawford et al., 1989; Vilupuru and Glasser, 2005; Ott, 2006; Dearworth et al., 2009). EW function has been discussed in the preceding historical review, and for more detailed accounts of the physiological and functional aspects of EWpg in oculomotor autonomic function see reviews by Gamlin, (1999, 2000).
Centrally projecting EW neurons
In the last fifteen years, work on CART, but especially on Ucn1 neurons in the rodent midbrain has markedly expanded our insights into the functional significance of centrally projecting EW neurons. An increasing body of evidence implies that EWcp neurons sex-dependently control and/or modulate stress-related physiological and behavioral responses, as well as possess a unique role in consumption of rewarding substances, suggesting that EWcp neurons are in a unique position to integrate stress- and reward-related processes. This intriguing notion, however, remains to be tested in future studies.
Ucn1 and stress (mal)adaptation
An increasing body of evidence suggests the involvement of the midbrain Ucn1 cells in stress adaptation. This intriguing hypothesis was first raised by the groups of Majzoub (Weninger et al., 2000), and Kozicz (Kozicz et al., 2001), who reported that acute restraint and pain stress recruited Ucn1 neurons, and up-regulated their Ucn1 mRNA expression. Subsequent studies have revealed that the activation of Ucn1 neurons is stressor-specific in rats (Gaszner et al., 2004). In addition, surgical intervention (a form of acute stress) also induces strong Fos and Fra-2 expression in EW indicating that EW neurons are sensitive to altered body homeostasis (Lantéri-Minet et al., 1994; Palkovits et al., 2009).
Restraint stress similarly increased the expression of egr-1 (another inducible transcription factor) in bird Ucn1 cells (Cunha et al., 2007). In mice, however, no induction of Fos or egr-1-ir has been reported following various acute stressors (Turek and Ryabinin, 2005; Spangler et al., 2009) suggesting that the stress responsiveness of EW neurons is species-specific. In agreement with this idea, electrolytic lesions of this brain area in the mouse, which produce several other robust behavioral effects, do not affect anxiety-like behavior in the plus maze, locomotor behavior in the open field, or levels of plasma corticosterone (Weitemier and Ryabinin, 2005b).
Interestingly, the dynamics of activation of the classical hypothalamic-pituitary-adrenal (HPA) axis and that of Ucn1 neurons are different (usually modulating in opposing directions) (Viau and Sawchenko, 2002; Korosi et al., 2005; Kozicz et al., 2008a). Consequently, the hypothesis has been put forward that the complementary dynamics of Ucn1 serves to terminate the central stress responses (Kozicz, 2007). Collaboration between the two systems would imply Ucn1 has an important role in adaptation to stress, and, as a consequence, in stress-related disorders.
To provide evidence for this notion, three mouse models with null mutations of the Ucn1 gene have been generated. One of these showed significantly heightened anxiety-like behavior (Vetter et al., 2002), but a normal HPA-axis response to stress, and no change in CRF mRNA expression (Vetter et al., 2002). These findings suggest endogenous Ucn1 may not be directly involved in regulation of the HPA system in acute stress, or has a minor, redundant role. Instead, its role may lie in regulating the HPA axis response to chronic challenges. In line with this, another model with a null mutation in the Ucn1 gene showed impaired adaptation to repeated restraint (Zalutskaya et al., 2007). However, it must be noted that a third mouse line, did not show any change in anxiety-like behavior (Wang et al., 2002). In a recent study, Neufeld-Cohen et al. (2010) demonstrated decreased anxiety and altered forebrain serotonergic functioning in Ucn1/Ucn2 double knock-out mice. This, together with the fact that Ucn1 neurons project to the dorsal raphe nucleus, clearly puts the EWcp urocortinergic neurons in a critical position to control dorsal raphe serotonergic activity, and thereby modulate the response of the HPA axis to stress (Kozicz, 2010).
The involvement of CRF in stress-related psychopathologies is beyond dispute (for reviews, see: Joels et al., 2008; de Kloet, 2008). However, increasing evidence suggests a role for EW Ucn1 neurons in the development of stress-related mood disorders (Kozicz et al., 2008b). In fact, stress-induced increases in neuronal activity in Ucn1 neurons may represent an element of the organism’s strategy to cope with the stressor (Weninger et al., 2000; Kozicz et al., 2001; Gaszner et al., 2004; Korosi et al., 2005; Kozicz et al., 2008a). There is also pharmacological evidence indicating specific roles for Ucn1 neurons in anxiogenesis. Specifically, the anxiolytic properties of benzodiazepines and selective agonists of the metabotropic glutamate receptors manifest, at least in part, via regulation of Ucn1 neuron activity (Skelton et al., 2000; Linden et al., 2004; 2006).
Sex differences
Recently, estrogen receptor (ER) beta, but not ER alpha, was found to co-exist with Ucn1 in mouse EW neurons (Derks et al., 2007; 2009). Haeger et al. (2005) demonstrated that estrogen decreases the transcriptional activity of the Ucn1 promoter acting via ERβ. These two observations have led to the notion that Ucn1 may be responsible, at least in part, for gender-differences in stress sensitivity in rodents, and possibly in humans. In agreement with this idea, recent studies showed that female rats possess significantly higher levels of Ucn1 immunoreactivity than male rats (Fonareva et al., 2009). In addition, Ucn1 immunoreactivity decreases during the course of pregnancy in rats (Fatima et al., 2007). The intriguing idea of regulation of Ucn1 by sex hormones needs further investigation.
In this light, it is useful to note that Ucn1 mRNA levels in EW neurons are significantly higher in male, but unchanged in female, depressed suicide victims. In addition, male suicide victims had significantly more Ucn1 in their EW neurons than female suicide victims (Kozicz et al., 2008b). These data strongly suggest that imbalanced activity of HPA axis and Ucn1 neurons may both contribute to the pathogenesis of depression and suicide in a sex-dependent manner (Kozicz, 2007).
Involvement of Ucn1 in food intake and addiction
Besides a role in anxiety and depression-like behavior, EW Ucn1 neurons appear to be involved in regulating intake of food, alcohol and addictive drugs. Several lines of evidence confirm this idea.
First, several genes involved in regulating intake of alcohol, drugs of abuse and food (e.g. Ucn, Cart, Cck, Ghsr1) are expressed in EW cells, and their expression occurs at levels higher than in areas traditionally associated with reward (Maciewicz et al., 1984; Douglass and Daoud, 1996; Koylu et al., 1998; Kozicz et al., 1998; Bittencourt et al., 1999; Ryabinin and Weitemier, 2006; Zigman et al., 2006).
Second, Ucn1 administration potently regulates food consumption (Spina et al., 1996), and rats fed high-fat diets showed a decrease in Ucn1 mRNA expression (Legendre et al, 2007). Double-labeling immunohistochemistry revealed that almost all rat Ucn1 neurons expressed leptin receptor, and 2-day fasting caused a marked upregulation of Ucn1 mRNA in male (but not in female) rats (Xu et al., 2009). The involvement of Ucn1 neurons in regulation of food consumption could be evolutionary conserved, as studies in Xenopus laevis show that food deprivation decrease CART immunoreactivity in EW neurons (Calle et al., 2006).
Third, Ucn1 cells are highly sensitive to administration of alcohol and drugs of abuse. Thus, administration of alcohol, ether, benzodiazepines, barbiturates, morphine, cocaine, amphetamine, methamphetamine and gamma-hydroxybutyric acid all result in increased Fos-ir in rodent EW neurons (Chang et al., 1995; Ryabinin et al., 1995; Bachtell et al., 2002b; Gaszner et al., 2004; Singh et al., 2004; Singh et al., 2006; van Nieuwenhuijzen et al., 2009). This response to drugs of abuse is independent of the stress of drug administration (Turek et al., 2005; Spangler et al., 2009). Thus, EW neurons show a robust and brain region-selective Fos response, not only after passive administration of drugs of abuse by an experimenter, but also when animals self-administer addictive substances, such as alcohol (Topple et al., 1998; Bachtell et al., 1999; Ryabinin, et al., 2001; Weitemier et al., 2001).
Fourth, multiple genetic studies have demonstrated that lines of mice and rats with high alcohol intake have higher Ucn1 levels than rodent lines with low alcohol intake. This suggests that the Ucn1 is involved in genetic predisposition to high alcohol consumption (Bachtell et al., 2002a; 2003; Fonareva et al., 2009; Turek et al., 2005; Weitemier and Ryabinin, 2005b)
Fifth, lesions of the rodent EW greatly attenuate ethanol preference, food and water consumption, but not preference for saccharine or saline solutions (Bachtell et al., 2004; Weitemier and Ryabinin, 2005b; Weitemier and Ryabinin, 2006). Interestingly, these anorexic effects of lesions are not accompanied by a decrease in body weight. The latter finding suggests that the effects of EW lesions on food are mediated by mechanisms that regulate energy metabolism.
Taken together, studies on addictive drugs and eating and drinking behaviors suggest that Ucn1 neurons of EW could have a unique role in consumption of highly rewarding substances.
Summary and solution for the nomenclature problem
Thus, to the surprise of many researchers, EW has been found to be involved in stress-related activity and regulation of alcohol and food consumption, in addition to being the final common pathway for control of lens accommodation and pupillary constriction. The designation EW has become confusing because it refers to two neuroanatomically and functionally distinct neuron populations, viz. a cholinergic, peripherally projecting motoneuron population that mediates pupil and lens control, as well as a peptidergic, centrally projecting cell group with no known role in ocular function. Consequently, adoption of new terminology that discriminates between these populations has become unavoidable. We propose a novel, connectivity-based, nomenclature solution in which the cholinergic group of cells with parasympathetic oculomotor function is termed EWpg, and the peptidergic group with consumption and stress-related functions is termed EWcp. Unlike the old name, these new terms alert the reader to the presence of the two populations adjacent to III, while at the same time acknowledging that they have different distributions, connections, chemistry and functions. Hopefully this new terminology will facilitate further investigation into the inputs and functions of these distinct populations. Such investigations may reveal whether their physical proximity in pIII along with other groups like the C- and S-group motoneurons is simply a side effect of development, or whether it reflects a need to share inputs.
Acknowledgments
T.K. would like to acknowledge the Nederlands Organization for Scientific Research NWO (#864.05.008) for giving support for this work. J.C.B. would like to thank Jeff Boyles for revising the manuscript. J.C.B. would also like to thank Joelcimar Martins and Amanda de Oliveira for the technical support and Dr. Luciane V. Sita for their help in the illustrations. J.C. B. acknowledges the support of The Fundação de Amparo à Pesquisa do Estado de Sao Paulo (FAPESP Grant # 2004/13849-5). J.C.B. is a CNPq Investigator. P.J.M. acknowledges the support of NIH grants EY07166 and EY066315 and the help of Susan Warren, PhD in proofing the manuscript. A.R. acknowledges the support of NIH Grant EY-05298. A.E.R acknowledges the support of NIH Grants AA013738 and AA016647. P.D.G acknowledges the support of NIH grants EY-07558, EY-09380, and NEI CORE grant P30 EY-03039. A.K.E.H. acknowledges the preparation of the double fluorescence preparations in rat by Christina Zeeh as part of her diploma thesis and the support by the Deutsche Forschungsgemeinschaft, DFG HO 1639/4-3. C.A.B.T. would like to thank Marcia Tsuruta and Renato Figueiredo de Santana for technical help and to the FAPESP for supporting the effort (04/11039-6 and 09/50623-9).
References
- Adler A. Zur Lokalisation des Konvergenzentrums und der Kerne der glatten Augenmuskeln. Ztschr ges Neurol Psychiat. 1933;145:185–207. [Google Scholar]
- Akert K, Glicksman MA, Lang W, Grob P, Huber A. The Edinger-Westphal nucleus in the monkey. A retrograde tracer study. Brain Res. 1980;184:491–498. doi: 10.1016/0006-8993(80)90816-1. [DOI] [PubMed] [Google Scholar]
- Bach L. Über das Verhalten der motorischen Kerngebiete nach Läsion der peripheren Nerven und uber die physiologische Bedeutung der Edinger-Westfalschen Kerne. Zbl Nervenheilk. 1906;29:140. [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Strain differences in urocortin expression in the Edinger-Westphal nucleus and its relation to alcohol-induced hypothermia. Neuroscience. 2002a;113:421–434. doi: 10.1016/s0306-4522(02)00174-4. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Alcohol-induced c-Fos expression in the Edinger-Westphal nucleus: pharmacological and signal transduction mechanisms. J Pharmacol Exp Ther. 2002b;302:516–524. doi: 10.1124/jpet.102.036046. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Wang YM, Freeman P, Risinger FO, Ryabinin AE. Alcohol drinking produces brain region-selective changes in expression of inducible transcription factors. Brain Res. 1999;847:157–165. doi: 10.1016/s0006-8993(99)02019-3. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Weitemier AZ, Galvan-Rosas A, Tsivkovskaia NO, Risinger FO, Phillips TJ, Grahame NJ, Ryabinin AE. The Edinger Westphal lateral septum urocortin pathway and its relationship to alcohol consumption. J Neurosci. 2003;23:2477–2487. doi: 10.1523/JNEUROSCI.23-06-02477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Weitemier AZ, Ryabinin AE. Lesions of the Edinger Westphal nucleus in C57BL/6J mice disrupt ethanol-induced hypothermia and ethanol consumption. Eur J Neurosci. 2004;20:1613–1623. doi: 10.1111/j.1460-9568.2004.03594.x. [DOI] [PubMed] [Google Scholar]
- Balaban CD. Vestibular nucleus projections to the Edinger-Westphal and anteromedian nuclei of rabbits. Brain Res. 2003;963:121–131. doi: 10.1016/s0006-8993(02)03955-0. [DOI] [PubMed] [Google Scholar]
- Baleydier C, Magnin M, Cooper HM. Macaque accessory optic system: II. Connections with the pretectum. J Comp Neurol. 1990;302:405–416. doi: 10.1002/cne.903020216. [DOI] [PubMed] [Google Scholar]
- Barbas-Henry HA, Lohman AHM. The motor nuclei and sesory neurons of the IIIrd, IVth, and VIth cranial nerves in the monitor lizard, Varanus exanthematicus . J Comp Neurol. 1988;267:370–386. doi: 10.1002/cne.902670307. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Rezak M, Santos A. An autoradiographic study of the projections of the pretectum in the rhesus monkey (Macaca mulatta): evidence for sensorimotor links to the thalamus and oculomotor nuclei. Brain Res. 1977;127:197–218. doi: 10.1016/0006-8993(77)90536-4. [DOI] [PubMed] [Google Scholar]
- Benjamin JW. The nucleus of the oculomotor nerve with special reference to innervation of the pupil and fibers from the pretectal region. J Nerv Ment Dis. 1939;89:294–310. [Google Scholar]
- Berman N. Connections of the pretectum in cats. J Comp Neurol. 1977;174:227–54. doi: 10.1002/cne.901740204. [DOI] [PubMed] [Google Scholar]
- Bernard C. Experiences sur les fonctions de la portion cephalique du grand sympathique. CR Soc Biol Paris. 1852;3:163–165. [Google Scholar]
- Bernheimer S. Experimentelle Studien zur Kenntnis der Innervation der inneren und äusseren vom Oculomotorius versorgten Muskeln des Auges. v Graefes Arch Ophthalmol. 1897;44:481–525. [Google Scholar]
- Bernheimer S. Die Lage des Sphinktercentrums. v Graefes Arch Ophthalmol. 1901;52:306–316. [Google Scholar]
- Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999;41:85–312. [PubMed] [Google Scholar]
- Breen LA, Burde RM, Loewy AD. Brainstem connections to the Edinger-Westphal nucleus of the cat: a retrograde tracer study. Brain Res. 1983;261:303–306. doi: 10.1016/0006-8993(83)90633-9. [DOI] [PubMed] [Google Scholar]
- Brouwer B. Klinisch-anatomische Untersuchung über den Oculomotoriuskern. Z Ges Neurol Psychiat. 1918;40:152–193. [Google Scholar]
- Buisseret-Delmas C, Angaut P, Compoint C, Diagne M, Buisseret P. Brainstem efferents from the interface between the nucleus medialis and the nucleus interpositus in the rat. J Comp Neurol. 1998;40:264–275. [PubMed] [Google Scholar]
- Budge J. Uber die Bewegung der Iris. Brauschweig: F Vieweg und Sohn; 1855. [Google Scholar]
- Burde RM. The visceral nuclei of the oculomotor complex. Tr. Am Ophth Soc. 1983;LXXXI:532–548. [PMC free article] [PubMed] [Google Scholar]
- Burde RM. Disparate visceral neuronal pools subserve spinal cord and ciliary ganglion in the monkey: a double labelling approach. Brain Res. 1988;440:177–180. doi: 10.1016/0006-8993(88)91173-0. [DOI] [PubMed] [Google Scholar]
- Burde RM, Loewy AD. Central origin of oculomotor parasympathetic neurons in the monkey. Brain Res. 1980;198:434–439. doi: 10.1016/0006-8993(80)90757-x. [DOI] [PubMed] [Google Scholar]
- Burde RM, Williams F. Parasympathetic nuclei. Brain Res. 1989;498:371–375. doi: 10.1016/0006-8993(89)91119-0. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Akert K. Medial rectus subgroups of the oculomotor nucleus and their abducens internuclear input in the monkey. J Comp Neurol. 1981;197:17–27. doi: 10.1002/cne.901970103. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Cohen B, Horn AK, Reisine H. Pretectal projections to the oculomotor complex of the monkey and their role in eye movements. J Comp Neurol. 1996;366:348–359. doi: 10.1002/(SICI)1096-9861(19960304)366:2<348::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Horn AKE, Scherberger H, D’Ascanio P. Motoneurons of twitch and nontwitch extraocular muscle fibers in the abducens, trochlear, and oculomotor nuclei of monkeys. J Comp Neurol. 2001;438:318–335. doi: 10.1002/cne.1318. [DOI] [PubMed] [Google Scholar]
- Cabot JB, Reiner A, Bogan N. Avian bulbospinal pathways: Anterograde and retrograde studies of cells of origin, funicular trajectories and laminar terminations. In: Kuypers HGJM, Martin GF, editors. Descending Pathways to the Spinal Cord. Vol. 57. Amsterdam: Elsevier; 1982. pp. 25–67. Prog Brain Res. [DOI] [PubMed] [Google Scholar]
- Calle M, Kozicz T, van der Linden E, Desfeux A, Veening JG, Barendregt HP, Roubos EW. Effect of starvation on Fos and neuropeptide immunoreactivities in the brain and pituitary gland of Xenopus laevis. Gen Comp Endocrinol. 2006;147:237–46. doi: 10.1016/j.ygcen.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Cavani JC, Reiner A, Cuthbertson SL, Bittencourt JC, Toledo CAB. Evidence of that urocortin is absent from neurons of the nucleus of Edinger-Westphal in pigeon. Braz J Med Biol Res. 2003;26:1695–1700. doi: 10.1590/s0100-879x2003001200011. [DOI] [PubMed] [Google Scholar]
- Chang SL, Patel NA, Romero AA. Activation and desensitization of Fos immunoreactivity in the rat brain following ethanol administration. Brain Res. 1995;679:89–98. doi: 10.1016/0006-8993(95)00210-h. [DOI] [PubMed] [Google Scholar]
- Chung RY, Mason P, Strassman A, Maciewicz R. Edinger-Westphal nucleus: cells that project to spinal cord contain corticotropin releasing factor. Neurosci Lett. 1987;83:13–19. doi: 10.1016/0304-3940(87)90208-4. [DOI] [PubMed] [Google Scholar]
- Clarke RJ, Coimbra CJ, Alessio ML. Distribution of parasympathetic motoneurones in the oculomotor complex innervating the ciliary ganglion in the marmoset (Callithrix jacchus) Acta Anat (Basel) 1985;121:53–58. doi: 10.1159/000145942. [DOI] [PubMed] [Google Scholar]
- Clarke RJ, Blanks RH, Giolli RA. Midbrain connections of the olivary pretectal nucleus in the marmoset (Callithrix jacchus): implications for the pupil light reflex pathway. Anat Embryol (Berl) 2003;207:149–155. doi: 10.1007/s00429-003-0339-0. [DOI] [PubMed] [Google Scholar]
- Crawford K, Terasawa E, Kaufman PL. Reproducible stimulation of ciliary muscle contraction in the cynomolgus monkey via a permanent indwelling midbrain electrode. Brain Res. 1989;503:265–272. doi: 10.1016/0006-8993(89)91673-9. [DOI] [PubMed] [Google Scholar]
- Crosby EC, Woodburne RT. The nuclear pattern of the nontectal portions of the midbrain and isthmus in primates. J Comp Neurol. 1943;78:441–482. [Google Scholar]
- Crouch RL. The efferent fibers of the Edinger-Westphal nucleus. J Comp Neurol. 1936;64:365–373. [Google Scholar]
- Cunha RP, Reiner A, Toledo CAB. Involvement of urocortinergic neurons below the midbrain central gray in the physiological response to restraint stress in pigeons. Brain Res. 2007;1147:175–183. doi: 10.1016/j.brainres.2007.01.122. [DOI] [PubMed] [Google Scholar]
- Dearworth JR, Jr, Brenner JE, Blaum JF, Littlefield TE, Fink DA, Romano JM, Jones MS. Pupil constriction evoked in vitro by stimulation of the oculomotor nerve in the turtle (Trachemys scripta elegans) Vis Neurosci. 2009;26:309–318. doi: 10.1017/S0952523809090099. [DOI] [PubMed] [Google Scholar]
- de Kloet ER. About stress hormones and resilience to psychopathology. J Neuroendocrinol. 2008;20:885–892. doi: 10.1111/j.1365-2826.2008.01707.x. [DOI] [PubMed] [Google Scholar]
- Derks NM, Roubos EW, Kozicz T. Presence of estrogen receptor beta in urocortin 1-neurons in the mouse non-preganglionic Edinger-Westphal nucleus. Gen Comp Endocrinol. 2007;153:228–234. doi: 10.1016/j.ygcen.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Derks NM, Gaszner B, Roubos EW, Kozicz LT. Sex differences in urocortin 1 dynamics in the non-preganglionic Edinger-Westphal nucleus of the rat. Neurosci Res. 2010;66:117–23. doi: 10.1016/j.neures.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Douglass J, Daoud S. Characterization of the human cDNA and genomic DNA encoding CART: a cocaine- and amphetamine-regulated transcript. Gene. 1996;169:241–245. doi: 10.1016/0378-1119(96)88651-3. [DOI] [PubMed] [Google Scholar]
- Dun SL, Brailoiu GC, Mizuo K, Yang J, Chang JK, Dun NJ. Neuropeptide B immunoreactivity in the central nervous system of the rat. Brain Res. 2005;1045:157–163. doi: 10.1016/j.brainres.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Edinger L. Über den Verlauf der centralen Hirnnervenbahnen mit Demonstrationen von Präparaten. Arch Psychiatr Nervenkrankheiten. 1885;16:858–859. [Google Scholar]
- Erichsen JT, May PJ. The pupillary and ciliary components of the cat Edinger-Westphal nucleus: a transsynaptic transport investigation. Vis Neurosci. 2002;19:15–29. doi: 10.1017/s0952523801191029. [DOI] [PubMed] [Google Scholar]
- Eustachius B. Tabulae Anatomicae. Romae: F. Gomzagae. Tab; 1714. p. XVIII. [Google Scholar]
- Fallopius G. Opera-Omnia. Frankfurti: apud. Haer. A. Welcheli. Tom. Sec. 1600:293–294. [Google Scholar]
- Fatima A, Haroon MF, Wolf G, Engelmann M, Spina MG. Reduced urocortin 1 immunoreactivity in the non-preganglionic Edinger-Westphal nucleus during late pregnancy in rats. Regul Pept. 2007;143:34–38. doi: 10.1016/j.regpep.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MEC, Gamlin PDR, Zagvazdin Y, Reiner A. Central neural circuits for the light-mediated reflexive control of choroidal blood flow in the pigeon eye: a laser Doppler study. Vis Neurosci. 1996;13:655–669. doi: 10.1017/s0952523800008555. [DOI] [PubMed] [Google Scholar]
- Fonareva I, Spangler E, Cannella N, Sabino V, Cottone P, Ciccocioppo R, Zorrilla EP, Ryabinin AE. Increased perioculomotor urocortin 1 immunoreactivity in genetically selected alcohol preferring rats. Alcohol Clin Exp Res. 2009 doi: 10.1111/j.1530-0277.2009.01033.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo KS, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience. 2008;156:563–579. doi: 10.1016/j.neuroscience.2008.07.054. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain atlas in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Gamlin PD. Subcortical neural circuits for ocular accommodation and vergence in primates. Ophthalmic Physiol Opt. 1999;19:81–89. doi: 10.1046/j.1475-1313.1999.00434.x. [DOI] [PubMed] [Google Scholar]
- Gamlin PD, Reiner A, Erichsen JT, Karten HJ, Cohen DH. The neural substrate for the pupillary light reflex in the pigeon (Columba livia) J Comp Neurol. 1984;226:523–543. doi: 10.1002/cne.902260407. [DOI] [PubMed] [Google Scholar]
- Gamlin PDR, Reiner A. The Edinger-Westphal nucleus: sources of input influencing accommodation, pupilloconstriction, and choroidal blood flow. J Comp Neurol. 1991;306:425–438. doi: 10.1002/cne.903060307. [DOI] [PubMed] [Google Scholar]
- Gamlin PD, Zhang Y, Clendaniel RA, Mays LE. Behavior of identified Edinger-Westphal neurons during ocular accommodation. J Neurophysiol. 1994;72:2368–2382. doi: 10.1152/jn.1994.72.5.2368. [DOI] [PubMed] [Google Scholar]
- Gamlin PD, Yoon K. An area for vergence eye movement in primate frontal cortex. Nature. 2000;407:1003–1007. doi: 10.1038/35039506. [DOI] [PubMed] [Google Scholar]
- Gamlin PDR. The Functions of the Edinger-Westphal Nucleus. In: Burnstock G, Sillito A, editors. Nervous Control of the Eye. Harwood Academic Publishers: 2000. pp. 117–154. [Google Scholar]
- Gaszner B, Csernus V, Kozicz T. Urocortinergic neurons respond in a differentiated manner to various acute stressors in the Edinger-Westphal nucleus in the rat. J Comp Neurol. 2004;480:170–179. doi: 10.1002/cne.20343. [DOI] [PubMed] [Google Scholar]
- Gaszner B, Korosi A, Palkovits M, Roubos EW, Kozicz T. Neuropeptide Y activates urocortin 1 neurons in the nonpreganglionic Edinger-Westphal nucleus. J Comp Neurol. 2007;500:708–719. doi: 10.1002/cne.21177. [DOI] [PubMed] [Google Scholar]
- Haeger P, Cuevas R, Forray MI, Rojas R, Daza C, Rivadeneira J, Gysling K. Natural expression of immature Ucn1 antisense RNA in the rat brain. Evidence favoring bidirectional transcription of the Ucn1 gene locus. Mol Brain Res. 2005;139:115–128. doi: 10.1016/j.molbrainres.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Hall CR. An experimental inquiry into the functions of the opthlamic ganglion/ Edinb Med Surg J. 1846;65:355–383. 66: 84–108 and 66 312 353. [PMC free article] [PubMed] [Google Scholar]
- Haller A. Iconum Anatomicum Corporis Humani. Gottingae: A. Vanderhoeck. Fig. VI and. 1743;50:46. [Google Scholar]
- Heesy CP, Hall MI. The nocturnal bottleneck and the evolution of mammalian vision. Brain, Behav Evol. 2010;75:195–203. doi: 10.1159/000314278. [DOI] [PubMed] [Google Scholar]
- Hensen V, Völkers C. Experimentaluntersuchung uber den Mechanismus der Accomodation. Kiel: Schwers; 1868. [Google Scholar]
- Hiraoka M, Shimamura M. The midbrain reticular formation as an integration center for the 'near reflex' in the cat. Neurosci Res. 1989;7:1–12. doi: 10.1016/0168-0102(89)90032-1. [DOI] [PubMed] [Google Scholar]
- Hogg ID. Observations on the development of the nucleus of Edinger-Westphal in man and the albino rat. J Comp Neurol. 1966;126:105–114. [PubMed] [Google Scholar]
- Hökfelt T, Johansson O, Fuxe K, Goldstein M, Park D. Immunohistochemical studies on the localization and distribution of monoamine neuron systems in the rat brain. I. Tyrosine hydroxylase in the mes- and diencephalon. Med Biol. 1976;54:427–453. [PubMed] [Google Scholar]
- Horn AK, Eberhorn A, Härtig W, Ardeleanu P, Messoudi A, Büttner-Ennever JA. Perioculomotor cell groups in monkey and man defined by their histochemical and functional properties: Reappraisal of the Edinger-Westphal nucleus. J Comp Neurol. 2008;507:1317–1335. doi: 10.1002/cne.21598. [DOI] [PubMed] [Google Scholar]
- Innis RB, Aghajanian GK. Cholecystokinin-containing and nociceptive neurons in rat Edinger-Westphal nucleus. Brain Res. 1986;363:230–238. doi: 10.1016/0006-8993(86)91008-5. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Sekiya H, Kondo Y. The center for controlling the near reflex in the midbrain of the monkey: a double labelling study. Brain Res. 1990;519:217–222. doi: 10.1016/0006-8993(90)90080-u. [DOI] [PubMed] [Google Scholar]
- Jacubowich NM. O tonchaishem stroenii cherepnigo i spinnogo mozga. Voenno-Meditsinksii Zhurnal 20, part. 1857;2:35–85. [Google Scholar]
- Jaeger RJ, Benevento LA. A horseradish peroxidase study of the innervation of the internal structures of the eye. Evidence for a direct pathway. Invest Ophthalmol Vis Sci. 1980;19:575–583. [PubMed] [Google Scholar]
- Jansen AS, Farwell DG, Loewy AD. Specificity of pseudorabies virus as a retrograde marker of sympathetic preganglionic neurons: implications for transneuronal labelling studies. Brain Res. 1993;617:103–112. doi: 10.1016/0006-8993(93)90619-x. [DOI] [PubMed] [Google Scholar]
- Joels M, Karst H, Derijk R, de Kloet ER. The coming out of the brain mineralocorticoid receptor. Trends Neurosci. 2008;31:1–7. doi: 10.1016/j.tins.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Purves D. Post-natal reduction of neural unit size in the rabbit ciliary anglion. J Physiol. 1981;318:143–159. doi: 10.1113/jphysiol.1981.sp013855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliusbergern O, Kaplan L. Anatomischer Befund bei einseitiger Oculomotoriuslähmung im Verlaufe von progressiver Paralyse Neurol Zbl. 1899;18:486–495. [Google Scholar]
- Klooster J, Beckers HJ, Vrensen GF, van der Want JJ. The peripheral and central projections of the Edinger-Westphal nucleus in the rat. A light and electron microscopic tracing study. Brain Res. 1993;632:260–273. doi: 10.1016/0006-8993(93)91161-k. [DOI] [PubMed] [Google Scholar]
- Knies M. Über die zentralen Störungen der willkürlichen Augenmuskeln. Arch f Augenheilk. 1891;22:19–51. [Google Scholar]
- Korosi A, Schotanus S, Olivier B, Roubos EW, Kozicz T. Chronic ether stress-induced response of urocortin 1 neurons in the Edinger-Westphal nucleus in the mouse. Brain Res. 2005;1046:172–179. doi: 10.1016/j.brainres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Kourouyan HD, Horton JC. Transneuronal retinal input to the primate Edinger-Westphal nucleus. J Comp Neurol. 1997;381:68–80. doi: 10.1002/(sici)1096-9861(19970428)381:1<68::aid-cne6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391:115–132. [PubMed] [Google Scholar]
- Kozicz T, Yanaihara H, Arimura A. Distribution on urocortin-like immunoreactivity in the central nervous system of the rat. J Comp Neurol. 1998;391:1–10. doi: 10.1002/(sici)1096-9861(19980202)391:1<1::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Min L, Arimura A. The activation of urocortin immunoreactive neurons in the Edinger-Westphal nucleus following acute pain stress in rats. Stress. 2001;4:85–90. doi: 10.3109/10253890109115724. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Arimura, Maderdrut JL, Lázár G. Distribution of urocortin-like immunoreactivity in the central nervous system of the frog Rana esculenta. J Comp Neurol. 2002;453:185–198. doi: 10.1002/cne.10403. [DOI] [PubMed] [Google Scholar]
- Kozicz T. On the role of urocortin 1 in the non-preganglionic Edinger-Westphal nucleus in stress adaptation. Gen Comp Endocrinol. 2007;153:235–240. doi: 10.1016/j.ygcen.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Bordewin LA, Czeh B, Fuchs E, Roubos EW. Chronic psychosocial stress affects corticotropin-releasing factor in the paraventricular nucleus and central extended amygdala as well as urocortin 1 in the non-preganglionic Edinger-Westphal nucleus of the tree shrew. Psychoneuroendocrinology. 2008a;33:741–754. doi: 10.1016/j.psyneuen.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Tilburg-Ouwens D, Faludi G, Palkovits M, Roubos E. Gender-related urocortin 1 and brain-derived neurotrophic factor expression in the adult human midbrain of suicide victims with major depression. Neuroscience. 2008b;152:1015–1023. doi: 10.1016/j.neuroscience.2007.12.050. [DOI] [PubMed] [Google Scholar]
- Kozicz T. The missing link; the significance of urocortin 1/urocortin 2 in the modulation of the dorsal raphe serotoninergic system. Mol Psychiatry. 2010;15:340–341. doi: 10.1038/mp.2009.134. [DOI] [PubMed] [Google Scholar]
- Kuchiiwa S, Kuchiiwa T, Suzuki T. Comparative anatomy of the accessory ciliary ganglion in mammals. Anat Embryol (Berl) 1989;180:199–205. doi: 10.1007/BF00309772. [DOI] [PubMed] [Google Scholar]
- Kuchiiwa S, Kuchiiwa T, Nakagawa S. Localization of preganglionic neurons of the accessory ciliary ganglion in the midbrain: HRP and WGA-HRP studies in the cat. J Comp Neurol. 1994;340:577–91. doi: 10.1002/cne.903400410. [DOI] [PubMed] [Google Scholar]
- Lantéri-Minet M, Weil-Fugazza J, de Pommery J, Menétrey D. Hindbrain structures involved in pain processing as revealed by the expression of c-Fos and other immediate early gene proteins. Neuroscience. 1994;58:287–98. doi: 10.1016/0306-4522(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Lázar G, Calle M, Roubos EW, Kozicz T. Immunohistochemical localization of cocaine-and amphetamine-regulated transcript peptide in the central nervous system of the frog Rana esculenta. J Comp Neurol. 2004;477:324–339. doi: 10.1002/cne.20264. [DOI] [PubMed] [Google Scholar]
- Leathart PW. The tabetic pupil. Br J Ophthalmol. 1941;25:111–116. doi: 10.1136/bjo.25.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre A, Papakonstantinou E, Roy MC, Richard D, Harris RB. Differences in response to corticotropin-releasing factor after short- and long-term consumption of a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1076–R1085. doi: 10.1152/ajpregu.00592.2006. [DOI] [PubMed] [Google Scholar]
- Le Gros Clark WE. The mammalian oculomotor nucleus. J Anat. 1926;60:426–448. [PMC free article] [PubMed] [Google Scholar]
- Lenz G. Untersuchungen über die anatomische Grundlage von Pupillenstörungen. Ber Dtsch Ophthalmol Ges. 1928;47:234–246. [Google Scholar]
- Lenz G. Über die anatomische Grundlage der Ophtalmoplegia interna. Z Augenheilgk. 1929;69:102–113. [Google Scholar]
- Levinson G. Beitrag zur Physiologie der Pupillenreflexes. V Graefes Arch Ophthal. 1904;59:191–220. and 436–458. [Google Scholar]
- Lim MM, Tsivkovskaia NO, Bai Y, Young LJ, Ryabinin AE. Corticotropin-releasing factor and urocortin 1 brain distribution among monogamous and promiscuous vole species. Brain Behav Evol. 2006;68:229–240. doi: 10.1159/000094360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden AM, Baez M, Bergeron M, Schoepp DD. Effects of mGlu2 or mGlu3 receptor deletions on mGlu2/3 receptor agonist (LY354740)-induced brain c-Fos expression: specific roles for mGlu2 in the amygdala and subcortical nuclei, and mGlu3 in the hippocampus. Neuropharmacology. 2006;51:213–228. doi: 10.1016/j.neuropharm.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Linden AM, Greene SJ, Bergeron M, Schoepp DD. Anxiolytic activity of the MGLU2/3 receptor agonist LY354740 on the elevated plus maze is associated with the suppression of stress-induced c-Fos in the hippocampus and increases in c-Fos induction in several other stress-sensitive brain regions. Neuropsychopharmacol. 2004;29:502–513. doi: 10.1038/sj.npp.1300321. [DOI] [PubMed] [Google Scholar]
- Longet FA. Anatomie ET Physiologie Du Systeme Nerveux. Vol. 2. Paris: Fortin, Masson ET Cie, T; 1842. pp. 377–390. [Google Scholar]
- Loewenfeld IE. The Pupil. Anatomy, Physiology and Clinical Applications. Detroit, MI: Wayne State University Press; 1993. [Google Scholar]
- Loewy AD, Araujo JC, Kerr FW. Pupillodilator pathways in the brain stem of the cat: anatomical and electrophysiological identification of a central autonomic pathway. Brain Res. 1973;60:65–91. doi: 10.1016/0006-8993(73)90851-2. [DOI] [PubMed] [Google Scholar]
- Loewy AD, Saper CB. Edinger-Westphal nucleus: projections to the brain stem and spinal cord in the cat. Brain Res. 1978;150:1–27. doi: 10.1016/0006-8993(78)90650-9. [DOI] [PubMed] [Google Scholar]
- Loewy AD, Saper CB, Yamodis ND. Re-evaluation of the efferent projections of the Edinger-Westphal nucleus in the cat. Brain Res. 1978;141:153–159. doi: 10.1016/0006-8993(78)90624-8. [DOI] [PubMed] [Google Scholar]
- Lyman D, Mugnaini E. The avian accessory oculomotor nucleus. Soc Neurosci Abstr. 1980;6:479. [Google Scholar]
- Maciewicz R, Phipps BS, Grenier J, Poletti CE. Edinger-Westphal nucleus: cholecystokinin immunocytochemistry and projections to spinal cord and trigeminal nucleus in the cat. Brain Res. 1984;299:139–145. doi: 10.1016/0006-8993(84)90796-0. [DOI] [PubMed] [Google Scholar]
- Majano N. Über Ursprung und Verlauf des Nervus oculomotorius im Mittelhirn Mschr Psychiat Neurol. 1903;13:291. [Google Scholar]
- Marina A. Das Neuron des Ganglion ciliare und die Centra der Pupillenbewegungen. Dtsch Z Nervenheilk. 1899;20:369–396. [Google Scholar]
- Marwitt R, Pilar G, Weakly JN. Characterization of two ganglion cell populations in avian ciliary ganglia. Brain Res. 1971;25:317–334. doi: 10.1016/0006-8993(71)90441-0. [DOI] [PubMed] [Google Scholar]
- May PJ, Porter JD, Gamlin PD. Interconnections between the primate cerebellum and midbrain near-response regions. J Comp Neurol. 1992;315:98–116. doi: 10.1002/cne.903150108. [DOI] [PubMed] [Google Scholar]
- May PJ, Reiner A, Ryabinin AE. Comparison of the distribution of urocortin containing and cholinergic neurons in the perioculomotor midbrain of the cat and the macaque. J Comp Neurol. 2008a;507:1302–1318. doi: 10.1002/cne.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ, Sun W, Erichsen JT. Defining the pupillary component of the perioculomotor preganglionic population within a unitary primate Edinger-Westphal nucleus. Prog Brain Res. 2008b;171:97–106. doi: 10.1016/S0079-6123(08)00613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo H. Anatomical and Physiological Commentaries. II. London: T.G. Underwood; 1823. p. 5. [Google Scholar]
- Mays LE, Porter JD. Neural control of vergence eye movements: activity of abducens and oculomotor neurons. J Neurophysiol. 1984;5:743–761. doi: 10.1152/jn.1984.52.4.743. [DOI] [PubMed] [Google Scholar]
- Mays LE, Gamlin PD. Neuronal circuitry controlling the near response. Curr Opin Neurobiol. 1995;5:763–768. doi: 10.1016/0959-4388(95)80104-9. [DOI] [PubMed] [Google Scholar]
- Meckel JF. De Quinto Pare Nervorum Cerebri. 1748:29–30. [Google Scholar]
- Medina L, Smeets WJAJ, Hoogland PV, Puelles L. Distribution of choline acetyltransferase in the brain of the lizard Gallotia galloti. J Comp Neurol. 1993;331:261–285. doi: 10.1002/cne.903310209. [DOI] [PubMed] [Google Scholar]
- Muck F. Dissertatio Anatomica de Ganglio Ophtalmico et Nervis Ciliaribus Animalium. Landshuti: Typis Josephi Thoman; 1815. p. 67. [Google Scholar]
- Narayanan CH, Narayanan Y. An experimental inquiry into the central source of preganglionic fibers to the chick ciliary ganglion. J Comp Neurol. 1976;166:101–109. doi: 10.1002/cne.901660107. [DOI] [PubMed] [Google Scholar]
- Neufeld-Cohen A, Evans AK, Getselter D, Spyroglou A, Hill A, Gil S, Tsoory M, Beuschlein F, Lowry CA, W Vale W, Chen A. Urocortin-1 and -2 double-deficient mice show robust anxiolytic phenotype and modified serotonergic activity in anxiety circuits. Mol Psychiatry. 2010;15:426–441. doi: 10.1038/mp.2009.115. [DOI] [PubMed] [Google Scholar]
- Olszewski J, Baxter D. Cytoarchitecture of the human brain stem. Basel: S Karger; 1982. [Google Scholar]
- Otake K. Cholecystokinin and substance P immunoreactive projections to the paraventricular thalamic nucleus in the rat. Neurosci Res. 2005;51(4):383–394. doi: 10.1016/j.neures.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Ott M. Visual accommodation in vertebrates: mechanisms, physiological response and stimuli. J Comp Physiol. 2006;192:97–111. doi: 10.1007/s00359-005-0049-6. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Sebekova K, Gallatz K, Boor P, Sebekova K, Jr, Klassen A, Bahner U, Heidland A. Neuronal activation in the CNS during different forms of acute renal failure in rats. Neuroscience. 2009;159:862–82. doi: 10.1016/j.neuroscience.2008.12.062. [DOI] [PubMed] [Google Scholar]
- Parelman JJ, Fay MT, Burde RM. Confirmatory evidence for a direct parasympathetic pathway to internal eye structures. Trans Am Ophthalmol Soc. 1984;82:371–380. [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain is Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Perlia R. Uber das Orbital-Nervensystem des Kaninchens mit specieller Berucksichtigung der Ciliarnerven. V Graefes Arch Opthal. 1893;39:1–44. [Google Scholar]
- Phipps BS, Maciewicz R, Sandrew BB, Poletti CE, Foote WE. Edinger-Westphal neurons that project to spinal cord contain substance P. Neurosci Lett. 1983;36:125–131. doi: 10.1016/0304-3940(83)90253-7. [DOI] [PubMed] [Google Scholar]
- Pierson RJ, Carpenter MB. Anatomical analysis of pupillary reflex pathway in the rhesus monkey. J Comp Neurol. 1974;158:121–144. doi: 10.1002/cne.901580202. [DOI] [PubMed] [Google Scholar]
- Pilar G, Tuttle JB. A simple neuronal system with a range of uses: The avian ciliary ganglion. In: Goldberg A, Hanin I, editors. Model Cholinergic Synapses. New York: Raven Press; 1982. pp. 213–247. [Google Scholar]
- Pong M, Fuchs AF. Characteristics of the pupillary light reflex in the macaque monkey: discharge patterns of pretectal neurons. J Neurophysiol. 2000;84(2):964–974. doi: 10.1152/jn.2000.84.2.964. [DOI] [PubMed] [Google Scholar]
- Porter JD, Guthrie BL, Sparks DL. Selective retrograde transneuronal transport of wheat germ agglutinin-conjugated horseradish peroxidase in the oculomotor system. Exp Brain Res. 1985;57:411–416. doi: 10.1007/BF00236549. [DOI] [PubMed] [Google Scholar]
- Powers AS, Reiner A. The distribution of cholinergic neurons in the cenral nervous system of turtles. Brain Behav Evol. 1993;41:326–345. doi: 10.1159/000113853. [DOI] [PubMed] [Google Scholar]
- Ranson SW, Magoun HW. The central path of the pupillo-constrictor response to light. Arch Neurol Psychiat. 1933;30:1193–1204. [Google Scholar]
- Reiner A, Erichsen JT, Cabot JB, Evinger C, Fitzgerald ME, Karten HJ. Neurotransmitter organization of the nucleus of Edinger-Westphal and its projection to the avian ciliary ganglion. Vis Neurosci. 1991;6:451–472. doi: 10.1017/s0952523800001310. [DOI] [PubMed] [Google Scholar]
- Reiner A, Karten HJ, Gamlin PDR, Erichsen JT. Parasympathetic ocular control: Functional subdivisions and circuitry of the avian nucleus of Edinger-Westphal. Trends Neurosci. 1983;6:140–145. [Google Scholar]
- Roste GK, Dietrichs E. Cerebellar cortical and nuclear afferents from the Edinger-Westphal nucleus in the cat. Anat Embryol (Berl) 1988;178:59–65. doi: 10.1007/BF00305015. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Bachtell RK, Freeman P, Risinger FO. ITF expression in mouse brain during acquisition of alcohol self-administration. Brain Res. 2001;890:192–195. doi: 10.1016/s0006-8993(00)03251-0. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Melia KR, Cole M, Bloom FE, Wilson MC. Alcohol selectively attenuates stress-induced c-fos expression in rat hippocampus. J Neurosci. 1995;15:721–730. doi: 10.1523/JNEUROSCI.15-01-00721.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Tsivkovskaia NO, Ryabinin SA. Urocortin 1-containing neurons in the human Edinger-Westphal nucleus. Neuroscience. 2005;134:1317–1317. doi: 10.1016/j.neuroscience.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Weitemier AZ. The urocortin 1 neurocircuit: ethanol-sensitivity and potential involvement in alcohol consumption. Brain Res Rev. 2006;52:368–380. doi: 10.1016/j.brainresrev.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–12. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- Scinto LFM, Wu CK, Firla KM, Daffner KR, Saroof D, Geula C. Focal pathology in the Edinger-Westphal nucleus explains pupillary hypersensitivity in Alzheimer's disease. Acta Neuropathol. 1999;97:557–64. doi: 10.1007/s004010051031. [DOI] [PubMed] [Google Scholar]
- Sekiya H, Kawamura K, Ishikawa S. Projections from the Edinger-Westphal complex of monkeys as studied by means of retrograde axonal transport of horseradish peroxidase. Arch Ital Biol. 1984;122:311–319. [PubMed] [Google Scholar]
- Schacher PC. De Cataracta. Lipsiae: typ. JC Brandenburgeri; 1701. p. 9. [Google Scholar]
- Sharpe AL, Tsivkovskaia NO, Ryabinin AE. Ataxia and c-Fos expression in mice drinking ethanol in a limited access session. Alcohol Clin Exp Res. 2005;29:1419–1426. doi: 10.1097/01.alc.0000174746.64499.83. [DOI] [PubMed] [Google Scholar]
- Singh ME, McGregor IS, Mallet PE. Perinatal exposure to delta(9)-tetrahydrocannabinol alters heroin-induced place conditioning and fos-immunoreactivity. Neuropsychopharmacology. 2006;31:58–69. doi: 10.1038/sj.npp.1300770. [DOI] [PubMed] [Google Scholar]
- Singh ME, Verty AN, Price I, McGregor IS, Mallet PE. Modulation of morphine-induced Fos-immunoreactivity by the cannabinoid receptor antagonist SR 141716. Neuropharmacology. 2004;47:1157–1169. doi: 10.1016/j.neuropharm.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Skelton KH, Nemeroff CB, Knight DL, Owens MJ. Chronic administration of the triazolobenzodiazepine alprazolam produces opposite effects on corticotropin-releasing factor and urocortin neuronal systems. J Neurosci. 2000;20:1240–1248. doi: 10.1523/JNEUROSCI.20-03-01240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeraski CA, Sollars PJ, Ogilvie MD, Enquist LW, Pickard GE. Suprachiasmatic nucleus input to autonomic circuits identified by retrograde transsynaptic transport of pseudorabies virus from the eye. J Comp Neurol. 2004;471:298–313. doi: 10.1002/cne.20030. [DOI] [PubMed] [Google Scholar]
- Spangler E, Cote DM, Anacker AM, Mark GP, Ryabinin AE. Differential sensitivity of the perioculomotor urocortin-containing neurons to ethanol, psychostimulants and stress in mice and rats. Neuroscience. 2009;160:115–125. doi: 10.1016/j.neuroscience.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina M, Merlo-Pich E, Chan RK, Basso AM, Rivier J, Vale W, Koob GF. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- Spitzka EC. The oculomotor centres and their co-oordinators. J Nerv Ment Dis. 1888:413–432. [Google Scholar]
- Steiger HJ, Büttner-Ennever J. Relationship between motoneurons and internuclear neurons in the abducens nucleus: a double retrograde tracer study in the cat. Brain Res. 1978;148:181–188. doi: 10.1016/0006-8993(78)90387-6. [DOI] [PubMed] [Google Scholar]
- Steiger HJ, Büttner-Ennever JA. Oculomotor nucleus afferents in the monkey demonstrated with horseradish peroxidase. Brain Res. 1979;160:1–115. doi: 10.1016/0006-8993(79)90596-1. [DOI] [PubMed] [Google Scholar]
- Stilling B. Disquisitiones de structure et functionibus cerebri. Jenae: sumt. F. Maukii. T.1; 1846. p. 36. [Google Scholar]
- Strassman A, Mason P, Eckenstein F, Baughman RW, Maciewicz R. Choline acetyltransferase immunocytochemistry of Edinger-Westphal and ciliary ganglion afferent neurons in the cat. Brain Res. 1987;423:293–304. doi: 10.1016/0006-8993(87)90852-3. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Itoh K, Mizuno N. Localization of neurons giving rise to the oculomotor parasympathetic outflow: an HRP study in cat. Neurosci Lett. 1977;7:301–305. doi: 10.1016/0304-3940(78)90217-3. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Itoh K, Mizuno N. Direct projections from the Edinger-Westphal nucleus to the cerebellum and spinal cord in the cat: an HRP study. Neurosci Lett. 1978;9:17–22. doi: 10.1016/0304-3940(78)90041-1. [DOI] [PubMed] [Google Scholar]
- Sun W, May PJ. Organization of the extraocular and preganglionic motoneurons supplying the orbit in the lesser Galago. Anat Rec. 1993;237:89–103. doi: 10.1002/ar.1092370109. [DOI] [PubMed] [Google Scholar]
- Szentágothai J. Die innere Gliederung des Oculomotoriuskernes. Arch Psychiat Nervenkrankh. 1942;115:127–135. [Google Scholar]
- Tanaka H, Yoshida T, Miyamato N, Motoike T, Kurosu H, Shibata K, Yamanaka A, Williams SC, Richardson JA, Tsujino N, Garry MG, Lerner MR, King DS, O'Dowd BF, Sakurai T, Yanagisawa M. Characterization of a family of endogenous neuroopeptide ligands for the G protein-couple receptors GPR7 and GPR8. Proc Natl Acad Sci U S A. 2003;100:6251–6256. doi: 10.1073/pnas.0837789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Donkelaar HJ, Kusuma A, de Bôer-Van Huizen R. Cells of origin of pathways descending to the spinal cord in some quadrupedal reptiles. J Comp Neurol. 1980;192:827–851. doi: 10.1002/cne.901920413. [DOI] [PubMed] [Google Scholar]
- Toledo CA, Britto LR, Pires RS, Veenman CL, Reiner A. Interspecific differences in the expression of the AMPA-type glutamate receptors and parvalbumin in the nucleus of Edinger-Westphal of chicks and pigeons. Brain Res. 2002;947:122–130. doi: 10.1016/s0006-8993(02)02919-0. [DOI] [PubMed] [Google Scholar]
- Topple AN, Hunt GE, McGregor IS. Possible neural substrates of beer-craving in rats. Neurosci Lett. 1988;252:99–102. doi: 10.1016/s0304-3940(98)00574-6. [DOI] [PubMed] [Google Scholar]
- Toyoshima K, Kawana E, Sakai H. On the neuronal origin of the afferents to the ciliary ganglion in cat. Brain Res. 1980;185:67–76. doi: 10.1016/0006-8993(80)90671-x. [DOI] [PubMed] [Google Scholar]
- Turek VF, Ryabinin AE. Expression of c-Fos in the mouse Edinger-Westphal nucleus following ethanol administration is not secondary to hypothermia or stress. Brain Res. 2005;1063:132–139. doi: 10.1016/j.brainres.2005.09.056. [DOI] [PubMed] [Google Scholar]
- Turek VF, Tsivkovskaia NO, Hyytia P, Harding S, Le AD, Ryabinin AE. Urocortin 1 expression in five pairs of rat lines selectively bred for differences in alcohol drinking. Psychopharmacology (Berl) 2005;181:511–517. doi: 10.1007/s00213-005-0011-x. [DOI] [PubMed] [Google Scholar]
- Vann VR, Atherton SS. Neural spread of herpes simplex virus after anterior chamber inoculation. Invest Ophthalmol Vis Sci. 1991;32:2462–2472. [PubMed] [Google Scholar]
- van Biervliet J. Noyau d’origine du nerf oculo-moteur commun du lapin. Cellule. 1899:7–29. [Google Scholar]
- van Nieuwenhuijzen PS, McGregor IS, Hunt GE. The distribution of gamma-hydroxybutyrate-induced Fos expression in rat brain: comparison with baclofen. Neuroscience. 2009;158:441–455. doi: 10.1016/j.neuroscience.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Vasconcelos LA, Donaldson C, Sita LV, Casatti CA, Lotfi CF, Wang L, Cadinouche MZ, Frigo L, Elias CF, Lovejoy DA, Bittencourt JC. Urocortin in the central nervous system of primate (Cebus apella): sequencing, immunohistochemical, and hybridization histochemical characterization. J Comp Neurol. 2003;463:157–175. doi: 10.1002/cne.10742. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotrophin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Li C, Zhao L, Contarino A, Liberman MC, Smith GW, Marchuk Y, Koob GF, Heinemann SF, Vale W, Lee KF. Urocortin-deficient mice show hearing impairment and increased anxiety-like behavior. Nat Genet. 2002;31:363–369. doi: 10.1038/ng914. [DOI] [PubMed] [Google Scholar]
- Viau V, Sawchenko PE. Hypophysiotropic neurons of the paraventricular nucleus respond in spatially, temporally, and phenotypically differentiated manners to acute vs. repeated restraint stress: rapid publication. J Comp Neurol. 2002;445:293–307. doi: 10.1002/cne.10178. [DOI] [PubMed] [Google Scholar]
- Vilupuru AS, Glasser A. The relationship between refractive and biometric changes during Edinger-Westphal stimulated accommodation in rhesus monkeys. Exp Eye Res. 2005;80:349–360. doi: 10.1016/j.exer.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bechterew A. Uber die Kerne der mit Augenbewegungen in Beziehung stehenden Nerven (des Oculomotorius, Abducens, und Trochlearis) und uber die Verbindung derelben unter einander. Arch Anat Physiol, Anat Abt. 1897:307–315. [Google Scholar]
- von Gudden B. Ueber die Kerne der Augenbewegungsnerven. Tagebl Vers Dtsch Naturf Aerzte. 1881;54:186. (Teil 2) [Google Scholar]
- Wang X, Su H, Copenhagen LD, Vaishnav S, Pieri F, Shope CD, Brownell WE, De Biasi M, Paylor R, Bradley A. Urocortin-deficient mice display normal stress-induced anxiety behavior and autonomic control but an impaired acoustic startle response. Mol Cell Biol. 2002;22:6605–6610. doi: 10.1128/MCB.22.18.6605-6610.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasicky R, Horn AK, Büttner-Ennever JA. Twitch and nontwitch motoneuron subgroups in the oculomotor nucleus of monkeys receive different afferent projections. J Comp Neurol. 2004;479:117–129. doi: 10.1002/cne.20296. [DOI] [PubMed] [Google Scholar]
- Warwick RB. The ocular parasympathetic nerve supply and its mesencephalic sources. J Anat. 1954;88:71–93. [PMC free article] [PubMed] [Google Scholar]
- Warwick R. The so-called nucleus of convergence. Brain. 1955;78:92–114. doi: 10.1093/brain/78.1.92. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Brain region-specific regulation of urocortin 1 innervation and corticotropin-releasing factor receptor type 2 binding by ethanol exposure. Alcohol Clin Exp Res. 2005a;29:1610–1620. doi: 10.1097/01.alc.0000179363.44542.05. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Lesions of the Edinger-Westphal nucleus alter food and water consumption. Behav Neurosci. 2005b;119:1235–1245. doi: 10.1037/0735-7044.119.5.1235. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Urocortin 1 the dorsal raphe regulates food and fluid consumption, but not ethanol preference in C57BL/6J mice. Neuroscience. 2006;137:1439–1445. doi: 10.1016/j.neuroscience.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Tsivkovskaia NO, Ryabinin AE. Urocortin 1 distribution in mouse brain of strain-dependent. Neuroscience. 2005;132:729–740. doi: 10.1016/j.neuroscience.2004.12.047. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Woerner A, Backstrom P, Hyytia P, Ryabinin AE. Expression of c-Fos in Alko alcohol rats responding for ethanol in an operant paradigm. Alcohol Clin Exp Res. 2001;25:704–710. [PubMed] [Google Scholar]
- Weninger SC, Peter LL, Majzoub JA. Urocortin expression in the Edinger-Westphal nucleus is up-regulated by stress and corticotropin-releasing hormone deficiency. Endocrinol. 2000;141:256–263. doi: 10.1210/endo.141.1.7277. [DOI] [PubMed] [Google Scholar]
- Westphal CFO. Ueber einen Fall von chronischer progressiver Lähmung der Augenmuskeln (Ophthalmoplegia externa) nebst Beschreibung von Ganglienzellengruppen im Bereiche des Oculomotoriuskerns. Arch Psychiat und Nervenkrankheiten. 1887;18:846–871. [Google Scholar]
- Willis T. Cerebri Anatome. Amstelodami: apud Casparum Commelinum. 1664;172 [Google Scholar]
- Winslow JB. Exposition Anatomique de la Structure du Corpus Humain T. III. Paris: Desprez et Desessartz; 1732. [Google Scholar]
- Wolters JG, de Boer-Van Huizen, ten Donkelaar, Leenen L. Collateralization of descending pathways from the brainstem to the spinal cord in a lizard, Varanus exanthematicus. J Comp Neurol. 1986;251:317–333. doi: 10.1002/cne.902510304. [DOI] [PubMed] [Google Scholar]
- Xu L, Bloem B, Gaszner B, Roubos EW, Kozicz T. Sex-specific effects of fasting on Urocortin 1, Cocaine- and Amphetamine-Regulated Transcript peptide and Nesfatin-1 expression in the rat Edinger-Westphal nucleus. Neuroscience. 2009;162:1141–1149. doi: 10.1016/j.neuroscience.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Maeda T, Fujimura M, Fujimiya M. Urocortin-like immunoreactivity in the substantia nigra, ventral tegmental area and Edinger Westphal nucleus of rat. Neurosci Lett. 1998;243:21–24. doi: 10.1016/s0304-3940(98)00071-8. [DOI] [PubMed] [Google Scholar]
- Zalutskaya AA, Arai M, Bounoutas GS, Abou-Samra AB. Impaired adaptation to repeated restraint and decreased response to cold in urocortin 1 knockout mice. Am J Physiol Endocrinol Metab. 2007;293:E259–E263. doi: 10.1152/ajpendo.00616.2006. [DOI] [PubMed] [Google Scholar]
- Zeri A. Studien zur vergleichenden Anatomie der zentralen Hohlengraus bei den Wirbeltieren. Jb Psychiat Neurol. 1895;41:18–38. [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig H. Studien zur vergleichenden Anatomie des zentralen Hohlengraus bei den Wirbelthieren. Jahrb Psychiat Neurol. 1921;41:18–38. [Google Scholar]