Abstract
PURPOSE
Polymethyl methacrylate (PMMA) is the most commonly used denture base material despite typically low in strength. The purpose of this study was to improve the physical properties of the PMMA based denture base resins (QC-20, Dentsply Ltd., Addlestone, UK; Stellon, AD International Ltd, Dentsply, Switzerland; Acron MC; GC Lab Technologies Inc., Alsip, Japan) by copolymerization mechanism.
MATERIALS AND METHODS
Control group specimens were prepared according to the manufacturer recommendations. In the copolymer groups; resins were prepared with 5%, 10%, 15% and 20% acrylamide (AAm) (Merck, Hohenbrunn, Germany) content according to the moleculer weight ratio, respectively. Chemical structure was characterized by a Bruker Vertex-70 Fourier transform infrared spectroscopy (FTIR) (Bruker Optics Inc., Ettlingen, Germany). Hardness was determined using an universal hardness tester (Struers Duramin, Struers A/S, Ballerup, Denmark) equipped with a Vickers diamond penetrator. The glass transition temperature (Tg) of control and copolymers were evaluated by Perkin Elmer Diamond DSC (Perkin Elmer, Massachusetts,USA). Statistical analyses were carried out using the statistical package SPSS for Windows, version 15.0 (SPSS, Chicago, IL, USA). The results were tested regarding the normality of distribution with the Shapiro Wilk test. Data were analyzed using ANOVA with post-hoc Tukey test (P<.01).
RESULTS
The copolymer synthesis was confirmed by FTIR spectroscopy. Glass transition temperature of the copolymer groups were higher than the control groups of the resins. The 10%, 15% and 20% copolymer groups of Stellon presented significantly higher than the control group in terms of hardness. 15% and 20% copolymer groups of Acron MC showed significantly higher hardness values when compared to the control group of the resin. Acrylamide addition did not affect the hardness of the QC-20 resin significantly.
CONCLUSION
Within the limitation of this study, it can be concluded that copolymerization of PMMA with AAm increased the hardness value and glass transition temperature of PMMA denture base resins.
Keywords: Acrylic resin, Copolymerization, Acrylamide, Hardness, FTIR, DSC
INTRODUCTION
The demand for complete dentures is high due to the increase in life expectancy, and studies on the reinforcement of dentures will continue to be required over the next decade.1 Although a variety of resins have been introduced into dental treatments for the construction of removable partial and complete dentures, polymethyl methacrylate (PMMA) is the most commonly used material, despite its low strength.2
The choice of a denture base material and polymerization method influence the final strength of the prostheses. Differences in processing techniques influence the physical and mechanical properties of polymers.3 Heat curing in a water bath is the most commonly applied method, and is considered to be the conventional method.4,5 Microwave polymerization was introduced by Nishii6 and has become a popular alternative to the conventional water bath method. This method provides easy and fast curing, resulting in improvements in the mechanical properties. Various findings have been reported regarding these two polymerization techniques.7,8 Some studies have shown no difference in mechanical strength of acrylic resins processed in either method.9 Microwave polymerization provides dielectric heating by electromagnetic irradiation. During the polymerization reaction, there is increased monomer activity which is produced by this motion.10 For conventional heat polymerization, concentration of the initiator, generally benzoyl peroxide influences the polymerization rate, in the polymer.11 Polymerization is initially performed at 60℃ to consume all of the free monomer, and then the temperature is increased to approximately 100℃ crosslinking the polymer.12 Depending on the polymerization conditions, it is usually impossible to obtain these temperatures using the conventional water bath technique. For this reason, thermostatically controlled devices provide high temperatures and decrease the number of unreacted monomers.13
The fracture and deformation of dentures are recurrent and common problems. Consequently, reinforcement materials are embedded in the denture base to prevent fracture and deformation.14,15 The methods to improve the base materials' properties have included; addition of metal wires and development of various types of fibers or crosslinking agents for modification of the chemical structure to develop stronger denture base materials that are resistant to fracture.16 Chemical modification of the resins to incorporate a monomer produces a copolymer structure. Both monomers can lend properties to the final product.
Copolymer additions have been used as reinforcement for acrylic resin dentures17 for some time and their validity has been investigated. Copolymerisation mechanism provide polymer network form, resulting a number of favorable changes like; increase in polymerization rate, superior mechanical and physical properties and decrease in water solubility when compared to those of linear polymers.18 Several types of monomers have been added to MMA and the prepared copolymer structure of PMMA. Fluoroalkyl, butadiene styrene, ethyl methacrylate, butyl methacrylate, butyl acrylate, tetramethyldisiloxane, narbonyl and phenyl methacrylate monomers have been used with MMA to produce a modified copolymer structure, and conflicting results have been reported.17,19,20
The majority of acrylamide has been used for manufacturing various polymers.21 The acrylamide monomer contains vinyl groups within its molecular backbone. Because of these polar pendant groups, polymers made with this monomer that are tended to be somewhat crosslinked with etylene bonds and reinforced the polymer against to stresses.22
Hardness is an important physical property of acrylic resins, which makes these materials to resist plastic deformation, measuring usually by penetration, and to be used for manufacturing denture bases that withstand forces, such as originating from occlusion and mechanical denture cleansing, increasing the long term clinical use of the dental prosthesis.23 Although several different test methods (Rockwell, Brinnel, Knoop, Vickers) are practically in use, Vickers hardness test are more suitable for dental polymers that can provide correct records, thoroughly adaptable, very precise and just one type of indenter is used for softest and hardest of materials under varying loads.24,25
Thermal analysis is defined as a group of techniques in which a physical property of a polymer is observed under a controlled temperature program. The changes in the properties of the sample during the heating process can be monitored using this analysis. Measurements are generally repeated, and the rate of heating is often linear over time. These measurements are shown thermal analysis curves, and thermal events in the sample are related to the trends of these curves. Differential scanning calorimetry (DSC) and thermogravimetry (TGA) are two well-known analytical methods for evaluating the thermal characteristics of polymers.26 DSC provides a fast method for determining the glass transition temperature (Tg). Tg is critical in understanding the upper use of the temperature and processing environs.27
The aim of this in vitro study was to evaluate the effect of the incorporation of the acrylamide monomer (AAm) on the hardness, glass transition temperature and chemical structure of three different denture base resins. We hypothesized that the addition of acrylamide monomer to PMMA-based denture base resins would improve the mechanical and thermal properties of the resins.
MATERIALS AND METHODS
The three PMMA-based acrylic resins used in this study included the following: two heat polymerized resins, QC-20 (Dentsply Ltd., Addlestone, UK) and Stellon (AD, International Limited, Dentsply, Switzerland); one microwave-polymerized resin, Acron MC (GC Lab Technologies Inc., Alsip, Japan); and also an acrylamide monomer Materials, manufacturers, polymerization process and the experimental group codes are given in Table 1.
Table 1.
Materials, manufacturer and experimental groups used in this study
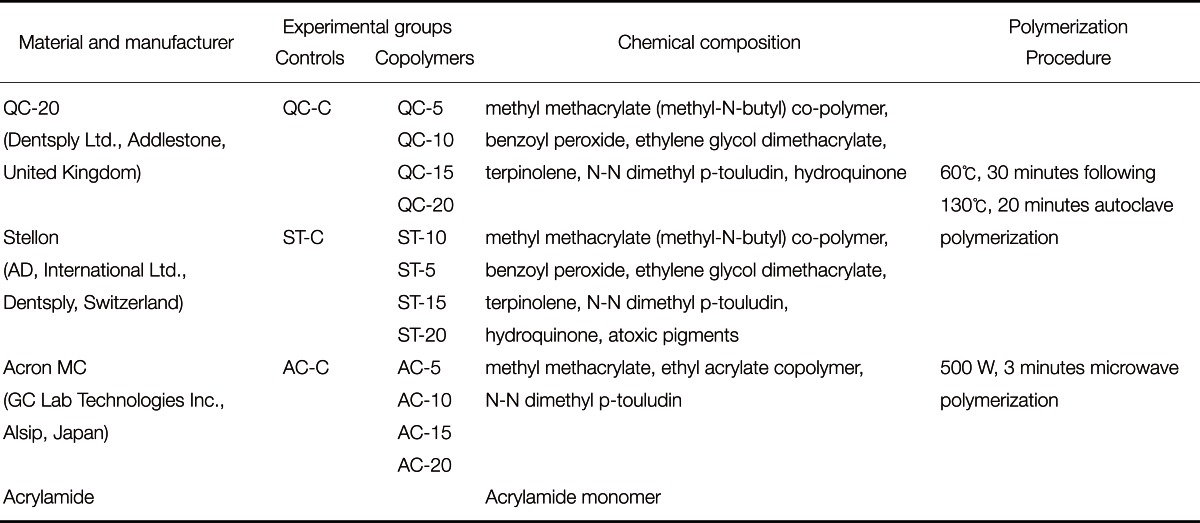
A total of 150, rectangularly shaped (64 × 10 × 3.3 mm) wax patterns were prepared for hardness test. The powder/liquid ratio was maintained as recommended by the manufacturers for the control groups. In the copolymer groups, preparation was carried out at various AAm contents with 5%, 10%, 15% and 20% (Merck, Hohenbrunn, Germany) according to the molecular weight ratio, respectively. First, the particulate acrylamide monomer was added to liquid MMA monomer, and dissolved monomer mixture was obtained. Then, the powder resin was added to the dissolved liquid and experimenters waited for the mixture to become doughy.
All flasks were compressed under hydraulic pressure for 15 minutes. Heat-polymerized resin specimens were processed in a thermally controlled autoclave device (QT 4060V, Nuve, Istanbul, Turkey) at 60℃ for 30 minutes followed by 130℃ for 20 minutes. The specimens of Acron MC were polymerized in a microwave oven (Vestel, Goldstar, Turkey) at 500 W for 3 minutes. Acrylic resins were bench-cooled before deflasking and then polished on both surfaces using an automatic polishing machine (Grin PO 2V grinder-polisher, Metkon A.Ş., Bursa, Turkey). All specimens were stored in a distilled water bath at 37℃ for 48 ± 2 hours before testing.
Control and copolymer resins were characterized using FTIR spectroscopy. A sample size of 1 mg (n=1) was used in each study group. After the polymerization, resin specimens were dried in a dry-heat oven for 12 hours at 70℃ to remove the H2 molecules from the structure, and then the dried resins were cut into small pieces to obtain a clear FTIR spectrum. The specimens were placed on the diamond set, and FTIR spectra of the samples were recorded using a Bruker Vertex-70 FTIR spectrometer (Bruker Optics Inc., Ettlingen, Germany).
The hardness of the resins was determined using rectangularly shaped specimens (n=10) with an universal hardness tester (Struers Duramin, Struers A/S, Ballerup, Denmark) equipped with a Vickers diamond penetrator. A 500 g load was applied to each specimen for 30 seconds at three points 15 mm apart. The average hardness among the indentations was considered as the final value of the Vickers hardness number.
The thermal properties of the samples were determined by differential scanning calorimetry (DSC). A sample size of 4 mg (n=1) was used in each study group. The glass transition temperature (Tg) of control and copolymers was evaluated by Perkin Elmer Diamond DSC (Perkin Elmer, Massachusetts, USA) in a nitrogen atmosphere with a heating rate of 10℃/min up to 250℃.
Additionally, 4-6 mg resin samples were placed into the pan with aluminum capsules. The pan containing the samples and an empty reference pan were placed on a thermoelectric disk surrounded by a furnace. As the temperature of the furnace was changed, heat was transferred to the samples and the reference through the thermoelectric disk. Area thermocouples on the underside of each platform measured the temperature of the sample and the reference. The mean and standard deviation of hardness were calculated for each measurement by SPSS, Windows 11.5 (SPSS Inc., Chicago, IL, USA). Distribution of the variables in terms of proximity to normal was detected by Shapiro Wilk test. Also homogeneity of variances were checked by Levene test. The data were analyzed using ANOVA with post-hoc Tukey Honestly Significant Difference (HSD) test. The differences between means were significant if the P value was less than 0.01. Bonferroni adjustment was used to counteract the problem of multiple comparisons.
RESULTS
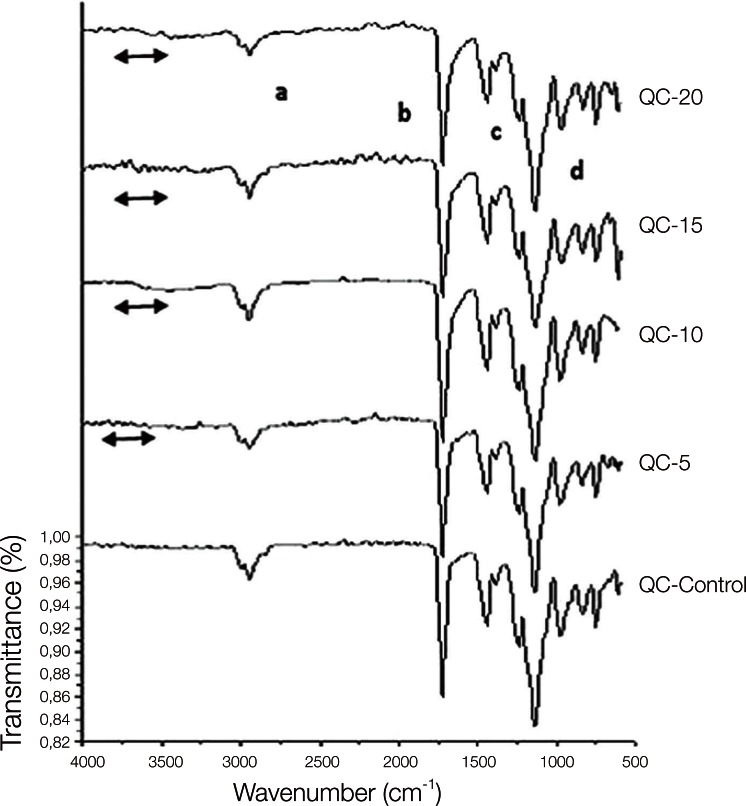
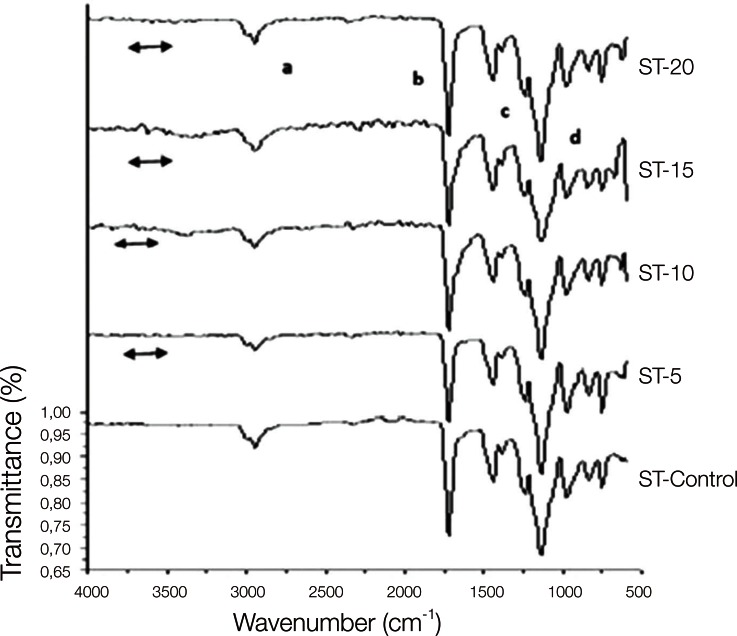
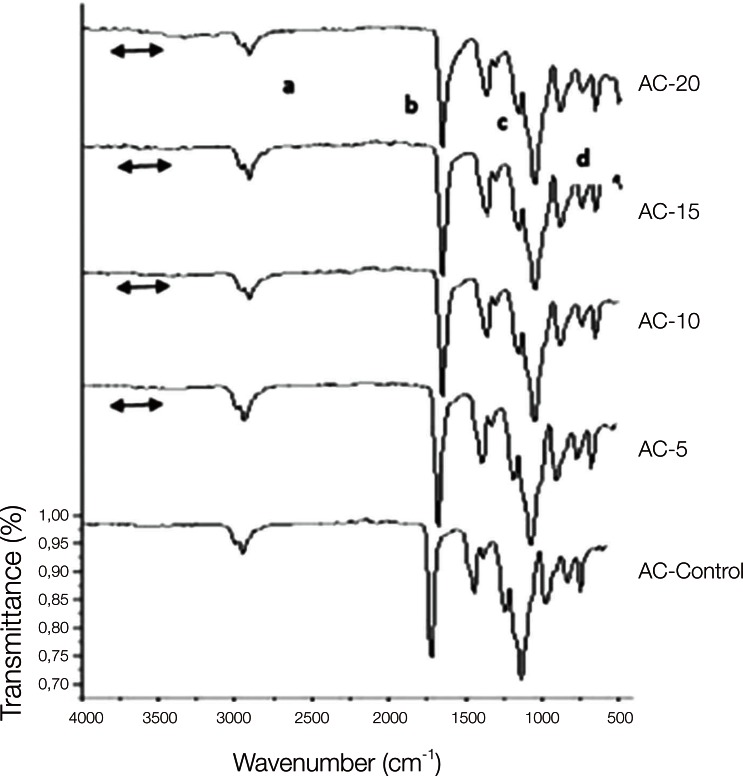
The copolymer synthesis was confirmed by FTIR spectroscopy, and the first group of QC control and QC-Acrylamide copolymers is shown in Fig. 1. In the FTIR spectr um of QC-C, a strong -C=O stretching was observed at 1720 cm-1 and the -C-O stretching vibrations of esters (C-C(=O) -O and O-C-C) were observed at in the range of 1249-1064 cm-1. Methyl and methylene -C-H stretchings were observed at approximately 2954 cm-1, whereas -C-H bendings of the same groups appeared at 1458 cm-1. In the FTIR spectra of copolymers with various molar percentages of acrylamide (AAm), the same vibrations were identified corresponding to the methyl methacrylate (MMA) units. Additionally, the wide peak at 3417 cm-1 can be ascribed to N-H stretchings. Fig. 2 reveals the characteristic absorption bands of both the MMA and AAm groups of Stellon resins. The -C-H stretching and bending vibrations appeared at 2947 and 1442 cm-1, respectively. The -C=O groups and ester groups appeared at 1720 and 1242-1056 cm-1. The methyl rocking vibrations are observed at 979 cm-1. Again, a broad band at approximately 3332 cm-1 was assigned to N-H stretchings. Similarly to Fig. 1 and Fig. 2, PMMA and PAAm characteristic groups can be observed in the FTIR spectra of the Acron MC groups in Fig. 3.
Fig. 1.
FTIR spectra of QC-20 control and QC-20-AAm copolymers. 'a' and 'c' show the C-H, 'b' shows the C=O and 'd' shows the O-C-C bonds of MMA which are observed in both control and copolymer of the resins. The line symbol indicates the NH2 peaks of acrylamide in the copolymer groups.
Fig. 2.
FTIR spectra of Stellon control and Stellon-AAm copolymers. 'a' and 'c' show the C-H, 'b' shows the C=O and 'd' shows the O-C-C bonds of MMA which are observed in both control and copolymer of the resins. The line symbol indicates the NH2 peaks of acrylamide in the copolymer groups.
Fig. 3.
FTIR spectra of Acron MC control and Acron MC-AAm copolymers. 'a' and 'c' show the C-H, 'b' shows the C=O and 'd' shows the O-C-C bonds of MMA which are observed in both control and copolymer of the resins. The line symbol indicates the NH2 peaks of acrylamide in the copolymer groups.
The means and standard deviations of the hardness of the control and copolymer groups are given in Table 2. There were significant interactions between the control and copolymer groups with respect to hardness according to the ANOVA.
Table 2.
Means and standard deviations (kg/mm2) of Vickers Hardness of the control and copolymer groups
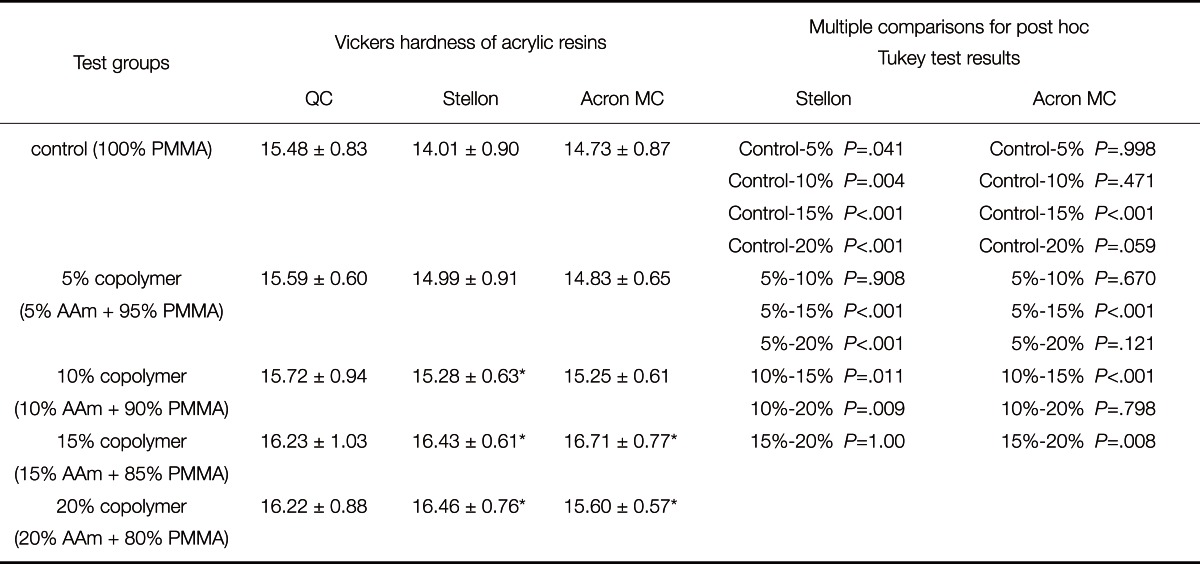
*Symbol indicates significant differences between control and copolymer groups in each column (P<.01).
For both polymerization types, the data showed that the mean hardness of all resins was not significant. A significant difference was found between the control groups of the QC (15.48 ± 0. 83) and Stellon (14.01 ± 0.90) resins.
Control groups showed the lowest hardness values, respectively (QC: 15.48 ± 0.83; Stellon: 14.01 ± 0.90; Acron MC: 14.73 ± 0.87). Acrylamide addition did not affect the hardness of the QC acrylic resin. Significant differences were found in the Stellon and Acron MC resins. The 10%, 15% and 20% copolymer groups of Stellon presented significantly higher hardness values compared to the control group, but there is not any significant difference between ST-5 and ST-c. Other significant differences were observed between ST-5 and ST-15, ST-5 and ST-20, ST-10 and ST-15 as well as ST-10 and ST-20. The hardness data for Acron MC showed significant differences between groups of AC-C (14.73 ± 0.87) and AC-15 (16.71 ± 0.77), AC-20 (15.60 ± 0.57). In addition, no significant differences were found between the control group and AC-5 and AC-10. For the copolymer groups of Acron MC resins, significant differences were observed between AC-5 and AC-15, AC-10 and AC-15, as well as AC-15 and AC-20.
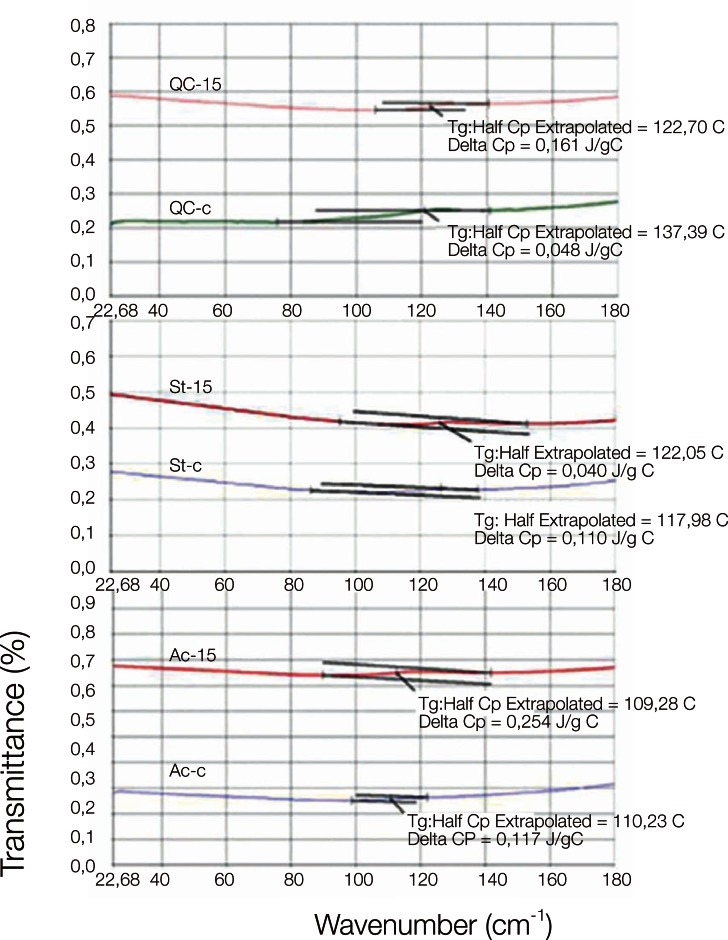
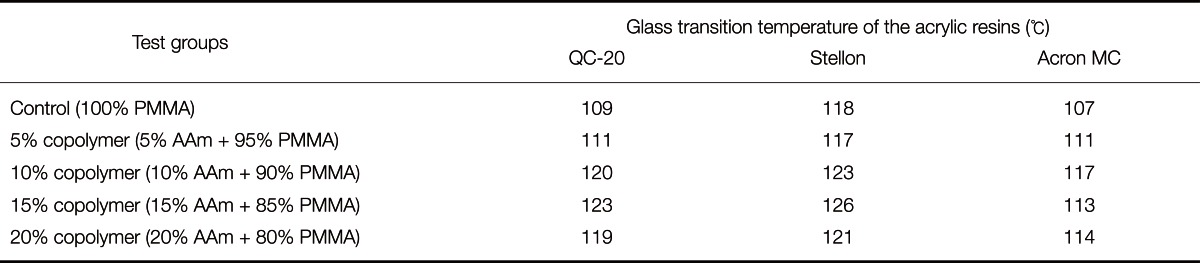
DSC curves of QC-C, QC-15; ST-C, ST-15 and AC-C, AC-15 are shown in Fig. 4. The glass transition temperatures (Tg) of the control and copolymer groups, according to the DSC thermograms, are given in Table 3. The Tg values of PMMA were increased by the incorporation of AAm units in the copolymer, and this trend was observed in both the heat and microwave-cured denture base resins. The highest Tg value was recorded for the Stellon 15% AAm copolymer group.
Fig. 4.
DSC thermogram of QC, Stellon and Acron MC control and 15% copolymer groups.
Table 3.
Glass transition temperature of the control and copolymer groups
DISCUSSION
There has been increasing interest in the incorporation of monomers into polymeric dental materials.17,20,28,29 To date, this is the first study to investigate the mechanical properties, chemical structure and thermal behavior of methyl methacrylate-co-acrylamide denture base polymers. A similar method was also tested for dental composites and adhesives by means of incorporation of the multifunctional monomers. Pavlinec and Moszner30 studied the effect of acrylamide copolymerization for dental filling materials. Conflicting results were reported for the properties of acrylamide monomers' excellent hydrolytic stability, very good mechanical properties and low cytotoxicity.
In this in vitro study, the structure of polymethyl methacrylate was modified with acylamide monomer to reinforce the denture base resin materials. As a result of this reinforcement method, the PMMA matrix was changed to a PMMA-co-AAm form that has different physical and mechanical behaviors. Acrylamide monomer particles show gel formation when there is a change in the temperature. The cohesive interactions of the polymeric chains are increased by this gel formation, providing augmentation in the mechanical stability of the final copolymers while affecting the phase, strength and crosslinking ability of the polymer.31 For these reasons, acrylamide monomer was selected for copolymerization of PMMA in the present study. The chemical structure of acrylamide compound is formulated as C3H5NO. It is a white, odorless, crystalline solid that is soluble in water, ethanol, ether, and chloroform.32 In the present study, mixture ratios of monomers were also adjusted according to the molecular weight because the MMA monomer was a liquid and the AAm monomer was in particle form, and it was impossible to mix the monomers according to a volumetric ratio. During the copolymer preparation stage, the monomer was also dissolved in MMA, which resulted in a homogenous structure that can be easily ground and polished. Additionally, the presence of the low molecular weight acrylamide monomer did not greatly change the viscosity and manipulability, and modified techniques were not required for polymerization.33
FTIR is one of the most widely used spectroscopic methods for analyzing the structure of polymers. Umemoto and Kurata19 studied the structure of PMMA-based polymers and copolymers using FTIR spectroscopy. The FTIR spectra of the control groups presented methyl and methylene peaks at approximately 2998-1245 cm-1, similarly to those observed in the control groups of this study.
Copolymerization synthesis was confirmed by FTIR spectroscopy. Methyl and methylene units of PMMA were recorded in the FTIR spectra for both the control and copolymer groups. In addition to the PMMA units, new peaks appeared in the copolymer spectrum from amine molecules of AAm. The 15% AAm-PAMM copolymer resins showed especially wide and strong peaks. These spectra confirm the free radical copolymerization of PMMA-co-AAm.
The hardness test has been considered a simple and useful method to determine the mechanical properties of polymer based materials because it is highly sensitive to the amount of monomer in dental polymers.34,35 The hardness test results showed that incorporation of increasing amounts of acrylamide into the PMMA increased the Vickers hardness value by 5%, 10%, 15% and 20% when compared to the control group of the resins. The present results support the hypothesis that copolymerization would affect the physical properties of acrylic denture resins. These results are in agreement with the study performed by Vuorinen et al.28 The authors evaluated the mechanical properties of denture base material with polyphenylene-based rigid rod polymers using the Vickers hardness test and reported that the addition of particulate rod polymers increased the surface microhardness compared to the control. Rodford36 also investigated the effect of copolymerization with butadiene styrene monomer on the mechanical properties of PMMA-based denture resins and reported an increase in the impact strength. In contrast with these results, Puri et al.29 modified the structure of PMMA denture base resin by adding ethylene glycol methacrylate phosphate monomer in increasing concentrations of 10%, 15% and 20% by volume and reported that no significant differences were found regarding the impact and fracture toughness property when compared with the control acrylic resin. As there are no reports in the literature concerning the hardness of acrylamide-monomer-added forms of the three denture base resins used in this study, it is not possible to compare the results; therefore, further studies need to be conducted.
The addition of acrylamide to PMMA increased the hardness property of Stellon and Acron MC resins significantly, but there was no significant increase for the QC resins. This result may be attributable to the fact that the monomer of QC is modified with a methyl/n butyl methacrylate copolymer. Therefore, the copolymerization mechanism did not affect the more saturated resin in term of hardness. In the present study, the hardness values of all resins became higher as the acrylamide content increased up to 15%. The 15% copolymer groups had the highest hardness values among all the groups. Increasing the AAm content ratio from 15% to 20% decreased the hardness of the copolymer resins. This may be explained by the fact that maximum saturated semi-IPN matrix formation between PMMA and AAm occurred in the 15% copolymeric structure; therefore, increasing the AAm content caused a decrease in the hardness of the material. Although this decrease occurred for the 20% copolymer groups, these groups showed significantly higher values than the controls.
DSC has been generally used in the research of dental materials for studying the polymerization or setting reactions of dental resins and for measuring the glass transition temperatures of polymers.26 The Tg value for PMMA varies from 117℃ to 122℃.37 In the present study, the Tg values of control groups are in accordance with these temperatures. The addition of acrylamide monomer increased the Tg values in the copolymer forms of all resins. This parameter was considered to be important because exposure to excessive temperatures, either inside or commonly outside of the mouth, may cause distortions of the dentures.38 It is known that a linear relationship exists between the additional monomer rate and the Tg of the polymer.31,39 The results of this study showed that increasing the acrylamide monomer ratio improved the glass transition phase of the polymers. The 15% copolymer group presented the highest Tg value. This may be because the best saturated semi-IPN structure occurred in the 15% copolymer groups of all denture base resins. This result is also comparable with the increase in hardness values for the same experimental group.
Amide moieties in the main chain or in pendant groups affect the physical and chemical properties when the structure becomes a copolymer.28 Copolymer particles are generally added to a polymer structure to form linear chains with crosslinking. The presence of crosslinking is known to improve the physical properties of the acrylic resins so that they become superior when compared to the linear polymers, and causes cross-linked polymer matrix not soluble in water.33 Copolymerization with acrylamide may result in a crosslinking polymer structure, which explains these increases in both the hardness and the glass transition temperature of the PMMA-based denture resins.
The Acron MC resins were polymerized by microwave energy, whereas the QC-20 and Stellon resins were polymerized by heat. Neppelenbroek et al.40 reported higher hardness values for microwave-polymerized acrylic resins compared to the conventional water bath. Although some studies showed better results for microwave polymerization compared with the conventional water bath method, the autoclave polymerization method had similar results to the microwave polymerization in the present study. In contrast, according to the DSC analysis, the lowest Tg values belonged to the Acron MC control group. This was most likely attributable to the prevention of the homogenous matrix form of the final product which results from the local heating within the material.12 Domestic microwave ovens have the disadvantage of not being able to control temperature precisely.41 This may create a superficial cure effect in the microwave-polymerized samples, resulting in lower Tg values.
Addition of acrylamide monomer PMMA resulted in improvements in some of the physical properties of these materials, but some characteristics remain to be evaluated, such as the mechanical and biological properties, before the addition can be recommended in the market.
CONCLUSION
Under the conditions of the present study, the following conclusions may be drawn:
FTIR spectras of the groups showed that the addition of acrylamide to PMMA created a copolymer structure.
Copolymerization of PMMA with AAm increased the hardness values and glass transition temperatures of PMMA denture base resins.
Heat and microwave polymerization techniques did not affect the hardness property significantly.
The microwave-polymerizing control group showed the lowest glass transition temperature.
The addition of 15% acrylamide may be recommended as an alternative method to increase some of the mechanical and physical properties of the PMMA-based denture resins.
Footnotes
This study was performed as the PhD thesis of the first author at the Karadeniz Technical University, Trabzon, Turkey and it was supported by Scientific Research Subjects of the Karadeniz Technical University by the Project number of 2009.127.001.3.
References
- 1.Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bull World Health Organ. 2005;83:661–669. [PMC free article] [PubMed] [Google Scholar]
- 2.Tang AT, Li J, Ekstrand J, Liu Y. Cytotoxicity tests of in situ polymerized resins: methodological comparisons and introduction of a tissue culture insert as a testing device. J Biomed Mater Res. 1999;45:214–222. doi: 10.1002/(sici)1097-4636(19990605)45:3<214::aid-jbm9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.Rached RN, Del-Bel Cury AA. Heat-cured acrylic resin repaired with microwave-cured one: bond strength and surface texture. J Oral Rehabil. 2001;28:370–375. doi: 10.1046/j.1365-2842.2001.00666.x. [DOI] [PubMed] [Google Scholar]
- 4.Wolfaardt JF, Cleaton-Jones P, Fatti P. The occurrence of porosity in a heat-cured poly (methyl methacrylate) denture base resin. J Prosthet Dent. 1986;55:393–400. doi: 10.1016/0022-3913(86)90128-9. [DOI] [PubMed] [Google Scholar]
- 5.Honorez P, Catalan A, Angnes U, Grimonster J. The effect of three processing cycles on some physical and chemical properties of a heat-cured acrylic resin. J Prosthet Dent. 1989;61:510–517. doi: 10.1016/0022-3913(89)90025-5. [DOI] [PubMed] [Google Scholar]
- 6.Nishii M. Curing of denture base resins with microwave irradiation: with particular reference to heat-curing resins. J Osaka Dent Univ. 1968;2:23–40. [PubMed] [Google Scholar]
- 7.Barbosa DB, Monteiro DR, Barão VA, Pero AC, Compagnoni MA. Effect of monomer treatment and polymerisation methods on the bond strength of resin teeth to denture base material. Gerodontology. 2009;26:225–231. doi: 10.1111/j.1741-2358.2008.00262.x. [DOI] [PubMed] [Google Scholar]
- 8.Jacob J, Chia LHL, Boey FYC. Comparative study of methyl methacrylate cure by microwave radiation versus thermal energy. Polym Test. 1995;14:343–354. [Google Scholar]
- 9.Smith LT, Powers JM, Ladd D. Mechanical properties of new denture resins polymerized by visible light, heat, and microwave energy. Int J Prosthodont. 1992;5:315–320. [PubMed] [Google Scholar]
- 10.De Clerck JP. Microwave polymerization of acrylic resins used in dental prostheses. J Prosthet Dent. 1987;57:650–658. doi: 10.1016/0022-3913(87)90353-2. [DOI] [PubMed] [Google Scholar]
- 11.Jerolimov V, Brooks SC, Huggett R, Bates JF. Rapid curing of acrylic denture-base materials. Dent Mater. 1989;5:18–22. doi: 10.1016/0109-5641(89)90086-9. [DOI] [PubMed] [Google Scholar]
- 12.Usanmaz A, Ateş J, Doğan A. Thermal and Mechanical Properties of Microwave- and Heat-Cured Poly(methyl methacrylate) Used as Dental Base Material. J Appl Polym Sci. 2003;90:251–256. [Google Scholar]
- 13.Bural C, Aktaş E, Deniz G, Ünlüçerçi Y, Bayraktar G. Effect of leaching residual methyl methacrylate concentrations on in vitro cytotoxicity of heat polymerized denture base acrylic resin processed with different polymerization cycles. J Appl Oral Sci. 2011;19:306–312. doi: 10.1590/S1678-77572011005000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi T, Gonda T, Maeda Y. Influence of reinforcing materials on strain of maxillary complete denture. Acta Odontol Scand. 2013;71:307–311. doi: 10.3109/00016357.2012.680903. [DOI] [PubMed] [Google Scholar]
- 15.Beyli MS, von Fraunhofer JA. An analysis of causes of fracture of acrylic resin dentures. J Prosthet Dent. 1981;46:238–241. doi: 10.1016/0022-3913(81)90206-7. [DOI] [PubMed] [Google Scholar]
- 16.Jagger DC, Harrison A, Jandt KD. The reinforcement of dentures. J Oral Rehabil. 1999;26:185–194. doi: 10.1046/j.1365-2842.1999.00375.x. [DOI] [PubMed] [Google Scholar]
- 17.Cunha TR, Regis RR, Bonatti MR, de Souza RF. Influence of incorporation of fluoroalkyl methacrylates on roughness and flexural strength of a denture base acrylic resin. J Appl Oral Sci. 2009;17:103–107. doi: 10.1590/S1678-77572009000200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moszner N, Fischer UK, Angermann J, Rheinberger V. Bis-(acrylamide)s as new cross-linkers for resin-based composite restoratives. Dent Mater. 2006;22:1157–1162. doi: 10.1016/j.dental.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Umemoto K, Kurata S. Basic study of a new denture base resin applying hydrophobic methacrylate monomer. Dent Mater J. 1997;16:21–30. doi: 10.4012/dmj.16.21. [DOI] [PubMed] [Google Scholar]
- 20.Umemoto K, Kurata S, Morishita K, Kawase T. Basic study of a new soft resin applied with bisfunctional siloxane oligomer. Dent Mater J. 2007;26:656–658. doi: 10.4012/dmj.26.656. [DOI] [PubMed] [Google Scholar]
- 21.Mortimer DA. Synthetic polyelectrolytes- A review. Polym Int. 1991;25:29–41. [Google Scholar]
- 22.Friedman M. Chemistry, biochemistry, and safety of acrylamide. A review. J Agric Food Chem. 2003;51:4504–4526. doi: 10.1021/jf030204+. [DOI] [PubMed] [Google Scholar]
- 23.Pinto Lde R, Acosta EJ, Távora FF, da Silva PM, Porto VC. Effect of repeated cycles of chemical disinfection on the roughness and hardness of hard reline acrylic resins. Gerodontology. 2010;27:147–153. doi: 10.1111/j.1741-2358.2009.00282.x. [DOI] [PubMed] [Google Scholar]
- 24.Philips RW. Science of Dental Materials. 9th ed. Philadelphia: W.B. Saunders Co; 1991. pp. 15–35. [Google Scholar]
- 25.Ping Chaing BK. Polymers in the service of prosthetic dentistry. J Dent. 1984;12:203–214. doi: 10.1016/0300-5712(84)90063-0. [DOI] [PubMed] [Google Scholar]
- 26.Sorai M. The Japan Society of Calorimetry and Thermal Analysis, authors. Comprehensive Handbook of Calorimetry and Thermal Analysis. J. Wiley; 2004. [Google Scholar]
- 27.Aouachria K, Bensemra NB. Miscibility of PVC/PMMA blends by vicat softening temperature, viscometry, DSC and FTIR analysis. Polym Test. 2006;25:1101–1108. [Google Scholar]
- 28.Vuorinen AM, Dyer SR, Lassila LV, Vallittu PK. Effect of rigid rod polymer filler on mechanical properties of polymethyl methacrylate denture base material. Dent Mater. 2008;24:708–713. doi: 10.1016/j.dental.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Puri G, Berzins DW, Dhuru VB, Raj PA, Rambhia SK, Dhir G, Dentino AR. Effect of phosphate group addition on the properties of denture base resins. J Prosthet Dent. 2008;100:302–308. doi: 10.1016/S0022-3913(08)60210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavlinec J, Moszner N. Monomers for Adhesive Polymers, 8a Crosslinking Polymerization of Selected N-Substituted bis(Acrylamide)s for Dental Filling Materials. J Appl Polym Sci. 2009;113:3137–3145. [Google Scholar]
- 31.Pai RK, Ng JB, Pillai S, Bergström L, Hedin N. Temperature-induced formation of strong gels of acrylamide-based polyelectrolytes. J Colloid Interface Sci. 2009;337:46–53. doi: 10.1016/j.jcis.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 32.Hacker MC, Klouda L, Ma BB, Kretlow JD, Mikos AG. Synthesis and characterization of injectable,thermally and chemically gelable, amphiphilic poly(N-isopropylacrylamide)-based macromers. Biomacromolecules. 2008;9:1558–1570. doi: 10.1021/bm8000414. [DOI] [PubMed] [Google Scholar]
- 33.Bhattacharya A, Rawlins J, Ray P. Polymer grafting and crosslinking. Wiley; 2009. [Google Scholar]
- 34.Azevedo A, Machado AL, Vergani CE, Giampaolo ET, Pavarina AC. Hardness of denture base and hard chair-side reline acrylic resins. J Appl Oral Sci. 2005;13:291–295. doi: 10.1590/s1678-77572005000300017. [DOI] [PubMed] [Google Scholar]
- 35.Rueggeberg FA, Craig RG. Correlation of parameters used to estimate monomer conversion in a light-cured composite. J Dent Res. 1988;67:932–937. doi: 10.1177/00220345880670060801. [DOI] [PubMed] [Google Scholar]
- 36.Rodford RA. Further development and evaluation of high impact strength denture base materials. J Dent. 1990;18:151–157. doi: 10.1016/0300-5712(90)90056-k. [DOI] [PubMed] [Google Scholar]
- 37.Ruyter IE, Svendsen SA. Flexural properties of denture base polymers. J Prosthet Dent. 1980;43:95–104. doi: 10.1016/0022-3913(80)90362-5. [DOI] [PubMed] [Google Scholar]
- 38.Blagojevic V, Murphy VM. Microwave polymerization of denture base materials. A comparative study. J Oral Rehabil. 1999;26:804–808. doi: 10.1046/j.1365-2842.1999.00456.x. [DOI] [PubMed] [Google Scholar]
- 39.Sperling LH. Introduction to physical polymer science. Wiley; 1986. [Google Scholar]
- 40.Neppelenbroek KH, Pavarina AC, Vergani CE, Giampaolo ET. Hardness of heat-polymerized acrylic resins after disinfection and long-term water immersion. J Prosthet Dent. 2005;93:171–176. doi: 10.1016/j.prosdent.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 41.Rao S, Rao GK. Cure studies on bifunctional epoxy matrices using a domestic microwave oven. Polym Test. 2008;27:645–652. [Google Scholar]