Abstract
PURPOSE
Maxillofacial silicone elastomer is usually colored intrinsically with color pigments to match skin colors. The purpose of this study was to investigate the color stability of a maxillofacial silicone elastomer, colored with a thermochromic, color changing pigment.
MATERIALS AND METHODS
Disc-shaped maxillofacial silicone specimens were prepared and divided into 3 groups: a conventionally colored control group, one group additionally colored with 0.2 wt% thermochromic pigment , and one group with 0.6 wt% thermochromic pigment. Half of the surface of each specimen was covered with an aluminium foil. All of the specimens were exposed to UV radiation in 6 hour cycles over 46 days. In between the UV exposures, half of the specimens were stored in darkness, at room temperature, and the other half was stored in an incubator, at a humidity of 97% and a temperature of +37℃. Color measurements were made with a spectrophotometer and registered according to the CIELAB L*a*b* color model system. The changes in L*, a* and b* values during artificial aging were statistically analyzed by using paired samples t-test and repeated measures ANOVA. P-values <.05 were considered as statistically significant.
RESULTS
The UV exposure resulted in visually noticeable and statistically significant color changes in the L*, a* and b* values in both of the test groups containing thermochromic pigment. Storage in the incubator lead to statistically significant color changes in the a* and b* values of the specimens containing thermochromic pigment, compared to those stored at room temperature.
CONCLUSION
The specimens containing thermochromic pigment were very sensitive to UV radiation, and the thermochromic pigment is not suitable, as such, to be used in maxillofacial prostheses.
Keywords: Maxillofacial silicone, Thermochromic pigment, Color stability
INTRODUCTION
Missing or damaged facial parts, which have been lost or changed due to genetic disorders or as a result of disease or trauma are, in many cases, rehabilitated using facial prostheses.1-3 An ideal material should have physical and mechanical properties that are comparable to those of human tissues and skin.
Extraoral maxillofacial prostheses are usually fabricated using maxillofacial silicone elastomer, colored either internally or externally using inorganic or organic pigments.4-8 Used pigments are dry earth pigments, or kaolin and rayon flocking, which act as solid fillers that do not bond to the silicone. Artist's oil and liquid cosmetics act as a liquid base without bonding to the silicone matrix.9 The mechanical and physical properties of facial prosthetic materials have improved over recent years, but the color instability of the facial prostheses still limits the serviceability and is often the reason for remaking the prosthesis.10-21 Environmental factors, such as weathering, normal aging and cleaning agents cause degradation of the optical and mechanical properties of the silicone elastomer.12,15,22-27 Polymeric materials usually have a low thermal stability and little resistance to solar radiation.28 Some factors that affect the color stability are UVA-UVB irradiation (sunlight), temperature, moisture, wind, dust and pollutants.29
Recently, the use of 0.2 wt% thermochromic, color-changing, pigment in maxillofacial silicone prostheses was suggested.30 The purpose of the pigment was to mimic the color change of the skin in cold winter weather conditions, for example in ear prostheses. Thermochromism is a process where the external temperature induces a change in the colors of certain compounds.31 Color-changing pigments have been used in thermometers, in toys,32 as temperature indicators in cables and wires to detect overheating,31 and in the pharmaceutical industry.33
The purpose of the present study was to measure the color stability of a maxillofacial silicone elastomer colored with thermochromic pigment. The thermochromic pigment used in this study consists of thermochromic microcapsules with a diameter of < 7 µm in a powder pigment form. It changes color from red to colorless as the temperature rises. In reverse, it is colorless in room temperature and changes to red as the temperature decreases. Spectrophotometric analysis was used to evaluate the color at baseline (before artificial aging) and after UV irradiation, also comparing storage in dry, room temperature to storage at +37℃ in moisture in an incubator.
The null hypothesis of the study was that artificial aging would not affect the color of the silicone elastomer containing the thermochromic pigment.
MATERIALS AND METHODS
The materials used in the study are listed in Table 1. Maxillofacial silicone elastomer (MDX4-4210; Dow Corning Corp., Midland, MI, USA) was manually mixed according to the manufacturer's instructions. Color pigments were added (Table 1) by using an intrinsic coloring technique to make natural, fair skin-colored silicone elastomer. The fair skin-colored silicone elastomer was divided into 3 groups. Thermochromic pigment was added to 2 groups: 0.2 wt% and 0.6 wt% thermochromic pigment was added respectively, fair skin-colored silicone elastomer without thermochromic pigment was used as control group.
Table 1.
Materials used in the study
Maxillofacial silicone elastomer specimens were prepared by using prefabricated stone molds with circular cavities. The silicone elastomer was poured into the stone molds and polymerized for 2 hours at 90℃. The resultant test specimens were disc-shaped, with a dimension of 7 × 35 mm. The test specimens were finished with a grinding paper (Struers Waterproof Silicon Carbide Paper FEPA p#1200; Struers, Ballerup, Denmark), and they were then gently cleaned and rinsed with water. After 20 days of storage at room temperature, in darkness, the baseline color measurements were carried out with a spectrophotometer (Konica Minolta M-700d/600d; Konica Minolta Sensing Inc., Tokyo, Japan).
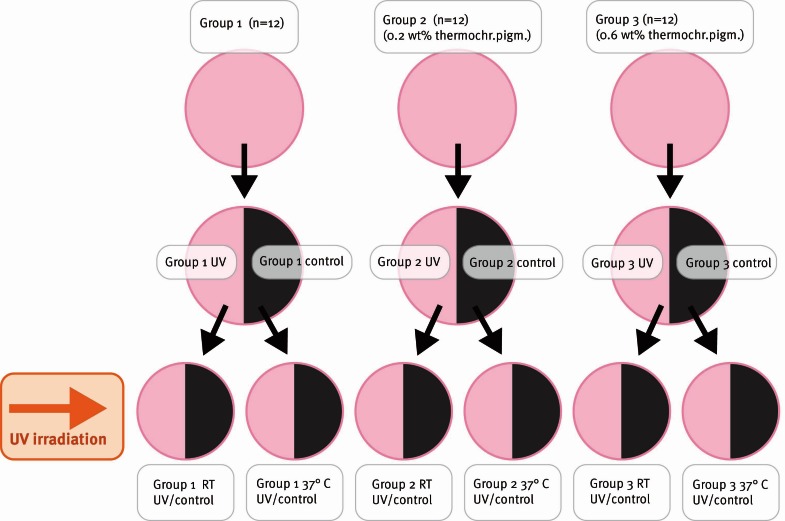
All specimens were covered with an aluminium-foil cover over half of their surface. The specimens were then divided into two main experimental groups (Fig. 1), regarding storage in between the color measurements and the 6 hour cycles of exposure to UVA-radiation: half of the specimens were stored in darkness at room temperature (RT group), +23℃, and the other half in an incubator at +37℃, with a humidity of 97% (37° group). The groups of specimens are listed in Table 2. The specimens were then exposed to UV-light (UVA lamp Blacklight, F 15W/350 BL-T8, 315-400 nm; Sylvania, Havells-Sylvania India Ltd) for 6 hours daily, apart from during the weekends. Color measurements were carried out in room temperature, at baseline (day 1), which was 20 days after fabrication of the specimens, and at day 2, 3, 4, 8, 24, 29, 38 and 46. The color-measurements were performed with the spectrophotometer described above, at three points of each half of the specimens, using a white background plate and according to the CIELAB coordinates. The specimens were thick enough, not to be translucent.
Fig. 1.
The division of specimens into groups prior to exposure to artificial aging. Each group consisted of 12 specimens, half of the surface was wrapped with aluminium foil to protect the surface from UV irradiation.
Table 2.
The division of silicone elastomer specimens into groups, according to the pigment concentration and storage. Each group consisted of 6 specimens
In the CIE (Comission Internationale d'Eclairage) L*a*b* three-dimensional color model system,34 the L* parameter stands for the degree of lightness and darkness (0 means ideal black, 100 means ideal white), the a* coordinate stands for red or green chroma : a high +a* value means an intense red chroma and a high absolute value of -a* means an intense green chroma. Correspondingly, the b* coordinate describes a yellow or blue chroma, where a high +b* means an intense yellow chroma, and a high absolute value of -b*means an intense blue chroma. The wavelength range of the measured spectrum was 400-700 nm. The color change ΔE within groups was calculated using the equation: ΔE = ([ΔL*]2 + [Δa*]2 + [ Δb*]2)½.
The changes in L*, a* and b* values during artificial aging were statistically analyzed by using repeated measures ANOVA and paired samples t-tests. Repeated measures ANOVA was used to analyze the differences between groups according to storage and concentration of the thermochromic pigment, and across time points. The two half surfaces of each specimen were compared to each other: the half that was exposed to UV irradiation was compared to the covered half by using paired samples t-tests. Distributions of the changes in L*, a*, and b* values during artificial aging were symmetrically distributed in each storage and concentration group. In the repeated measures ANOVA, sphericity assumption was violated and ε < 0.75, so Greenhouse-Geisser correction was applied. All of the used tests were two-sided, and P values <.05 were considered to be statistically significant. The data analyses were performed with SPSS statistical software (SPSS 19.0; SPSS Inc., Chicago, IL, USA).
RESULTS
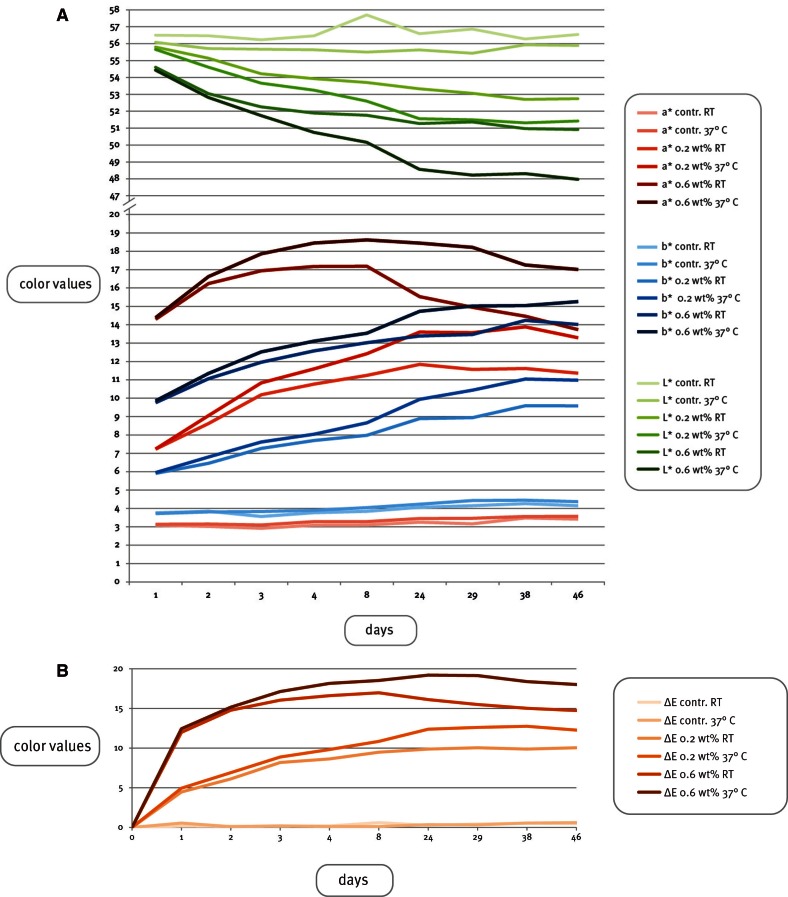
The color difference, ΔE, comparing the color of each non-irradiated half of the maxillofacial silicone elastomer specimen to the UV-irradiated half, and the change of the color values L*, a* and b* during the artificial aging process, are presented in Table 3, Table 4, Fig. 2A and Fig. 2B.
Table 3.
The mean color values of the maxillofacial silicone elastomer specimens after artificial aging
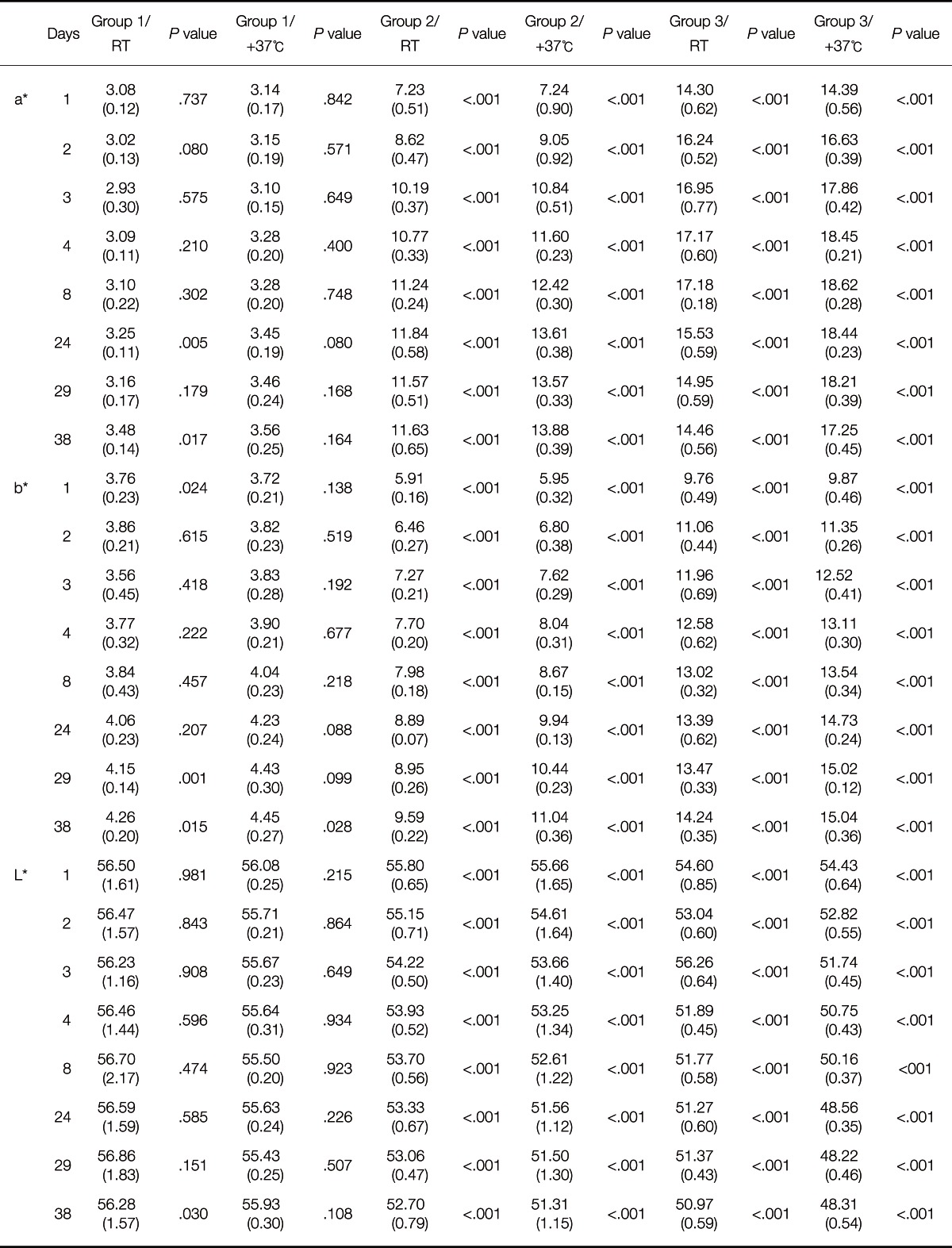
Table 4.
The ΔE values of the maxillofacial silicone elastomer specimens after artificial aging. ΔE describes the color difference between the UV irradiated and non-irradiated surfaces of each specimen, presented as the mean value of all the specimens
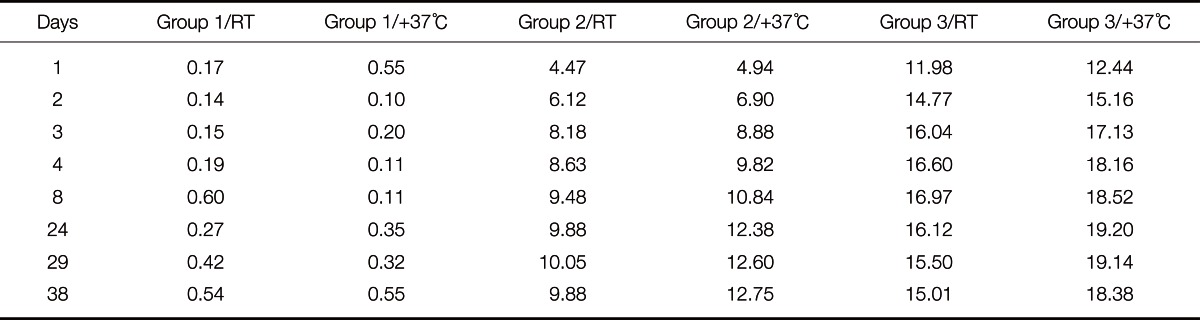
Fig. 2.
A: Color change in L*, a* and b* values after exposure to UV irradiation, B: The overall color change of the specimens, ΔE, after exposure to UV irradiation.
The results revealed that just one 6 hour cycle of UV irradiation caused considerable color changes in the specimens containing thermochromic pigment. This was demonstrated as high ΔE values in Group 2 and 3 - both in those specimens that had been stored at room temperature and those stored in an incubator at 37℃. Group 2 presented the mean ΔE values of 4.47 (RT) and 4.94 (incubator), respectively, after one cycle of UV irradiation, while the color change in group 3 was as large as 11.98 (RT) and 12.44 (incubator). In this study, the a* coordinate is the most interesting color coordinate, as it describes the redness.
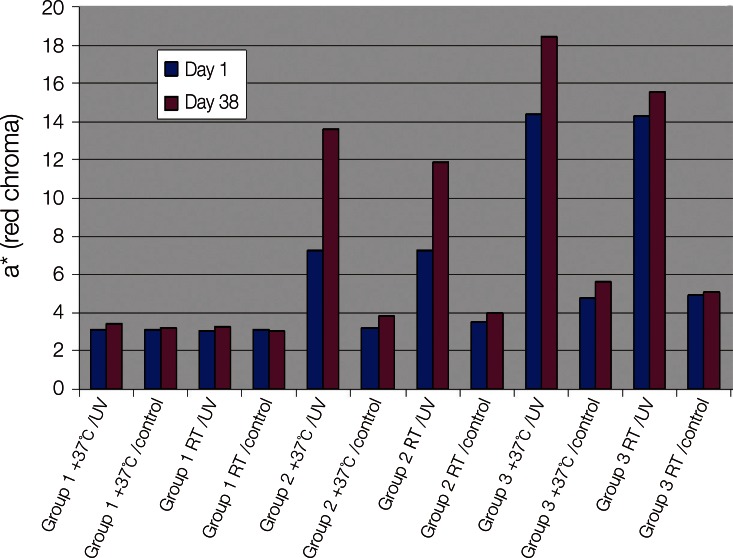
Statistical analysis revealed that UV irradiation had a significant effect (P<.001) on all color values, L*, a* and b*, of the specimens containing thermochromic pigment, already after one cycle of UV irradiation. In the test group 2/RT the mean a* value of the UV exposed surfaces changed from 7.23 to 11.62 during 37 days. In group 2/37℃, the a* value changed from 7.24 to 13.88. The corresponding a* values in Group 3/RT and Group 3/37℃ were 14.30 and 14.46. Though, the highest a*values in Group 3/RT are seen as soon as on days 3-8 and in group 1/RT, colored only with conventional pigments, mean a* value changed from 3.08 at the baseline to 3.48 at day 46. The a* value is shown in Fig. 3.
Fig. 3.
Comparison of the color value a* (red chroma) of the specimens at day 1 and day 38 of the study.
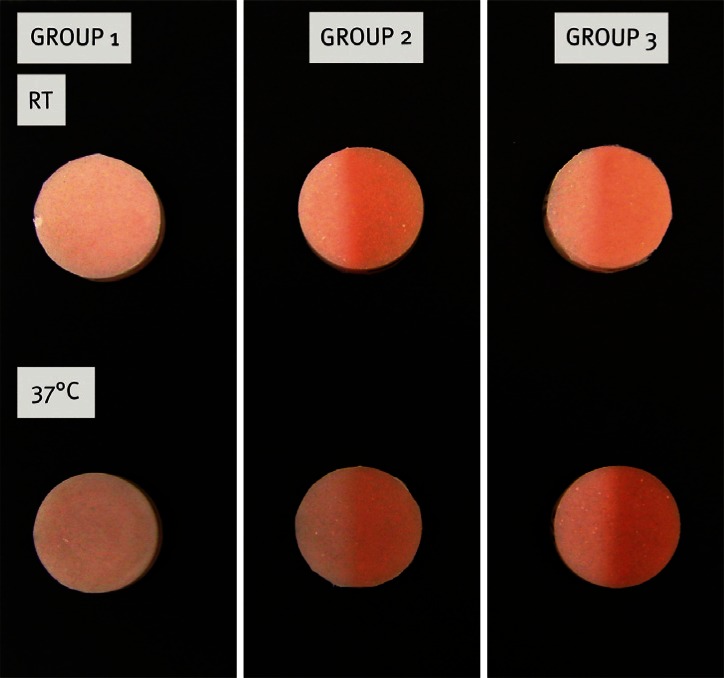
After 24 days (11 × 6 hours of UV irradiation), both the ΔE value and a* seemed to cease rising in the specimens containing 0.2 wt% thermochromic pigment (Group 2). The same trend was observed even earlier in Group 3, which contained a higher concentration of thermochromic pigment (0.6 wt%): after 4 days the thermochromic pigment seemed to have reached the maximum redness, and showed no higher ΔE values after that. The visible color changes after 46 days can be seen in Fig. 4. When comparing the groups stored in room temperature with corresponding groups stored in an incubator, it is concluded that ΔE, throughout the aging process, is consistently higher in the specimens containing thermochromic pigment and which were stored in the incubator. When comparing the specimens stored in room temperature with those stored in an incubator at 37℃ in 97% humidity, the higher a* and b* values of the specimens stored in an incubator were statistically significant (P<.001) among the specimens containing thermochromic pigment.
Fig. 4.
The color change of the UV exposed specimens after 46 days.
DISCUSSION
Three groups of maxillofacial silicone elastomer specimens were investigated with respect to the effect of UV irradiation and storage on the color stability. Three groups of specimens were compared: the silicone of the control group was only intrinsically colored with conventional color pigments, while the test groups also contained a thermochromic pigment, 0.2 wt% and 0.6 wt% respectively. Registration of the color according to the L*a*b* color system showed that the most remarkable color changes during the aging process occurred in the a*values, which means that the color change of the UV irradiated specimens containing thermochromic pigment was towards red. The used thermochromic pigment changed from colorless at room temperature, to red in cold environment.
The color stability has been thoroughly investigated as a part of the primary physical property studies of maxillofacial elastomers. Aging and environmental factors cause a general degradation of polymers, color changes of the silicone elastomer itself, and in some cases, color changes of the pigments. The weathering of polymers can cause changes in physical and chemical characteristics, which result in alterations in their mechanical properties. For most polymeric materials the primary cause of deterioration is a photo-oxidative attack, the combined action of oxygen and sunlight, on their chemical structure.23,29,35 In outdoor exposure experiments, the degradation of polymers is not always explained on the basis of meteorological data on factors that are generally held responsible for polymer degradation (solar radiation, temperature and moisture). There are differences in weathering test results at different geographical locations, even though the meteorological data seems to be the same. The constituents of the atmosphere, such as sulphur dioxide, nitrogen oxide and ozone or rain and/or wind may also alter the photo-oxidative process.
In this study, the artificial, accelerated aging was represented by UV irradiation, a raised temperature (+37℃) and 97% humidity. As each specimen was divided into one half that was not exposed to UV irradiation, and the environmental circumstances remained the same, the effect of the UV irradiation alone was seen.36 According to the manufacturer, the pigment should be sensitive to UV irradiation, but - surprisingly - the color change was observed as early as after just one 6 hours period of UV exposure. That means that the thermochromic pigment was extremely sensitive to UV irradiation. The color change after one cycle of UV irradiation was remarkable. Even if the artificial UV exposure does not correspond to 6 hours of exposure to natural sunlight, we can draw the conclusion that the thermochromic pigment is extremely sensitive to UV irradiation.
The color change of the conventional pigments that are used in maxillofacial elastomers, has been studied by Beatty et al.14,37 They studied how UV irradiation caused color changes in a silicone elastomer that was colored with 5 oil pigments, which were applied either as base colorants (intrinsic) or surface tints (extrinsic). Samples containing oil pigments showed susceptibility to UV radiation. After 1,800 hours of exposure to UV irradiation, the greatest changes occurred for cadmium red, cadmium yellow and yellow ochre (7.1 ≤ ΔE ≤ 9.4).The same amount of UV exposure lead to significantly lower color change (maximum ΔE = 4.2; P≤.05) when the oil pigments were used as surface tints in higher concentrations. The authors postulated that either a chemical reaction or a chemical incompatibility between the pigments and the elastomer was responsible for the observed color changes.14
According to Craig et al.,10 accelerated aging of silicones containing pigments of yellow, orange yellow, white or light orange displayed the greatest color changes. Accelerated aging leads to fading of the color.10 Haug et al. concluded that the addition of pigments altered the effect of weathering on color. Inorganic colorants had the best color stability. The addition of pigments could even have a stabilizing effect on the color of the elastomer. Colorless silicone itself, changes color over time, and even without exposure to UV irradiation.9 Kiat-amnuay et al.38 studied different dry earth pigments in maxillofacial silicone and found that red dry earth pigment had poor color stability after exposure to microwave energy.
Attempts have been made to protect silicone elastomers from color degradation. Lemon et al. investigated the efficacy of UV light absorber (Spectra-sorb UV-5411), a benzotriazole compound with a broad range of UV-absorption properties, used in plastics and cosmetics industry, on the color stability of maxillofacial silicone elastomer MDX4-4210. The silicone elastomer was colored with kaolin (10 vol%) and oil-base pigments. Spectrophotometric analysis was performed before and after artificial weathering in an aging chamber, or direct outdoor exposure. The changes in ΔE were greater for the artificially aged than for the outdoor-aged samples. The addition of a UV light absorber did not protect the samples from experiencing a change.13 Han et al., tested UV-shielding nano-oxides (TiO2, ZnO, CeO2) as opacifiers for silicone A-2186 maxillofacial prostheses. After artificial aging the best color stability was reached with mixed pigments and 1% nano-CeO2 , and 2% and 2,5% nano-TiO2 used as opacifiers. Yellow silicone pigment mixed with all three nano-oxides significantly affected the color stability of A-2186 silicone elastomer.39 In another study, dry earth opacifiers artskin white and titanium white pigment (10% and 15%) were found to protect maxillofacial silicone A-2000 from color degradation.40 According to Li et al.41 nanoparticles of zinc oxide-doped ceria (3-5 nm in diameter) displayed excellent UV absorption and transparency. Fine particles of titania and zinc oxide are effective inorganic sunscreens used in cosmetics. They have, however, high refractive indices which make the skin look white, and they have a high photocatalytic activity, which may oxidize and degrade other ingredients in the formulation. Ceria is relatively transparent to visible light and has excellent UV radiation absorption properties, but has high catalytic activity for oxidation of organic materials. Doping with zinc oxide resulted in a decrease in the catalytic activity; it showed excellent UV absorption and transparency.
The thermochromic pigment that was used in this study has the character of a leuco dye incorporated in microcapsules. According to Lötzsch and Seeboth,42 the light stability of leuco dyes is rather poor. They are sensitive to both visible and UV light. Particular leuco dye-matrix-stabilizer systems, which can inhibit the photofading process almost completely, have been discovered. However, none of the stabilizer systems has reversible thermochromic properties.43,44
This is the first study to investigate the effect of artificial aging on changes in color values, using thermochromic pigment in maxillofacial silicone elastomer MDX4-4210. That means that there were no previous results to make a power calculation prior to the study. The sample size in this study was decided according to convenience and looking at the sample sizes in other studies in this field. However, as the statistical analyses showed statistically significant p-values, the sample size should be large enough to draw conclusions about the investigated thermochromic pigment.
A color change corresponding to a ΔE value of > 1 is claimed to be detectable by a trained human eye. Human observers judged ΔE ≈ 1 as a color match 50% of the time (ASTM Int. Std practice for calcul. 2011).45 In this study, the color changes of the maxillofacial silicone elastomer specimens were even as high as ΔE 18 (Group 3/+37℃) after 46 days. The thermochromic pigment concentration of 0.2%, which makes a "natural" reddish appearance after storage in cold environment,30 shows a ΔE value of over 4 even after one 6 hour exposure of UVA radiation. Even if this amount of UV radiation does not correspond to natural solar radiation, ΔE value is far too high and the specimens show an extremely high a* value. The thermochromic pigment that was tested in this study is not suited to be used in maxillofacial prostheses as such, because of this rapid color change after exposure to UV irradiation. The idea of mimicking the redness of cooled skin of for instance the outer region of external ear is still worth developing to make the prosthesis more natural looking. External coloration, as suggested by Beatty et al.,14 might be a better alternative than the intrinsic coloration technique that was used in this study. With an external coloration technique, used with suitable and effective UV absorbers, and perhaps with leuco dye-matrix-stabilizer systems, it might be possible to use thermochromic pigment in maxillofacial silicone elastomer in the future. This would enhance the natural appearance and quality of the facial prostheses at least in those countries with pronounced seasonal variations in the climate.
CONCLUSION
The thermochromic pigment used in this study is very sensitive to UV irradiation, and is not suitable, as such, to be used in the fabrication of maxillofacial silicone elastomer prostheses.
References
- 1.Watson RM, Coward TJ, Forman GH. Results of treatment of 20 patients with implant-retained auricular prostheses. Int J Oral Maxillofac Implants. 1995;10:445–449. [PubMed] [Google Scholar]
- 2.Huber H, Studer SP. Materials and techniques in maxillofacial prosthodontic rehabilitation. Oral Maxillofac Surg Clin North Am. 2002;14:73–93. doi: 10.1016/s1042-3699(02)00018-3. [DOI] [PubMed] [Google Scholar]
- 3.Leonardi A, Buonaccorsi S, Pellacchia V, Moricca LM, Indrizzi E, Fini G. Maxillofacial prosthetic rehabilitation using extraoral implants. J Craniofac Surg. 2008;19:398–405. doi: 10.1097/SCS.0b013e318163e443. [DOI] [PubMed] [Google Scholar]
- 4.Craig RG, Koran A, Yu R. Elastomers for maxillofacial applications. Biomaterials. 1980;1:112–117. doi: 10.1016/0142-9612(80)90010-1. [DOI] [PubMed] [Google Scholar]
- 5.Ryan JE. Twenty-five years of clinical application of a heat-cured silicone rubber. J Prosthet Dent. 1991;65:658–661. doi: 10.1016/0022-3913(91)90201-7. [DOI] [PubMed] [Google Scholar]
- 6.Gary JJ, Smith CT. Pigments and their application in maxillofacial elastomers: a literature review. J Prosthet Dent. 1998;80:204–208. doi: 10.1016/s0022-3913(98)70111-8. [DOI] [PubMed] [Google Scholar]
- 7.Seelaus R, Troppmann RJ. Facial Prosthesis Fabrication: Coloration Techniques. In: Taylor TD, editor. Clinical Maxillofacial Prosthetics. Carol Stream: Quintessence Publishing Co.; 2000. pp. 245–264. [Google Scholar]
- 8.Leow ME, Ow RK, Lee MH, Huak CY, Pho RW. Assessment of colour differences in silicone hand and digit prostheses: perceptible and acceptable thresholds for fair and dark skin shades. Prosthet Orthot Int. 2006;30:5–16. doi: 10.1080/03093640500465096. [DOI] [PubMed] [Google Scholar]
- 9.Haug SP, Andres CJ, Moore BK. Color stability and colorant effect on maxillofacial elastomers. Part III: weathering effect on color. J Prosthet Dent. 1999;81:431–438. doi: 10.1016/s0022-3913(99)80010-9. [DOI] [PubMed] [Google Scholar]
- 10.Craig RG, Koran A, Yu R, Spencer J. Color stability of elastomers for maxillofacial appliances. J Dent Res. 1978;57:866–871. doi: 10.1177/00220345780570090401. [DOI] [PubMed] [Google Scholar]
- 11.Koran A, Yu R, Powers JM, Craig RG. Color stability of a pigmented elastomer for maxillofacial appliances. J Dent Res. 1979;58:1450–1454. doi: 10.1177/00220345790580050301. [DOI] [PubMed] [Google Scholar]
- 12.Mohite UH, Sandrik JL, Land MF, Byrne G. Environmental factors affecting mechanical properties of facial prosthetic elastomers. Int J Prosthodont. 1994;7:479–486. [PubMed] [Google Scholar]
- 13.Lemon JC, Chambers MS, Jacobsen ML, Powers JM. Color stability of facial prostheses. J Prosthet Dent. 1995;74:613–618. doi: 10.1016/s0022-3913(05)80314-2. [DOI] [PubMed] [Google Scholar]
- 14.Beatty MW, Mahanna GK, Jia W. Ultraviolet radiation-induced color shifts occurring in oil-pigmented maxillofacial elastomers. J Prosthet Dent. 1999;82:441–446. doi: 10.1016/s0022-3913(99)70031-4. [DOI] [PubMed] [Google Scholar]
- 15.Haug SP, Moore BK, Andres CJ. Color stability and colorant effect on maxillofacial elastomers. Part II: weathering effect on physical properties. J Prosthet Dent. 1999;81:423–430. doi: 10.1016/s0022-3913(99)80009-2. [DOI] [PubMed] [Google Scholar]
- 16.Hulterström AK, Ruyter IE. Changes in appearance of silicone elastomers for maxillofacial prostheses as a result of aging. Int J Prosthodont. 1999;12:498–504. [PubMed] [Google Scholar]
- 17.Polyzois GL. Color stability of facial silicone prosthetic polymers after outdoor weathering. J Prosthet Dent. 1999;82:447–450. doi: 10.1016/s0022-3913(99)70032-6. [DOI] [PubMed] [Google Scholar]
- 18.Kiat-Amnuay S, Lemon JC, Powers JM. Effect of opacifiers on color stability of pigmented maxillofacial silicone A-2186 subjected to artificial aging. J Prosthodont. 2002;11:109–116. [PubMed] [Google Scholar]
- 19.Kiat-Amnuay S, Mekayarajjananonth T, Powers JM, Chambers MS, Lemon JC. Interactions of pigments and opacifiers on color stability of MDX4-4210/type A maxillofacial elastomers subjected to artificial aging. J Prosthet Dent. 2006;95:249–257. doi: 10.1016/j.prosdent.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Visser A, Raghoebar GM, van Oort RP, Vissink A. Fate of implant-retained craniofacial prostheses: life span and aftercare. Int J Oral Maxillofac Implants. 2008;23:89–98. [PubMed] [Google Scholar]
- 21.Hatamleh MM, Watts DC. Effect of extraoral aging conditions on color stability of maxillofacial silicone elastomer. J Prosthodont. 2010;19:536–543. doi: 10.1111/j.1532-849X.2010.00627.x. [DOI] [PubMed] [Google Scholar]
- 22.Veres EM, Wolfaardt JF, Becker PJ. An evaluation of the surface characteristics of a facial prosthetic elastomer. Part II: The surface texture. J Prosthet Dent. 1990;63:325–331. doi: 10.1016/0022-3913(90)90206-r. [DOI] [PubMed] [Google Scholar]
- 23.Dootz ER, Koran A, 3rd, Craig RG. Physical properties of three maxillofacial materials as a function of accelerated aging. J Prosthet Dent. 1994;71:379–383. doi: 10.1016/0022-3913(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 24.Polyzois GL, Tarantili PA, Frangou MJ, Andreopoulos AG. Physical properties of a silicone prosthetic elastomer stored in simulated skin secretions. J Prosthet Dent. 2000;83:572–577. doi: 10.1016/s0022-3913(00)70017-5. [DOI] [PubMed] [Google Scholar]
- 25.Eleni PN, Krokida MK, Polyzois GL. The effect of artificial accelerated weathering on the mechanical properties of maxillofacial polymers PDMS and CPE. Biomed Mater. 2009;4:035001. doi: 10.1088/1748-6041/4/3/035001. [DOI] [PubMed] [Google Scholar]
- 26.Eleni PN, Katsavou I, Krokida MK, Polyzois GL, Gettleman L. Mechanical behavior of facial prosthetic elastomers after outdoor weathering. Dent Mater. 2009;25:1493–1502. doi: 10.1016/j.dental.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Haug SP, Andres CJ, Munoz CA, Bernal G. Effects of environmental factors on maxillofacial elastomers: Part IV--Optical properties. J Prosthet Dent. 1992;68:820–823. doi: 10.1016/0022-3913(92)90210-2. [DOI] [PubMed] [Google Scholar]
- 28.Rosa DS, Angelini JMG, Agnelli JAM, Mei LHI. The use of optical microscopy to follow thedegradation of isotactic polyprophylene (iPP) subjected to natural and artificial aging. Polym Test. 2005;24:1022–1026. [Google Scholar]
- 29.Davis A, Sims D. Weathering of polymers. London: Applied Science Publishers; 1983. pp. 1–41. [Google Scholar]
- 30.Kantola R, Kurunmäki H, Vallittu PK, Lassila LVJ. Use of thermochromic pigment in maxillofacial silicone elastomer. J Prosthod Dent. 2013 doi: 10.1016/S0022-3913(13)60382-0. In press. [DOI] [PubMed] [Google Scholar]
- 31.Ogrodnik W. Use of color-changing pigment to detect wire and cable hazards. Wire J Int. 2008;41:170–175. [Google Scholar]
- 32.Hallcrest Inc. Handbook of thermochromic liquid crystal technology. Glenview: Hallcrest; 1991. [Google Scholar]
- 33.Lakio S, Heinämäki J, Yliruusi J. Colorful drying. AAPS PharmSciTech. 2010;11:46–53. doi: 10.1208/s12249-009-9351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Commission Internationale de l'Éclairage (CIE) Colorimetry - technical report. CIE Pub. No. 15. 3rd ed. Vienna: Bureau Central de la CIE; 2004. [Google Scholar]
- 35.Gijsman P, Hennekens J, Janssen K. Comparison of UV degradation chemistry in accelerated (xenon) aging tests and outdoor tests (II) Polym Degrad Stab. 1994;46:63–74. [Google Scholar]
- 36.Sampers J. Importance of weathering factors other than UV radiation and temperature in outdoor exposure. Polym Degrad Stab. 2002;76:455–465. [Google Scholar]
- 37.Beatty MW, Mahanna GK, Dick K, Jia W. Color changes in dry-pigmented maxillofacial elastomer resulting from ultraviolet light exposure. J Prosthet Dent. 1995;74:493–498. doi: 10.1016/s0022-3913(05)80351-8. [DOI] [PubMed] [Google Scholar]
- 38.Kiat-amnuay S, Johnston DA, Powers JM, Jacob RF. Color stability of dry earth pigmented maxillofacial silicone A-2186 subjected to microwave energy exposure. J Prosthodont. 2005;14:91–96. doi: 10.1111/j.1532-849X.2005.00017.x. [DOI] [PubMed] [Google Scholar]
- 39.Han Y, Zhao Y, Xie C, Powers JM, Kiat-amnuay S. Color stability of pigmented maxillofacial silicone elastomer: effects of nano-oxides as opacifiers. J Dent. 2010;38(Suppl 2):e100–e105. doi: 10.1016/j.jdent.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Kiat-amnuay S, Beerbower M, Powers JM, Paravina RD. Influence of pigments and opacifiers on color stability of silicone maxillofacial elastomer. J Dent. 2009;37(Suppl 1):e45–e50. doi: 10.1016/j.jdent.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Li R, Yabe S, Yamashita M, Momose S, Yoshida S, Yin S, Sato T. UV-shielding properties of zinc oxide-doped ceria fine powders derived via soft solution chemical routes. Mater Chem Phys. 2002;75:39–44. [Google Scholar]
- 42.Löetzsch D, Seeboth A. Thermochromic Phenomena in Polymers. 1st ed. Shawbury, UK: Smithers Rapra Press; 2008. pp. 17–47. [Google Scholar]
- 43.Caine MA, McCabe RW, Wang L, Brown RG, Hepworth JD. The inhibition of triphenylmethane primary dye fading in carbonless copying paper systems by singlet oxygen quenching bis(dithiocarbamato)nickel(II) complexes. Dyes Pigm. 2002;52:55–65. [Google Scholar]
- 44.Oda H. Photostabilization of organic thermochromic pigments. Part 2: Effect of hydroxyarylbenzotriazoles containing an amphoteric counter-ion moiety on the light fastness of color formers. Dyes Pigm. 2008;76:400–405. [Google Scholar]
- 45.Standard practice for calculation of color tolerances and color differences from instrumentally measured color coordinates. Pennsylvania, USA: ASTM International; 2011. ASTM D2244-11; pp. 1–12. [Google Scholar]