Abstract
A 50-year-old man, who underwent a procedure for an implantable cardioverter defibrillator (ICD), visited the outpatient department of our clinic after suffering multiple ICD shocks. The ICD interrogation revealed recurrent shock due to a high frequency of noise that is sensed by the device as ventricular fibrillation. Chest radiography revealed a significant split in the insulation of the lead allowing the inner wire to protrude. We considered the removal of the failed lead, but the removal of ICD lead is potentially a high risk procedure, so we cut and capped a proximal part of the failed lead and inserted a new lead. This is the first report of a St. Jude Riata® dual coil defibrillator lead failure with clinical and radiologic evidence of a defect in lead insulation in Korea.
Keywords: Implantable cardioverter-defibrillators, Noise, Equipment failure
Introduction
An implantable cardioverter defibrillator (ICD) is indicated for patients who were resuscitated from sudden cardiac death due to fatal ventricular tachyarrhythmias. In the past decade, several studies showed the superiority of an ICD over antiarrthythmic drug therapy1),2) and nowadays, ICD has proven to be effective in the prevention of primary and secondary sudden cardiac deaths.
However, several ICD lead insulation defect cases leading to ICD failure have been reported. The insulation defect of the ICD lead presents with impedance changes or inappropriate ventricular sensing and ICD shock delivery. Recently, St. Jude Medical, Inc. (St. Paul, MN, USA) recalled Riata®, and Riata® ST silicone defibrillation leads because of a peculiar insulation defect regarding the ICD lead.
A radiologically proven ICD lead insulation defect case has not yet been reported in Korea. We report a case of an outer insulation break in the implanted dual coil defibrillator which led to inappropriate sensing and shock deliver.
Case
A 50-year-old man with a history of acute myeloid leukemia and Wolff-Parkinson-White (WPW) syndrome was brought to the emergency room of a local hospital with chest discomfort and syncope in 2001. His initial electrocardiography showed atrial fibrillation with rapid ventricular response and pre-excited QRS complexes, which was converted to sinus rhythm with direct arrent (DC) cardioversion. He had taken amiodarone for two years but stopped the medication by himself.
In 2007, he experienced chest discomfort again and presented to the emergency room of another university hospital. He took a 12-lead electrocardiography, which showed atrial fibrillation with rapid ventricular response and pre-excited QRS complexes. Sinus rhythm resumed with DC cardioversion.
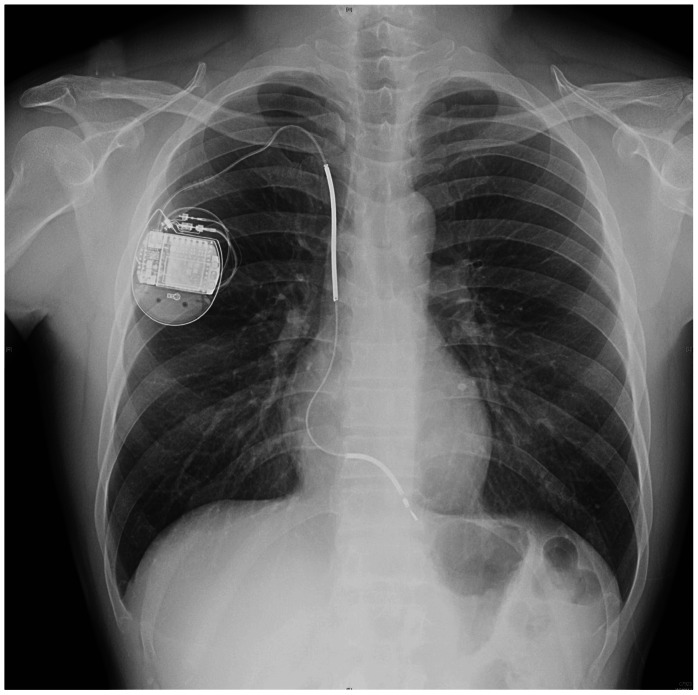
He was referred to our institute for catheter ablation of WPW syndrome. Contrary to expectations, ventricular fibrillation was reproducibly induced with programmed ventricular stimulation during an electrophysiologic study. Considering his episode of unexplained syncope, he underwent ICD implantation.3) A single dual coil ICD lead (St. Jude Medical, Inc., RIATA® 1570) was percutaneously introduced via the right subclavian vein because the left subclavian vein was not visible, then advanced and placed in RV apex. Intraoperative lead parameters including sensing amplitude, threshold, and lead impedance were all within appropriate limits. The ICD lead was connected to the ICD device (Atlas™+VR®, St. Jude Medical, Inc., St. Paul, MN, USA). They were placed in the right infraclavicular subcutaneous site. He had chest radiography after ICD implantation (Fig. 1).
Fig. 1.
Chest radiography two weeks later after implantable cardioverter defibrillator implantation.
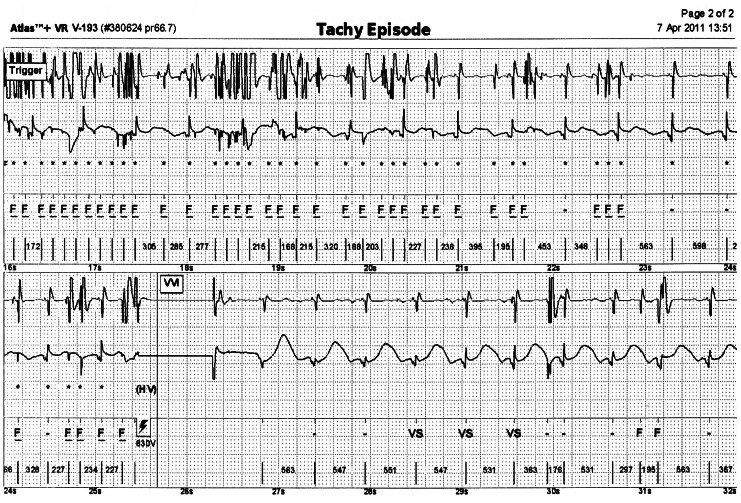
Approximately 3 years after the ICD implant, he received multiple shocks in the same day. He had never received a shock over 3 years of follow-up (Table 1). The ICD interrogation revealed recurrent shock due to a high frequency of noise that is sensed by the device as ventricular fibrillation (Fig. 2). There was no evidence of other ICD malfunction, with a ventricular sensitivity of 0.3 mV as well as ventricular lead and shock impedances of 315 Ohms and 60 Ohms, respectively. After ICD interrogation, ventricular sensitivity was raised up to 0.6 mV. Chest radiography was not done at that time.
Table 1.
Serial follow-up of implantable cardioverter defibrillator interrogation
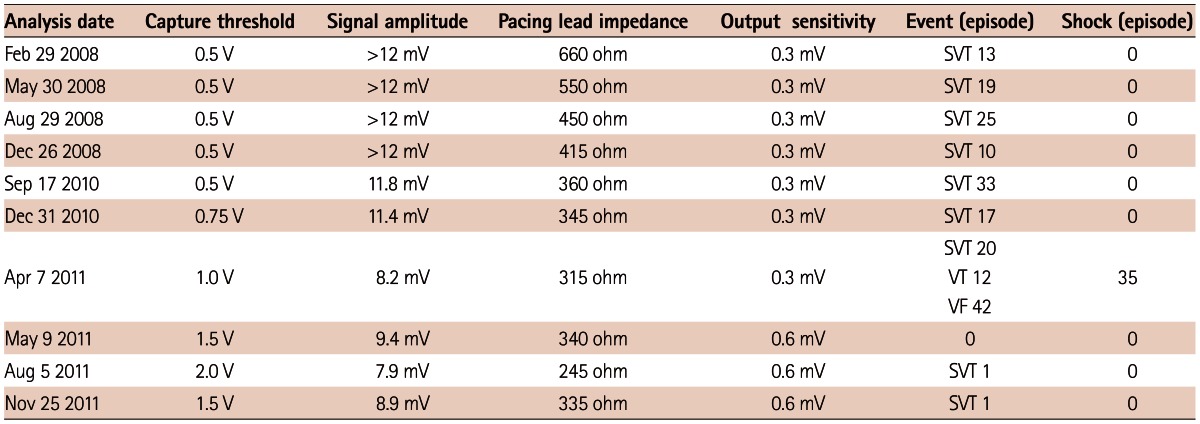
SVT: supraventricular tachycardia, VT: ventricular tachycardia, VF: ventricular fibrillation
Fig. 2.
Multiple noise sensing is marked as 'F's.
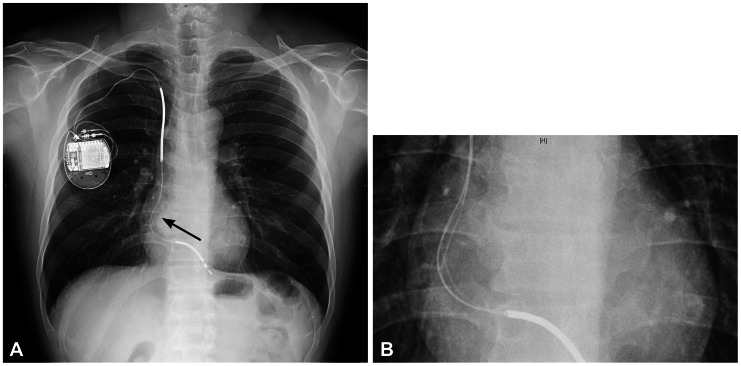
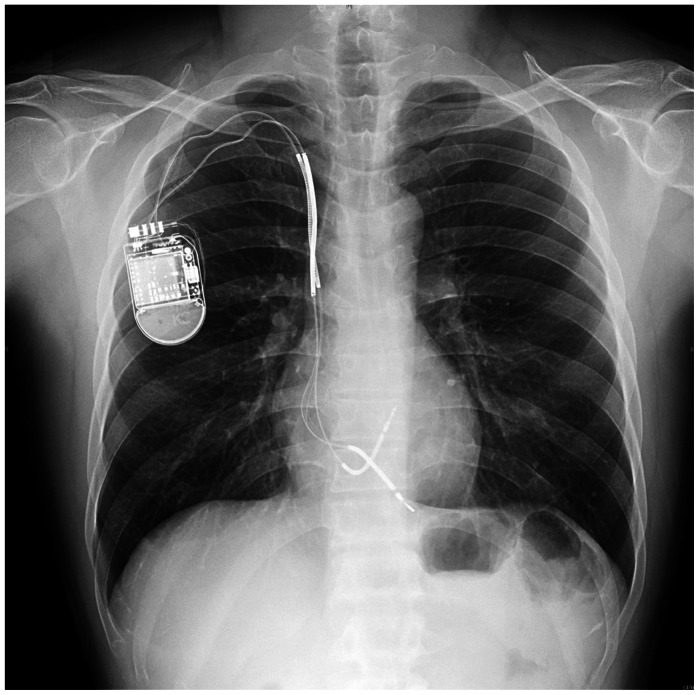
After 6 months of follow-up, routine chest radiography revealed a significant split in the insulation of the ICD lead allowing the inner wire to protrude (Fig. 3). He underwent a fluoroscopic evaluation for conductor external canalization. Before fluoroscopy, we considered removing the lead. But, we decided to leave a failed lead and insert a new ICD lead instead because of the high risk of complication during lead extraction. Under local anesthesia and fluoroscopic guidance, a previously inserted generator was removed and the proximal part of the old failed lead was cut and capped with encap®. A new single dual coil lead (DURATA® 7120Q/58, St. Jued Medical, Inc., St. Paul, MN, USA) with active fixation was inserted through the right subclavian vein and placed to the right ventricular mid-septum. After intraoperative measurements of pacing threshold, signal, and impedance, the lead was connected to a new single chamber generator (FORTIFY VR® CD1231-40Q, St. Jued Medical, Inc., St. Paul, MN, USA). The device and lead were placed appropriately in the preexisted ICD pocket site, which were verified via postoperative chest radiography (Fig. 4). He has been followed up regularly with no further episode of inappropriate shock via the outpatient department for 6 months.
Fig. 3.
A: chest radiography 3.5 years later after ICD implantation. An arrow indicates the defect of ICD lead. B: a significant outer insulation defect of ICD lead, allowing the inner wire to protrude, was noted. A magnified view of defected lead is shown. ICD: implantable cardioverter defibrillator.
Fig. 4.
We cut and capped proximal part of failed lead and inserted new lead.
Discussion
Recently, a medical device advisory board reported the prevalence and predictors of cable extrusion and loss of electrical integrity with the Riata® defibrillator lead.4) A large, multicenter retrospective analyses revealed that the long-term electrical failure rate of Riata/ST® leads is significantly higher than Quattro® or Endotak® leads.5)
This issue is important because of the potential risk of serious injury to the patient or death if the malfunction in the affected device cannot be appropriately managed.
Case reports have previously provided examples of leads with extruded cables without evidence of electrical malfunction, but the absolute numbers reported were small.6-9) The evidence of a higher prevalence in a larger population came from Northern Ireland and Switzerland. Through the screening program, they reported 15% and 11.5% of ICD coil extrusion, respectively. In the Northern Ireland study, a clinically significant event was noted in 20% of lead extrusion patients.10),11) According to St. Jude Medical data, the most common form of insulation abrasion was lead-to-can abrasion occurring in the pocket area. Externalization of conductors is another manifestation of insulation abrasion. Approximately 85% of confirmed externalized conductors were caused by inside-out abrasion, while 15% resulted from external sources of abrasion. A recent retrospective study found that 65% of patients with cable extrusion received a high-voltage shock within 12 months of detection of cable extrusion.4) No electrical abnormalities were seen in almost all cases, except one case. This suggests that leads with cable extrusion may not necessarily manifest overt electrical dysfunction, even in the setting of high-voltage shock.4) On the other hand, there is a study where externalized leads had a significantly pronounced decrease in R-wave amplitude.12)
In Korea, this is the first case of a St. Jude Riata® dual coil defibrillator lead failure with clinical and radiologic evidence of a break in lead insulation. In our case, we did not remove a failed lead because of the high risk of complication during ICD lead extraction. Some failed lead extraction cases were reported but most of them were clinically suspected cases which were not proven as a definite lead extrusion problem by chest radiography or fluoroscopy. Contrary to these cases, a definite outer insulation defect with an inner coil extrusion was shown in our case. Forceful removal of a failed lead with inner coil extrusion can cause severe complication including bleeding, rupture, and remnant lead caused by incomplete removal since the Riata® 1500 series has more ingrowth at the coils. Therefore, a failed Riata® lead extraction should not be considered when definite inner coil extrusion was proven by chest radiography.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 2.The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 3.Gregoratos G, Abrams J, Epstein AE, et al. ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines) J Am Coll Cardiol. 2002;40:1703–1719. doi: 10.1016/s0735-1097(02)02528-7. [DOI] [PubMed] [Google Scholar]
- 4.Shen S, Bhave P, Giedrimas E, et al. Prevalence and predictors of cable extrusion and loss of electrical integrity with the Riata defibrillator lead. J Cardiovasc Electrophysiol. 2012;23:1207–1212. doi: 10.1111/j.1540-8167.2012.02378.x. [DOI] [PubMed] [Google Scholar]
- 5.Sung RK, Massie BM, Varosy PD, et al. Long-term electrical survival analysis of Riata and Riata ST silicone leads: National Veterans Affairs experience. Heart Rhythm. 2012;9:1954–1961. doi: 10.1016/j.hrthm.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Valk S, Luijten R, Jordaens L. Insulation damage in a shock wire: an unexpected fluoroscopic image. Pacing Clin Electrophysiol. 2010;33:770–772. doi: 10.1111/j.1540-8159.2009.02664.x. [DOI] [PubMed] [Google Scholar]
- 7.Chan CW, Chiang CS. An ICD lead with failure of outer insulation goes undetected by regular measurements. Pacing Clin Electrophysiol. 2012;35:e261–e262. doi: 10.1111/j.1540-8159.2011.03164.x. [DOI] [PubMed] [Google Scholar]
- 8.Erkapic D, Duray GZ, Bauernfeind T, De Rosa S, Hohnloser SH. Insulation defects of thin high-voltage ICD leads: an underestimated problem? J Cardiovasc Electrophysiol. 2011;22:1018–1022. doi: 10.1111/j.1540-8167.2011.02055.x. [DOI] [PubMed] [Google Scholar]
- 9.Krebsbach A, Alhumaid F, Henrikson CA, Calkins H, Berger RD, Cheng A. Premature failure of a Riata defibrillator lead without impedance change or inappropriate sensing: a case report and review of the literature. J Cardiovasc Electrophysiol. 2011;22:1070–1072. doi: 10.1111/j.1540-8167.2011.02042.x. [DOI] [PubMed] [Google Scholar]
- 10.Kodoth V, Cromie N, Lau E, McEneaney D, Wilson C, Roberts MJ. Riata lead failure; a report from Northern Ireland Riata lead screening programme. Eur Heart J. 2011;32:1838. Abstract. [Google Scholar]
- 11.Schmutz M, Delacrétaz E, Schwick N, et al. Prevalence of asymptomatic and electrically undetectable intracardiac inside-out abrasion in silicon-coated Riata® and Riata® ST implantable cardioverter-defibrillator leads. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2011.12.076. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Rattan R, Adelstein E, et al. Fluoroscopic screening of asymptomatic patients implanted with the recalled Riata lead family. Circ Arrhythm Electrophysiol. 2012;5:809–814. doi: 10.1161/CIRCEP.112.973081. [DOI] [PubMed] [Google Scholar]