Abstract
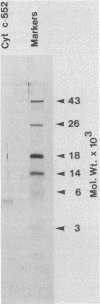
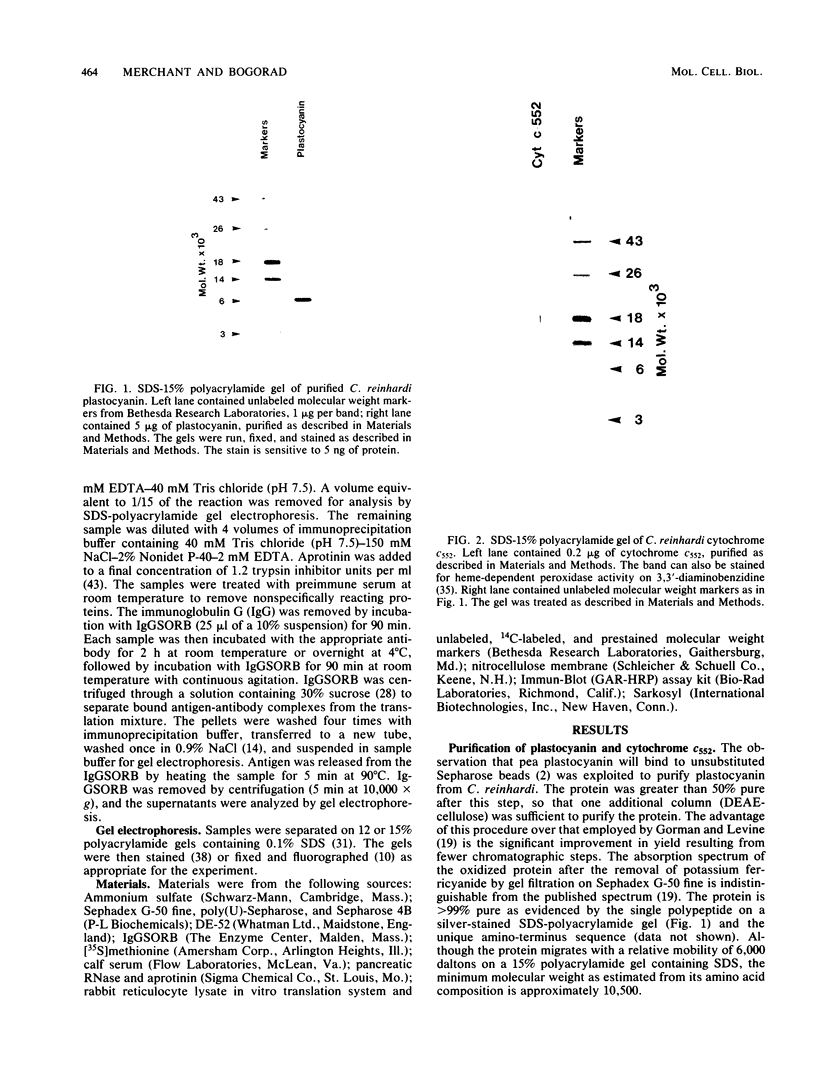
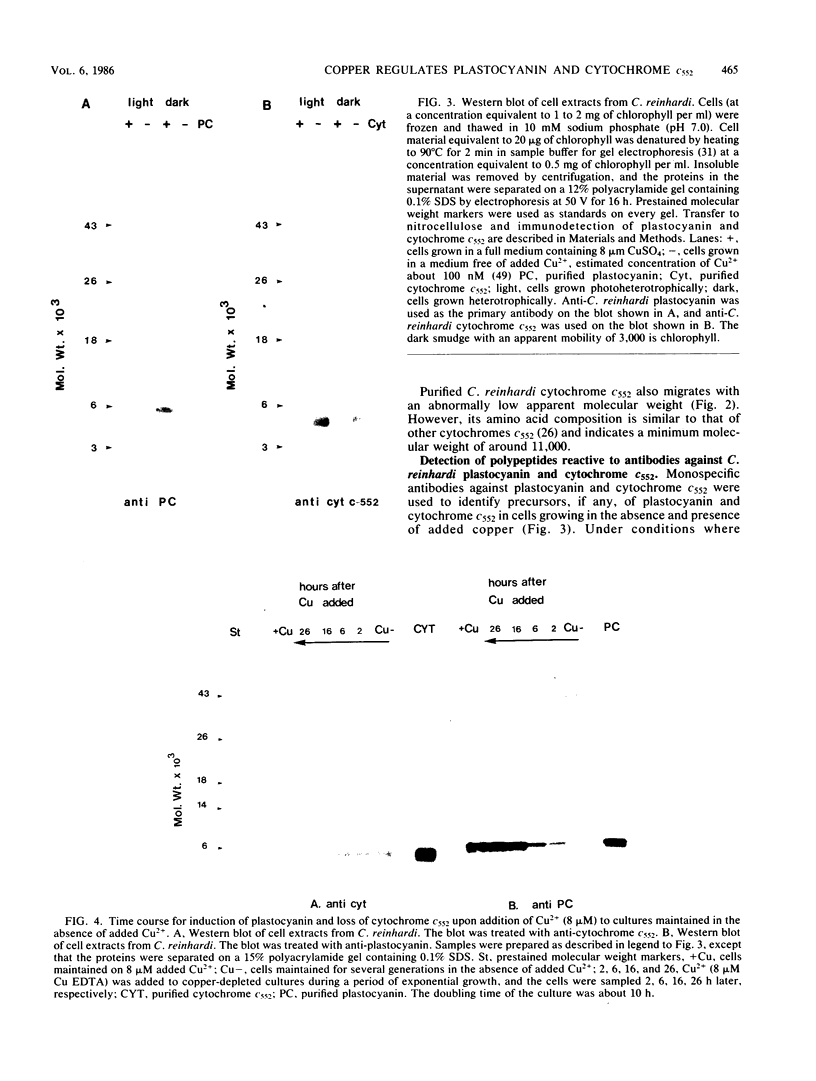
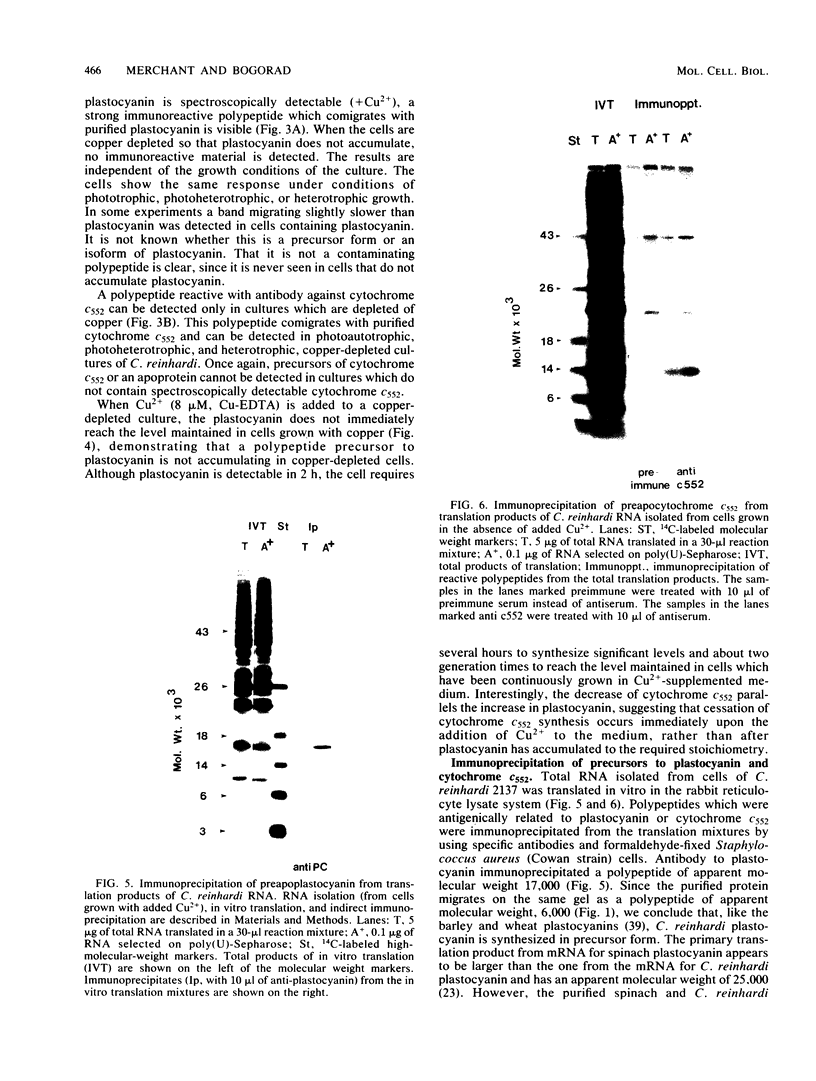
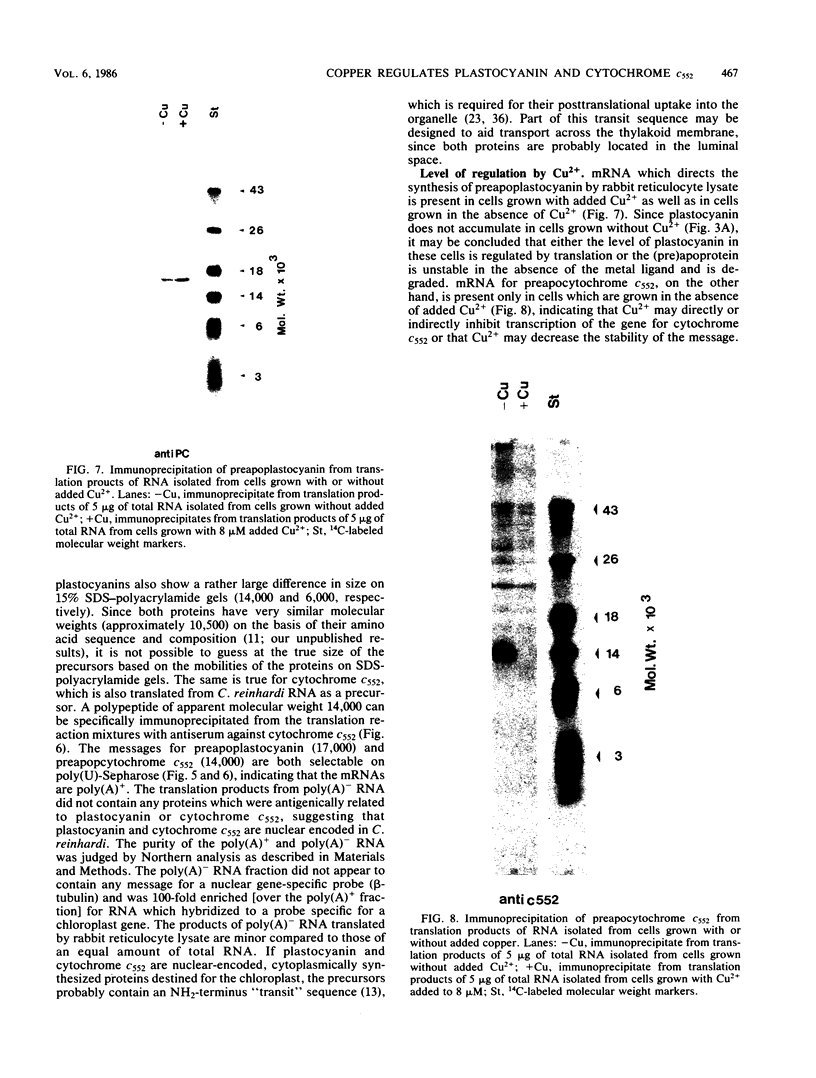
Plastocyanin and cytochrome c552 are interchangeable electron carriers in the photosynthetic electron transfer chains of some cyanobacteria and green algae (P. M. Wood, Eur. J. Biochem. 87:9-19, 1978; G. Sandmann et al., Arch. Microbiol. 134:23-27, 1983). Chlamydomonas reinhardi cells respond to the availability of copper in the medium and accordingly accumulate either plastocyanin (if copper is available) or cytochrome c552 (if copper is not available). The response occurs in both heterotrophically and phototrophically grown cells. We have studied the molecular level at which this response occurs. No immunoreactive polypeptide is detectable under conditions where the mature protein is not spectroscopically detectable. Both plastocyanin and cytochrome c552 appear to be translated (in vitro) from polyadenylated mRNA as precursors of higher molecular weight. RNA was isolated from cells grown either under conditions favorable for the accumulation of plastocyanin (medium with Cu2+) or for the accumulation of cytochrome c552 (without Cu2+ added to the medium). Translatable mRNA for preapoplastocyanin was detected in both RNA preparations, although mature plastocyanin was detected in C. reinhardi cells only when copper was added to the culture. Translatable mRNA for preapocytochrome, on the other hand, was detected only in cells grown under conditions where cytochrome c552 accumulates (i.e., in the absence of copper). We conclude that copper-mediated regulation of plastocyanin and cytochrome c552 accumulation is effected at different levels, the former at the level of stable protein and the latter at the level of stable mRNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André E., Kessler M., Briquel M. E., Alexandre P., Hurault de Ligny B., Huriet C. Intérêt pratique du dosage des produits de dégradation de la fibrine urinaire dans la surveillance précoce des transplantés rénaux. Pathol Biol (Paris) 1983 Jan;31(1):23–27. [PubMed] [Google Scholar]
- Ashton A. R., Anderson L. E. A novel procedure for the rapid purification of plastocyanin from pea (Pisum sativum) leaves. Biochem J. 1981 Jan 1;193(1):375–378. doi: 10.1042/bj1930375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedbrook J. R., Link G., Coen D. M., Bogorad L. Maize plastid gene expressed during photoregulated development. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3060–3064. doi: 10.1073/pnas.75.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohner H., Böger P. Reciprocal formation of cytochrome c-553 and plastocyanin in Scenedesmus. FEBS Lett. 1978 Jan 15;85(2):337–339. doi: 10.1016/0014-5793(78)80486-4. [DOI] [PubMed] [Google Scholar]
- Bohner H., Merkle H., Kroneck P., Böger P. High variability of the electron carrier plastocyanin in microalgae. Eur J Biochem. 1980 Apr;105(3):603–609. doi: 10.1111/j.1432-1033.1980.tb04538.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burkey K. O., Gross E. L. Effect of carboxyl group modification on redox properties and electron donation capability of spinach plastocyanin. Biochemistry. 1981 Sep 15;20(19):5495–5499. doi: 10.1021/bi00522a023. [DOI] [PubMed] [Google Scholar]
- Böhme H., Pelzer B., Böger P. Purification and characterization of cytochrome f-556.5 from the blue-green alga Spirulina platensis. Biochim Biophys Acta. 1980 Oct 3;592(3):528–535. doi: 10.1016/0005-2728(80)90097-3. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Transport of proteins into mitochondria and chloroplasts. J Cell Biol. 1979 Jun;81(3):461–483. doi: 10.1083/jcb.81.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. J., Krogmann D. W., Pietro A. S. Electron donation to photosystem I. Plant Physiol. 1980 Apr;65(4):697–702. doi: 10.1104/pp.65.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. M., Rahire M., Rochaix J. D. Chlamydomonas reinhardii gene for the 32 000 mol. wt. protein of photosystem II contains four large introns and is located entirely within the chloroplast inverted repeat. EMBO J. 1984 Dec 1;3(12):2753–2762. doi: 10.1002/j.1460-2075.1984.tb02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Photosynthetic Electron Transport Chain of Chlamydomonas reinhardi VI. Electron Transport in Mutant Strains Lacking Either Cytochrome 553 or Plastocyanin. Plant Physiol. 1966 Dec;41(10):1648–1656. doi: 10.1104/pp.41.10.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Photosynthetic Electron Transport Chain of Chlamydomonas reinhardi. IV. Purification and Properties of Plastocyanin. Plant Physiol. 1966 Dec;41(10):1637–1642. doi: 10.1104/pp.41.10.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Photosynthetic Electron Transport Chain of Chlamydomonas reinhardi. V. Purification and Properties of Cytochrome 553 and Ferredoxin. Plant Physiol. 1966 Dec;41(10):1643–1647. doi: 10.1104/pp.41.10.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graan T., Ort D. R. Quantitation of the rapid electron donors to P700, the functional plastoquinone pool, and the ratio of the photosystems in spinach chloroplasts. J Biol Chem. 1984 Nov 25;259(22):14003–14010. [PubMed] [Google Scholar]
- Grossman A. R., Bartlett S. G., Schmidt G. W., Mullet J. E., Chua N. H. Optimal conditions for post-translational uptake of proteins by isolated chloroplasts. In vitro synthesis and transport of plastocyanin, ferredoxin-NADP+ oxidoreductase, and fructose-1,6-bisphosphatase. J Biol Chem. 1982 Feb 10;257(3):1558–1563. [PubMed] [Google Scholar]
- Kessler S. W. Use of protein A-bearing staphylococci for the immunoprecipitation and isolation of antigens from cells. Methods Enzymol. 1981;73(Pt B):442–459. doi: 10.1016/0076-6879(81)73084-2. [DOI] [PubMed] [Google Scholar]
- Kunert K. J., Böhme H., Böger P. Reactions of plastocyanin and cytochrome 553 with photosystem I of Scenedesmus. Biochim Biophys Acta. 1976 Dec 6;449(3):541–553. doi: 10.1016/0005-2728(76)90163-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine R. P., Gorman D. S. Photosynthetic electron transport chain of Chlamydomonas reinhardi. 3. Light-induced absorbance changes in chloroplast fragments of the wild type and mutant strains. Plant Physiol. 1966 Oct;41(8):1293–1300. doi: 10.1104/pp.41.8.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews F. S. The structure, function and evolution of cytochromes. Prog Biophys Mol Biol. 1985;45(1):1–56. doi: 10.1016/0079-6107(85)90004-5. [DOI] [PubMed] [Google Scholar]
- McDonnel A., Staehelin L. A. Detection of cytochrome f, a c-class cytochrome, with diaminobenzidine polyacrylamide gels. Anal Biochem. 1981 Oct;117(1):40–44. doi: 10.1016/0003-2697(81)90688-6. [DOI] [PubMed] [Google Scholar]
- Mishkind M. L., Wessler S. R., Schmidt G. W. Functional determinants in transit sequences: import and partial maturation by vascular plant chloroplasts of the ribulose-1,5-bisphosphate carboxylase small subunit of Chlamydomonas. J Cell Biol. 1985 Jan;100(1):226–234. doi: 10.1083/jcb.100.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Robinson C., Ellis R. J. Transport of proteins into chloroplasts. Partial purification of a chloroplast protease involved in the processing of important precursor polypeptides. Eur J Biochem. 1984 Jul 16;142(2):337–342. doi: 10.1111/j.1432-1033.1984.tb08291.x. [DOI] [PubMed] [Google Scholar]
- Schmidt R. J., Myers A. M., Gillham N. W., Boynton J. E. Chloroplast ribosomal proteins of Chlamydomonas synthesized in the cytoplasm are made as precursors. J Cell Biol. 1984 Jun;98(6):2011–2018. doi: 10.1083/jcb.98.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz B. E. Clinical observations on telekinesis. Part III. J Am Soc Psychosom Dent Med. 1980;27(3):87–96. [PubMed] [Google Scholar]
- Sigel M. B., Sinha Y. N., VanderLaan W. P. Production of antibodies by inoculation into lymph nodes. Methods Enzymol. 1983;93:3–12. doi: 10.1016/s0076-6879(83)93031-8. [DOI] [PubMed] [Google Scholar]
- Sueoka N. MITOTIC REPLICATION OF DEOXYRIBONUCLEIC ACID IN CHLAMYDOMONAS REINHARDI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):83–91. doi: 10.1073/pnas.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. M. Interchangeable copper and iron proteins in algal photosynthesis. Studies on plastocyanin and cytochrome c-552 in Chlamydomonas. Eur J Biochem. 1978 Jun 1;87(1):9–19. doi: 10.1111/j.1432-1033.1978.tb12346.x. [DOI] [PubMed] [Google Scholar]
- Wood P. M. The roles of c-type cytochromes in algal photosynthesis. Extraction from algae of a cytochrome similar to higher plant cytochrome f. Eur J Biochem. 1977 Feb;72(3):605–612. doi: 10.1111/j.1432-1033.1977.tb11283.x. [DOI] [PubMed] [Google Scholar]
- Youngblom J., Schloss J. A., Silflow C. D. The two beta-tubulin genes of Chlamydomonas reinhardtii code for identical proteins. Mol Cell Biol. 1984 Dec;4(12):2686–2696. doi: 10.1128/mcb.4.12.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]