Abstract
Aims
Mesenchymal stem cells (MSCs) can ameliorate myocardial infarction (MI) injury. However, older-donor MSCs seem less efficacious than those from younger donors, and the contributing underlying mechanisms remain unknown. Here, we determine how age-related expression of pigment epithelium-derived factor (PEDF) affects MSC therapeutic efficacy for MI.
Methods and results
Reverse transcriptase–polymerized chain reaction and enzyme-linked immunosorbent assay analyses revealed dramatically increased PEDF expression in MSCs from old mice compared to young mice. Morphological and functional experiments demonstrated significantly impaired old MSC therapeutic efficacy compared with young MSCs in treatment of mice subjected to MI. Immunofluorescent staining demonstrated that administration of old MSCs compared with young MSCs resulted in an infarct region containing fewer endothelial cells, vascular smooth muscle cells, and macrophages, but more fibroblasts. Pigment epithelium-derived factor overexpression in young MSCs impaired the beneficial effects against MI injury, and induced cellular profile changes in the infarct region similar to administration of old MSCs. Knocking down PEDF expression in old MSCs improved MSC therapeutic efficacy, and induced a cellular profile similar to young MSCs administration. Studies in vitro showed that PEDF secreted by MSCs regulated the proliferation and migration of cardiac fibroblasts.
Conclusions
This is the first evidence that paracrine factor PEDF plays critical role in the regulatory effects of MSCs against MI injury. Furthermore, the impaired therapeutic ability of aged MSCs is predominantly caused by increased PEDF secretion. These findings indicate PEDF as a promising novel genetic modification target for improving aged MSC therapeutic efficacy.
Keywords: Myocardial infarction, Mesenchymal stem cells, Paracrine, Pigment epithelium-derived factor
Introduction
Myocardial infarction (MI) remains the most common cause of cardiac morbidity and mortality in the developed world. Acute myocardial ischaemia causes rapid death of cardiomyocytes and vasculature, leading to left ventricular (LV) remodelling, including progressive fibrotic myocardium replacement, LV dilation, and heart failure.1 In the last decade, stem cell therapy has been shown to be a promising method for treating MI,2 and mesenchymal stem cells (MSCs) have been considered a good option because of their unique biological attributes.3,4 When introduced to an infarcted heart, MSCs prevent deleterious remodelling and improve recovery.3,4 Increasing evidence demonstrates that the beneficial effect of MSCs is significantly mediated through incompletely understood indirect paracrine actions,4–8 supplying multiple therapeutic growth factors and cytokines regulatory of the ongoing MI pathological processes.4–6 In recent studies, pigment epithelium-derived factor (PEDF), a member of the serpin protease inhibitor family lacking protease inhibitory activity, was identified as a major MSC-secreted protein.9,10 Pigment epithelium-derived factor is a multifunctional, pleiotropic protein with antiangiogenic, anti-oxidant, anti-inflammatory, anti-tumourigenic, and neuroprotective properties, with cell-type-dependent biological function.11 Thus, PEDF might be contributive to MSC paracrine actions regulating ongoing MI pathologic processes, but has not been investigated.
Most clinical studies have used autologous stem cells for MI treatment. Patients suffering MI typically are of advanced age. However, the impact of donor age on MSCs therapeutic efficacy remains uncertain.12 Clarification of this issue and underlying mechanisms are of utmost importance, as their elucidation will give insight regarding MSC genetic modification with age and any potential reversibility. In the present study, MSCs from older mice secreted significantly greater PEDF levels compared to young mice. We hypothesized that PEDF plays a critical role in MSC paracrine functioning during the pathological processes associated with MI. Furthermore, age-related PEDF expression may critically influence MSC therapeutic efficacy in MI treatment.
Methods
Detailed methodology is available in the Online Data Supplement.
Laboratory animals, isolation, culture, and characterization of mesenchymal stem cells
All experimental animal procedures were approved by the Animal Experiment Administration Committee of our institution. Mesenchymal stem cells were isolated and expanded from young (8-week-old) and older (18-month-old) male C57BL/6 mice via standard protocols.13 Third-passage cells were used for experiments. To validate the enriched MSCs population, flow cytometry characterized the surface antigens, and in vitro differentiation of cultured MSCs was performed as previously reported.13
Detection of pigment epithelium-derived factor expression in vitro
Reverse transcriptase–polymerized chain reaction (RT–PCR) analysis was performed as previously described.9 Pigment epithelium-derived factor concentrations released by MSCs were measured via mouse PEDF enzyme-linked immuno sorbent assay (ELISA) kit (USCN Life. Science & Technology Company, Double Lake, MO, USA) per manufacturer's instructions.
Adenoviral vectors and mesenchymal stem cells transduction
Briefly, human type 5 adenoviral vectors (SinoGenoMax Co., Ltd, Beijing, China) expressing human-PEDF (Ad.PEDF) from a cytomegalovirus (CMV) immediate early promoter were generated, and the E1 and E3 regions of the viral genome were replaced with a reporter gene [green fluorescent protein (GFP)]. Control vectors (Ad.Null) not expressing PEDF were constructed and produced concomitantly. In addition, the Ad.shPEDF vector, in which a shRNA was driven by the U6 promoter, and GFP was driven by an independent CMV promoter, which resulted in dramatically attenuated PEDF levels, was generated for further experiments. Ad.shctrl (Ad-Scramble) vector served as a control.
Mesenchymal stem cells were incubated with adenoviruses at infection multiplicity of 3000 for 2 h. Transduction efficiency was analysed 24 h after transduction by flow cytometry and inverted microscopy.
Model of myocardial infarction and mesenchymal stem cells transplantation
Myocardial infarction was established as described previously.14 Briefly, 8-week-old male C57BL/6 mice (under general anesthaesia, with 2% isoflurane) were subjected to small left thoracotomy, temporary cardio-exteriorization, and placement of 6.0 silk suture below origin of the left anterior descending coronary artery (LAD). The heart was replaced immediately into the thoracic cavity, with thoracic air evacuation to avoid pneumothorax. 4.0 × 106 MSCs/mouse were tail-vein injected 0.5–1 h post-MI. Control animals underwent LAD ligation with only saline injection.
Detection of mesenchymal stem cells recruitment and pigment epithelium-derived factor expression in vivo
To investigate MSC recruitment, alternate sets of 8 µm-thick serial vertical sections of the infarcted heart were mounted on gelatin-coated slides for fluorescent microscope examination, and images were analysed by Image-Pro Plus, Media Cybernetics Inc., Carlsbad, CA, USA. Infarct region PEDF expression was identified by immunofluorescence. Sections were sequentially incubated with goat anti-human PEDF primary antibodies (R&D Systems, Minneapolis, MN, USA) or rat anti-mouse PEDF primary antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and Texas Red-conjugated secondary antibody. Furthermore, PEDF levels were also determined by a human or mouse PEDF ELISA kit (USCN Life. Science & Technology Company).
Analysis of cellular profile in the MI area
On Day 7 post-MI, hearts were fixed and sectioned. Slides were incubated with primary antibodies for CD31 (Chemicon, Temecula, CA, USA), αSMA (Neomarkers, Fremont, CA, USA), vimentin (Abcam, Cambridge, MA, USA), Cardiac Troponin I (Abcam, Raleigh, NC, USA), and F4/80 (Serotec, Raleigh, NC, USA). After PBS rinsing, slides were incubated overnight with biotinylated goat anti-mouse IgG (Sigma-Aldrich, St. Louis, MO, USA) or donkey anti-rat IgG antibodies (Jackson, West Grove, PA, USA), further incubated with Texas red-conjugated streptavidin in PBS. Cell nuclei were stained with 4′, 6′-diamino-2-phenylindole (DAPI; Molecular Probes, Eugene, OR, USA).
Measurement of left ventricular fibrosis and left ventricular function
Left ventricle cross sections (4 µm thick) at the mid-papillary muscle level were Trichrome-Masson stained. For LV functional analysis, mice were anesthaetized by intraperitoneal 1% sodium pentobarbital injection (50 mg/kg body weight) before a micro-catheter was inserted into the LV via the right carotid artery. Left ventricular function and electrocardiogram data were recorded using a Biopac Data Acquisition System (Biopac Systems Inc., Goleta, CA, USA).
Co-culture of cardiac fibroblasts with mesenchymal stem cells for proliferation and migration assays
Young MSCs, old MSCs, Y&Ad.Null, Y&Ad.PEDF, O&Ad.shctrl, or O&Ad.shPEDF cells were plated (1.5 × 105 cells/cm2) in a transwell insert (Millipore, Billerica, MA, USA) with 0.4 µm pores, while neonatal mouse cardiac fibroblasts (CFs) were plated as control. The insert was then placed into 24-well plates, where CFs were plated (2 × 105 cells/cm2). Cardiac fibroblast proliferation was evaluated 48 h after co-culture under hypoxia using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cardiac fibroblast migration assay was carried out using a transwell insert of 8 µm pore size. Chemotaxis was induced by MSCs in the lower compartment. Cardiac fibroblasts were serum-starved overnight, and 5 × 104 cells were seeded in the upper compartment. Cells were allowed to migrate for 8 h under hypoxic conditions. The filters were stained with crystal violet for microscopy. A blinded, single person counted the number of cells that migrated to the lower filter surface.
The direct effects of pigment epithelium-derived factor on cardiac fibroblasts
Cardiac fibroblasts were stimulated for 8 h with 0–200 ng/mL recombinant human (rh) PEDF (Peprotech Inc., Rocky Hill, NJ, USA) under hypoxia, and the MTT assay was performed. Cell cycle distribution was detected by a Calibur flow cytometer (Becton–Dickinson, San Jose, CA, USA). For western blotting analysis, 50 µg total proteins from each cell extract was resolved with sodium dodecyl sulfate–polyacrilamide gel electrophoresis and transferred onto a polyvinylidence difluoride membrane (Immobillon-P; Millipore). The membrane was then incubated sequentially with primary anti-cyclin D1, anti-p27, or anti-glyceraldehyde-3-phosphate dehydrogenase (Santa Cruz Biotechnology, Inc.) and horseradish peroxidase-conjugated secondary antibodies; bands were detected with enhanced chemiluminescence (Pierce, Rockford, IL, USA). The CF migration assay was carried out using a transwell insert as described above, except that rh-PEDF was used for chemotaxis in the lower compartment. AnnexinV-FITC apoptosis detection kit (Beckman Coulter, Fullerton, CA, USA) determined apoptotic cell percentage, and flow cytometry analysis (BD Biosciences, San Diego, CA, USA) was performed. Moreover, CFs were stained with Hoechst 33258. Apoptotic CFs were identified on the basis of their nuclear morphology (presence of condensed chromatin and fragmentation). The nuclei from five random fields were counted by a single blinded person, and the percentage of apoptotic nuclei relative to total nuclei was calculated.
Statistical analyses
Data were expressed as mean ± standard error of the mean (SEM). Multiple group comparisons were evaluated via one-way analysis of variance followed by least significant difference -t-test for post hoc analysis. Comparisons between two independent groups were analysed using the Student t-test. Analyses were performed using SPSS software, Chicago, IL, USA. P values less than 0.05 were considered to be statistically significant and tests were performed two sided.
Results
Mesenchymal stem cells from older donors express and secrete more pigment epithelium-derived factor than younger donors
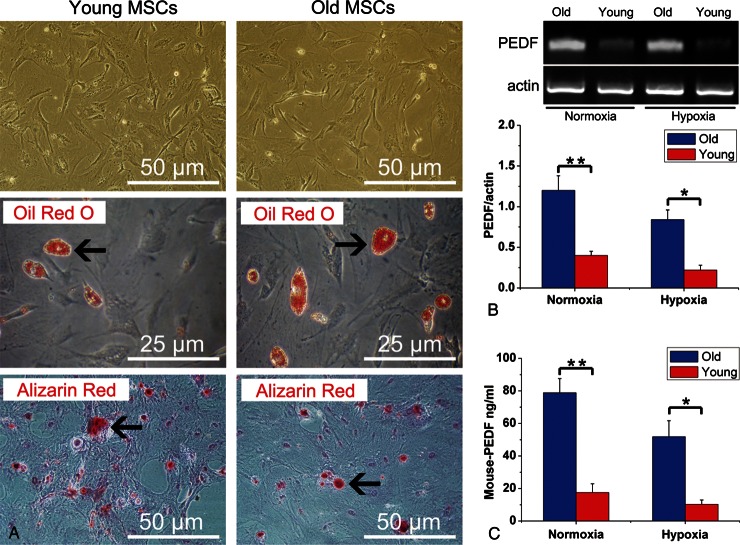
After three passages, MSC pluripotency was confirmed by capacity to differentiate into adipogenic and osteogenic lineages in vitro (Figure 1A). By flow cytometry analysis, cultured MSCs expressed CD29, CD44, CD73, CD90, and CD105, and were devoid of haematopoietic markers CD11b, CD34, and CD45 (Supplementary material online, Table S1). Notably, RT–PCR (Figure 1B) and ELISA (Figure 1C) revealed that, in comparison to young MSCs, significantly greater PEDF expression (for both mRNA and protein levels) was found in older MSCs under both normoxic and hypoxic conditions.
Figure 1.
Characterization of mesenchymal stem cells and expression of pigment epithelium-derived factor. (A) Mesenchymal stem cells (passage 3) from both young and older donor mice display fibroblast-like morphology in culture. In vitro adipocyte differentiation was confirmed by Oil Red O staining (arrows show accumulation of lipid droplets in vacuoles). In vitro osteogenic differentiation was demonstrated by alizarin red staining, demonstrating Ca2+ deposition (arrows). (B) Reverse transcriptase–polymerized chain reaction results demonstrated pigment epithelium-derived factor mRNA levels in older mesenchymal stem cells and young mesenchymal stem cells under both normoxic/hypoxic conditions (n= 5 mice/group). (C ) Enzyme-linked immuno sorbent assay experiment demonstrated pigment epithelium-derived factor protein levels in older mesenchymal stem cells and young mesenchymal stem cells culture supernatants (n= 5 mice/group) Data expressed as means ± SEM. *P< 0.01, **P< 0.001.
Adenoviral vector-transduced mesenchymal stem cells recruit to the infarct region, and regulate pigment epithelium-derived factor levels
We used adenoviral vectors carrying human-PEDF and or short hairpin RNA (shRNA) targeting mouse-PEDF to overexpress PEDF in young MSCs or knock down PEDF expression in older MSCs respectively. All adenoviral vectors were designed to carry a GFP reporter. The transduction efficiency of these adenoviral vectors was analysed (Supplemental Results). Utilizing our mouse MI model, we intravenously injected either: (i) saline, (ii) young MSCs, (iii) old MSCs, (iv) Y&Ad.Null, (v) Y&Ad.PEDF, (vi) O&Ad.shctrl, or (vii) O&Ad.shPEDF. On Day 1 after MI, a large number of GFP positive cells were found dispersed in the infarct region (Figure 2A and B). Furthermore, no significant differences were observed in the green fluorescent intensity of infarcted hearts among Y&Ad.Null, Y&Ad.PEDF, O&Ad.shctrl, and O&Ad.shPEDF groups (Figure 2C), indicating similar numbers of MSCs dispersal in the infarct region among these groups. Subsequently, we performed immunofluorescent staining and ELISA to identify the ability of genetically modified MSCs in the infarct region to regulate local PEDF levels. Greater PEDF levels were found in the infarct region after administration of MSCs of older compared to young origin. Adenoviral vectors were used to overexpress human-PEDF or block mouse-PEDF expression in young or old MSCs in vivo, respectively, thereby leading to PEDF level significantly changes in the infarct region (Supplemental Results).
Figure 2.

Localization of engrafted mesenchymal stem cells in the infarcted heart. (A) The representative image of infarcted heart 1 day post-injection of adenoviral vector transduced mesenchymal stem cells (haematoxylin and eosin staining). (B) The fluorescent image of consecutive sections of Figure 3A. Many green cells were dispersed in the infarct region. (C) Bar graph shows no significant differences (P= 0.708) in the green fluorescent intensity of infarcted hearts among Y&Ad.Null, Y&Ad.PEDF, O&Ad.shctrl, and O&Ad.shPEDF groups. Data expressed as means ± SEM (n= 5 mice/group).
Decreased efficacy of old mesenchymal stem cells for myocardial infarction treatment is associated with increased pigment epithelium-derived factor expression
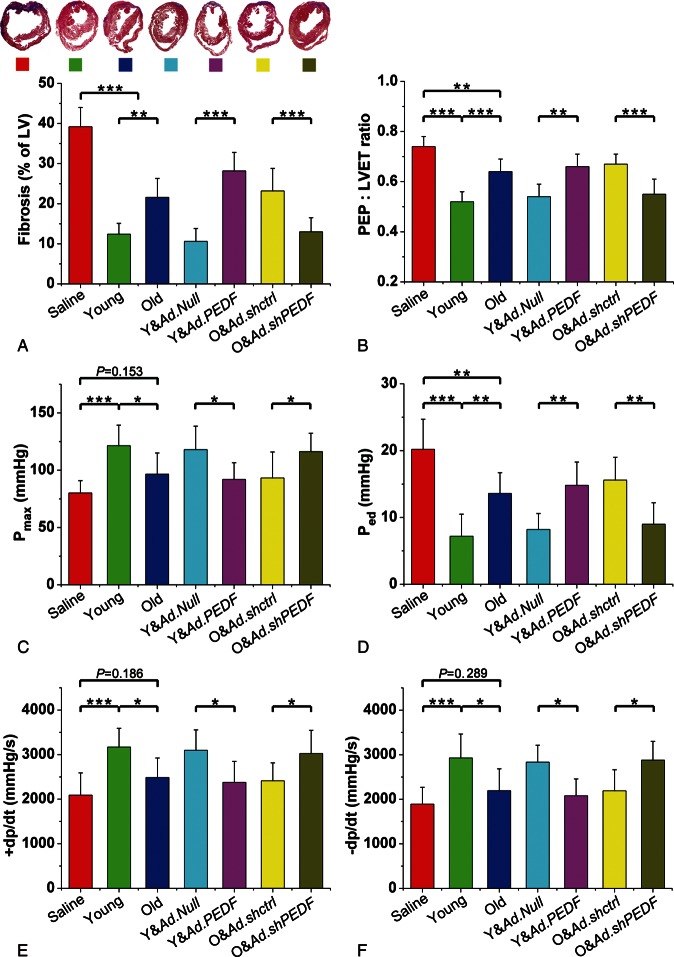
In order to study whether different PEDF levels could affect the efficacy of MSCs for MI, we performed gross cardiac morphology and function analyses. As illustrated in Figure 3A, compared to saline treatment, both young and old MSCs significantly attenuated fibrosis post-MI, but to greater extent after young MSC treatment. Compared to saline treatment, administration of young MSCs after MI markedly improved LV functional parameters. Compared to young MSC treatment, administration of old MSCs after MI increased the ratio of the pre-ejection period/left ventricular ejection time (Figure 3B) and LV end-diastolic pressure (Figure 3D), and decreased maximum LV pressure (Figure 3C), as well as the + dp/dt and –dp/dt (Figure 3E and F), all data suggestive of decreased therapeutic ability of old MSCs compared to young MSCs. Importantly, compared with Y&Ad.Null cells, Y&Ad.PEDF not only significantly increased LV fibrosis (Figure 3A), but also impaired LV function (Figure 3B–F). In contrast, O&Ad.shPEDF decreased the LV fibrosis area (Figure 3A), and ameliorated LV function compared with old MSCs transduced with Ad.shctrl (Figure 3B–F), supporting the notion that impaired therapeutic efficacy of old MSCs is associated with increased PEDF secretion.
Figure 3.
Measurement of left ventricular fibrosis and function. (A) Representative Masson trichrome-stained myocardial sections and bar graph showing fibrotic region measurement (n= 5 mice/group). (B–E) Bar graphs illustrating left ventriculum function parameters (n= 5 mice/group). Data expressed as means ± SEM. *P< 0.05, **P< 0.01, ***P≤ 0.001. Pre-ejection period: left ventricular ejection time ratio, the ratio of the pre-ejection period/left ventricular ejection time. Pmax, maximum pressure. Ped, end-diastolic pressure.
Mesenchymal stem cells regulate the cellular profile in the myocardial infarction area via paracrine factor pigment epithelium-derived factor
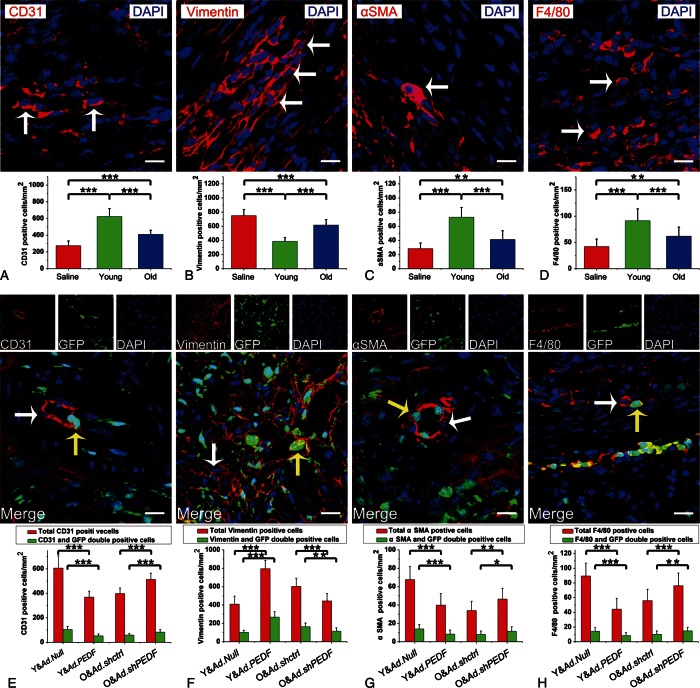
To investigate in the underlying mechanism contributing to age-diminished efficacy against MI injury, we analysed the cellular profile of the infarct region after administration of saline, young MSCs, or old MSCs. In infarcted myocardial sample regions 7 days after MI, confocal microscopy images demonstrated significantly increased density of vascular endothelial cells (ECs and CD31-positive), vascular smooth muscle cells (VSMCs and αSMA-positive), and macrophages (F4/80-positive) in mice having received young or old MSCs compared to saline. In contrast, fibroblast (vimentin-positive) density was significantly lower in mice receiving young or old MSCs compared to saline (Figures 4A and 5D). The infarct area of hearts subjected to old MSCs contained significantly fewer ECs, VSMCs, and macrophages, but more fibroblasts compared to those subjected to young MSCs (Figures 4A and 5D). To investigate whether the differential cellular profile between young and old MSCs was caused by variant PEDF level expression, we assessed the resultant cellular profile following administration of genetically modified MSCs. Interestingly, we observed only a small proportion of ECs, fibroblasts, VSMCs, and macrophages in the infarct region of hearts receiving CD31-, vimentin-, αSMA-, or F4/80- cells (each with GFP double positivity, confirmatory of viral infectivity, Figures 4E and 5H). Importantly, PEDF overexpression in young MSCs (Y&Ad.PEDF group vs Y&Ad.Null group) not only decreased the total number of ECs, VSMCs, and macrophages (total CD31- or αSMA- or F4/80-positive cells), but also the number of MSC-derived ECs, VSMCs, and macrophages in the infarct region, whereas both fibroblast (all vimentin-positive cells) and MSC-derived fibroblast populations were increased (Figures 4E and 5H). Furthermore, knocking down PEDF expression in old MSCs (O&Ad.shPEDF group vs. O&Ad.shctrl group) increased the total number of ECs, VSMCs, and macrophages, as well as MSC-derived ECs, VSMCs, and macrophages in the infarct region, whereas both fibroblast population and MSC-derived fibroblast density was decreased (Figures 4E and 5H). These results suggest that MSCs can alter the cellular profile within a MI area through PEDF paracrine factor, and furthermore, this cellular profile alteration can be influenced by the age-specific type PEDF expression present.
Figure 4.
Mesenchymal stem cells alter the cellular profile in the myocardial infarction area through pigment epithelium-derived factor. (A–D) Representative confocal microscopic images of CD31- or vimentin-, or αSMA- or F4/80-positive cells (red fluorescence, white arrows point to representative positive cells) in infarct region 7 days after administration of saline, young mesenchymal stem cells, or older mesenchymal stem cells (bar: 20 µm). Nuclei were stained by DAPI (blue fluorescence). The bar graph shows the cell density (n= 5 mice/group). (E–H) Representative confocal microscopic images of CD31- or vimentin- or αSMA- or F4/80-positive cells (red fluorescence) in infarct region 7 days after administration of Y&Ad.Null, Y&Ad.PEDF, O&Ad.shctrl, or O&Ad.shPEDF groups (bar: 20 µm). Injected mesenchymal stem cells were green fluorescent protein-positive (green fluorescence). Nuclei were stained by DAPI (blue fluorescence). White arrows point to representative CD31-, vimentin-, αSMA-, or F4/80 positive cells. Yellow arrows show representative CD31- or vimentin- or αSMA- or F4/80 and green fluorescent protein double positive cells. Bar graph shows the cell density (n= 5 mice/group). All data are expressed as means ± SEM. *P< 0.05, **P< 0.01, ***P≤ 0.001.
Figure 5.
Co-culture of cardiac fibroblasts with mesenchymal stem cells and the direct effects of pigment epithelium-derived factor on cardiac fibroblasts. (A) Bar graph illustrates proliferation assays of cardiac fibroblasts co-cultured with mesenchymal stem cells. Cardiac fibroblasts co-cultured with cardiac fibroblasts served as control. (B) Bar graph illustrates migration assays of cardiac fibroblasts co-cultured with mesenchymal stem cells. Cardiac fibroblasts co-cultured with cardiac fibroblasts served as control. (C) Bar graph shows the direct effect of pigment epithelium-derived factor on cardiac fibroblasts proliferation. (D) Representative flow cytometry analyses for cell cycle and the bar graph shows the effect of pigment epithelium-derived factor on cardiac fibroblasts' cell cycle. (E) Representative western blotting analyses and bar graphs demonstrate that pigment epithelium-derived factor regulated cardiac fibroblasts expression of cyclin D1 and P27. (F) The effect of pigment epithelium-derived factor on cardiac fibroblasts apoptosis. Representative Hoechest stained images and bar graphs illustrating degree of apoptosis for cardiac fibroblasts analysed by Hoechest staining or flow cytometry. White arrows show representative apoptotic cells with nuclear fragmentation. (G) The effect of pigment epithelium-derived factor on cardiac fibroblast migration. Representative crystal violet staining of migrated cardiac fibroblasts in the lower compartments of the transwell system and bar graph illustrating the effect of pigment epithelium-derived factor on cardiac fibroblasts migration in transwell system. All data expressed as means ± SEM. n = 6 per group. *P< 0.05, **P< 0.01, ***P≤ 0.001.
Pigment epithelium-derived factor secreted by mesenchymal stem cells stimulates cardiac fibroblast proliferation and migration in dose dependent manner
It has been reported that PEDF can induce EC and macrophage apoptosis, and inhibit the proliferation and migration of ECs and VSMCs.11,15–18 Our results demonstrating increased PEDF levels in the infarct region associated with decreased ECs, VSMCs, and macrophages are consistent with such reports. Additionally, increased PEDF levels in the infarct region were associated with increased fibroblast presence via an elusive underlying mechanism. To provide further evidence that MSC-secreted PEDF can affect fibroblast populations in the infarct region, we performed a transwell co-culture experiment with CFs and MSCs. We demonstrated that CF proliferation was slower when they were cultured with young MSCs than with old MSCs. Furthermore, this inhibitory effect was markedly weaker when CFs were co-cultured with Y&Ad.PEDF group (Figure 5A). Moreover, CF proliferation was much slower when they were cultured with old MSCs after PEDF expression had been knocked down (Figure 5A). In fact, our data showed that PEDF protein alone stimulated CF proliferation under hypoxic conditions in a dose-dependent fashion (Figure 5C). Pigment epithelium-derived factor (200 ng/mL) significantly increased the number of CFs in S phase (Figure 5D), up-regulated cyclin D1 expression, and down-regulated P27 (Figure 5E). However, 200 ng/mL PEDF did not have a pronounced effect on CF apoptosis during hypoxia (Figure 5F). Additionally, we demonstrated that either old MSCs with naturally high PEDF expression, or young MSCs with overexpressed PEDF, attracted more CFs in the transwell system. In contrast, knocking down PEDF expression in old MSCs attenuated CF chemotaxis (Figure 5B). Furthermore, by using PEDF protein to substitute MSCs in the lower compartments of the transwell system, PEDF alone was consistently shown to be an important chemoattractant of the MSC secretome (Figure 5G).
Discussion
Mesenchymal stem cells isolated from adult bone marrow provide excellent biomaterial source for therapeutic stem cell development. Although the present findings and previous study demonstrated MSCs could restore cardiac function partly via cell-type differentiation potential after MI,19 the population contributions of such cell differentiation subtypes were limited. Compelling evidence has shown that the secretion of a broad range of bioactive molecules by MSCs contributes to their cardiac repair role after ischaemic onslaught.4–6 In this study, we identified the paracrine factor PEDF as a significant mediator of the resultant cellular profile of the post-MI infarct region. Moreover, we demonstrated that the decline in therapeutic efficacy of old MSCs was associated with age-dependent paracrine PEDF release.
Encoded by a single gene, PEDF is a 50 kDa glycoprotein with strong conservation across phyla from fish to mammals, and is widely expressed among many tissues.11,15,16 Most secreted PEDF deposit within the extracellular matrix, with cell-type-specific functions.11,15,16 PEDF is known as a strong anti-angiogenic factor due to its ability to induce EC apoptosis by activation of the Fas/Fas ligand transduction cascade and p38 MAP kinas dependent cleavage of caspases 3, 8, and 9, and is inhibitive of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF)-induced EC migration and proliferation.11,15,16 Conversely, it exerts anti-apoptotic effects on neuronal or hepatoma cells by activating the nuclear factor-κB signaling cascade or inhibiting lysosomal Bcl-xL degradation, respectively.15,16,20 It can also inhibit platelet-derived growth factor-induced VSMC proliferation and migration by blocking reactive oxygen species generation,18 although we recently demonstrated that PEDF stimulated both proliferation and migration of retinal pigment epithelial cells in a laser-induced choroidal neovascularization model.21 It is speculated that the elusive functions of PEDF may attribute to multiple unknown receptors, the immediate cellular state, as well as pathologic signals and factors present in the cellular microenvironment.16 Furthermore, age-related differential PEDF expression has been observed previously, but with inconsistent results among different tissues.22,23 We found presently that PEDF expression in older mice MSCs was significantly greater than from young mice. Notably, our results evidenced the negative correlation between MSC-secreted PEDF quantity and therapeutic potency against MI injury.
Angiogenesis is induced by MSC secretion of bioactive factors, such as VEFG, bFGF, and hepatocyte growth factor, which promote both EC and VSMC proliferation and migration.24 Conversely, MSCs also secrete PEDF, which can potentially induce apoptosis, and inhibit EC and VSMC proliferation and migration via above-mentioned mechanisms. Compared to young MSCs, old MSCs secrete more PEDF in the infarct region. It is therefore conceivable that increased PDEF levels exert more significant biological effects resulting in an infarct region containing fewer ECs and VSMCs; hence, angiogenesis is inhibited. Moreover, CFs are key effector cells in the pathogenesis of post-MI cardiac fibrosis, and it has been shown that MSCs can attenuate CF proliferation via a paracrine mechanism resulting in an anti-fibrotic effect.25 Our in vivo experimental results are confirmatory, demonstrating both young and old MSCs administration, compared to saline, decreased fibroblast number and fibrotic region. Furthermore, the anti-fibrotic effect of MSCs attenuated with age. Our results suggested that this phenomenon was associated with the ability of PEDF to stimulate CF proliferation. The underlying mechanism likely involves up-regulation of cyclin D1 expression and P27 down-regulation, a cyclin-dependent kinase inhibitor arresting G1. Consequently, an increased number of cells were in S phase, whereas there was no effect upon apoptosis during hypoxia. Additionally, we demonstrated PEDF stimulated CF chemotaxis in dose-dependent fashion, as previously reported.9 Thus, our study implies that, compared to younger MSCs, older MSCs increased the fibroblast population in the infarct region at least partly due to increased PEDF secretion. After MI, damaged cardiomyocytes are removed largely by macrophages and replaced by scar tissue.2 In the present study, regardless of which cell type received which genetic alteration of PEDF expression, the more PEDF in the infarct region, the fewer macrophages were observed. This phenomenon may be attributed to the findings of Ho et al.,17 which demonstrated that PEDF induces macrophage apoptosis and necrosis via peroxisome proliferator-activated receptor γ signaling. In addition, PEDF can inhibit macrophage activation.26 Notably, reduction in macrophage infiltration and decreased macrophage activation are associated with significantly delayed MI healing, principally due to deficient phagocytosis of injured cardiomyocytes and delayed granulation tissue formation,27 yet another aetiology for older MSCs’ decreased therapeutic efficacy.
The MSC delivery route having greatest impact on prevention of remodelling post-MI has not been determined.28 In the current study, we utilized peripheral venous injection of MSCs post-MI for the following reasons. Firstly, MSCs can be infused intravenously due to their ability to gravitate to injured regions within the myocardium, stimulated, at least in part, by stromal cell-derived factor-1/CXCR4 axis.29,30 Secondly, intravenous infusion remains one of the safe, convenient, clinically relevant delivery approaches in patients.31 Thirdly, it has been reported that the plastic adherent-derived MSCs are not a homogeneous cell population, which might be contaminated by a fibroblasts, osteoblasts, and adipocytes.4 We speculate that these cellular contaminants (which exhibit no chemotactic properties) are filtered out by the peripheral circulation. Lastly, we endeavored to assess the possible impact of PEDF on MSC chemotaxis. Our results revealed the rapid recruitment of MSCs to the MI area. Additionally, no significant difference between groups was demonstrated in the relative fluorescent intensity of the infarcted heart after infusion of GFP-labeled MSCs, suggesting that PEDF has no significant impact upon MSC chemotaxis. However, there is still a huge gap between small animal models and practical clinical application, and acknowledge the limitations of this current study that do not address several major problems of stem cell-based therapy, including targeted and effective MSCs delivery route, the most appropriate time point for cell transplantation, and other risk control issues.
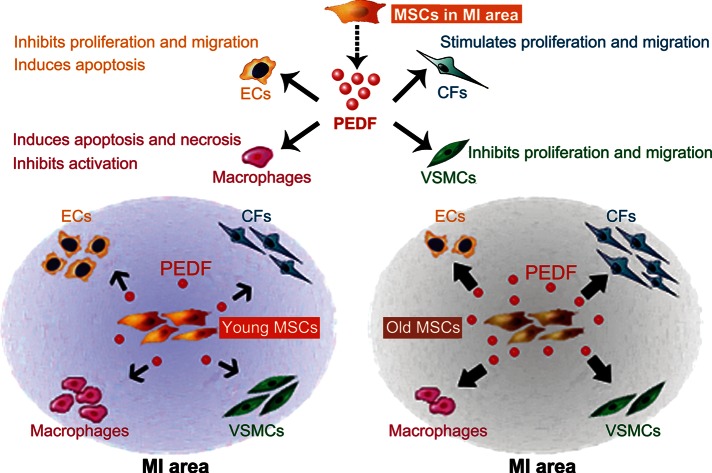
Overall, the current study confirmed that MSCs can regulate MI pathological processes through paracrine actions. Thus, MSCs could be considered an “organizer” in the MI area, manipulating tissue cellular profile by secreting various growth factors and cytokines. We demonstrated that PEDF was one of the most important factors involved in MSC paracrine function. Pigment epithelium-derived factor secreted by MSCs can act upon several cell types in the infarct region (Figure 6). However, our experiments were not designed to address all discrepancies in the paracrine profile between young and older MSCs. Further experiments defining the exact alteration of the aged MSCs paracrine profile are clearly necessary. One method for increasing injury repair efficiency, and minimizing undesirable outcomes, would be directly manipulating the cell types controlling the pathological processes at work.32 Therefore, our results imply that the most efficacious aged-donor MSC utilization must focus upon optimizing paracrine profile to favorably manipulate the cellular profile in the infarct region. For such means, our study supports PEDF as a promising novel genetic modification target for augmenting aged MSC therapeutic efficacy.
Figure 6.
Schematic illustrating mesenchymal stem cells can regulate the cellular profile in the myocardial infarction area via paracrine factor pigment epithelium-derived factor. Older mesenchymal stem cells express and secrete more pigment epithelium-derived factor in infarct region than young mesenchymal stem cells, leading to fewer endothelial cells, vascular smooth muscle cells, and macrophages, but more fibroblasts in the infarct region after older mesenchymal stem cell administration. (Biological effects of pigment epithelium-derived factor upon endothelial cells, vascular smooth muscle cells, macrophages, and fibroblasts mentioned in schematic are based on present study results and previous literatures).
Supplementary material
Supplementary material is available at European Heart Journal online.
Conflict for interest: none declared.
Funding
This work was supported by National Natural Science Foundation of China (81070183) and Foundation for Excellent Doctoral Level Students of the Fourth Military Medical University (2009019). Funding to pay the Open Access publication charges for this article was provided by the laboratory of Institute of Cardiovascular Disease of Chinese PLA.
Supplementary Material
Acknowledgements
We acknowledge Xiaozhao Lu, Liang Jin, Haiyan Fu, Mengying Wei (Department of Biochemistry and Molecular Biology, the Fourth Military Medical University), and Yueming Wang (Department of Physiology, the Fourth Military Medical University), for their technical help.
References
- 1.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 2.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–329. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 4.Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells. 2008;26:2201–2210. doi: 10.1634/stemcells.2008-0428. [DOI] [PubMed] [Google Scholar]
- 5.Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salem HK, Thiemermann C. Mesenchymal Stromal Cells - Current Understanding and Clinical Status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarojini H, Estrada R, Lu H, Dekova S, Lee MJ, Gray RD, et al. PEDF from mouse mesenchymal stem cell secretome attracts fibroblasts. J Cell Biochem. 2008;104:1793–1802. doi: 10.1002/jcb.21748. [DOI] [PubMed] [Google Scholar]
- 10.Chiellini C, Cochet O, Negroni L, Samson M, Poggi M, Ailhaud G, et al. Characterization of human mesenchymal stem cell secretome at early steps of adipocyte and osteoblast differentiation. BMC Mol Biol. 2008;9:26. doi: 10.1186/1471-2199-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rychli K, Huber K, Wojta J. Pigment epithelium-derived factor (PEDF) as a therapeutic target in cardiovascular disease. Expert Opin Ther Targets. 2009;13:1295–1302. doi: 10.1517/14728220903241641. [DOI] [PubMed] [Google Scholar]
- 12.Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102:1319–1330. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, et al. Jacobsen SE, Fleischmann BK. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–1369. doi: 10.1182/blood-2006-12-063412. [DOI] [PubMed] [Google Scholar]
- 14.Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, et al. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 2010;107:1445–1453. doi: 10.1161/CIRCRESAHA.110.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becerra SP. Focus on Molecules: Pigment epithelium-derived factor (PEDF) Exp Eye Res. 2006;82:739–740. doi: 10.1016/j.exer.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Barnstable CJ, Tombran-Tink J. Neuroprotective and antiangiogenic actions of PEDF in the eye: molecular targets and therapeutic potential. Prog Retin Eye Res. 2004;23:561–577. doi: 10.1016/j.preteyeres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Ho TC, Yang YC, Chen SL, Kuo PC, Sytwu HK, Cheng HC, et al. Pigment epithelium-derived factor induces THP-1 macrophage apoptosis and necrosis by the induction of the peroxisome proliferator-activated receptor gamma. Mol Immunol. 2008;45:898–909. doi: 10.1016/j.molimm.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura K, Yamagishi S, Matsui T, Yoshida T, Takenaka K, Jinnouchi Y, et al. Pigment epithelium-derived factor inhibits neointimal hyperplasia after vascular injury by blocking NADPH oxidase-mediated reactive oxygen species generation. Am J Pathol. 2007;170:2159–2170. doi: 10.2353/ajpath.2007.060838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci USA. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawaguchi T, Yamagishi S, Itou M, Okuda K, Sumie S, Kuromatsu R, et al. Pigment epithelium-derived factor inhibits lysosomal degradation of Bcl-xL and apoptosis in HepG2 cells. Am J Pathol. 2010;176:168–176. doi: 10.2353/ajpath.2010.090242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou HY, Liang HL, Wang YS, Zhang ZX, Wang BR, Shi YY, et al. A therapeutic strategy for choroidal neovascularization based on recruitment of mesenchymal stem cells to the sites of lesions. Mol Ther. 2010;18:1837–1845. doi: 10.1038/mt.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pina AL, Kubitza M, Brawanski A, Tombran-Tink J, Kloth S. Expression of pigment-epithelium-derived factor during kidney development and aging. Cell Tissue Res. 2007;329:329–338. doi: 10.1007/s00441-007-0420-8. [DOI] [PubMed] [Google Scholar]
- 23.Francis MK, Appel S, Meyer C, Balin SJ, Balin AK, Cristofalo VJ. Loss of EPC-1/PEDF expression during skin aging in vivo. J Invest Dermatol. 2004;122:1096–1105. doi: 10.1111/j.0022-202X.2004.22510.x. [DOI] [PubMed] [Google Scholar]
- 24.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 25.Ohnishi S, Sumiyoshi H, Kitamura S, Nagaya N. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Lett. 2007;581:3961–3966. doi: 10.1016/j.febslet.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 26.Zamiri P, Masli S, Streilein JW, Taylor AW. Pigment epithelial growth factor suppresses inflammation by modulating macrophage activation. Invest Ophthalmol Vis Sci. 2006;47:3912–3918. doi: 10.1167/iovs.05-1267. [DOI] [PubMed] [Google Scholar]
- 27.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, et al. CCL2/Monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 28.Hatzistergos KE, Quevedo H, Oskouei BN, Hu QH, Feigenbaum GS, Margitich IS, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 30.Cheng ZK, Ou LL, Zhou X, Li F, Jia XH, Zhang YG, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571–579. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 31.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–1669. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.