Abstract
Background
Oral anticoagulation in addition to antiplatelet treatment after an acute coronary syndrome might reduce ischaemic events but increase bleeding risk. We performed a meta-analysis to evaluate the efficacy and safety of adding direct thrombin or factor-Xa inhibition by any of the novel oral anticoagulants (apixaban, dabigatran, darexaban, rivaroxaban, and ximelagatran) to single (aspirin) or dual (aspirin and clopidogrel) antiplatelet therapy in this setting.
Methods and results
All seven published randomized, placebo-controlled phase II and III studies of novel oral anticoagulants in acute coronary syndromes were included. The database consisted of 30 866 patients, 4135 (13.4%) on single, and 26 731 (86.6%) on dual antiplatelet therapy, with a non-ST- or ST-elevation acute coronary syndrome within the last 7–14 days. We defined major adverse cardiovascular events (MACEs) as the composite of all-cause mortality, myocardial infarction, or stroke; and clinically significant bleeding as the composite of major and non-major bleeding requiring medical attention according to the study definitions. When compared with aspirin alone the combination of an oral anticoagulant and aspirin reduced the incidence of MACE [hazard ratio (HR) and 95% confidence interval 0.70; 0.59–0.84], but increased clinically significant bleeding (HR: 1.79; 1.54–2.09). Compared with dual antiplatelet therapy with aspirin and clopidogrel, adding an oral anticoagulant decreased the incidence of MACE modestly (HR: 0.87; 0.80–0.95), but more than doubled the bleeding (HR: 2.34; 2.06–2.66). Heterogeneity between studies was low, and results were similar when restricting the analysis to phase III studies.
Conclusion
In patients with a recent acute coronary syndrome, the addition of a new oral anticoagulant to antiplatelet therapy results in a modest reduction in cardiovascular events but a substantial increase in bleeding, most pronounced when new oral anticoagulants are combined with dual antiplatelet therapy.
Keywords: Oral anticoagulants, Antiplatelet therapy, Acute coronary syndrome, Myocardial infarction, Meta-analysis
See page 1618 for the editorial comment on this article (doi:10.1093/eurheartj/eht075)
Introduction
Despite contemporary evidence-based care,1–4 including revascularization and dual antiplatelet therapy, patients with acute coronary syndromes (ACSs) remain at risk of recurrent ischaemic events. Oral vitamin K antagonists have been shown to prevent recurrent ischaemic events after ACS, both as mono-therapy and in combination with aspirin, but substantially increase bleeding and are cumbersome to use because of multiple interactions with food and drugs, and the need for frequent laboratory monitoring.5,6 Evidence regarding the efficacy and safety of the combination of dual antiplatelet therapy and old oral anticoagulants, i.e. vitamin K antagonists, is limited but registry data indicate a high risk of major bleeding.7
New oral anticoagulants offer advantages to warfarin for stroke prevention in atrial fibrillation.8–10 Their potential as antithrombotic therapy after ACSs has been investigated during the last years. The first experience in this setting used an oral direct thrombin inhibitor, ximelagatran, later withdrawn from the market because of liver toxicity, and showed a reduction in the composite of death, myocardial infarction, and stroke when added to aspirin for 6 months after an ACS.11 Another oral direct thrombin inhibitor, dabigatran, and three novel oral direct factor Xa inhibitors—apixaban, rivaroxaban, and darexaban—added to single and/or dual antiplatelet therapy have recently been evaluated in phase II trials in ACS patients.12–15 All phase II trials have shown a definite increase in the incidence of bleeding with the combination of oral anticoagulation and antiplatelet therapy, but were under-powered for the evaluation of efficacy. The phase III study with apixaban16 in high-risk ACS patients, in doses shown to be effective for stroke prevention in atrial fibrillation, was terminated prematurely due to increased bleeding without evidence for efficacy. The phase III study with rivaroxaban,13 in doses lower than evaluated for stroke prevention in atrial fibrillation, showed significant reductions in the composite of death, myocardial infarction, and stroke overall and at each dose and reductions in mortality, but also an increased rate of major bleeding. Given the consistent increases in bleeding and the less consistent reduction in ischaemic events, the overall benefit–risk profile of adding new oral anticoagulants to antiplatelet treatment after an ACS is unknown.
We hypothesized that the addition of a novel oral anticoagulant to antiplatelet therapy after an ACS would decrease subsequent major adverse cardiovascular events (MACEs) but increase bleeding; that these effects would vary by the number of concomitant antiplatelet drugs; and that the effects on bleeding would be inversely related to the effects on MACEs.
Methods
Identification of studies
The present meta-analysis did not have a registered review protocol. Information sources for identification of studies were PubMed, Scopus, Web of Knowledge, reference lists of articles, and personal knowledge of the field through participation in the studies. The search expression used in PubMed was ((‘antithrombins “[MeSH Terms] OR ‘antithrombins’[All Fields]) OR (‘factor Xa’[MeSH Terms] OR factor Xa[Text Word]) AND inhibitor[All Fields]) OR ximelagatran[Text Word] OR dabigatran[Text Word] OR apixaban[Text Word] OR rivaroxaban[Text Word] OR darexaban[Text Word] OR (‘N-(2-(((4-(aminoiminomethyl)phenyl)amino)methyl)-1-methyl-1H-benzimidazol-5-yl)carbonyl)-N-2-pyridinyl-beta-alanine’[Supplementary Concept] OR [‘N-(2-hydroxy-6-(4-methoxybenzamido)phenyl)-4-(4-methyl-1,4-diazepan-1-yl)benzamide’[Supplementary Concept]) AND ‘Acute Coronary Syndrome’[Majr] OR ‘Myocardial infarction’[Majr] AND ‘Randomized Controlled Trial’ [Publication Type:NoExp]. Similar expressions were used in the other databases. Two researchers (J.S. and B.J.) performed the literature review independently of each other. For this study, data from all published phase II and III studies (seven in total) of new oral anticoagulants after a recent ACS were included (the ESTEEM,11 REDEEM,14 RUBY-1,15 APPRAISE-112 and APPRAISE-2,16 ATLAS ACS-TIMI 46,13 and ATLAS ACS 2–TIMI 5117 studies), irrespective of other study or report characteristics.
Outcomes, data collection, and quality assessment
The collected data items included inclusion criteria, exclusion criteria, interventions (the randomized anticoagulant treatment as well as treatment with antiplatelet drugs aspirin and/or clopidogrel), effect estimates from the comparisons made, follow-up time, and definition of outcomes (MACEs and major bleeding events).
In the ESTEEM11 study only single antiplatelet therapy with aspirin was allowed, while the APPRAISE-1,12 APPRAISE-2,16 and ATLAS ACS-TIMI 4613 recruited patients either on single (aspirin) or dual antiplatelet therapy with aspirin and clopidogrel, see Table 1, and these patients were analysed stratified on their antiplatelet therapy status at the time of randomization in the present study. In the REDEEM,14 RUBY-1,15 and ATLAS ACS 2–TIMI 5117 studies, the vast majority of patients received dual antiplatelet therapy at randomization, i.e. 98, 95, and 93%, respectively, and all patients in these studies were thus considered as receiving dual antiplatelet therapy in the present analysis.
Table 1.
Study characteristics
| Study | No. of study patients | No. of patientsa in meta-analysis | Duration | Agec | Per cent women | Study treatment | Antiplatelet drugs |
Bleeding events used in the present study | |
|---|---|---|---|---|---|---|---|---|---|
| Single (%) | Dual (%) | ||||||||
| ESTEEM11 | 1900 | 1883 | 6 months | 68 | 32 | Ximelagatran 24, 36, 48, or 60 mg b.i.d., or placebo | 100 | – | ISTH major and clinically relevant non-major bleeds |
| APPRAISE-112 | 1715 | 1210b | 6 months | 61 | 24 | Apixaban 2.5 mg b.i.d., 10 mg o.d., or placebo | 24 | 76 | ISTH major and clinically relevant non-major bleeds |
| ATLAS ACS-TIMI4613 | 3462 | 1997b | 6 months | 57 | 23 | Rivaroxaban 5 mg o.d., 5 mg b.i.d., 10 mg o.d., or placebo | 25 | 75 | TIMI clinically significant bleeding (TIMI major bleeding, TIMI minor bleeding, or bleeding requiring medical attention) |
| REDEEM14 | 1878 | 1861 | 6 months | 62 | 24 | Dabigatran 50, 75, 110. or 150 mg b.i.d. or placebo | 2 | 98 | ISTH major and clinically relevant non-major bleeds |
| RUBY-115 | 1279 | 1258 | 6 months | 57 | 20 | Darexaban 5, 15 or 30 mg b.i.d., 10, 30 or 60 mg o.d., or placebo | 5 | 95 | ISTH major and clinically relevant non-major bleeds |
| APPRAISE-216 | 7392 | 7315 | 8 months | 67 | 32 | Apixaban 5 mg (or 2.5 mgd) b.i.d., or placebo | 19 | 81 | TIMI major bleeds; ISTH major and clinically relevant non-major bleeds |
| ATLAS ACS2-TIMI5117 | 15 526 | 15 342 | 13 months | 62 | 25 | Rivaroxaban 2.5 or 5 mg b.i.d., or placebo | 7 | 93 | TIMI major (non-CABG related) bleeds, TIMI bleeding requiring medical attention |
o.d., once daily; b.i.d., twice daily; ISTH, International Society on Thrombosis and Haemostasis; TIMI, Thrombolysis in Myocardial Infarction.
See Methods for description of inclusion and exclusion criteria. Single antiplatelet therapy includes patients not receiving any antiplatelet medication at randomization, dual antiplatelet treatment consists of aspirin and clopidogrel at randomization.
aNo. of patients who received at least one dose of study drug.
bNo. of patients treated with daily doses also evaluated in phase III study.
cMean or median.
dApixaban 2.5 mg b.i.d. in 8.5% of the patients with estimated creatinine clearance <40 mL/min.
Eligible patients were men or women with ACS with or without ST-elevation within 7–14 days before randomization. In addition, patients had one or more of following additional risk factors; age ≥65years, prior myocardial infarction, diabetes mellitus, congestive heart failure or a left ventricular ejection fraction <40%, no revascularization for the index event, mild to moderate renal insufficiency, peripheral vascular disease, or previous ischaemic stroke in all studies, except the ATLAS ACS-TIMI 46 in which no additional risk factor was required and the ATLAS ACS 2–TIMI 51 study in which subjects who were 18–54 years of age had to have either diabetes mellitus or a prior myocardial infarction in addition to the presenting ACS event. The main exclusion criteria for all seven trials were; planned percutaneous coronary intervention, ongoing or planned treatment with vitamin K antagonist, recent stroke, conditions associated with an increased bleeding risk, e.g. anaemia, thrombocytopenia, and a history of severe bleeding.
The groups of patients receiving at least one dose of the studied dosages of the novel oral anticoagulants ximelagatran, dabigatran, and darexaban in the phase II trials ESTEEM,11 REDEEM,14 and RUBY-1,15 respectively, were included in the meta-analysis. To avoid the evaluation of doses deemed to have too high bleeding risk, we only included the 1210 (out of 1715) patients in the phase II study APPRAISE-112 receiving at least one dose of the apixaban doses later evaluated in the phase III study APPRAISE-2,16 i.e. apixaban 5 and 10 mg daily. Likewise, only the 1997 (out of 3491) patients receiving at least one dose of the rivaroxaban doses in the phase II study ATLAS ACS-TIMI 46,13 i.e. rivaroxaban 5 and 10 mg daily, later taken forward to the phase III study ATLAS ACS 2–TIMI 5117 study were included.
All the phase II studies aimed at 6 months of treatment and follow-up. The phase III studies had a longer follow-up, in the APPRAISE-2 study the mean follow-up was 8.2 months, while the ATLAS ACS 2–TIMI 51 had treatment duration of the mean 13 months and up to 31 months; we therefore also analysed associations of treatment with risk of ischaemic and bleeding events in a subgroup of these phase III studies.
The MACE outcome was defined as the composite of all-cause mortality, myocardial infarction, or stroke in all studies in this meta-analysis. Myocardial (re-)infarction was in all trials defined as elevated cardiac biomarkers together with ischaemic symptoms or ECG-changes (ST-elevation or -depression, new left bundle branch block, or new Q-waves). Stroke was defined as an acute onset of focal neurological deficit of presumed vascular origin lasting for 24 h or more and further categorized as haemorrhagic or ischaemic after imaging. We retrieved data for some non-published composite outcomes for dabigatran, apixaban, and darexaban studies after contact with the original study authors.
Major bleeding events assessed by the International Society of Thrombosis and Haemostasis (ISTH) definition18 were reported in the trials with ximelagatran, dabigatran, apixaban, and darexaban. An ISTH major bleeding is defined as a bleeding that was fatal, occurred in a critical location (intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, intramuscular with compartment syndrome, or pericardial), or was associated with a fall in haemoglobin of 2 g/dL or more, or a transfusion of two units or more of whole blood or packed red blood cells. Clinically relevant minor bleeding was in these studies defined as a clinically overt bleed that did not meet the criteria for major bleed but prompted a clinical response, i.e. hospital admission for bleeding, medical or surgical treatment, or a change in antithrombotic therapy including interruption or discontinuation of study drug. In the present meta-analysis, a clinically significant bleeding was in all studies, except those with rivaroxaban, defined as the composite of major (ISTH) and clinically relevant minor bleeds, i.e. the primary outcome event for the phase II studies with apixaban, dabigatran, and darexaban and a secondary safety outcome in the phase III study with apixaban.
The studies with rivaroxaban,13,17 and also the studies with apixaban,12,16 reported bleeding events by the Thrombolysis in Myocardial Infarction (TIMI) definitions.19 A TIMI major bleeding is defined as any intracranial bleeding or clinically overt bleeding associated with a decrease in haemoglobin of ≥5 g/dL or an absolute drop in haematocrit of ≥15%. TIMI minor bleeding is defined as any clinically overt bleeding event, including bleeding that is evident on imaging studies, that is associated with a decrease in haemoglobin ≥3 g/dL but <5g/dL. Bleeding requiring medical attention is defined as any bleeding requiring medical or surgical treatment or laboratory evaluation without meeting criteria for TIMI major or minor bleeding. In the present meta-analysis, a clinically significant bleeding was defined as a the composite of TIMI major bleeding, TIMI minor bleeding, or bleeding requiring medical attention in ATLAS ACS-TIMI 46 study,13 and a TIMI bleeding requiring medical attention in the ATLAS ACS 2–TIMI 51 study.17
In the subgroup analysis of the two phase III studies, the MACE outcome was similarly defined as the composite of all-cause mortality, myocardial infarction, or stroke, while only major bleeding events were evaluated, defined as TIMI major bleeding in the APPRAISE-2 study16 with apixaban, and TIMI major bleeding not related to coronary-artery bypass grafting in the ATLAS ACS 2–TIMI 51 study17 with rivaroxaban, which were the primary safety outcomes in these studies.
Risk of bias within studies was judged by proper blinding and the presence/absence of a placebo-controlled arm.
Statistical analysis
The principal summary measure was the hazard ratio (95% confidence interval), supplemented by absolute risk reduction (risk difference) (95% confidence interval). Because numbers needed to treat for an additional beneficial (NNTB) or harmful (NNTH) outcome must be calculated using a homogenous follow-up time, these properties were calculated from pooled hazard ratios using only the phase II studies with 6 months follow-up, and using the pooled 6-month risks of control arms of these studies as assumed control risks. We did not have access to individual participant data. Data were summarized across treatment arms using the DerSimonian and Laird random effects model.20 Subgroups based on single vs. dual concomitant antiplatelet therapy, and treatment with direct thrombin inhibitors vs. direct factor Xa inhibitors, were compared using fixed-effects models with inverse variance weighting. We evaluated heterogeneity of effects using the I2 statistic. We assessed risk of publication bias using a funnel plot and Egger's test,21 involving all of the treatment arms and the primary outcome MACEs. To investigate the association between the intended effects on MACEs and the side effects of bleeding, we performed a pre-specified random-effects meta-regression with the hazard ratio of MACEs as independent and hazard ratio HR of bleeding as dependent variable (hazard ratios for both outcomes are comparing the active arm against placebo). This meta-regression was stratified on the number of antiplatelet drugs (the total weight was 100% within each stratum). We used Stata 12.0 for all analyses.
Results
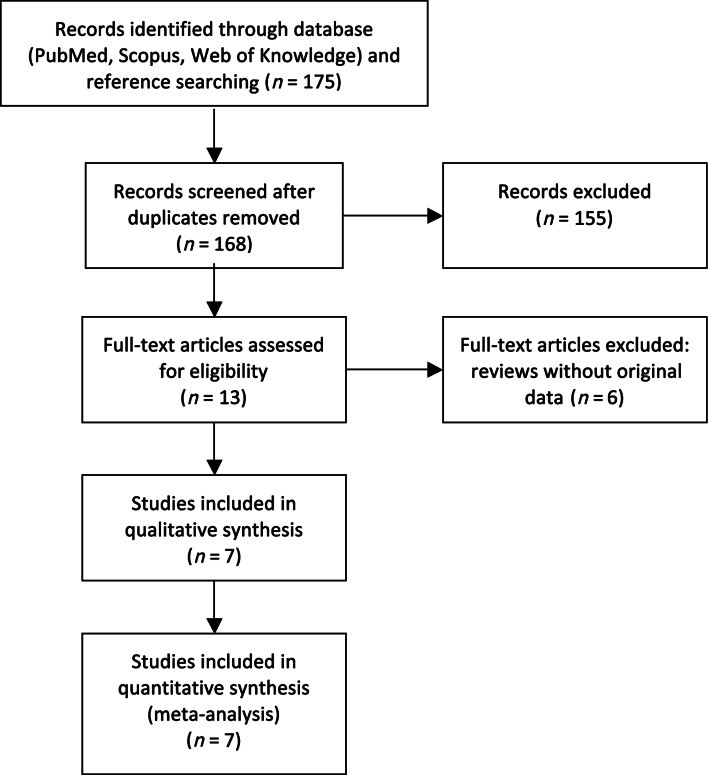
The search strategy identified 168 potential articles, of which 13 were read in full text (Figure 1). Among these, seven phase II and III studies with new oral anticoagulants after a recent ACS were identified and included in the analysis. Data from all seven studies (ESTEEM,11 REDEEM,14 RUBY-1,15 APPRAISE-112 and APPRAISE-2,16 ATLAS ACS-TIMI 46,13 and ATLAS ACS 2–TIMI 5117) rendered a study base of 30 866 patients with a recent ACSs, 4135 (13.4%) in the single antiplatelet therapy group and 26 731 (86.6%) in the dual antiplatelet therapy group. Details of these studies are outlined in Table 1 and Supplementary material online, Table S1.
Figure 1.
Flow chart of literature review.
All of the studies were double blind, placebo-controlled, with outcome assessment by independent adjudication committees unaware of treatment assignment, and had descriptions of number and reasons for losses to follow-up; therefore, risk of bias within studies was considered low. Because all studies had very similar inclusion and exclusion criteria and length of follow-up, risk of bias across studies was also low. No evidence of publication bias was observed (Egger's bias coefficient 0.04, P = 0.86; Supplementary material online, Figure S1). All trials were sponsored by the pharmaceutical industry. Statistical analyses in the apixaban and rivaroxaban studies were performed by research organizations independent of the sponsors, i.e. Duke Clinical Research Institute,12,16 Durham, NC, USA and the TIMI study group,13,17 Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA, respectively. Statistical analyses in the ximelagatran,11 dabigatran,14 and darexaban15 studies were performed by the sponsors but the principal investigators of the studies had access to all data.
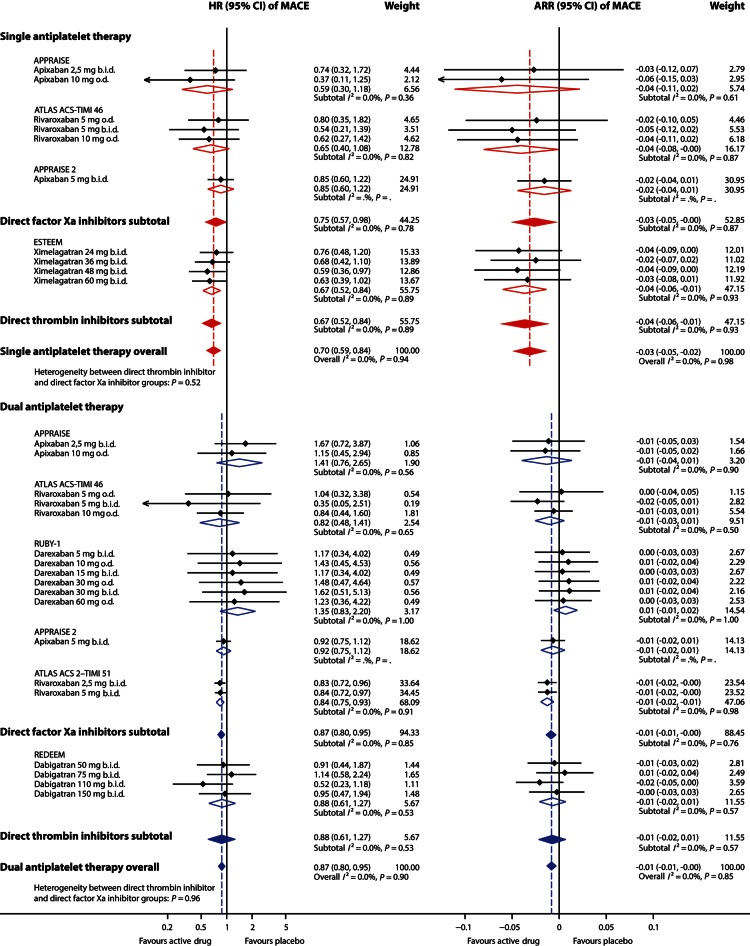
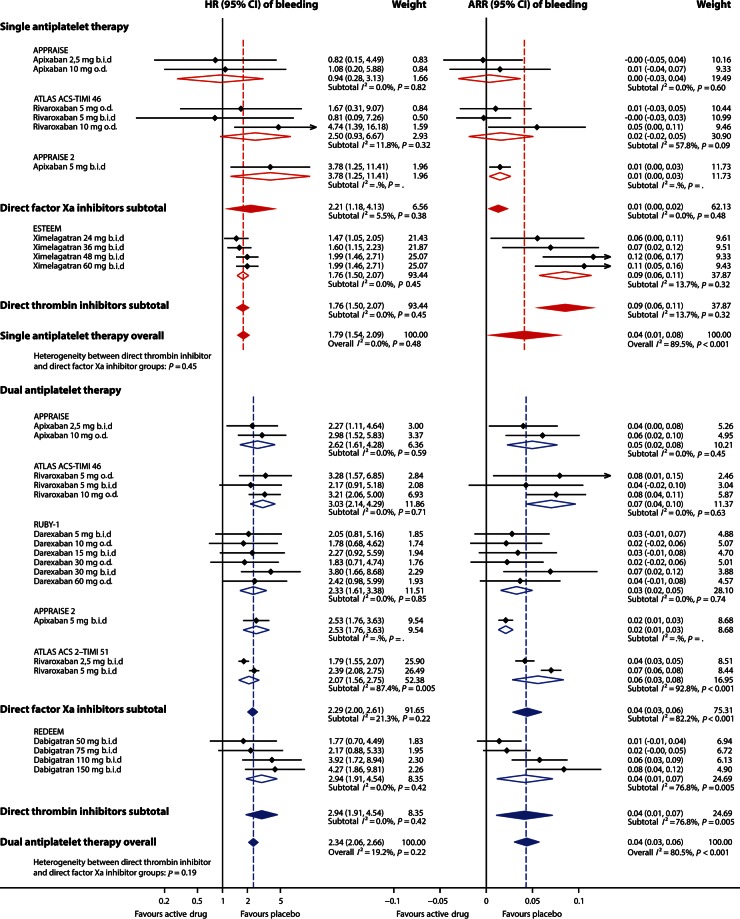
In patients receiving single antiplatelet therapy (aspirin), additional treatment with an oral anticoagulant on average decreased the rate of MACEs by 30% (Figure 2), with similar effects for ximelagatran, apixaban, and rivaroxaban (I2= 0%). In those studies, the rate of clinically significant bleeding events increased with 79% (Figure 3). No heterogeneity of effects on bleeding could be discerned (I2 = 0%). In patients on single antiplatelet therapy, 6-month rates of MACEs were ∼15% (149/1000) and 6-month rates of clinically significant bleeding were ∼9% (89/1000). Additional treatment with an oral anticoagulant for 6 months prevented ∼44 MACEs (NNTB 22) and caused ∼70 additional clinically significant bleeding events (NNTH 14) per 1000 patients.
Figure 2.
Effect of adding an oral anticoagulant to single (aspirin) or dual (aspirin and clopidogrel) antiplatelet therapy on rate of major adverse cardiovascular events after an acute coronary syndrome. Effect estimates of individual study arms with horizontal lines representing 95% CI; coloured diamonds, subtotal effects and 95% CI from random effects models for direct thrombin inhibitors and factor Xa inhibitors, respectively, and overall effects in addition to single and dual antiplatelet therapy; Open diamonds, subtotal summary effects and 95% CI from random effects models per trial; HR, hazard ratio; CI, confidence interval; ARR, absolute risk reduction (risk difference); b.i.d., twice daily; o.d., once daily; MACE, major adverse cardiovascular events, i.e. a composite of all-cause mortality, myocardial infarction and stroke.
Figure 3.
Effect of adding an oral anticoagulant to single (aspirin) or dual (aspirin and clopidogrel) antiplatelet therapy on rate of clinically significant bleeding events after an acute coronary syndrome. Effect estimates of individual study arms with horizontal lines representing 95% CI; coloured diamonds, subtotal effects, and 95% CI from random effects models for direct thrombin inhibitors and factor Xa inhibitors, respectively, and overall effects in addition to single and dual antiplatelet therapy; open diamonds, subtotal summary effects, and 95% CI from random effects models per trial; HR, hazard ratio; CI, confidence interval; ARR, absolute risk reduction (risk difference); b.i.d., twice daily; o.d., once daily.
In patients treated with dual antiplatelet therapy (aspirin and clopidogrel), the addition of an oral anticoagulant decreased the rate of MACEs by 13% (Figure 2), with negligible heterogeneity between the drugs apixaban, rivaroxaban, dabigatran, and darexaban (I2 = 0%). In contrast, additional treatment with an anticoagulant led to an increase in clinically significant bleeding rate by 134% (Figure 4), with low heterogeneity between treatment arms (I2 = 19%, mainly explained by heterogeneity between the two rivaroxaban arms in the ATLAS ACS 2–TIMI 51 study). In patients on dual antiplatelet therapy, 6-month rates of MACEs were ∼4% (43/1000) and 6-month rates of clinically significant bleeding were ∼3% (31/1000). Additional treatment with an oral anticoagulant for 6 months prevented ∼5 MACEs (NNTB 187) and caused ∼42 additional clinically significant bleeding events (NNTH 24) per 1000 patients.
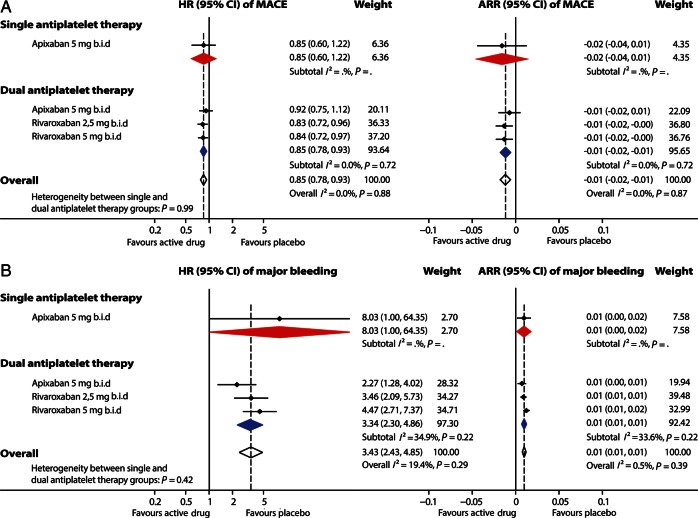
Figure 4.
Effect of adding an oral anticoagulant to single (aspirin) or dual (aspirin and clopidogrel) antiplatelet therapy on rates of major adverse cardiovascular events (A) and TIMI major bleeding events (B) after an acute coronary syndrome in a subgroup of phase III studies. Effect estimates of individual study arms with horizontal lines representing 95% CI; coloured diamonds, subtotal effects, and 95% CI from random effects models for direct thrombin inhibitors and factor Xa inhibitors, respectively, and overall effects in addition to single and dual antiplatelet therapy; open diamonds, subtotal summary effects, and 95% CI from random effects models per trial; HR, hazard ratio; CI, confidence interval; ARR, absolute risk reduction (risk difference); b.i.d., twice daily; o.d., once daily; MACE, major adverse cardiovascular events, i.e. a composite of all-cause mortality, myocardial infarction and stroke.
In the subgroup of phase III studies, the addition of apixaban 5 mg b.i.d. to single or dual antiplatelet therapy or the addition of rivaroxaban 2.5 or 5 mg b.i.d. to dual antiplatelet therapy on average decreased the rate of MACEs by 15% and increased the rate of TIMI major bleeding more than three-fold (Figure 4), with low heterogeneity between treatment arms (I2 = 0 and 19%, respectively) although with wide confidence intervals for the smaller group of patients receiving apixaban and only one antiplatelet drug.
The gain in protection against MACEs was larger when adding an anticoagulant to single than to dual antiplatelet therapy (Figure 2), P = 0.03 for heterogeneity between effects. Effects on bleeding were smaller when adding an anticoagulant to single than to dual antiplatelet therapy (Figure 3), P = 0.02. No consistent differences were observed between subgroups of direct thrombin inhibitors and direct factor Xa inhibitors regarding effects on MACEs or bleeding, either as addition to single or dual antiplatelet therapy, all P ≥ 0.19.
There was a non-significant tendency to an inverse association between the effect on MACEs and the effect on clinically significant bleeding when adding an anticoagulant to single antiplatelet therapy, but no association when adding an anticoagulant to dual antiplatelet therapy (Figure 5).
Figure 5.
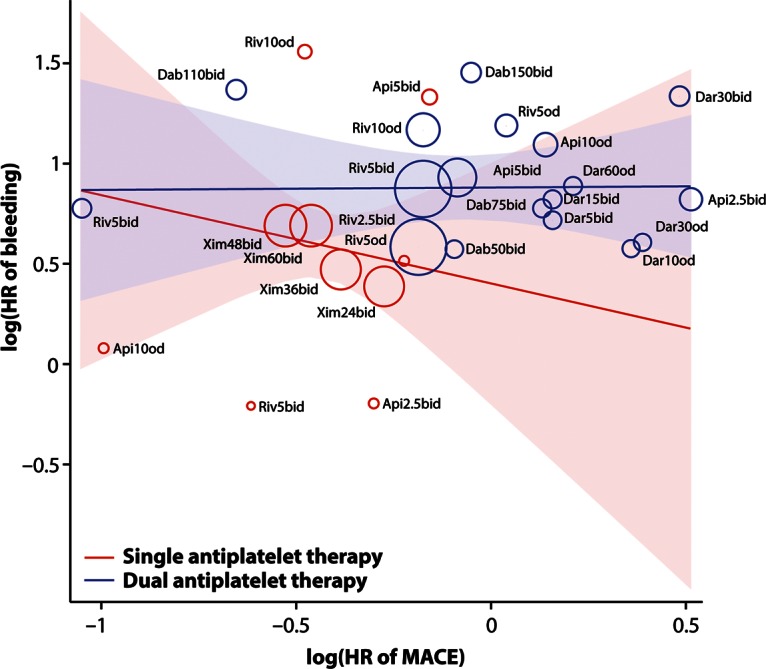
Association of effects of adding an oral anticoagulant to single (aspirin) or dual (aspirin and clopidogrel) antiplatelet therapy on rate of MACE with effects on rate of clinically significant bleeding events after an acute coronary syndrome. Random-effects meta-regression, stratified on number of antiplatelet drugs. The area of a circle representing a study arm is proportional to its weight in the random-effects model. Shaded areas are 95% confidence intervals. HR, hazard ratio; MACE, major adverse cardiovascular events; Riv, rivaroxaban; Dab, dabigatran, Api, apixaban; Dar, darexaban; Xim, ximelagatran; b.i.d., twice daily; o.d., once daily; number denotes strength in milligram.
Discussion
In this meta-analysis of patients with a recent ACS, the addition of a new oral anticoagulant to antiplatelet therapy led to a modest reduction in cardiovascular events but a substantial increase in bleeding, most pronounced in patients receiving dual antiplatelet therapy. The strength of the evidence was high for both outcomes and in patients using one as well as two antiplatelet drugs.
There was little heterogeneity between the different anticoagulant treatments and the different trials. The results were similar in the phase II trials and in the phase III trials that provided the majority of events because of higher patient numbers and longer treatment exposure and follow-up. No differences were observed between direct thrombin inhibitors and direct factor Xa inhibitors. There was no significant association between the oral anticoagulant treatments' effects on MACEs and effects on clinically significant bleeding, implying that there is no simple trade-off between protective and adverse effects of these drugs after ACSs in patients receiving single or dual antiplatelet therapy. Under the questionable assumption of equal clinical importance of bleedings and the components of MACEs, the net clinical benefit (NNTB minus NNTH) was unfavourable overall. It tended to be better when adding an anticoagulant to single than to dual antiplatelet therapy, but some overlap between those two groups of studies was seen. The results support the concept that treatment with new oral anticoagulants may reduce ischaemic events after an ACS. However, in combination with dual anti-platelet therapy, no specific treatment or dosing regimen avoided a substantial increase in major bleeding.
The reduction of ischaemic events by the new oral anticoagulants was most promising when added to single antiplatelet therapy with aspirin. However, single antiplatelet treatment is rarely used because many ACS patients are treated with percutaneous coronary interventions and stents, and current guidelines1–4 recommend that all post-ACS patients should receive up to 12 months of dual antiplatelet treatment.22 Thus, the most striking results of this meta-analysis concern the high bleeding rates in combination with only modest reductions in MACEs in the large groups of patients receiving combinations of new oral anticoagulants with dual antiplatelet therapy.
The reviewed trials were all designed and performed at a time when clopidogrel was the only available P2Y12 inhibitor. Recently, acute and long-term treatment combining aspirin and new P2Y12 inhibitors, either prasugrel23 or ticagrelor,24 have been shown more effective than clopidogrel for the prevention of MACEs during and after ACS, and prasugrel or ticagrelor is now considered the preferred P2Y12 inhibitors in patients with ACS.3,25 Long-term treatment with any of these new antiplatelet agents was, compared with clopidogrel, associated with a modest increase, up to 35%, in major non-CABG-related bleedings, in contrast to the findings of the present study of more than three-fold increase in major bleeding events when adding the new oral anticoagulants to aspirin and clopidogrel. The addition of a new oral anticoagulant to aspirin and any of ticagrelor or prasugrel has not been thoroughly evaluated and might expose the patients to even larger risks of major bleeding with even more limited expectations for further reductions in MACEs. Therefore, in patients receiving oral anticoagulant therapy the combination of aspirin and clopidogrel will probably still be the most widely used dual antiplatelet strategy after an ACS. It will be challenging to identify settings where the incremental benefits of an oral anticoagulant in addition to aspirin in combination with prasugrel or ticagrelor can be utilized without exposing the patient to unacceptable risks of major bleeding, unless doses are drastically reduced. Further studies evaluating this concept are clearly warranted.
The majority of the patients and events in this meta-analysis were accrued from the two phase III trials. The bleeding results in these two large trials were consistent with the phase II experiences. The bleeding outcomes were also very similar between the two phase III trials, albeit with numerically larger relative increase in bleeding in the concluded ATLAS ACS 2–TIMI 51 trial17 than the prematurely terminated APPRAISE-2 trial.16 A modest reduction in MACEs was also seen in both these phase III trials, significant only in the ATLAS ACS 2–TIMI 51 trial17 but not in the APPRAISE-2 trial,16 although the premature termination and shorter duration of treatment in the latter trial may have limited the statistical power to detect associations with both bleeding and MACE outcomes. As both rivaroxaban and apixaban are factor Xa inhibitors, it is likely that the meta-analysis result is the most plausible outcome when taking these treatments into real-life health care. This information might be useful when considering patients with a combination of atrial fibrillation and ACS, where a combination of anticoagulation and dual antiplatelet treatment is currently recommended for at least 1–6 months.26,27 Notably, the rivaroxaban doses, 2.5 or 5 and 5 mg twice daily, used in the ATLAS ACS 2–TIMI 51 trial17 in combination with ASA and clopidogrel were lower than the 20 mg once daily dose shown to be efficacious for stroke prevention in atrial fibrillation,9 a setting where most patients are not receiving antiplatelet therapy.
Strengths and limitations
The strengths of this study are the inclusion of all published randomized, placebo-controlled phase II and III studies of novel oral anticoagulants; that risk of publication bias was very low as assessed by two methods; that the very few data points that were unavailable in the publications were successfully retrieved from the authors of the studies. Studies were all of high quality with low risk of within-study bias; and a pre-specified subgroup analysis of the phase III studies with longer follow-up gave similar results as the main analyses. The latter is particularly important since phase II dose ranging trials purposely explore the bleeding risk in relation to dose range and, therefore, may somewhat overestimate bleeding risk compared with the doses selected for testing in phase III studies. Supportive of the combination of these studies in a meta-analysis is the fact that all studies had nearly identical inclusion and exclusion criteria, definitions of MACEs, and length of follow-up in the phase II studies; and that heterogeneity of effects, estimated using the I2-statistic, was negligible in all comparisons. In a pooled analysis of several agents, it is nevertheless difficult to rule out an impact of the dose of these anticoagulants on clinical outcomes. Moreover, cross-trial comparisons should despite the similarities in inclusion and exclusion criteria and outcome definitions always be done with caution, e.g. regarding absolute risk reductions, and NNTB or NNTH.
A potential limitation is the variation between studies in the definitions of bleeding outcomes. The definition of clinically significant bleeds as the composite of major (ISTH) and clinically relevant minor bleeding was identical in the studies with apixaban, darexaban, dabigatran, and ximelagatran and this composite was judged to be sufficiently comparable with the composite of TIMI major, TIMI minor, and bleeding requiring medical attention in the phase II study with rivaroxaban. Since the latter composite of bleedings based on the TIMI scale was not available from the phase III study with rivaroxaban, only TIMI bleedings requiring medical attention in this study were used in the present meta-analysis. This limitation is in part overcome by the use of hazard ratios instead of absolute event rates, and the results are further strengthened by the similar results in the subgroup analysis of TIMI major bleeding outcomes in the phase III studies. We did not separately analyse the MACE components, i.e. all-cause mortality, myocardial infarction or stroke in this meta-analysis since the event rates were deemed too low, mainly in the phase II studies.
Single or dual antiplatelet therapy was in this analysis based on treatment at randomization, but antiplatelet therapy was persistent throughout most of the observation periods for these studies including the phase III studies.16,17 Another limitation is the lack of separately evaluable results for the small proportions of patients on single antiplatelet treatment, i.e. 2, 5, and 7%, in REDEEM,14 RUBY-1,15 and ATLAS ACS 2–TIMI 5117 studies, respectively.
Conclusions
In patients with a recent ACS, the addition of a new oral anticoagulant to antiplatelet therapy leads to a modest reduction in cardiovascular events but a substantial increase in bleeding. These results are most pronounced when oral anticoagulants are combined with dual anti-platelet therapy with aspirin and clopidogrel. No new oral anticoagulant has been evaluated in addition to dual antiplatelet therapy with aspirin and any of the more efficacious novel P2Y12 inhibitors ticagrelor or prasugrel, a combination which might expose the patients to even larger risks of major bleeding with even lower expectations for further reductions in ischaemic events. Further studies evaluating new oral anticoagulants in combination with effective single antiplatelet therapy or shorter duration of triple antithrombotic therapy are warranted.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
L.W. has received funds from the Swedish Heart-Lung Foundation. J.S. was funded by the Swedish Heart-Lung Foundation (grant 20041151) and the Swedish Research Council (grants 2007–5942 and 2010–1078).
Conflict of interest: J.O. reports institutional research grant from Boehringer-Ingelheim; and consulting and lecture fees from Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, and Pfizer. L.W. has received consultant fees from AstraZeneca, Athera Biotechnologies, Boehringer-Ingelheim, Bristol-Myers Squibb, CSL Behring, Evolva, GlaxoSmithKline, Portola, Regado Bitoechnologies, and Schering-Plough/Merck; lecture fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, and Schering-Plough/Merck; and institutional research grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Pfizer, and Schering-Plough. J.H.A. has received consultant fees and honoraria from Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Janssen Pharmaceuticals, Orexigen, Pfizer and Xoma, and institutional research grants from Bristol-Myers Squibb, CSL, the U.S. National Institutes of Health, Phyxius Pharmaceuticals, and Regado Biosciences. S.J. has received institutional research grants from AstraZeneca, Bristol-Myers Squibb, and Eli Lilly; and consultant/speaking fees from AstraZeneca, Eli Lilly, and Merck. B.J. reports no conflicts of interest. Dr Steg has received research grant (to INSERM U-698) from NYU School of Medicine, Sanofi, and Servier; and consultant/speaking fees from Ablynx, Amarin, Amgen, Astellas, AstraZeneca, Bayer, Boehringer-Ingelheim, BMS, Daiichi/Sankyo, Eisai, GlaxoSmithKline, Eli Lilly, Medtronic, Merck Sharpe & Dohme, Novartis, Otsuka, Pfizer, Roche, Sanofi, Servier, The Medicines Company, and Vivus; and reports stockholding in Aterovax.
Supplementary Material
References
- 1.Steg PG, James SK, Atar D, Badano LP, Blomström-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van't Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 2.Kushner FG, Hand M, Smith SC, Jr, King SB, III, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE, Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. 2009 Focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction. Circulation. 2009;120:2271–2306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 3.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 4.Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE, Jr, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Jacobs AK. 2011 ACCF/AHA focused update of the guidelines for the management of patients with unstable angina/ non-ST-elevation myocardial infarction. Circulation. 2011;123:2022–2060. doi: 10.1161/CIR.0b013e31820f2f3e. [DOI] [PubMed] [Google Scholar]
- 5.Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347:969–974. doi: 10.1056/NEJMoa020496. [DOI] [PubMed] [Google Scholar]
- 6.van Es RF, Jonker JJ, Verheugt FW, Deckers JW, Grobbee DE. Aspirin and coumadin after acute coronary syndromes (the ASPECT-2 study): a randomised controlled trial. Lancet. 2002;360:109–113. doi: 10.1016/S0140-6736(02)09409-6. [DOI] [PubMed] [Google Scholar]
- 7.Sorensen R, Hansen ML, Abildstrom SZ, Hvelplund A, Andersson C, Jorgensen C, Madsen JK, Hansen PR, Kober L, Torp-Pedersen C, Gislason GH. Risk of bleeding in patients with acute myocardial infarction treated with different combinations of aspirin, clopidogrel, and vitamin K antagonists in Denmark: a retrospective analysis of nationwide registry data. Lancet. 2009;374:1967–1974. doi: 10.1016/S0140-6736(09)61751-7. [DOI] [PubMed] [Google Scholar]
- 8.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 9.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 10.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 11.Wallentin L, Wilcox RG, Weaver WD, Emanuelsson H, Goodvin A, Nystrom P, Bylock A. Oral ximelagatran for secondary prophylaxis after myocardial infarction: the ESTEEM randomised controlled trial. Lancet. 2003;362:789–797. doi: 10.1016/S0140-6736(03)14287-0. [DOI] [PubMed] [Google Scholar]
- 12.Alexander JH, Becker RC, Bhatt DL, Cools F, Crea F, Dellborg M, Fox KA, Goodman SG, Harrington RA, Huber K, Husted S, Lewis BS, Lopez-Sendon J, Mohan P, Montalescot G, Ruda M, Ruzyllo W, Verheugt F, Wallentin L. Apixaban, an oral, direct, selective factor Xa inhibitor, in combination with antiplatelet therapy after acute coronary syndrome: results of the Apixaban for Prevention of Acute Ischemic and Safety Events (APPRAISE) trial. Circulation. 2009;119:2877–2885. doi: 10.1161/CIRCULATIONAHA.108.832139. [DOI] [PubMed] [Google Scholar]
- 13.Mega JL, Braunwald E, Mohanavelu S, Burton P, Poulter R, Misselwitz F, Hricak V, Barnathan ES, Bordes P, Witkowski A, Markov V, Oppenheimer L, Gibson CM. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374:29–38. doi: 10.1016/S0140-6736(09)60738-8. [DOI] [PubMed] [Google Scholar]
- 14.Oldgren J, Budaj A, Granger CB, Khder Y, Roberts J, Siegbahn A, Tijssen JG, Van de Werf F, Wallentin L. Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double-blind, phase II trial. Eur Heart J. 2011;32:2781–2789. doi: 10.1093/eurheartj/ehr113. [DOI] [PubMed] [Google Scholar]
- 15.Steg PG, Mehta SR, Jukema JW, Lip GY, Gibson CM, Kovar F, Kala P, Garcia-Hernandez A, Renfurm RW, Granger CB. RUBY-1: a randomized, double-blind, placebo-controlled trial of the safety and tolerability of the novel oral factor Xa inhibitor darexaban (YM150) following acute coronary syndrome. Eur Heart J. 2011;32:2541–2554. doi: 10.1093/eurheartj/ehr334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander JH, Lopes RD, James S, Kilaru R, He Y, Mohan P, Bhatt DL, Goodman S, Verheugt FW, Flather M, Huber K, Liaw D, Husted SE, Lopez-Sendon J, De Caterina R, Jansky P, Darius H, Vinereanu D, Cornel JH, Cools F, Atar D, Leiva-Pons JL, Keltai M, Ogawa H, Pais P, Parkhomenko A, Ruzyllo W, Diaz R, White H, Ruda M, Geraldes M, Lawrence J, Harrington RA, Wallentin L. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699–708. doi: 10.1056/NEJMoa1105819. [DOI] [PubMed] [Google Scholar]
- 17.Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FW, Gibson CM. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19. doi: 10.1056/NEJMoa1112277. [DOI] [PubMed] [Google Scholar]
- 18.Schulman S, Kearon C Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 19.Gibson CM, Mega JL, Burton P, Goto S, Verheugt F, Bode C, Plotnikov A, Sun X, Cook-Bruns N, Braunwald E. Rationale and design of the Anti-Xa therapy to lower cardiovascular events in addition to standard therapy in subjects with acute coronary syndrome-thrombolysis in myocardial infarction 51 (ATLAS-ACS 2 TIMI 51) trial: a randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of rivaroxaban in subjects with acute coronary syndrome. Am Heart J. 2011;161:815–821. doi: 10.1016/j.ahj.2011.01.026. e6. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 23.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 24.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Investigators P, Freij A, Thorsen M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 25.Vandvik PO, Lincoff AM, Gore JM, Gutterman DD, Sonnenberg FA, Alonso-Coello P, Akl EA, Lansberg MG, Guyatt GH, Spencer FA. ACCP Guidelines for primary and secondary prevention of cardiovascular disease. Chest. 2012;141(2 Suppl):e637S–e668S. doi: 10.1378/chest.11-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohnloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. ESC Guidelines for the management of atrial fibrillation. Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 27.You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, Fang MC, Hylek EM, Schulman S, Go AS, Hughes M, Spencer FA, Manning WJ, Halperin JL, Lip GY. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e531S–e575S. doi: 10.1378/chest.11-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.