Abstract
Recently, a novel H7N9 avian influenza A virus has led to a human influenza outbreak in China. Here we report a 64-year old man with possible history of chronic bronchitis died from the H7N9 infection in Huzhou City, Zhejiang Province in Eastern China. The patient had been exposed to poultry before disease onset. Phylogenetic analyses of hemagglutinin and neuraminidase genes showed a close genetic relationship between viruses from the patient and from poultry booths where he had visited, indicating that the patient may have been exposed from the infected poultry. Two poultry venders and close contacts of the patient were negative for H7N9, suggesting that there are some unknown mechanisms to prevent them from being infected by the novel H7N9 virus. Furthermore, we found five novel H7N9 virus-specific sequence variations in receptor-binding site of hemagglutinin, which may be associated with the acquisition of the ability to infect humans.
Keywords: A/H7N9 influenza virus, susceptibility, chronic bronchitis, poultry, hemagglutinin, neuraminidase
INTRODUCTION
Since February 2013, a novel avian-origin influenza A (H7N9) virus has emerged in clinical patients in Yangtze River Delta of China (including Shanghai, Jiangsu, Zhejiang and Anhui Provinces).1,2 It represents the first time of human infection with the H7N9 avian influenza virus. Up to April 22nd, it has caused 104 infected cases including 21 deaths. Soon after the identification of the H7N9 virus in humans,3 it was further detected in some poultry samples (including chicken and pigeon), but not from other animal sources such as pigs, indicating that the poultry may be the reservoir of this new reassortant influenza virus. However, epidemiological surveillance data showed that not all the patients had a history of close contact with poultry.1,2,4 Therefore, whether the H7N9 virus was transmitted directly from poultry to human remains to be determined.
Here we report a 64-year old man died from the H7N9 infection in Huzhou City, Zhejiang Province. The patient had been exposed to H7N9 infected poultry before disease onset. Phylogenetic analyses of hemagglutinin (HA) and neuraminidase (NA) genes showed a close genetic relationship between viruses from the patient and from the poultry booth where he had visited, supporting the possible acquisition of the infection from the infected poultry. Furthermore, two poultry venders and close contacts of this patient were negative for H7N9, suggesting that there are some unknown mechanisms in selecting who may be susceptible to H7N9 virus infection.
MATERIALS AND METHODS
The patient
A 64-year old man in Huzhou City, Zhejiang Province, felt sick with cough, occasionally with low grade fever from March 29th to 30th, 2013. He had been engaged in mining work and had a history of smoking more than 20 cigarettes per day for over 20 years. Initially, it was not unusual for him to have the respiratory symptom, especially in spring and autumn, and he believed that the symptom was caused by the smoking-related chronic respiratory illness. On March 31st, he was admitted to hospital due to dyspnea. On the same day, the patient was intubated in Intensive Care Unit due to severe pneumonia and respiratory failure, and treated with antibiotics and steroids. On April 4th, he died from acute respiratory distress syndrome and multi-organ failure.
RNA extraction and real-time RT-PCR
On April 3rd, swab of respiratory tract from this patient was collected and sent to the Huzhou Centers for Disease Control and Prevention for the detection of avian influenza A/H7N9 virus. A simple questionnaire on demographic characteristics, recent exposures to poultry and/or other animals, recent visits to a live animal market, and clinical signs and symptoms was conducted. Since the patient had visited two poultry booths nearby before his hospitalization, 39 samples including avian feces, waste, and sewage from two poultry booths near the patient's home and the upstream wholesale market that directly supplies poultry for the booths were also collected.
RNA was extracted from the swab from the patient and the samples from the poultry booths and wholesale market using QIAamp Viral RNA Mini Kit. A real-time reverse transcription-polymerase chain reaction (RT-PCR) for the detection of avian influenza A (H7N9) virus was performed according to the protocol recommended by the World Health Organization (available at: http://www.who.int/influenza/gisrs_laboratory/a_h7n9/en/). In addition, HA and NA genomic fragments were amplified from the patient's respiratory tract swab and the samples from the poultry booths and wholesale market using universal primer sets as described previously,5 and subjected to sequencing.
Phylogenetic analysis
Nucleotide sequences of HA and NA were aligned with available sequences from avian influenza HA subtype 7 and NA subtype 9 using Clustal W program implemented in MEGA 5.05. The H7N9 sequences reported recently were included in the analyses. Phylogenetic trees of HA and NA sequences were constructed with MEGA 5.05 by using the neighbor-joining method. The stability of the nodes was assessed by using maximum likelihood with a bootstrap value of 1000 replications.
RESULTS
Clinical presentation
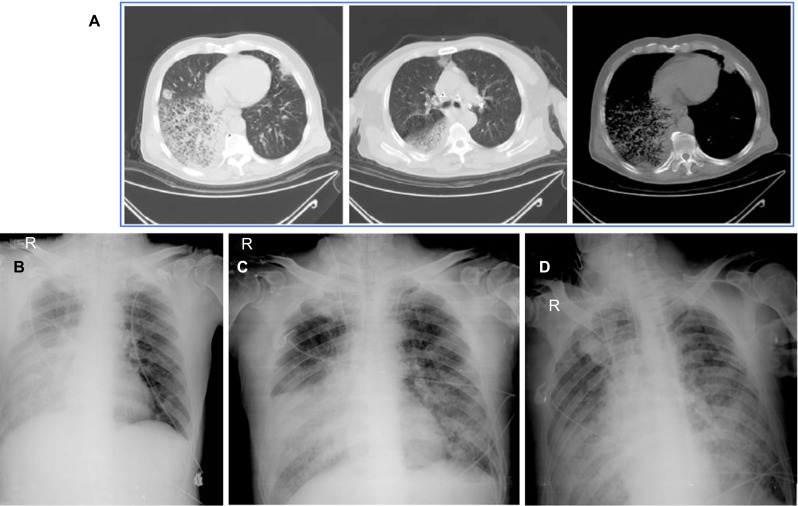
At admission (March 31st), the patient was conscious with a heart rate of 138/min, body temperature 38.8 °C, breath rate 33/min, blood pressure 218/103 mmHg, SaO2 88%, PaO2 56 mmHg and PaCO2 58.3 mmHg. White blood cell count was 7.0×109/L, lymphocyte count was 0.1×109/L, and C-reactive protein was 177.6 mg/L. Prothrombin time was 15.1 seconds. Prothrombin ratio was 1.26 and D-dipolymer was 1235.0 ng/L. Occult blood was positive in stool and urine, and urine protein was also positive. Moist rales from both lungs were noted. The chest radiograph revealed severe infiltration, especially in the right lower lobe (Figure 1), and hydrothorax in right lung.
Figure 1.
Chest radiographs. Computed tomographic scans of the chest of the patient on March 31st (panel A); Chest X-rays on April 1st–3rd (panels B–D, respectively).
During the five days of hospitalization before the patient died on April 4th, the patient was closely monitored for clinical symptoms and with a wide range of lab studies, including biochemical, immunological and cytological parameters. Fifty-nine parameters were measured daily. Of them, 13 appeared unusual compared to the normal reference ranges (Table 1). The levels of serum glucose, glutamic oxalacetic transaminase, glutamic-pyruvic transaminase, adenosine deaminase, neutrophil percentage, mean corpuscular hemoglobin, and high-sensitivity C-reactive protein continued to be above the higher limits of the reference ranges, while the other six indexes including calcium concentration, retinol conjugated protein, total protein, albumin, albumin/globulin ratio, and lymphocyte percentage were substantially below the lower limit of the reference ranges (Table 1).
Table 1. Dynamic changes of 13 clinical parameters possibly associated with the death.
| Variable | Mar. 31 | Apr. 1 | Apr. 2 | Apr.3 | Apr. 4 (death) | Reference range | Unit |
|---|---|---|---|---|---|---|---|
| Calcium | 1.88 | 1.89 | 1.92 | 1.97 | 2.54 | 2.17–2.75 | mmol/L |
| Serum glucose | NA | 14.71 | 7.69 | 8.77 | 8.34 | 3.90–6.10 | mmol/L |
| Retinol conjugated protein | NA | 2.8 | 7.7 | 7.4 | NA | 18.0–70.0 | mg/L |
| Glutamic oxalacetic transaminase | NA | 41.7 | 56.8 | 96.1 | NA | 8.0–10.0 | U/L |
| Glutamic-pyruvic transaminase | NA | 44.9 | 59.7 | 87.2 | 57 | 5.0–10.0 | U/L |
| Total protein | NA | 48.4 | 48.3 | 59.9 | NA | 60.0–85.0 | g/L |
| Albumin | NA | 25.6 | 26 | 28.1 | NA | 35.0–50.0 | g/L |
| Albumin/globulin ratio | NA | 1.12 | 1.17 | 0.88 | NA | 1.20–2.50 | — |
| Adenosine deaminase | NA | 22.6 | 24.4 | 27.9 | NA | 0.0–21.0 | U/L |
| Neutrophil percentage | NA | 93.8 | 91.8 | 96.6 | NA | 51.0–75.0 | % |
| Lymphocyte percentage | NA | 3.9 | 3.6 | 3 | NA | 20.0–40.0 | % |
| Mean Corpuscular Hemoglobin | NA | 32.6 | 32.4 | 33.1 | NA | 27.0–31.0 | pg |
| High-sensitivity C-reactive protein | NA | 175.3 | 151.9 | 159 | NA | 0.0–10.0 | mg/L |
NA: not available.
Detection of avian influenza A/H7N9 virus
On April 1st, the patient's sputum smear was tested negative for acid-fast bacilli, fungus and haemophilus. It was suspected that the patient might be infected with the novel H7N9 influenza virus based on reports from other similar cases.1,2,3 On April 3rd, we performed specific real-time RT-PCR to determine the presence of avian influenza A/H7N9 virus in patient's swab of respiratory tract. The result showed that the patient was positive for the novel H7N9 virus. At the same time, 11 samples, including five from one poultry booth, and six from the upstream wholesale market, were also found to contain H7N9 virus using the real time RT-PCR.
Phylogenetic analysis and comparison of HA amino acid sequences
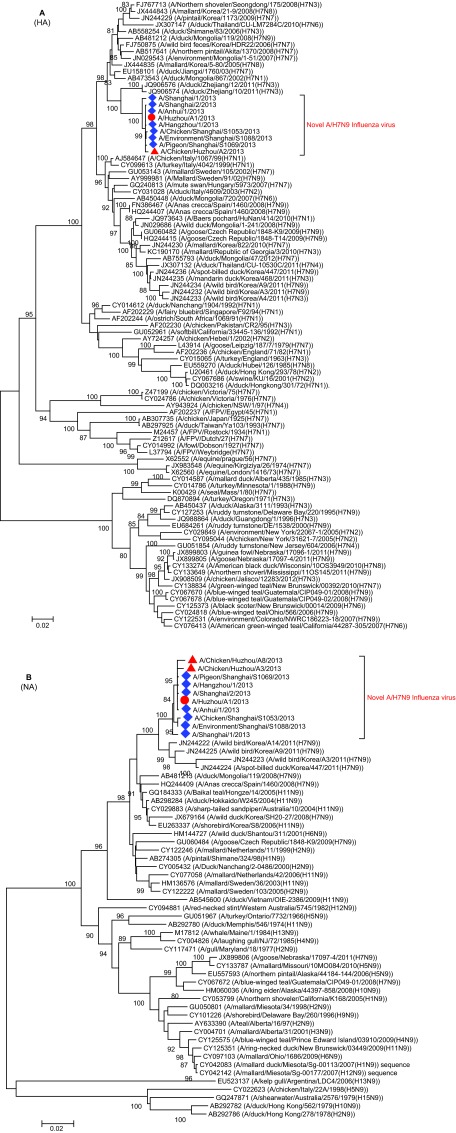
Phylogenetic trees of HA and NA fragments showed that the novel H7N9 strains form a well-supported clade (with a bootstrap value of 100%) and the sequence from this patient clusters closely with those from the patients in Shanghai, Auhui and Zhejiang, as well as those from the poultry (including chickens and pigeon) from the local area and Shanghai (Figure 2). These results not only support that this patient was infected by the novel H7N9 virus, but also suggest that the novel H7N9 virus was present in the environment where the patient was exposed.
Figure 2.
Phylogenetic tree of influenza HA (A) and NA (B) sequences. The phylogenetic trees were constructed with MEGA 5.0 using the neighbor-joining method. The stability of the nodes was assessed by bootstrap analysis with 1000 replications, and only bootstrap values of ≥80 were shown at the corresponding nodes. The sequences from this patient and the poultry in Huzhou are highlighted by red circle and triangles, respectively. The blue diamonds indicate the sequences of A/H7N9 viruses from other regions of China.
Comparison of amino acid sequences of the receptor-binding site (RBS) (amino acids 105–319) in HA showed that there are five variants K181R, D183S/N, I188V, I211V and V298I occurring between H7N3 and H7N9 (Figure 3). In addition, three additional variants G195V, Q235L/I and D285N were found in all the novel H7N9 strains except A/Shanghai/1/2013, which has identical amino acids to H7N3 virus in the three sites.
Figure 3.
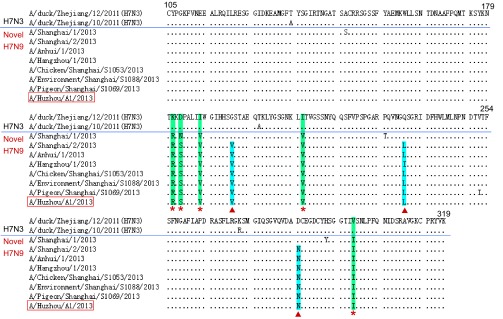
Sequence comparison of RBS in HA between the novel H7N9 and avian H7N3. The red stars indicate the H7N9 virus-specific variants. The red triangles indicate variants occurring in all H7N9 viruses except A/Shanghai/1/2013.
DISCUSSION
HA of influenza virus is the receptor-binding and membrane fusion glycoprotein that is responsible for viral attachment and entry into target cells by binding to sialic acids, the functional receptor of influenza virus.6,7 The RBS in HA plays a dominant role in determining the forms of receptors and thus the host tropism. Most avian influenza viruses preferentially bind to sialic acids in the α-2,3 linkage, whereas human viruses preferentially bind to sialic acids in the α-2,6 linkage.8 Sporadic human infection with avian influenza virus (e.g. highly pathogenic H5N1, H7N2, H7N3, H7N7 and H9N2) had been observed since 1997.9 To acquire the potential to infect humans, these avian viruses evolve a binding affinity of HA for the α-2,6 linkage, which is preferentially involved in the mutations in the RBS regions of HA.8
Since February 2013, the infection with the novel H7N9 virus has led to at least 21 deaths. The patient reported in this study was confirmed to be infected with the novel H7N9 virus by possible exposure to H7N9 infected poultry before disease onset. Recently, the novel H7N9 virus was demonstrated to originate from the assortment between avian H7N3, H7N9 and H9N2 viruses, which were circulating in Korea and East China including Zhejiang, respectively.3 The HA genomic fragment of the novel H7N9 was directly derived from H7N3. Comparison of amino acid sequences showed that there was no difference in the RBS sequence of HA between the H7N9 virus from this patient and those from other patients. However, the comparison between H7N3 and the novel H7N9 suggested that variants K181R, D183S/N, I188V, I211V and V298I in RBS of HA may be crucial for the novel H7N9 virus to evolve the ability to infect humans (Figure 3). Furthermore, we observed three additional variants G195V, Q235L/I and D285N occurring in all the novel H7N9 viruses except A/Shanghai/1/2013, which has identical amino acids to H7N3 virus in the three sites. Among them, at least Q235L had been demonstrated to be involved in a change of HA receptor specificity from the α-2,3 linkage to the α-2,6 linkage.7,10 Whether the variants mentioned above contribute to the acquisition of HA binding to α-2,6 linkage needs to be determined by further experiments.
A/Shanghai/1/2013 was isolated from the first H7N9 infected patient3 and located in the basal position of the clade of the novel H7N9 virus in the phylogenetic trees (Figure 2). These findings not only indicate that A/Shanghai/1/2013 represents a relative early form of the novel H7N9 virus after accomplishing poultry-to-human transmission, but also imply that the novel H7N9 has a too short history to adapt human host and to evolve the ability of human-to-human transmission, which provides a possible explanation for the reason why no human-to-human transmission was observed so far.
We tested the samples from two poultry venders and 55 close contacts of the patient (including his family) using the real time RT-PCR. Interestingly, none was tested positive for the H7N9 virus. This result confirmed two observations. First, two venders were not infected by the virus in despite of their extremely frequent exposure to the infected poultry. Second, the patient did not spread the virus to his close contacts. These further imply that there are some unknown mechanisms which determine why only some people are more readily infected by the novel H7N9 virus but not others.4 The patient reported here was 64 years old, had a long history (over 20 years) of heavy smoking and a clinical history compatible with the chronic bronchitis. His relative poor health condition may have contributed to the infection or clinical outcome. However, it is yet to be proved whether the susceptibility to H7N9 infection is depended on a person's health condition.
Acknowledgments
This work was supported by grants from the China National Mega-projects for Infectious Diseases (2012ZX10004211-002 and 2013ZX10004101-005) to Ke Lan and the Li Ka-Shing Foundation to Qibin Leng.
References
- Parry J. H7N9 avian flu infects humans for the first time. BMJ. 2013;346:f2151. doi: 10.1136/bmj.f2151. [DOI] [PubMed] [Google Scholar]
- Wen YM, Klenk HD. H7N9 avian influenza virus - search and re-search. Emerg Microbes Infect. 2013;2:e18. doi: 10.1038/emi.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Cao B, Hu Y, et al. Human Infection with a Novel Avian-Origin Influenza A (H7N9) Virus N Engl J Med 2013. Apr 11; doi: 10.1056/NEJMoa1304459. [DOI] [PubMed]
- Yang F, Wang J, Jiang L, et al. A fatal case caused by novel H7N9 avian influenza A virus in China. Emerg Microbes Infect. 2013;2:e19. doi: 10.1038/emi.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. Universal primer set for the full-length amplification of all influenza A viruses. Arch Virol. 2001;146:2275–2289. doi: 10.1007/s007050170002. [DOI] [PubMed] [Google Scholar]
- Stevens J, Blixt O, Tumpey TM, et al. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science. 2006;312:404–410. doi: 10.1126/science.1124513. [DOI] [PubMed] [Google Scholar]
- Liu Q, Lu L, Sun Z, et al. Genomic signature and protein sequence analysis of a novel influenza A (H7N9) virus that causes an outbreak in humans in China Microbes Infect(In press). [DOI] [PubMed]
- Watanabe Y, Ibrahim MS, Suzuki Y, Ikuta K. The changing nature of avian influenza A virus (H5N1) Trends Microbiol. 2012;20:11–20. doi: 10.1016/j.tim.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Kalthoff D, Globig A, Beer M. (Highly pathogenic) avian influenza as a zoonotic agent. Vet Microbiol. 2010;140:237–245. doi: 10.1016/j.vetmic.2009.08.022. [DOI] [PubMed] [Google Scholar]
- Wan H, Perez DR. Amino acid 226 in the hemagglutinin of H9N2 influenza viruses determines cell tropism and replication in human airway epithelial cells. J Virol. 2007;81:5181–5191. doi: 10.1128/JVI.02827-06. [DOI] [PMC free article] [PubMed] [Google Scholar]