Chronic myelogenous leukemia (CML) is a hematological malignancy that expresses the BCR-ABL fusion oncogene. It has three distinct clinicopathological phases: chronic and accelerated phase and blast crisis. Tyrosine kinase inhibitors, which target the BCR-ABL fusion protein, can induce complete cytogenic remission (CCR) in more than 70% of newly diagnosed chronic CML patients. However, complete molecular response is a rare event in these CML patients with CCR, suggesting that they are not effective in eliminating CML leukemia initiating cells (LIC), although there are differences between the trosine kinase inhibitors. Once CML progresses to blast crisis, it becomes resistant to most treatment approaches, and survival rapidly declines. The exact underlying mechanism of transformation of chronic phase CML to blast crisis is still unclear; however, it is thought to be supported by self-renewing LIC. Therefore, identifying genes or signaling pathways involved in the self-renewal of LIC may promote more effective leukemia treatments. So far, two key regulators in self-renewal of LIC have been linked to CML progression, Wnt/β-catenin and Bmi-1(1–4).
Recently, several research groups, including ours, have demonstrated that SALL4 plays an essential role in the maintenance of pluripotent and self-renewal properties of embryonic stem cells (ESC) by interacting with Nanog and Oct4 (5). In addition, our group has shown that constitutive expression of SALL4 contributes to leukemogenesis in adult mice by interacting with two other key regulators in LIC, Wnt/β-catenin and Bmi-1 (6, 7). It appears that SALL4 is a unique gene involved in self-renewal in ESC and LIC. Therefore, we examined SALL4 expression in CML to determine whether it could be involved in the pathogenesis of this disease.
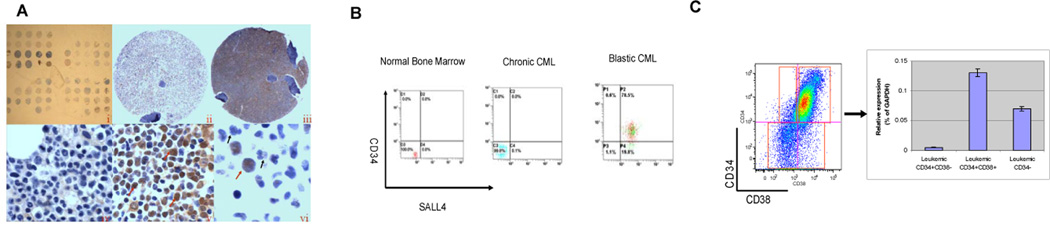
Using immunohistochemistry staining, we observed that SALL4 expression was present in blast-crisis CML (9/12, 75%) but not in chronic phase (0/11, 10%). In accelerated phase (1/6, 16.7%), wherein the blast count was 10–19%, immature blasts expressing SALL4 were observed in a background of negative, more mature myeloid cells (Figure 1A). Similar results were observed when we performed FACS analysis on whole bone marrow cells from normal human and CML samples at different disease phases using a conjugated SALL4 antibody (Figure 1B). No SALL4 positive population was detectable in normal whole bone marrow or chronic phase CML samples. A distinct SALL4 positive population was present in accelerated phase and blast crisis CML marrows, which correlated well with the blast count and overlapped with the CD34+ population. The CD34+CD38+ cells had the highest SALL4 RNA expression when tested by qRT-PCR (Figure 1C and Supplemental Table 1). This finding is of particular interest since Granulocyte-Macrophage Progenitors, which are CD34+CD38+, have been proposed as candidate LIC in CML, that transforms to acute myeloid leukemia (AML)(1).
Figure 1. SALL4 expression correlates with disease progression of human CML.
(A) SALL4 protein expression is present in blast crisis (N=12) but not in chronic phase (N=11) CML, as demonstrated by immunohistochemistry staining. (i) Lower power magnification of tissue array of CML cells at different phases of the disease. (ii) SALL4 expression was absent in CML chronic phase with all cell nuclei remaining blue in color (ii, ×4 & iv, ×100); while it was strongly positive in CML blast crisis as indicated by brown nuclear staining of SALL4 (iii, ×4 & v, ×60). In accelerated CML, while there was an increased but less than 20% blast count present, only blasts stained positive for SALL4 expression; in contrast, the mature neutrophils remained negative (vi, N=6). Red arrows indicate positive staining of blasts while the black arrows indicate negative, non-staining mature neutrophils. (B) Representative flow cytometry studies of SALL4 protein expression in normal bone marrow (N=3), chronic phase CML (N=3) and blast crisis CML samples (N=5). Consistent with the results obtained by immunohistochemistry staining, there were no SALL4 positive cells in normal and chronic phase CML bone marrow samples. In contrast, a distinct SALL4 positive population was observed in blast crisis, and a sub-population of these cells was CD34 positive as well. (C) SALL4 RNA expression in CML cells. QRT-PCR quantification of SALL4 RNA expression was normalized to GAPDH. As shown, CD34+CD38+ cells had the highest SALL4 expression (N=3). Y axis: SALL4 relative expression (percent of GAPDH).
To explore the role of SALL4 in CML, we first tested whether overexpression of SALL4B could block myeloid differentiation and cooperate with BCR-ABL in, consequently, promoting blastic transformation of chronic phase CML. For induction of CML-like leukemia, we harvested bone marrow cells from SALL4B transgenic and wild-type donor mice 4 days post intravenous administration of 200 mg per kg (body weight) 5-fluorouracil (5-FU), transduced with BCR-ABL-GFP retrovirus as described (8), and injected 4×105 cells intravenously into sublethally irradiated (750cGy) C57BL/6 recipients. Slightly sublethal irradiation in this mouse model has been shown not to inhibit induction of CML-like disease. Both, recipients of BCR-ABL induced SALL4B transgenic and wild-type bone marrow, succumbed to fatal CML-like leukemia within 3–6 weeks with increased WBC counts (Supplemental Table 2). Flow cytometry analysis demonstrated that 80% of BCR-ABL-positive bone marrow cells from both groups were Mac1+and Gr-1+ neutrophils (Supplemental Figure 1A). In addition, the percentage of CD34+ or c-Kit BCR-ABL-positive cells from bone marrow and spleen increased about 2- and 3-fold in leukemic SALL4B transgenic recipients compared to wild-type counterparts (Supplemental Figure 1B and 1C and data not shown). This suggests that BCR-ABL- transduced SALL4B leukemic cells were more immature and less differentiated.
We next investigated the functional role of SALL4 in CML progression using a loss-of-function approach by knocking down SALL4 expression in the human CML cell line KBM5. Two shRNA retroviral constructs targeting different regions of the SALL4 mRNA as we previously reported (6), were shown by qRT-PCR to reduce SALL4 and Bmi-1 mRNA level in KBM5 cells (Figure. 2A).
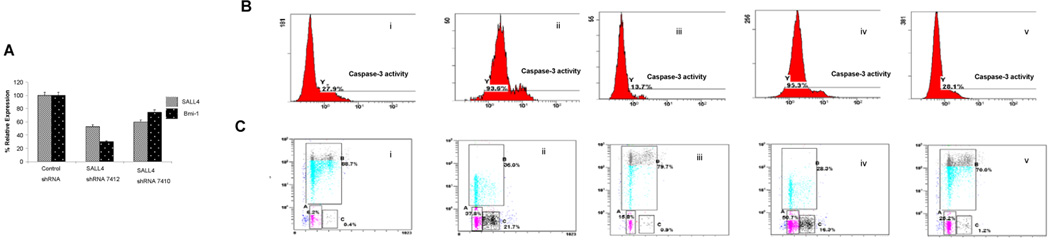
Figure 2. SALL4 expression is essential for CML cell survival.
(A) Generation of SALL4 Knockdown KBM5 cells. Two shRNA retroviral constructs that target different regions of SALL4 were made (termed as #7410 and #7412), and their abilities to reduce SALL4 and Bmi-1 mRNA levels in KBM5 cells were confirmed by qRT-PCR. The shRNA retroviral construct, #7412, was more effective in the suppression of SALL4 and Bmi-1 expression and was used in all subsequent experiments. (B) Increased apoptosis was observed in SALL4 knockdown KBM5 cells by FACS analysis of caspase-3 activity. This could be rescued by overexpression of Bmi-1. (i) KBM5 cells transfected with control retroviruses; (ii) KBM5 cells transfected with SALL4 shRNA retroviruses; (iii) SALL4 knockdown KBM5 cells transfected with Bmi-1; (iv) SALL4 knockdown KBM5 cells transfected with control vector for overexpression of Bmi-1; and (v) WT KBM5 cells transfected with Bmi-1. (C) Cell cycle arrest and decreased proliferation observed in SALL4 knockdown KBM5 cells demonstrated by BrdU incorporation assay and FACS assays. This phenotype can be rescued by overexpression of Bmi-1. (i) KBM5 cells transfected with control retroviruses; (ii) KBM5 cells transfected with SALL4 siRNA retroviruses; (iii) SALL4 knockdown KBM5 cells transfected with Bmi-1; (iv) SALL4 knockdown KBM5 cells transfected with control vector for overexpression of Bmi-1; and (v) WT KBM5 cells transfected with Bmi-1. All experiments were conducted in triplicates.
The KBM5 cells expressing reduced levels of SALL4 grew slowly. To better explain this phenomenon, we measured the level of caspase-3, which is a marker for the apoptosis signaling pathway. In KBM5 cells that retained 50% of the wild–type (WT) levels of SALL4, there was a 3-fold increase of caspase-3 activity from 27.9 % in WT cells to 93.6% in cells with reduced SALL4 levels as measured by FACS (Figure 2B).
We have previously shown that Bmi-1 is a major downstream target of SALL4 in leukemic cells (6). To determine if overexpression of Bmi-1 could rescue SALL4-induced apoptosis, we transfected SALL4 shRNA treated KBM5 cells with an expression vector containing Bmi-1. As shown in figure 2B, SALL4-induced caspase-3 activity (Fig. 2B-ii) was restored to a near normal level by overexpression of Bmi-1 (Fig. 2B-iii).
To further study the role of SALL4 in cell growth in a leukemic cell line, we monitored cell-cycle changes and cellular DNA synthesis in SALL4-reduced and WT KBM5 cells using the BrdU incorporation assay by FACS. A 50% reduction of SALL4 expression level in KBM5 cells resulted in G0/G1 phase (37.8%) and G2 phase (21.7%) arrests (Fig 2C-ii). A more than two-fold decrease in S phase cells was also observed, paralleling the drop in DNA synthesis measured by the level of BrdU incorporation. In contrast, no significant change in the cell-cycle profile was observed when WT- KBM5 cells were infected with control viruses (Fig. 2C-i). Reintroduction of Bmi-1 in SALL4-reduced KBM5 cells can rescue this phenotype (Fig. 2C-iii).
Our data suggest that the stem cell factor SALL4 plays an important role in the proliferation and survival of CML cells, and its expression is associated with an advanced stage of CML disease. The mechanism of SALL4-mediated proliferation and survival of CML cells, at least in part, is mediated by the Bmi-1 pathway. In support of this concept, downregulation of SALL4 leads to apoptosis and cell cycle arrest in CML cells, which is rescued by restoring its downstream target gene Bmi-1. This finding suggests that targeting SALL4 may provide a novel therapeutic modality for advanced-stage CML disease.
Supplementary Material
Acknowledgments
This work was supported in part through NIH grants NIH R01HL087948 (to Dr. Yupo Ma ), RO1HL092437, RO1HL092437-A1S1, PO1 DK080665 (to Dr. Li Chai ), and PO1HL095489 (to Dr. Li Chai and Leslie Silberstein)
Footnotes
"Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)"
Conflict of interest:
References
- 1.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004 Aug 12;351(7):657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 2.Mohty M, Yong AS, Szydlo RM, Apperley JF, Melo JV. The polycomb group BMI1 gene is a molecular marker for predicting prognosis of chronic myeloid leukemia. Blood. 2007 Jul 1;110(1):380–383. doi: 10.1182/blood-2006-12-065599. [DOI] [PubMed] [Google Scholar]
- 3.Raaphorst FM. Self-renewal of hematopoietic and leukemic stem cells: a central role for the Polycomb-group gene Bmi-1. Trends Immunol. 2003 Oct;24(10):522–524. doi: 10.1016/s1471-4906(03)00241-2. [DOI] [PubMed] [Google Scholar]
- 4.Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003 May 15;423(6937):302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Tam WL, Tong GQ, Wu Q, Chan HY, Soh BS, et al. Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat Cell Biol. 2006 Oct;8(10):1114–1123. doi: 10.1038/ncb1481. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Chai L, Liu F, Fink LM, Lin P, Silberstein LE, et al. Bmi-1 is a target gene for SALL4 in hematopoietic and leukemic cells. Proc Natl Acad Sci U S A. 2007 Jun 19;104(25):10494–10499. doi: 10.1073/pnas.0704001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Y, Cui W, Yang J, Qu J, Di C, Amin HM, et al. SALL4, a novel oncogene, is constitutively expressed in human acute myeloid leukemia (AML) and induces AML in transgenic mice. Blood. 2006 Oct 15;108(8):2726–2735. doi: 10.1182/blood-2006-02-001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, Ilaria RL, Jr, Million RP, Daley GQ, Van Etten RA. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J Exp Med. 1999 May 3;189(9):1399–1412. doi: 10.1084/jem.189.9.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.