Abstract
CD4+ T-helper type 2 (Th2) cells, characterized by their expression of interleukin (IL)-4, IL-5, IL-9 and IL-13, are required for immunity to helminth parasites and promote the pathological inflammation associated with asthma and allergic diseases. Recent reports from a number of laboratories have indicated that basophils can influence the induction and/or effector stages of Th2 cytokine-mediated inflammation. However, the impact of basophils appears to depend on the anatomical location and nature of the infectious or inflammatory stimulus. This review will highlight the factors that regulate basophil development and activation and will describe known basophil effector functions. Further, we will discuss the recent identification of phenotypic and functional heterogeneity within murine and human basophil populations and discuss how these findings may explain the context-dependent influence of basophils on either the propagation, regulation or effector phases of Th2 cytokine-associated inflammation.
Introduction
Basophils are the least abundant granulocyte population, accounting for less than 1% of leukocytes in the blood and spleen. Although originally described by Paul Erlich in 1879, their effector functions were not appreciated until 1972 when basophils were shown to bind immunoglobulin (Ig)E and release histamine1, 2. Despite these findings, basophils were thought to be a redundant cell population with effector functions similar to those of mast cells. However, subsequent studies directly comparing mast cells and basophils illustrated that these cell populations differ in their differentiation from progenitor cells, life span, anatomical location, surface marker expression and release of effector molecules3–5. Although these data demonstrated that basophils represent a unique cell population, the lack of an animal model prevented further interrogation of their functional properties in vivo. In 1981, a histamine containing basophil-like population termed a “persisting cell” or P cell was identified in mice6. However, the first cell population recognized as mouse basophils was not reported until 1982, when Dvorak et. al. characterized a granular cell population in murine bone marrow with ultrastructural characteristics similar to other mammalian basophil populations7. Advances in basophil biology were further aided by the development of interleukin (IL)-4/eGFP reporter mice and the determination that basophils acquire constitutive IL-4 mRNA expression during their development and can be easily identified by flow cytometric analysis8–10. These technical advances facilitated the identification of murine basophils as expressing surface markers consistent with that of human basophils (FcεRI+, CD49b+, CD69+, Thy-1.2+, CD123+, CD200R+, CD117−, CD19−, CD14−, CD122−, CD11c−, Gr-1−, NK1.1−, B220−, CD3−, αβTCR−, γδTCR−)2, 8, 9, 11, 12.
Methods of depleting murine basophils, including delivery of monoclonal antibodies targeting the high-affinity IgE receptor or the membrane glycoprotein CD200R3, lineage restricted expression of the diphtheria toxin receptor (DTR) or lineage restricted expression of Cre recombinase have also been developed13–22. The ability to deplete basophils has allowed for a series of in vivo studies that significantly advanced the understanding of basophil functions. Specifically, these studies identified a previously unrecognized role for basophils in contributing to optimal Th2 cytokine responses and prompted a renewed interest in the factors that regulate basophil development, activation and function.
This review will provide an overview of the molecules and pathways that regulate the development, activation and functions of murine and human basophil populations. In addition, we will highlight the recent discovery of phenotypic and functional heterogeneity in basophil populations and discuss how these findings may explain some of the paradoxical reports regarding the influence of basophils on the development to Th2 cytokine-mediated immunity or inflammation following exposure to either helminth parasites or allergens.
Basophil development
Like other myeloid lineages, basophils are thought to develop from hematopoietic stem cells in the bone marrow. However, many of the cytokines and growth factors that regulate basophil lineage commitment remain unknown. This section will discuss known progenitor cell populations and the molecular mechanisms that regulate basophil development. In addition, this section will highlight the recent discovery of basophil precursors in the periphery and the previously unrecognized ability of thymic stromal lymphopoietin (TSLP) to promote the development of a distinct basophil population from bone marrow-resident progenitor cells23.
Basophils are reported to arise from a common granulocyte-monocyte precursor in the bone marrow that has the capacity to develop into eosinophils, basophil-mast cell precursors (BMCP), mast cell precursors (MCP) and basophil precursors (BaP)24. Bone marrow-resident MCPs and BaPs are reported to give rise to mast cells and basophils respectively, while BMCPs are reported to exit to the periphery but maintain the capacity to migrate back to the bone marrow where they can develop into basophils25. Unlike mast cells which mature in peripheral tissues, basophils are reported to enter the periphery with a fully mature phenotype. However, recent studies have also demonstrated that an IL-25-responsive multipotent progenitor cell population termed MPPtype2 cells is present in the periphery, undergoes population expansion in response to heminth infection and has the capacity to mature into basophils26. The lineage commitment of basophil precursor populations is reported to be dependent on the expression of the transcription factors GATA-2 and the CCATT enhancer-binding protein C/EBPα25. In addition, studies have also demonstrated that mast cells, eosinophils and basophils initiate and maintain expression of IL-4 and IL-13 mRNA transcripts during their maturation10. Despite our understanding of these events, the cytokines and growth factors that initiate basophil development and whether plasticity exists between precursor populations remain poorly characterized.
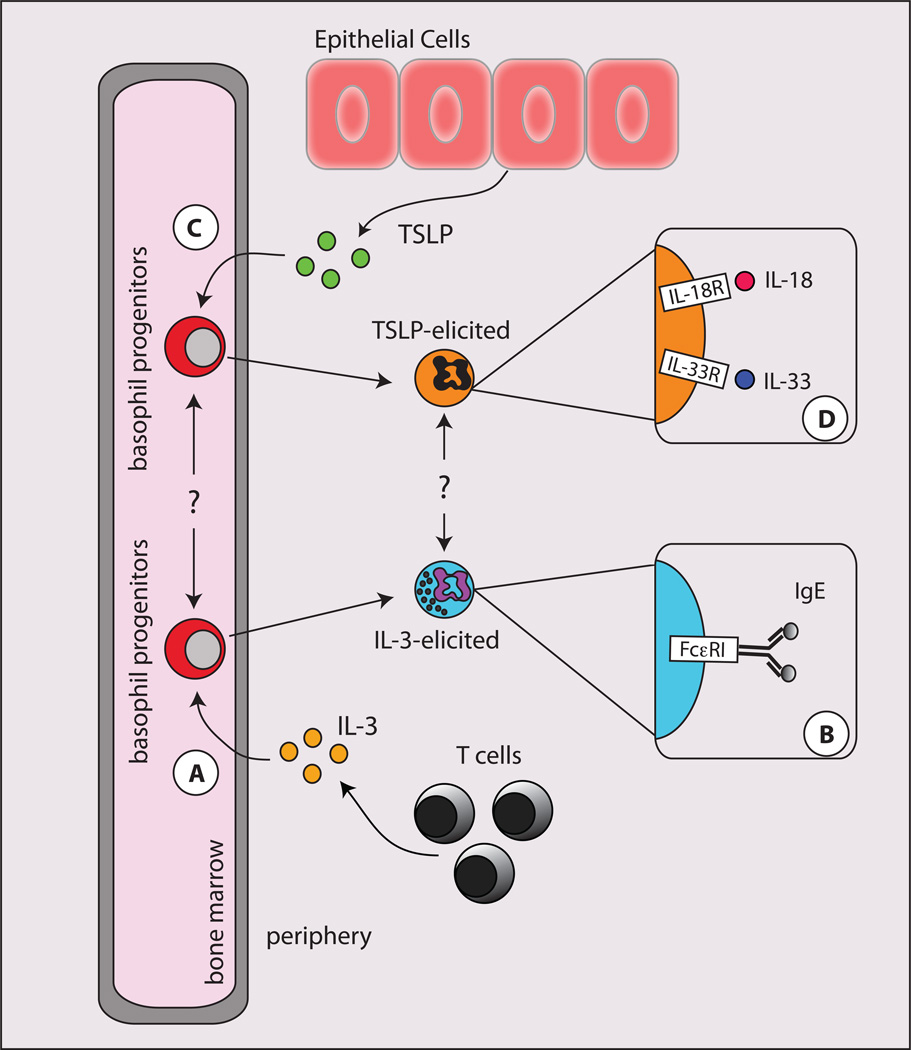
The life span of a mature basophil is relatively short and is estimated to be on the order of 60–70 hours25, 27, 28. Thus, it is thought that basophil differentiation from precursor populations is continually occurring in order to maintain basophil populations in the periphery. Although present in very small numbers at baseline levels, basophil populations can significantly expand in response to certain factors, such as IL-3 (IL-3)29–32. IL-3 promotes the differentiation of basophils from bone marrow cells (Fig. 1A), induces the generation of basophils in mice, and IL-3 signals are necessary for infectioninduced basophilia following Nippostrongylus brasiliensis and Strongyloides venezuelensis infections29, 31, 32. In addition, IL-3 can augment many aspects of basophil function including the release of effector molecules after IgE-dependent stimulation (Fig. 1B)33, 34. Collectively, these studies supported the hypothesis that basophil responses were critically dependent on IL-3 signaling.
Figure 1. Developmental and functional heterogeneity in basophils populations.
A, In the context of an ongoing inflammatory response, CD4+ Th2 cells produce IL-3. IL-3 promotes the generation of mature basophils from bone marrow-resident precursor cells. B, Mature IL-3-elicited basophils enter the periphery and acquire surface bound IgE via FcεRI and are potent producers of histamines and leukotrienes. C, In the context of inflammation at barrier surfaces, epithelial cells produce TSLP. TSLP promotes the generation of mature basophils from bone marrow-resident precursor cells. D, Mature TSLP-elicited basophils express elevated levels of IL-18Rαand IL-33R and are potent producers of IL-4 in response to IL-18 or IL-33 stimulation.
In recent studies IL-3-IL-3R-independent basophil development and peripheral basophil responses were observed in vivo, and the epithelial cell-derived cytokine TSLP was critical in promoting these responses23. Previous studies have shown that murine TSLP is critical for the development of Th2 cytokine-mediated immunity and inflammation35, 36, 37. Consistent with these findings, studies in patients identified that gain-of-function mutations in the gene encoding TSLP and elevated TSLP expression are associated with the allergic diseases asthma, atopic dermatitis and eosinophilic esophagitis38–41. In in vitro studies, TSLP promoted the selective population expansion of murine basophils from bone marrow-resident progenitor cells (Fig. 1C). In addition, overexpression of TSLP resulted in the population expansion of basophils in vivo23. Critically, TSLP was capable of promoting murine basophil responses in the absence of IL-3-IL-3R signaling, demonstrating a previously unrecognized pathway of basophil development and activation23. The phenotypic and functional heterogeneity that distinguish TSLP-elicited versus IL-3-elicited basophils will be discussed in greater detail below.
Basophil activation and effector functions: the traditional view
Basophils can be activated by an array of stimuli including those mediated by antibodies, cytokines, proteases, TLR ligands and complement factors. Activated basophils are known to produce cytokines (IL-4, IL-13, IL-6, and TNFα), effector molecules (histamine, leukotriene C4 (LTC4) and antimicrobial peptides) and chemotactic factors2, 42, 43. The following sections will highlight current knowledge of the mediators of basophil activation and the effector functions they promote.
Some populations of basophils rapidly produce preformed effector molecules such as histamines, LTC4 and cytokines in response to crosslinking of FcεRI via surface bound IgE2. The rapid release of effector molecules in response to antibody-mediated crosslinking allows basophils to contribute to the development of systemic anaphylaxis. Several studies of human basophils suggest they degranulate via surface bound IgE stimulation and contribute to anaphylaxis when exposed to blood-borne antigens44. Although studies in mouse models have failed to identify a similar role for basophils during IgE-mediated anaphylaxis, reports have demonstrated that basophils produce platelet-activating factor and contribute to anaphylaxis mediated by IgG145. Collectively, these studies indicate that some basophils are capable of contributing to anaphylaxis in both mice and humans.
Basophils can also contribute to a type of chronic allergic inflammation in mice46. Following subcutaneous injection of multivalent antigens into the ear, basophils were required for the development of IgE-mediated chronic allergic inflammation (IgE-CAI) in the skin. IgE-CAI was found to be mast cell- and T cell-independent, but was dependent on an FcεRI+, CD49b+ basophil population46. Although basophils comprised only a small percentage of the cellular infiltrate at the site of the chronic lesion in the skin, their depletion led to a dramatic reduction in inflammation. Specifically, basophil depletion resulted in a decrease in the number of eosinophils and neutrophils present at the site of the lesion and a marked reduction in ear thickness. The loss of infiltrating eosinophils and neutrophils suggests that basophils produce cytokines and/or other factors that directly result in cell recruitment, or indirectly induce the production of chemokines from tissue-resident cells46. Taken together, these results illustrate the potent inflammatory effects of small numbers of basophils and indicate a novel, non-redundant role for basophils in the initiation and maintenance of chronic IgE-mediated inflammatory responses in mice.
In addition to IgG1- and IgE-mediated activation, human basophils have been reported to selectively bind IgD, a class of antibody produced early in B cell development43. Although the biological function of IgD remains unclear, this study demonstrates that IgD is highly expressed in the human upper respiratory tract and can bind the respiratory bacteria Haemophilus influenzae and Moraxella catarrhalis. IgD-activated basophils exhibited distinct effector functions from IgE-activated basophils and expressed a broad spectrum of antimicrobial peptides, IL-4 and B cell activating factor43. Furthermore, IgD-activated basophils induced IgM, IgD, and IgA class switching from B cells, and supernatants from IgD-activated B cells were capable of preventing the replication of M. catarhalis and H. influenzae. Collectively, these data illustrate that IgD-activated basophils are functionally distinct from IgE-activated basophils and may play a protective role against respiratory pathogens43.
In addition to antibody-mediated activation, some basophils can be activated by the cytokines IL-18 and IL-3347, 48. IL-18 is produced by innate immune cells such as macrophages and Kupffer cells and is known to play a role in allergic disease and immunity to helminth parasites49–52. IL-33 is expressed by dermal fibroblasts, airway epithelial cells and bronchial smooth muscle cells and is associated with IL-4, IL-13 and IgE production53, 54. Consistent with their ability to enhance Th2 cytokine-associated immune responses, IL-18 and IL-33 are also capable of activating basophil populations. IL-18 induces IL-4 production from bone marrow-derived basophils in vitro, and administration of IL-18 in vivo enhanced the production of IL-4 and histamine in mice51. Interestingly, although human blood basophils express the IL-18R at high levels, a role for IL-18 in the activation of human basophils has yet to be reported47, 55. Similar to IL-18, IL-33 induces IL-4 production from murine bone marrow-derived basophils, and IL-33 stimulation induces the production of IL-4, IL-5, IL-6 and IL-13 from human blood-derived basophils56. Furthermore, recent studies demonstrate that IL-33-responsive basophils produce IL-4 and are protective in a model of murine arthritis57. Collectively, these data demonstrate that the IL-1 family cytokines IL-18 and IL-33 are capable of inducing and/or enhancing human and murine basophil responses.
Emerging functions of basophils
Historically, basophils have been thought of as late phase effector cells that migrate into inflamed tissues after Th2 cytokine-dependent inflammation is established. As such, basophil population expansion is associated with chronic allergic inflammation and helminth infections. In the context of ongoing inflammatory responses, basophils are known to incorporate surface bound IgE via FcεRI and degranulate in response to FcεRI crosslinking2. In addition to IgE-mediated late phase effector functions, some basophil populations were reported to contribute to the induction and propagation of Th2 cytokine-mediated immunity and inflammation in the context of either allergen exposure or helminth infection13, 14, 17, 19, 20, 23. Subsequent reports in other model systems demonstrated that although basophils contribute, they are not necessary for the development of optimal Th2 cytokine-mediated immunity and inflammation, indicating that the requirement for basophils may be stimulus- or tissue location-dependent. [Hammad, #47][Phythian-Adams, #54]. Collectively, these reports have provoked many questions and generated some controversies regarding the ability of basophils to function as initiators of Th2 cell responses. The following sections will review recent reports on murine models of infection or allergic inflammation and highlight the recent discovery of functional heterogeneity between IL-3-elicited versus TSLP-elicited basophil populations. We will discuss how basophil heterogeneity may inform the interpretation of these conflicting studies. Lastly, we will introduce a model illustrating how basophil heterogeneity within the basophil lineage may explain the differential requirement for basophils in the induction of Th2 cytokine responses in the context of some murine models of helminth infections or allergic disease but not others.
Influence of basophils on Th2 cell responses
It is well established that basophils are potent producers of IL-4 and as such may contribute to the induction and propagation of CD4+ Th2 cell responses. Consistent with this hypothesis, in vitro studies demonstrated that IL-4-producing basophils isolated from the spleen, liver or bone marrow are capable of initiating Th2 cell responses in the presence of DCs and antigen58, 59. In addition, IL-3-elicited bone marrow-derived basophils are also able to induce Th2 cell differentiation in the presence of DCs and antigen59. These data provided the proof of principle that IL-4-producing basophils can promote Th2 cell differentiation in the context of DCs and antigen. In vivo studies have further confirmed these data and demonstrated that CD4+ T cells stimulated in the presence of basophil populations preferentially adopt a Th2 phenotype. Collectively, these studies implicated basophils as a critical source of innate IL-4 required for the development of optimal Th2 cytokine-mediated immunity and inflammation.
In vivo basophil populations are primarily found in the blood and spleen, but are recruited to the tissues during inflammatory responses initiated by exposure to allergens or helminth parasites23. Although previously reported to be excluded from LNs, recent studies demonstrated that IL-4/eGFP+, MHC class II+ murine basophils migrate to the draining LNs following exposure to papain, house dust mite antigen (HDM), Schistosoma mansoni eggs or N. brasiliensis infection13, 14, 17, 60. Consistent with these data, MHC class II+ human basophils are also found in the LNs of patients suffering from systemic lupus erythematosus61. These data further suggested that IL-4-producing basophils might be capable of interacting with T cells and influencing their activation and/or differentiation. The ability of basophils to interact with T cells in the LN was recently confirmed using two-photon imaging techniques. Specifically, Basoph8 mice, which express yellow fluorescent protein under the basophil-specific gene Mcpt8, were used to demonstrated that basophils make transient interactions with CD4+ T cells in the LN post-S. mansoni egg challenge62. However, unlike DC-T cell interactions, basophil-T cell interactions were of short duration and did not appear to form stable conjugates62. Similar results were also observed in the LN post-papain immunization. In addition, these studies also indicated that basophils failed to produce IL-4 in the secondary lymphoid tissues, but were capable of producing IL-4 in the lung tissue post- N. brasiliensis infection in a CD4+ T cell-dependent, IL-3-dependent manner62. Collectively, these data demonstrate that basophils are capable of interacting with CD4+ T cells in secondary lymphoid tissues, but that these interactions differ from those made by DC populations.
Antigen presenting cell capacity of basophils
Consistent with the ability of MCH class II+, IL-4/eGFP+ basophils to migrate into peripheral LNs, three independent laboratories identified a previously unappreciated function for basophils as APCs in the context of helminth infections or exposure to allergens. In these studies, basophils were shown to endocytose IgE-allergen complexes, express MHC class II and costimulatory molecules, migrate to draining LNs and promote Th2 cell differentiation in vitro and in vivo13, 20, 63. For example, antigenpulsed basophils were sufficient to promote papain-specific Th2 cell differentiation after adoptive transfer into an MHC class II deficient host20. Consistent with these findings, adoptively transferred IL-4/eGFP+, MHC class II expressing basophils were capable of augmenting Th2 cell differentiation in response to S. mansoni egg antigens13. Further demonstrating a role for some basophil populations in the induction of Th2 cytokine responses, basophil depletion impaired protective immunity to Trichuris and eliminated Th2 cell development post-papain challenge [Sokol, 2009 #44][Perrigoue, 2009 #40]. Collectively, these studies demonstrated that in the context of some allergens and helminth infections, basophils function as inducers of Th2 cytokine-mediated inflammation13.
It is well established that DCs can directly promote Th2 cell responses. For example, in a murine model of asthma using intratracheal instillation of ovalbumin (OVA), pulsed DCs were sufficient to sensitize mice to airway hyper-responsiveness, and depletion of DCs prior to OVA challenge resulted in decreased Th2 cytokine production and reduced lung pathology64. DCs were also reported to play a critical role in the induction of Th2 cell responses during S. mansoni infection. In addition, work from our own lab demonstrated that DCs are sufficient to promote Th2 cytokine responses and protective immunity to Trichuris in the absence of IFNγ13. Further strengthening these previous reports, a series of additional studies were published directly addressing the relative contributions of DCs and basophil populations in induction of Th2 cytokine responses21. These studies demonstrated that FcεRI+, CD11c+, MHC class II+ inflammatory DCs were both necessary and sufficient for the induction of Th2 cytokine responses in an HDM model of airway inflammation; however, depletion of basophils in this model still resulted in a significant reduction in IL-4-expressing T cells suggesting that basophils also contribute to optimal Th2 cell responses in vivo19. In addition, in vivo depletion of CD11c+ DCs following S. mansoni infection severely disrupted Th2 cytokine responses, while depletion of basophils had no effect on Th2 cell development in response to subcutaneous challenge with S. mansoni-eggs21. A recent study also showed that Th2 cell development in response to immunization with the cysteine protease papain is dependent on cooperative responses between DCs and basophil populations22. Specifically, immunization with papain and OVA resulted in the release of reactive oxygen species (ROS) by DC populations. These studies reported that the release of ROS by DC populations initiated events that mediated the recruitment of basophil populations to the LN, and these basophils worked in concert with DCs to promote optimal Th2 cytokine responses22. Thus, these reports indicate that there are likely to be multiple pathways leading to Th2 cell differentiation, some of which may be DC-dependent, some of which may be basophil-dependent and some of which may require cooperation between DC and basophil populations.
Functional heterogeneity in basophil populations
Despite the likely existence of multiple pathways that promote the development of optimal of Th2 cell responses, conflicting reports describing the differential requirement for basophils in contributing to the induction of Th2 cytokine responses have been attributed to the limitations associated with the methods of basophil depletion employed. Multiple methods of basophil depletion have been developed including administration of mAb targeting the high affinity IgE receptor (FcεRI) or the membrane glycoprotein CD200R, basophil-specific expression of the DTR or lineage restricted expression of Cre recombinase (which was demonstrated to be toxic to basophils)13–22. As is commonly found with many cellular depletion strategies, different methods of basophil depletion have limitations. For example, treatment of mice with anti- FcεRI is capable of depleting basophil, mast cell and inflammatory dendritic cell populations, while lineage restriction of Cre-recombinase to basophils results in an incomplete loss of basophil populations17, 19. These limitations, coupled with the use of multiple model systems, has created some controversies regarding the functions of basophils as initiators or regulators of Th2 cytokines responses. The recent discovery of phenotypic and functional heterogeneity in basophil populations provides an additional explanation for the differential requirements for basophils in different disease models. The following section will highlight functional heterogeneity between IL-3-elicited versus TSLP-elicited basophil populations and discuss how these data could influence the interpretations of conflicting reports on basophil function.
Functional heterogeneity in basophil populations
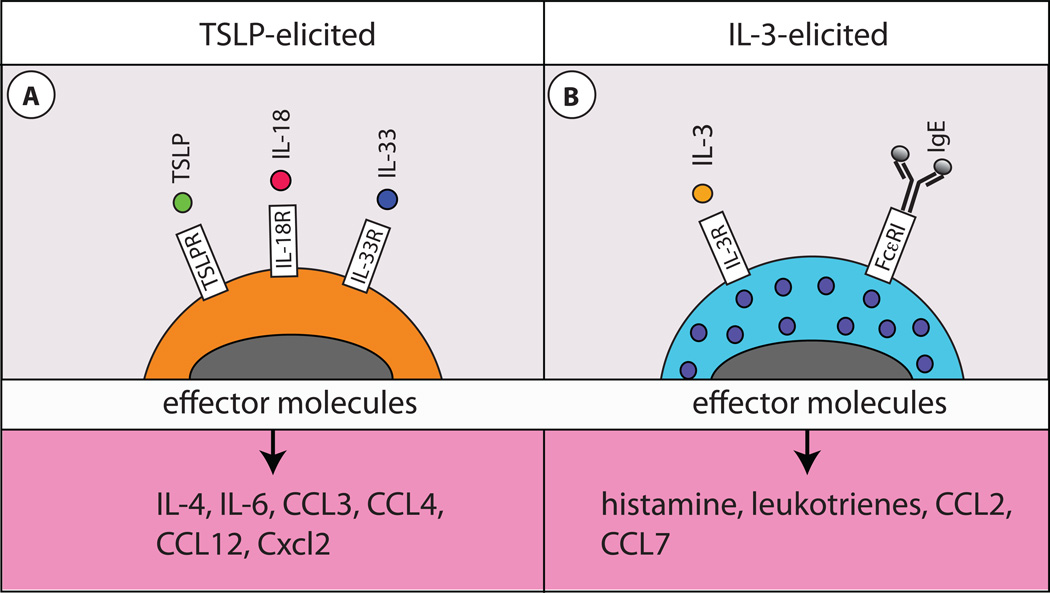
As mentioned above, a previously unrecognized pathway of IL-3-independent, TSLP-dependent basophil development and activation was recently described. TSLP-elicited basophils were demonstrated to be phenotypically and functionally distinct from IL-3-elicited basophils23. Further, genome-wide transcriptional profiling illustrated that TSLP-elicited basophils expressed genes associated with biological functions of linoleic acid metabolism, cell adhesion and cell communication, while IL-3-elicited basophils expressed genes consistent with the biological functions of dendritic cell (DC) and macrophage maturation. Interestingly, increased linoleic acid metabolism has previously been associated with atopic disorders of the skin65. Further, TSLP-elicited basophils expressed higher levels of CD123, IL-18Ra and T1/ST2 compared to IL-3-elicited basophils, while IL-3-elicited basophils were larger and more granular that TSLP-elicited basophils23. Consistent with phenotypic data, TSLP-elicited basophils demonstrated an enhanced ability to produce IL-4, IL-6 and other cytokines and chemokines compared to IL-3-elicited basophils (Fig. 2A). In contrast, IL-3-elicited basophils exhibited a significantly enhanced ability to degranulate in response to FcεRI crosslinking via surface bound IgE compared to TSLP-elicited basophils (Fig. 2B)23. These data demonstrate that heterogeneity exists in basophil populations and suggests that TSLP-elicited basophils may produce more cytokines and chemokines following stimulation, while IL-3-elicited basophils exhibit more classical basophil functions such as degranulation and histamine production in response to FceRI crosslinking23.
Figure 2. Effector mechanism of IL-3-elicited and TSLP-elicited basophils.
A, TSLP-elicited basophils are smaller and less granular than IL-3-elicited basophils and express elevated levels of IL-18Rα and IL-33R on their surface. TSLP-elicited basophils produce exaggerated amounts of IL-4, IL-6, CCL3, CCL4, CCL12 and Cxcl2 compared to IL-3-elicited basophils. B, IL-3-elicited basophils are larger and more granular than TSLP-elicited basophils and are potent producer of histamines, leukotrienes, CCL2 and CCL7 in response to IgE-mediated FcεRI crosslinking.
IL-3-dependent models of basophil activation
Many of the studies investigating the role of basophils as inducers of Th2 cytokine responses have employed the helminth infection models N. brasiliensis or S. mansoni17, 18, 21, 62. Critically, Nippostrogylus and Schistosoma infections are known to initiate Th2 cytokine-mediated immunity and inflammation independently of TSLP-TSLPR signaling66, 67. This suggests that the basophil populations elicited by N. brasiliensis and S. mansoni may be IL-3-dependent and exhibit more classical IgE-mediated effector functions. This hypothesis is supported by previous studies demonstrating that N. brasiliensis-induced basophilia and basophil recruitment to secondary lymphoid tissues are CD4+ T cell- and IL-3-dependent events29, 60. Further, the ability of basophils to produce IL-4 in the lung following N. brasiliensis infection is also dependent on CD4+ T cells and IL-362.
In the case of S. mansoni, the directly dependence of infection-induced basophilia on CD4+ T cells and IL-3 has not been investigated. However, it has been reported that the production of IL-4 from an FcεRI+, non B, non T (NBNT) cell population in the spleen of infected mice is dependent on CD4+ T cells and IL-368. Further, studies from our own lab demonstrated that the recruitment of basophils to the draining LN post- S. mansoni egg challenge is dependent on CD4+ T cells and IL-3-IL-3R signaling (Siracusa, Pearce and Artis, unpublished). Collectively, these data suggest that S. mansoni also promotes IL-3-elicited basophil populations.
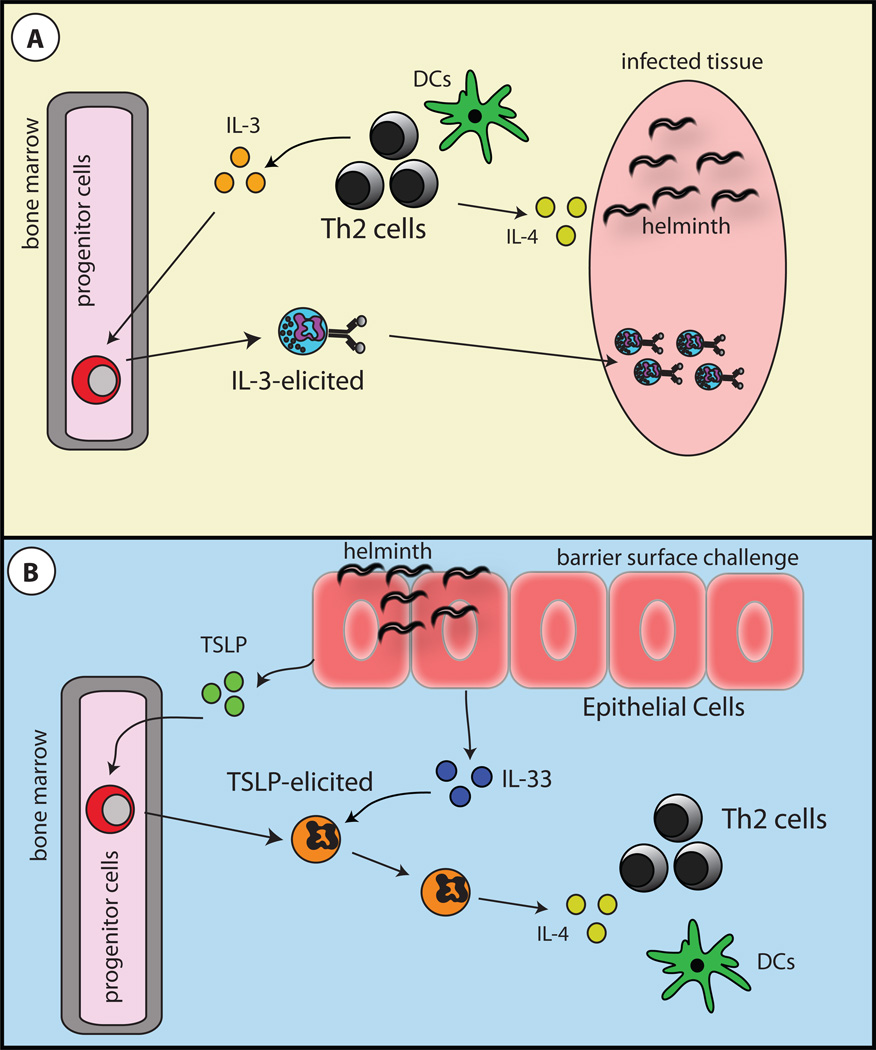
The demonstration that IL-3-elicited basophils appear to specialize in IgE-mediated effector functions suggests that N. brasiliensis- and S. mansoni induced-basophils may exhibit the same functions at TSLP-elicited basophils. Thus, it is likely that N. brasiliensis- and S. mansoni-induced basophils function more as late phase effector cells that migrate to tissues after Th2 cytokine responses have been initiated. This hypothesis is supported by the recent demonstration that basophils migrate to the lungs and produce IL-4 after CD4+ T cell activation has occurred17, 21, 62. In addition, depletion of basophils in the context of N. brasiliensis infection or S. mansoni egg challenge did not prevent the induction of Th2 cytokines responses21. Further, it has also been reported that basophil populations initiated in response to a primary N. brasiliensis infection are capable of providing protective immunity to a secondary challenge in an IgE-dependent manner18. These data further suggest that IL-3-elicited basophils are late phase effector cells that mediate their in vivo effector functions via antigen-specific IgE (Fig. 3A).
Figure 3. Basophil functions during Th2 cytokine-mediated immunity and inflammation.
A, In the context of an ongoing inflammatory response such as that initiated by tissue dwelling helminth infections, activated CD4+ Th2 cells produce IL-3. IL-3 promotes the development of classical basophil populations from bone marrow-resident precursor cells. Mature IL-3-elicited basophils enter the periphery and function as late phase effector cells that contribute to chronic inflammation. B, In the context of an infection or insult at a barrier surface, the epithelial cell-derived cytokines TSLP and IL-33 are produced. TSLP promotes the development of basophils from bone marrow-resident precursor cells. Mature TSLP-elicited basophils enter the periphery where they are actvated by IL-33. TSLP-elicited, IL-33-activated basophils are potent sources of IL-4 and along with DC populations, contribute the induction of CD4+ Th2 cell responses.
TSLP-dependent models of basophil activation
In contrast to N. brasiliensis and S. mansoni infections, Th2 cytokine responses and protective immunity to Trichuris muris is dependent on TSLP-TSLPR signaling36. Consistent with these data, Trichuris-induced basophilia was shown to be dependent on TSLP-TSLPR interactions. Further, depletion of basophils via anti-FcεRI treatment resulted in a loss of Th2 cytokine production and protective immunity while TSLPR+ basophils could restore immunity in TSLPR−/− mice, suggesting Trichuris-induced TSLP-dependent basophils play a critical role in the induction of optimal Th2 cytokine responses and protective immunity in the context of Trichuris infection13.
The importance of TSLP-elicited basophils in the induction of Th2-cytokine responses has also been demonstrated in a model of TSLP-TSLPR-dependent atopic dermatitis. Topical treatment with the vitamin D analogue MC903 resulted in the robust accumulation of basophils in the skin and the induction of significant atopic dermatitis-like pathology. MC903-induced basophilia was shown to be IL-3-IL-3R-independent, but was dependent on TSLP, as treatment with neutralizing anti-TSLP antibody prevented skin basophilia23. Further, blocking TSLP-TSLPR interactions resulted in reduced expression of Th2 cytokines and less cutaneous inflammation69. Critically, Th2 cytokine responses were also reduced when basophils were depleted via lineage-specific expression of DTR23. Together, these data are consistent with a role for TSLP-elicited basophils as potent producers of IL-4 and suggest that unlike IL-3-elicited basophils, TSLP-elicited basophils may function as initiators of Th2 cytokine responses (Fig. 3B).
Summary and future challenges
Recent studies regarding the relative contributions of DCs and basophils to the induction and propagation of CD4+ Th2 cells have resulted in conflicting reports and controversy. Many of the varied reports on the ability of basophils to contribute to Th2 cytokine mediated immunity and inflammation have been attributed to different experimental designs and basophil depletion strategies. However, the recent identification of phenotypic and functional heterogeneity in basophil populations provokes a fundamental reassessment of basophil effector functions and how they may vary depending on the infectious agent or antigen being used, the route of exposure and the cytokine environment induced. Much like M1 and M2 macrophages, basophils may exhibit distinct functional qualities based on the cytokine milieu in which they develop or are activated, and these differences should be considered when evaluating the ability of basophils to contribute to the induction of optimal Th2 cytokine-mediated immunity and inflammation.
However, much remains to be clarified regarding the differentiation, regulation, and effector functions of heterogeneous basophil populations. For instance, future studies will be required to determine whether IL-3-elicited versus TSLP-elicited basophils represent two distinct cell lineages that arise from distinct precursors or whether they represent different activation states of the same cell (Fig. 1A,C). Future studies will also be needed to delineate whether IL-3-elicited versus TSLP-elicited basophils develop in the bone marrow, or if these populations differentiate in the periphery in response to ongoing inflammation. In addition, it remains unclear whether IL-3-elicited versus TSLP-elicited basophils function in different contexts or tissues (Fig. 3A,B). Some of the data reviewed here suggest that TSLP-elicited basophils may function during the development of allergic responses at epithelial surfaces, while IL-3-elicited basophils may function as late phase effectors in the tissues in the context of helminth infection (Fig. 3A,B). Finally, further functional analysis will be required to determine if other factors, particularly epithelial-derived cytokines such as IL-25 and IL-33 can also shape the differentiation and function of basophil populations. A recent report described an IL 33-responsive IL-4 producing basophil population that was critical for the suppression of experimentally-induced arthritis57, further suggesting that epithelial cell-derived cytokines may regulate basophil activation. Further exploration of the effects of these cytokines on basophil populations will be integral in gaining a more complete understanding of the regulation of basophil differentiation and effector function, which in turn could inform efforts to develop treatment strategies to target allergic diseases and helminth infections.
Summary
Recent studies regarding the relative contributions of DCs and basophils to the induction and propagation of CD4+ Th2 cells have resulted in conflicting reports and controversy. Many of the varied reports on the ability of basophils to contribute to Th2 cytokine mediated immunity and inflammation have been attributed to different experimental designs and basophil depletion strategies. However, the recent identification of phenotypic and functional heterogeneity in basophil populations provokes a fundamental reassessment of basophil effector functions and how they may vary depending on the infectious agent or antigen being used, the route of exposure and the cytokine environment induced. Much like M1 and M2 macrophages, basophils may exhibit distinct functional qualities based on the cytokine milieu in which they develop or are activated, and these differences should be considered when evaluating the ability of basophils to contribute to the induction of optimal Th2 cytokine-mediated immunity and inflammation.
Acknowledgements
We thank members of the Artis laboratory for helpful discussions and critical reading of the manuscript. Research in the Artis laboratory is supported by the NIH (AI61570, AI74878, AI087990, AI074878, AI095608, AI091759, AI095466, F32-A1085828, F31- GM082187, T32-AI060516, T32-AI007532, T32-CA09140, T32-AI055438, T32-AI05528, and S10RR024525), the Burroughs Wellcome Fund (Investigator in Pathogenesis of Infectious Disease Award), the Crohn's and Colitis Foundation of America, and pilot grants from the University of Pennsylvania (CID, PGFI, and URI).
References
- 1.Ishizaka T, De Bernardo R, Tomioka H, Lichtenstein LM, Ishizaka K. Identification of basophil granulocytes as a site of allergic histamine release. J Immunol. 1972;108:1000–1008. [PubMed] [Google Scholar]
- 2.Schroeder JT. Basophils beyond effector cells of allergic inflammation. Adv Immunol. 2009;101:123–161. doi: 10.1016/S0065-2776(08)01004-3. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstein LM, Bochner BS. The role of basophils in asthma. Ann N Y Acad Sci. 1991;629:48–61. doi: 10.1111/j.1749-6632.1991.tb37960.x. [DOI] [PubMed] [Google Scholar]
- 4.MacGlashan DW, Jr, et al. Comparative studies of human basophils and mast cells. Fed Proc. 1983;42:2504–2509. [PubMed] [Google Scholar]
- 5.Arock M, Schneider E, Boissan M, Tricottet V, Dy M. Differentiation of human basophils: an overview of recent advances and pending questions. J Leukoc Biol. 2002;71:557–564. [PubMed] [Google Scholar]
- 6.Schrader JW, Lewis SJ, Clark-Lewis I, Culvenor JG. The persisting (P) cell: histamine content, regulation by a T cell-derived factor, origin from a bone marrow precursor, and relationship to mast cells. Proc Natl Acad Sci U S A. 1981;78:323–327. doi: 10.1073/pnas.78.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dvorak AM, et al. Ultrastructural identification of the mouse basophil. Blood. 1982;59:1279–1285. [PubMed] [Google Scholar]
- 8.Min B, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 10.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 11.Mack M, et al. Identification of antigen-capturing cells as basophils. J Immunol. 2005;174:735–741. doi: 10.4049/jimmunol.174.2.735. [DOI] [PubMed] [Google Scholar]
- 12.Dzionek A, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 13.Perrigoue JG, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denzel A, et al. Basophils enhance immunological memory responses. Nat Immunol. 2008;9:733–742. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 16.Kojima T, et al. Mast cells and basophils are selectively activated in vitro and in vivo through CD200R3 in an IgE-independent manner. J Immunol. 2007;179:7093–7100. doi: 10.4049/jimmunol.179.10.7093. [DOI] [PubMed] [Google Scholar]
- 17.Ohnmacht C, et al. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 33:364–374. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Ohnmacht C, Voehringer D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J Immunol. 184:344–350. doi: 10.4049/jimmunol.0901841. [DOI] [PubMed] [Google Scholar]
- 19.Hammad H, et al. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sokol CL, et al. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phythian-Adams AT, et al. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 207:2089–2096. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang H, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 11:608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siracusa MC, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arinobu Y, et al. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci U S A. 2005;102:18105–18110. doi: 10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwasaki H, Akashi K. Myeloid lineage commitment from the hematopoietic stem cell. Immunity. 2007;26:726–740. doi: 10.1016/j.immuni.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Saenz SA, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood. 2009;113:2816–2825. doi: 10.1182/blood-2008-05-154773. [DOI] [PubMed] [Google Scholar]
- 28.Mellblom L. A quantitative cytochemical study of the growth of individual mast cells. Cell Tissue Res. 1980;208:485–497. doi: 10.1007/BF00233880. [DOI] [PubMed] [Google Scholar]
- 29.Lantz CS, et al. IL-3 is required for increases in blood basophils in nematode infection in mice and can enhance IgE-dependent IL-4 production by basophils in vitro. Lab Invest. 2008;88:1134–1142. doi: 10.1038/labinvest.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen T, et al. T cell-derived IL-3 plays key role in parasite infection-induced basophil production but is dispensable for in vivo basophil survival. Int Immunol. 2008;20:1201–1209. doi: 10.1093/intimm/dxn077. [DOI] [PubMed] [Google Scholar]
- 31.Lantz CS, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 32.Ohmori K, et al. IL-3 induces basophil expansion in vivo by directing granulocyte-monocyte progenitors to differentiate into basophil lineage-restricted progenitors in the bone marrow and by increasing the number of basophil/mast cell progenitors in the spleen. J Immunol. 2009;182:2835–2841. doi: 10.4049/jimmunol.0802870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibbs BF, et al. Purified human peripheral blood basophils release interleukin-13 and preformed interleukin-4 following immunological activation. Eur J Immunol. 1996;26:2493–2498. doi: 10.1002/eji.1830261033. [DOI] [PubMed] [Google Scholar]
- 34.MacGlashan D, Jr, et al. Secretion of IL-4 from human basophils. The relationship between IL-4 mRNA and protein in resting and stimulated basophils. J Immunol. 1994;152:3006–3016. [PubMed] [Google Scholar]
- 35.Al-Shami A, et al. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor BC, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothenberg ME, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. 42:289–291. doi: 10.1038/ng.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ying S, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 40.Mou Z, et al. Overexpression of thymic stromal lymphopoietin in allergic rhinitis. Acta Otolaryngol. 2009;129:297–301. doi: 10.1080/00016480802225884. [DOI] [PubMed] [Google Scholar]
- 41.Soumelis V, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 42.Schroeder JT, MacGlashan DW, Jr, Lichtenstein LM. Human basophils: mediator release and cytokine production. Adv Immunol. 2001;77:93–122. doi: 10.1016/s0065-2776(01)77015-0. [DOI] [PubMed] [Google Scholar]
- 43.Chen K, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10:889–898. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golden DB. What is anaphylaxis? Curr Opin Allergy Clin Immunol. 2007;7:331–336. doi: 10.1097/ACI.0b013e3281f8290c. [DOI] [PubMed] [Google Scholar]
- 45.Tsujimura Y, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28:581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Obata K, et al. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood. 2007;110:913–920. doi: 10.1182/blood-2007-01-068718. [DOI] [PubMed] [Google Scholar]
- 47.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kroeger KM, Sullivan BM, Locksley RM. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38alpha-dependent pathway. J Leukoc Biol. 2009;86:769–778. doi: 10.1189/jlb.0708452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sasaki Y, et al. IL-18 with IL-2 protects against Strongyloides venezuelensis infection by activating mucosal mast cell-dependent type 2 innate immunity. J Exp Med. 2005;202:607–616. doi: 10.1084/jem.20042202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshimoto T, et al. IL-18 induction of IgE: dependence on CD4+ T cells, IL-4 and STAT6. Nat Immunol. 2000;1:132–137. doi: 10.1038/77811. [DOI] [PubMed] [Google Scholar]
- 51.Yoshimoto T, et al. IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc Natl Acad Sci U S A. 1999;96:13962–13966. doi: 10.1073/pnas.96.24.13962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Helmby H, Grencis RK. IL-18 regulates intestinal mastocytosis and Th2 cytokine production independently of IFN-gamma during Trichinella spiralis infection. J Immunol. 2002;169:2553–2560. doi: 10.4049/jimmunol.169.5.2553. [DOI] [PubMed] [Google Scholar]
- 53.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 54.Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 55.Florian S, et al. Detection of novel leukocyte differentiation antigens on basophils and mast cells by HLDA8 antibodies. Allergy. 2006;61:1054–1062. doi: 10.1111/j.1398-9995.2006.01171.x. [DOI] [PubMed] [Google Scholar]
- 56.Smithgall MD, et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 57.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 475:110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hida S, Tadachi M, Saito T, Taki S. Negative control of basophil expansion by IRF-2 critical for the regulation of Th1/Th2 balance. Blood. 2005;106:2011–2017. doi: 10.1182/blood-2005-04-1344. [DOI] [PubMed] [Google Scholar]
- 59.Oh K, Shen T, Le Gros G, Min B. Induction of Th2 type immunity in a mouse system reveals a novel immunoregulatory role of basophils. Blood. 2007;109:2921–2927. doi: 10.1182/blood-2006-07-037739. [DOI] [PubMed] [Google Scholar]
- 60.Kim S, et al. Cutting edge: Basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol. 184:1143–1147. doi: 10.4049/jimmunol.0902447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Charles N, Hardwick D, Daugas E, Illei GG, Rivera J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med. 16:701–707. doi: 10.1038/nm.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sullivan BM, et al. Genetic analysis of basophil function in vivo. Nat Immunol. 12:527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshimoto T, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 64.van Rijt LS, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calder PC. Abnormal fatty acid profiles occur in atopic dermatitis but what do they mean? Clin Exp Allergy. 2006;36:138–141. doi: 10.1111/j.1365-2222.2006.02433.x. [DOI] [PubMed] [Google Scholar]
- 66.Ramalingam TR, et al. Regulation of helminth-induced Th2 responses by thymic stromal lymphopoietin. J Immunol. 2009;182:6452–6459. doi: 10.4049/jimmunol.0900181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Massacand JC, et al. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc Natl Acad Sci U S A. 2009;106:13968–13973. doi: 10.1073/pnas.0906367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kullberg MC, et al. T cell-derived IL-3 induces the production of IL-4 by non-B, non-T cells to amplify the Th2-cytokine response to a non-parasite antigen in Schistosoma mansoni-infected mice. J Immunol. 1996;156:1482–1489. [PubMed] [Google Scholar]
- 69.Li M, et al. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A. 2006;103:11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]