Abstract
CRAC (Calcium Release-Activated Calcium) channels represent the primary pathway for so-called “store-operated calcium entry” – the cellular entry of calcium induced by depletion of intracellular calcium stores. These channels play a key role in diverse cellular activities, most noticeably in the differentiation and activation of Tcells, and in the response of mast cells to inflammatory signals. CRAC channels are formed by members of the recently discovered Orai protein family, with previous studies indicating that the functional channel is formed by a tetramer of Orai subunits. However, a recent report has shown that crystals obtained from the purified Drosophila Orai protein display a hexameric channel structure. Here, by comparing the biophysical properties of concatenated hexameric and tetrameric human Orai1 channels expressed in HEK293 cells, we show that the tetrameric channel displays the highly calcium-selective conductance properties consistent with endogenous CRAC channels, whilst the hexameric construct forms an essentially non-selective cation channel.
The concept of a pathway for Ca2+ entry that was activated upon depletion of internal Ca2+ stores, specifically the endoplasmic reticulum, was first proposed by Putney some 25 years ago1, and the biophysical properties of the underlying calcium release-activated calcium channels (CRAC channels) providing such an entry pathway were characterized a few years later2,3,4,5. However, the molecular identity of the CRAC channel was only revealed relatively recently6,7,8. As a result, it is now clear that the store-operated CRAC channels are formed by members of the Orai protein family. Although only one member of this family is present in non-vertebrates, three members are expressed in mammals (Orai1–3), and it is now clear that the endogenous CRAC channels in mammals are formed by a homomeric assembly of Orai1 proteins. As to the actual stoichiometric assembly of these proteins to form the functional channel, examination of various concatenated assemblies of different numbers of wild-type Orai1 subunits led us to propose that the CRAC channel was formed as a tetramer of Orai1 subunits9. Subsequently, this conformation was confirmed by other groups using a variety of different approaches including single-molecule photobleaching10,11 or brightness analysis12, and high-resolution electron microscopic examination of purified Orai proteins13, although debate remains regarding whether functional tetramers only form following activation-induced dimerization of resting dimers11,14. Recently, the proposed tetrameric stoichiometry of the functional CRAC channel has been challenged by a study examining the crystal structure formed by purified Drosophila Orai proteins15, where the authors concluded that the Orai subunits assembled as a hexamer. This proposed stoichiometry was further supported by cross-linking and size-exclusion chromatography, using partial sequences of the Drosophila Orai, specifically lacking most of the cytosolic N-terminus (119–351, and 132–341, respectively). Similar studies on the structure of crystals formed by purified proteins of other channel types, especially various K+ channel subtypes16,17, have shown that this approach can reveal important details on the structural elements determining the conductance and selectivity properties of the channel in question. However, as is common in such studies, successful crystal formation involved making certain deletions and mutations in the native Orai protein sequence. Given this, it would seem to be critical to confirm that the conductance properties of channels formed using this approach display features consistent with the native channel in question. In this context, Hou et al.15 incorporated a point mutation (V174A, equivalent to V102A in Orai1) into their expressed protein that enables constitutive activity in the absence of STIM18. Incorporation of the purified mutant protein into liposomes enabled them to demonstrate a clear flux of sodium ions in the absence of external divalent cations which was inhibited by Gd3+, and was absent in experiments using purified Orai proteins bearing the K163W mutation (equivalent to the R91W mutation in Orai1).
Results
Given the above contradictory findings, we chose to directly examine the functional properties of hexameric Orai1 channels in more detail. For this we generated a construct consisting of a concatenated hexameric assembly of full-length (i.e. unmutated) Orai1 subunits, and expressed this in HEK cells stably expressing STIM1. La3+-sensitive store-operated currents in these cells were evaluated in standard whole-cell patch clamp recordings using a Ca2+-free, Cs+-based (140 mM), internal (patch pipette) solution containing the potent InsP3-receptor agonist adenophostin A (2 μM). Under these conditions, maximal activation of store-operated Ca2+ currents occurred within approximately 85–120 s after achieving whole-cell conditions (see Supplementary Fig. S1), consistent with previous studies involving either Orai1 monomers, or concatenated Orai1 tetramers9,19. Examination of the resulting store-operated conductance measured in a Na+/Ca2+ based external solution ([Na+] = 140 mM, [Ca2+] = 10 mM) revealed a current-voltage relationship showing inward rectification, with clear outward currents observed at voltages above the reversal potential of +20 mV (Fig. 1a). Such outward currents are not seen with endogenous CRAC channel currents3,20, suggesting the presence of a significant permeability of the internal cation (Cs+). We next examined the effect of reducing extracellular Ca2+ from 10 mM to 0.1 mM, a concentration that is sufficient to render Ca2+ currents through CRAC channels to negligible levels, but is still sufficient, along with the presence of external Mg2+ (1.2 mM), to prevent any development of divalent-free currents3,21,22,23,24. This procedure resulted in only a modest reduction (less than 25%) in the inward current magnitude measured at −80 mV, and shifted the reversal potential to 0 mV (Fig. 1a). Together, these features are consistent with the presence of a significant permeability to the external Na+ ions, and only a modest overall contribution of Ca2+ ion flux. In addition, a significant (2-to-3-fold) increase in the outward currents recorded at positive potentials was seen, indicating that the reduction in extracellular Ca2+ concentration resulted in an increased permeability of the channel to Cs+ ions.
Figure 1. Selectivity properties of expressed hexameric and tetrameric Orai1 concatamers.
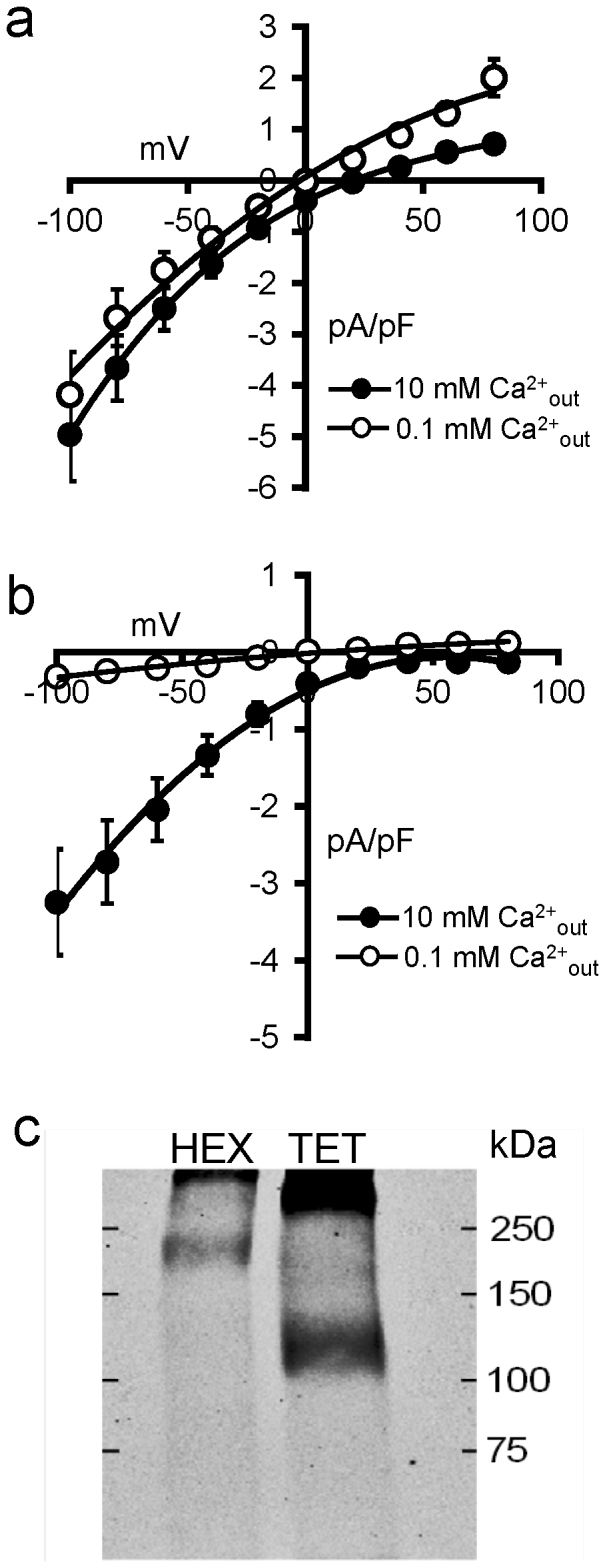
(a) Mean (±SE, n = 6) I/V curves for store-operated currents recorded between −100 mV and +80 mV in cells expressing the concatenated Orai1 hexamer in an external medium containing 140 mM Na+ and either 10 mM Ca2+, or 0.1 mM Ca2+. (b) The corresponding mean (±SE, n = 5) I/V curves for store-operated currents in cells expressing the concatenated Orai1 tetramer. (c) Western blot showing the expressed FLAG-tagged concatamers running at their appropriate expected molecular masses (approximately 195 kDa for the hexamer, and 130 kDa for the tetramer).
As a comparison, we performed the same experiments in the STIM1-stable HEK cells expressing a concatenated tetrameric assembly of Orai1 subunits. Here, store-operated currents obtained with the normal extracellular solution ([Na+] = 140 mM, [Ca2+] = 10 mM) showed strong inward-rectification with no detectible outward currents at voltages up to +80 mV (Fig. 1b). Moreover, reducing the extracellular Ca2+ concentration from 10 mM to 0.1 mM virtually eliminated all currents (inward currents at −80 mV were less than 10% of those recorded in extracellular media containing 10 mM Ca2+). Clearly, the characteristics of the current obtained upon expression of the tetrameric Orai1 construct differ markedly from those seen with the hexameric construct. More importantly, the properties of the current displayed by the tetramer closely match those reported for endogenous CRAC channels2,3,4,5, as well as those seen on simple overexpression of monomeric Orai1 and STIM125,26,27.
A recent study from the Prakriya group18 has indicated that the calcium selectivity of the channels formed by expression of Orai1 (either the wild-type Orai1, or the V102 mutant equivalent to that used in the study by Hou et al.15) can be markedly influenced, in a dose-dependent manner, by the level of co-expressed STIM1. This raises the possibility that the apparent lack of significant calcium-selectivity shown by the expressed concatenated hexamer might result from inadequate levels of cellular STIM1. To examine this, we transiently over-expressed STIM1 in our cells that were already stably expressing STIM1. Examination of the current-voltage relationship of the store-operated currents in these cells compared to those seen in the STIM1-stable cells without STIM1 overexpression and measured in the same Na+/Ca2+ based external solution ([Na+] = 140 mM, [Ca2+] = 10 mM) used above (see Fig. 1a), revealed that the overexpression of STIM1 failed to significantly affect either the overall shape of the I/V curve, the inward current magnitude measured at −80 mV (P = 0.56), the outward current measured at +60 mV (P = 0.42), or the reversal potential (P = 0.42) (see Supplementary Fig. S2). Consequently, we conclude that the relatively nonselective features of the expressed concatenated Orai1 hexamer were not a result of inadequate cellular STIM1 levels. In addition, unlike the endogenous CRAC channel3,28,29, and the expressed Orai1 concatenated tetramer19, the expressed concatenated hexamer did not display any obvious Ca2+-dependent fast-inactivation (CDI), even in the presence of 110 mM external calcium (Supplementary Fig. S3). Previous studies have shown that such a loss of CDI in Orai1 channels can result from inadequate levels of cellular STIM118. However, the demonstrated inability of overexpression of STIM1 in the STIM1-stable cells to affect the selectivity of the hexamer noted above, indicates that inadequate STIM1 is unlikely to be responsible for the absence of CDI.
The concatenated construct approach we have used is not without potential issues, particularly in relation to the correct processing of the expressed protein. However, the basic validity of this approach for Orai channels has been demonstrated in previous studies9,19,30. Nevertheless, to evaluate the intact expression of the concatenated constructs used, we performed Western blot analysis of the concatenated hexamer and tetramer constructs (Fig. 1c). The data obtained clearly indicate that each of these constructs ran as a single band of the appropriate relevant molecular size.
To further examine the nature of the currents induced on expression of the hexameric Orai1 construct, we examined the effect of changing the major external cation from Na+ to NMDG+ ([NMDG+] = 140 mM, [Ca2+] = 10 mM). Store-operated currents recorded under these conditions displayed characteristics similar to those seen with the Na+-containing extracellular solution, namely an inwardly-rectifying current-voltage relationship with significant outward currents above the reversal potential of +20 mV. As noted above, this is indicative of the presence of a modest permeability to Cs+ ions (Fig. 2a). However, in marked contrast to the currents recorded in the Na+-based external solution, reducing extracellular Ca2+ from 10 mM to 0.1 mM now virtually eliminated this current (greater than 90% reduction at −80 mV) (Fig. 2a). These results indicate that the inward current seen with NMDG+ as the extracellular monovalent cation predominantly reflects the inward flux of Ca2+ ions. The fundamental differences in conductance properties of the channels formed by these various constructs are summarized in Fig. 2b which compares the effect of changing the external Ca2+ concentration from 10 mM to 0.1 mM, on the magnitude of inward store-operated currents measured at −80 mV in cells expressing either the concatenated hexamer or the concatenated tetramer.
Figure 2. The apparent selectivity properties of the expressed Orai hexamer are influenced by the major external cation.
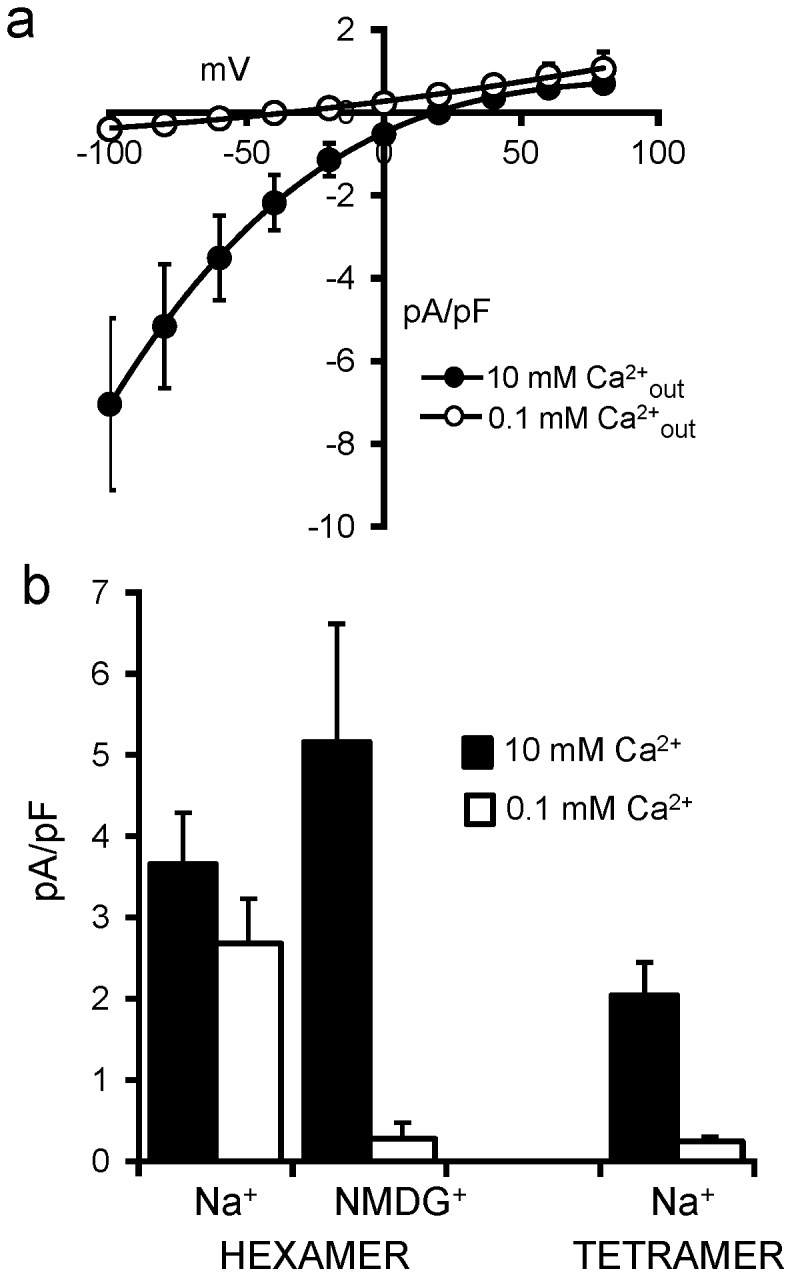
(a) Mean (±SE, n = 5) I/V curves for store-operated currents in cells expressing the concatenated Orai1 hexamer in an external medium containing 140 mM NMDG+ and either 10 mM Ca2+, or 0.1 mM Ca2+. (b) Histogram comparing of the effects of changing external calcium concentrations from 10 mM to 0.1 mM on inward currents measured at −80 mV, in cells expressing either the concatenated Orai1 hexamer or the Orai1 tetramer.
Discussion
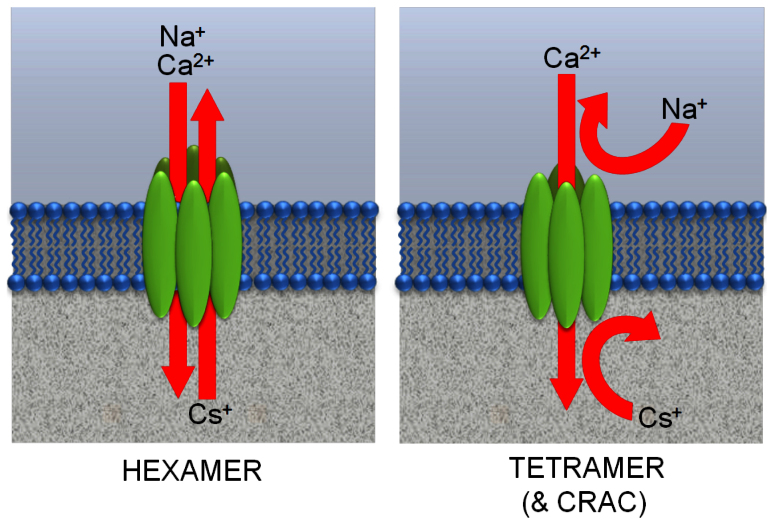
Together, these results indicate that the concatenated hexamer forms a store-operated channel that, in addition to being permeable to Ca2+ ions, is also permeable to both Na+ and Cs+ (see Fig. 3), but not to NMDG+. This permeability to both Na+ and Cs+ in the presence of external Ca2+ stands in marked contrast to the highly Ca2+-selective properties of both the concatenated Orai1 tetramer9 and native CRAC channels under the same conditions, whether examined in mammalian cells3,31,32, or in Drosophila cells7,33. We can offer no explanation as to why the crystals obtained from the purified protein used by Hou et al.15 should have assembled to form what is a clearly organized hexameric structure – a finding supported by both cross-linking and size-exclusion chromatography. Of course, our approach involving expressed concatenated proteins differs markedly from that used by Hou et al.15. However, it should be noted that the selectivity properties of the hexameric channel, as assessed by Hou et al.15 – namely significant permeability to Na+ ions (even in the presence of divalent cations), but little or no permeability to NMDG+ – are entirely duplicated by our expressed concatenated Orai1 hexamer. Critically however, further examination of the selectivity properties of the concatenated hexamer revealed marked differences from those of the endogenous CRAC channels. Consequently, based on the data obtained, we conclude that the expression of a concatenated hexameric Orai1 channel results in an essentially non-selective cation channel whose biophysical “fingerprint” fails to replicate that of endogenous CRAC channels, or that seen on expression of a concatenated tetramer of Orai1.
Figure 3. Comparison of the different selectivity properties of the channels formed by the concatenated constructs.
Diagram illustrating the distinct properties of the expressed Orai1 hexamer (left), and those of the expressed Orai1 tetramer, or endogenous CRAC channels (right).
Methods
The HEK293 cells stably expressing STIM1 were generated using the Flp-In™-293 system (Invitrogen) as previously described9. Concatenated constructs comprising the appropriate number of intact Orai1 subunits were prepared as previously described9.
Cells were transiently transfected with 0.75 μg of the Orai1 tetramer M070 or hexamer M070 along with 0.25 μg YFP using an Amaxa Nucleofector. In some experiments, 0.5 μg of STIM1pcmv6 was co-transfected with the Orai1 hexamer M070. Transfected cells were incubated in EMEM + 10% FBS in a 5% CO2 incubator for 40–48 hours before electrophysiological experiments were performed.
Electrophysiology
Patch clamp experiments were performed at room temperature (20–22°C) on cells 18–24 hours after plating on coverslips. Whole-cell current recordings were obtained using 250 ms voltage pulses to −80 mV delivered every 2 s from a holding potential of 0 mV. To obtain current-voltage relationships, 10 ms pulses to potentials between −100 mV and +80 mV were applied at 20 mV intervals. The standard internal (pipette) solution contained 140 mM CsC2H3O2, 7.0 mM MgCl2, 10 mM EGTA, and 10 mM Hepes (pH 7.2). Maximal activation of store-operated currents was achieved by inclusion of the potent InsP3 receptor agonist adenophostin A (2 μM) in this Ca2+-free solution. In addition, the free Mg2+ concentration in the pipette solution was set at 5 mM to prevent any contamination from the so-called MIC or MagNuM (TRPM7) currents32,34,35. The standard extracellular solution contained 140 mM NaCl, 5 mM CsCl, 1.2 mM MgCl2, 10 mM CaCl2, 20 mM glucose, and 10 mM Hepes (pH 7.4). All currents were corrected by leak subtraction using currents obtained at the end of each experiment in an external solution containing La3+ (100 μM).
Western blot of concatenated Orai1 tetramers and hexamers
Cells stably expressing STIM1 were transfected with 2.5 μg of either the Orai1 tetramer or Orai1 hexamer in the M070 vector using Lipofectamine LTX (Invitrogen) for 48 hours. The cells were washed twice with ice-cold Tris buffered saline (TBS) and then lysed in 500 μl IP lysis buffer (Pierce) plus a “complete Mini protease inhibitor tablet” (Roche), transferred to an ice cold dounce homogenizer with a tight pestle and ground 25 times on ice. The samples were left on ice for 30 minutes. The lysate was then spun at 13000 g at 4°C for 10 minutes to spin down the nuclei and cell debris. 7.5 μl of a FLAG polyclonal antibody (Cell signaling #2368) was added to the supernatant and incubated at 4°C, rotating for 2 hours. 80 μl of lysis buffer prewashed A/G beads were added and rotated for 2 hours at 4°C, followed by washing five times with lysis buffer, and a final wash with TBS. The samples were run on a 7% SDS acrylamide gel. 2× SDS loading buffer plus DTT was added to the samples and heated to 80°C for 5 minutes. 20 μl of each sample was loaded on to the gel along with a Precision Plus dual color standard ladder (BioRad). Gels were transferred onto nitrocellulose in transfer buffer containing 25 mM Tris Base, 192 mM glycine, 10% methanol and 0.04% SDS in a wet transfer cassette at 23 mA constant current. The blot was probed with 1:1000 anti-FLAG polyclonal antibody (Cell Signaling #2368) followed by IRDye 800CW anti-rabbit secondary antibody at 1:10,000 dilution. Blots were analyzed on an Odyssey Scanner (Licor).
Author Contributions
Both TJS and JLT contributed to the conception and analysis of the experiments, which were mainly carried out by JLT. TJS wrote the manuscript, with assistance from JLT.
Supplementary Material
Supplemental Information
Acknowledgments
We thank Dr. R. Dirksen for helpful comments on an early version of this manuscript. This study was supported National Institutes of Health grant GM040457 to TJS.
References
- Putney J. W. Jr A model for receptor-regulated calcium entry. Cell Calcium 7, 1–12 (1986). [DOI] [PubMed] [Google Scholar]
- Hoth M. & Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 355, 353–356 (1992). [DOI] [PubMed] [Google Scholar]
- Hoth M. & Penner R. Calcium release-activated calcium current in rat mast cells. J. Physiol. 465, 359–386 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. S. & Cahalan M. D. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul. 1, 99–112 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A. & Lewis R. S. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc. Natl. Acad. Sci. USA 90, 6295–6299 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S. et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185 (2006). [DOI] [PubMed] [Google Scholar]
- Zhang S. L. et al. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc. Natl. Acad. Sci. USA 103, 9357–9362 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M. et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312, 1220–1223 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignen O., Thompson J. L. & Shuttleworth T. J. Orai1 subunit stoichiometry of the mammalian CRAC channel pore. J. Physiol. 586, 419–425 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W. et al. Functional stoichiometry of the unitary calcium-release-activated calcium channel. Proc. Natl. Acad. Sci. USA 105, 13668–13673 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna A. et al. The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature 456, 116–120 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madl J. et al. Resting state Orai1 diffuses as homotetramer in the plasma membrane of live mammalian cells. J. Bio. l Chem. 285, 41135–41142 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y. et al. Tetrameric Orai1 Is a teardrop-shaped molecule with a long, tapered cytoplasmic domain. J. Biol. Chem. 284, 13676–13685 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuro A. et al. Subunit stoichiometry of human Orai1 and Orai3 channels in closed and open states. Proc. Natl. Acad. Sci. USA 108, 17832–17837 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X., Pedi L., Diver M. M. & Long S. B. Crystal structure of the calcium release-activated calcium channel Orai. Science 338, 1308–1313 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L., Kolmakova-Partensky L. & Miller C. Functional reconstitution of a prokaryotic K+ channel. J. Gen. Physiol. 111, 741–749 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Morais-Cabral J. H., Kaufman A. & MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature 414, 43–48 (2001). [DOI] [PubMed] [Google Scholar]
- McNally B. A., Somasundaram A., Yamashita M. & Prakriya M. Gated regulation of CRAC channel ion selectivity by STIM1. Nature 482, 241–245 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. L., Mignen O. & Shuttleworth T. J. The Orai1 severe combined immune deficiency mutation and calcium release-activated Ca2+ channel function in the heterozygous condition. J. Biol. Chem. 284, 6620–6626 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A. B. & Putney J. W. Jr Store-operated calcium channels. Physiol. Rev. 85, 757–810 (2005). [DOI] [PubMed] [Google Scholar]
- Bakowski D. & Parekh A. B. Monovalent cation permeability and Ca2+ block of the store-operated Ca2+ current ICRAC in rat basophilic leukemia cells. Pflugers Archiv. 443, 892–902 (2002). [DOI] [PubMed] [Google Scholar]
- Bakowski D. & Parekh A. B. Permeation through store-operated CRAC channels in divalent-free solution: potential problems and implications for putative CRAC channel genes. Cell Calcium 32, 379–391 (2002). [DOI] [PubMed] [Google Scholar]
- Lepple-Wienhues A. & Cahalan M. D. Conductance and permeation of monovalent cations through depletion-activated Ca2+ channels (ICRAC) in Jurkat T cells. Biophys. J. 71, 787–794 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M., Navarro-Borelly L., McNally B. A. & Prakriya M. Orai1 mutations alter ion permeation and Ca2+-dependent fast inactivation of CRAC channels: evidence for coupling of permeation and gating. J. Gen. Physiol 130, 525–540 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinelt C. et al. Amplification of CRAC current by STIM1 and CRACM1 (Orai1). Nat. Cell Biol. 8, 771–773 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboloff J. et al. Orai1 and STIM reconstitute store-operated calcium channel function. J. Biol. Chem. 281, 20661–20665 (2006). [DOI] [PubMed] [Google Scholar]
- Mercer J. C. et al. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J. Biol. Chem. 281, 24979–24990 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A. & Lewis R. S. Rapid inactivation of depletion-activated calcium current (ICRAC) due to local calcium feedback. J. Gen. Physiol. 105, 209–226 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro L. & Parekh A. B. Fast calcium-dependent inactivation of calcium release-activated calcium current (CRAC) in RBL-1 cells. J. Membr. Biol. 168, 9–17 (1999). [DOI] [PubMed] [Google Scholar]
- Mignen O., Thompson J. L. & Shuttleworth T. J. The molecular architecture of the arachidonate-regulated Ca2+-selective ARC channel is a pentameric assembly of Orai1 and Orai3 subunits. J. Physiol. 587, 4181–4197 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A. B. & Penner R. Store depletion and calcium influx. Physiol. Rev. 77, 901–930 (1997). [DOI] [PubMed] [Google Scholar]
- Prakriya M. & Lewis R. S. Separation and characterization of currents through store-operated CRAC channels and Mg2+-inhibited cation (MIC) channels. J. Gen. Physiol. 119, 487–507 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeromin A. V., Roos J., Stauderman K. A. & Cahalan M. D. A store-operated calcium channel in Drosophila S2 cells. J. Gen. Physiol 123, 167–182 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermosura M. C., Monteilh-Zoller M. K., Scharenberg A. M., Penner R. & Fleig A. Dissociation of the store-operated calcium current ICRAC and the Mg-nucleotide-regulated metal ion current MagNuM. J. Physiol. 539, 445–458 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak J. A., Kerschbaum H. H. & Cahalan M. D. Distinct properties of CRAC and MIC channels in RBL cells. J. Gen. Physiol. 120, 221–235 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Information