Abstract
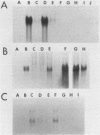
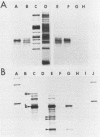
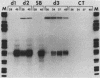
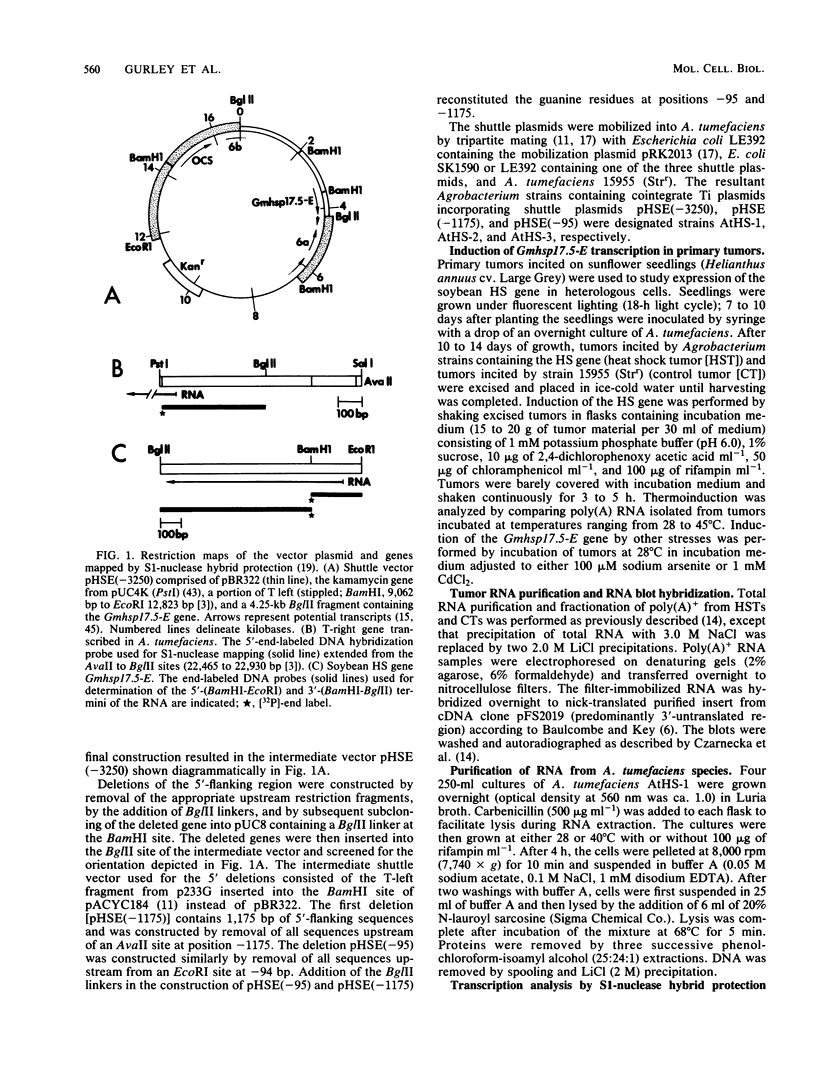
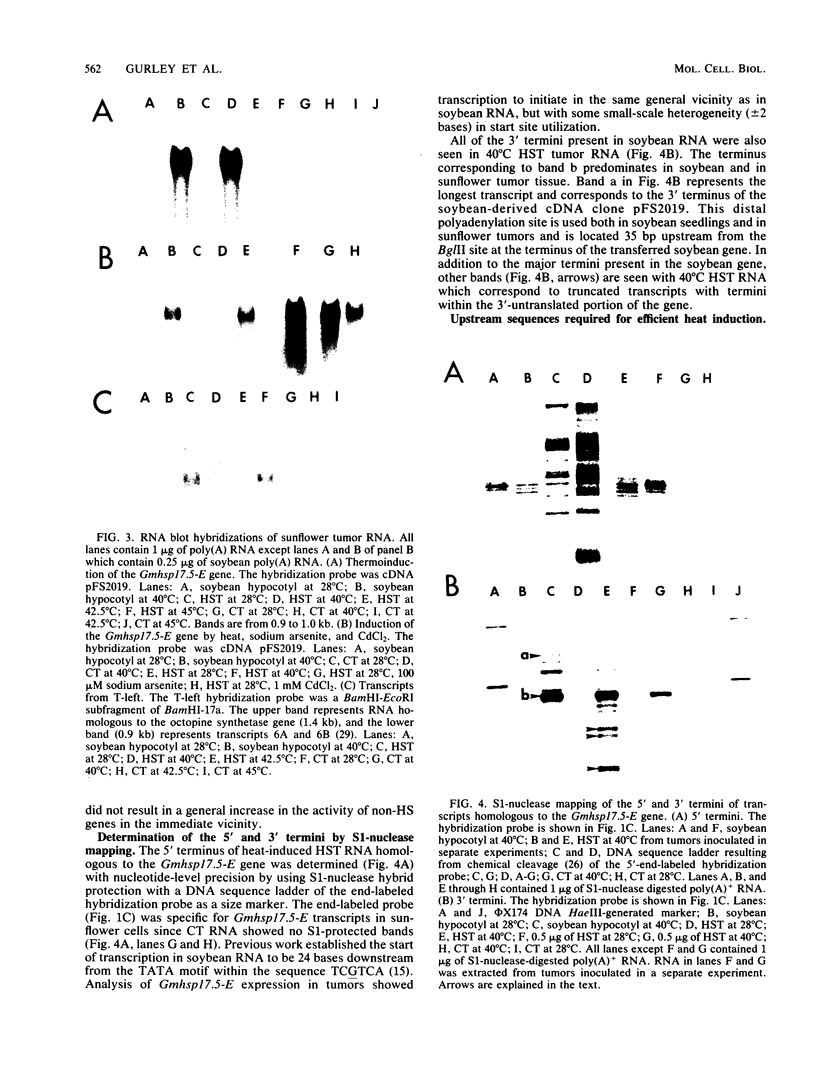
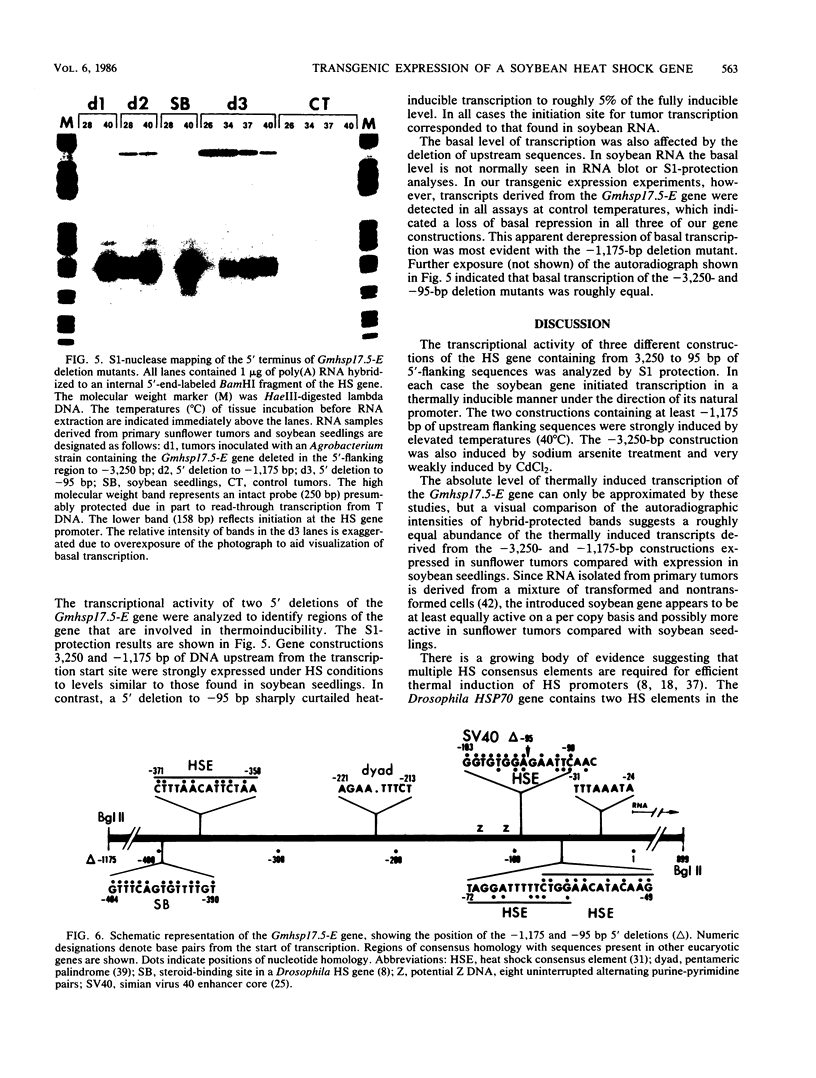
A soybean gene (Gmhsp17.5-E) encoding a small heat shock protein was introduced into primary sunflower tumors via T-DNA-mediated transformation. RNA blot hybridizations and S1-nuclease hybrid protection studies indicated that the heat shock gene containing 3.25 kilobases of 5'-flanking sequences was strongly transcribed in a thermoinducible (40 degrees C) manner. Transcriptional induction also occurred to a lesser extent upon treatment of whole tumors with sodium arsenite and CdCl2. Basal (26 degrees C) transcription was not detected in soybean seedlings, but it was quite evident in transformed tumor tissue. A 5' deletion to -1,175 base pairs with respect to the CAP site had no effect on the levels of thermoinducible transcription, but it resulted in a large increase in basal transcription. Further removal of DNA sequences (including the TATA-distal heat shock consensus element) to -95 base pairs reduced thermoinducible transcription by 95% and also greatly decreased basal transcription. The termini of the Gmhsp17.5-E RNA in the tumor were generally the same as those present in soybean RNA, with the exception of several additional 3' termini.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Baszczynski C. L., Walden D. B., Atkinson B. G. Regulation of gene expression in corn (Zea Mays L.) by heat shock. Can J Biochem. 1982 May;60(5):569–579. doi: 10.1139/o82-070. [DOI] [PubMed] [Google Scholar]
- Bienz M. Developmental control of the heat shock response in Xenopus. Proc Natl Acad Sci U S A. 1984 May;81(10):3138–3142. doi: 10.1073/pnas.81.10.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Burke J. F., Ish-Horowicz D. Expression of Drosophila heat shock genes is regulated in Rat-cells. Nucleic Acids Res. 1982 Jul 10;10(13):3821–3830. doi: 10.1093/nar/10.13.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Schilling-Cordaro C., Mergia A., Houck C. M. A new technique for genetic engineering of Agrobacterium Ti plasmid. Plasmid. 1983 Jul;10(1):21–30. doi: 10.1016/0147-619x(83)90054-9. [DOI] [PubMed] [Google Scholar]
- Cooper P., Ho T. H., Hauptmann R. M. Tissue specificity of the heat-shock response in maize. Plant Physiol. 1984 Jun;75(2):431–441. doi: 10.1104/pp.75.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corces V., Pellicer A., Axel R., Meselson M. Integration, transcription, and control of a Drosophila heat shock gene in mouse cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7038–7042. doi: 10.1073/pnas.78.11.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecka E., Gurley W. B., Nagao R. T., Mosquera L. A., Key J. L. DNA sequence and transcript mapping of a soybean gene encoding a small heat shock protein. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3726–3730. doi: 10.1073/pnas.82.11.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beuckeleer M., Lemmers M., De Vos G., Willmitzer L., Van Montagu M., Schell J. Further insight on the transferred-DNA of octopine crown gall. Mol Gen Genet. 1981;183(2):283–288. doi: 10.1007/BF00270630. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudler R., Travers A. A. Upstream elements necessary for optimal function of the hsp 70 promoter in transformed flies. Cell. 1984 Sep;38(2):391–398. doi: 10.1016/0092-8674(84)90494-x. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Key J. L., Lin C. Y., Chen Y. M. Heat shock proteins of higher plants. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3526–3530. doi: 10.1073/pnas.78.6.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Merlo D. J., Nutter R. C., Montoya A. L., Garfinkel D. J., Drummond M. H., Chilton M. D., Gordon M. P., Nester E. W. The boundaries and copy numbers of Ti plasmid T-DNA vary in crown gall tumors. Mol Gen Genet. 1980;177(4):637–643. doi: 10.1007/BF00272674. [DOI] [PubMed] [Google Scholar]
- Mirault M. E., Southgate R., Delwart E. Regulation of heat-shock genes: a DNA sequence upstream of Drosophila hsp70 genes is essential for their induction in monkey cells. EMBO J. 1982;1(10):1279–1285. doi: 10.1002/j.1460-2075.1982.tb00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao R. T., Czarnecka E., Gurley W. B., Schöffl F., Key J. L. Genes for low-molecular-weight heat shock proteins of soybeans: sequence analysis of a multigene family. Mol Cell Biol. 1985 Dec;5(12):3417–3428. doi: 10.1128/mcb.5.12.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor binds to the regulatory site of an hsp 70 gene. Cell. 1984 May;37(1):273–283. doi: 10.1016/0092-8674(84)90323-4. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Bienz M. A synthetic heat-shock promoter element confers heat-inducibility on the herpes simplex virus thymidine kinase gene. EMBO J. 1982;1(11):1473–1477. doi: 10.1002/j.1460-2075.1982.tb01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russnak R. H., Jones D., Candido E. P. Cloning and analysis of cDNA sequences coding for two 16 kilodalton heat shock proteins (hsps) in Caenorhabditis elegans: homology with the small hsps of Drosophila. Nucleic Acids Res. 1983 May 25;11(10):3187–3205. doi: 10.1093/nar/11.10.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon F., Deblaere R., Leemans J., Hernalsteens J. P., Van Montagu M., Schell J. Genetic identification of functions of TR-DNA transcripts in octopine crown galls. EMBO J. 1984 Jan;3(1):141–146. doi: 10.1002/j.1460-2075.1984.tb01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F., Baumann G. Thermo-induced transcripts of a soybean heat shock gene after transfer into sunflower using a Ti plasmid vector. EMBO J. 1985 May;4(5):1119–1124. doi: 10.1002/j.1460-2075.1985.tb03748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F., Key J. L. An analysis of mRNAs for a group of heat shock proteins of soybean using cloned cDNAs. J Mol Appl Genet. 1982;1(4):301–314. [PubMed] [Google Scholar]
- Schöffl F., Raschke E., Nagao R. T. The DNA sequence analysis of soybean heat-shock genes and identification of possible regulatory promoter elements. EMBO J. 1984 Nov;3(11):2491–2497. doi: 10.1002/j.1460-2075.1984.tb02161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. A., Sutton C. A., Lobell R. B., Glaser R. L., Lis J. T. Determinants of heat shock-induced chromosome puffing. Cell. 1985 Apr;40(4):805–817. doi: 10.1016/0092-8674(85)90340-x. [DOI] [PubMed] [Google Scholar]
- Southgate R., Ayme A., Voellmy R. Nucleotide sequence analysis of the Drosophila small heat shock gene cluster at locus 67B. J Mol Biol. 1983 Mar 25;165(1):35–57. doi: 10.1016/s0022-2836(83)80241-1. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Montoya A. L., Gordon M. P., Nester E. W. Integration and organization of Ti plasmid sequences in crown gall tumors. Cell. 1980 Mar;19(3):729–739. doi: 10.1016/s0092-8674(80)80049-3. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Voellmy R., Rungger D. Transcription of a Drosophila heat shock gene is heat-induced in Xenopus oocytes. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1776–1780. doi: 10.1073/pnas.79.6.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmitzer L., Simons G., Schell J. The TL-DNA in octopine crown-gall tumours codes for seven well-defined polyadenylated transcripts. EMBO J. 1982;1(1):139–146. doi: 10.1002/j.1460-2075.1982.tb01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. Two protein-binding sites in chromatin implicated in the activation of heat-shock genes. Nature. 1984 May 17;309(5965):229–234. doi: 10.1038/309229a0. [DOI] [PubMed] [Google Scholar]