Abstract
The pR plasmid, which enhances the survival of Escherichia coli C600 exposed to UV light by induction of the SOS regulatory mechanism, showed the same effect when it transformed mouse LTA cells (tk-, aprt-). With Tn5 insertion mutagenesis which inactivates UV functions in the pR plasmid, we recognized two different regions of the plasmid, uvp1 and uvp2. These pR UVR- mutants exhibited the same effect in LTA transformed cells, demonstrating that resistance to UV light, carried by the pR plasmid, was really due to the expression of these two regions, which were also in the mouse cells. Statistical analysis showed that the expression of the uvp1 and uvp2 regions significantly increased (P less than 0.01) the survival upon exposure to UV light in mouse cells and bacteria. These results might suggest the presence of an inducible repair response to DNA damage in mouse LTA cells.
Full text
PDF
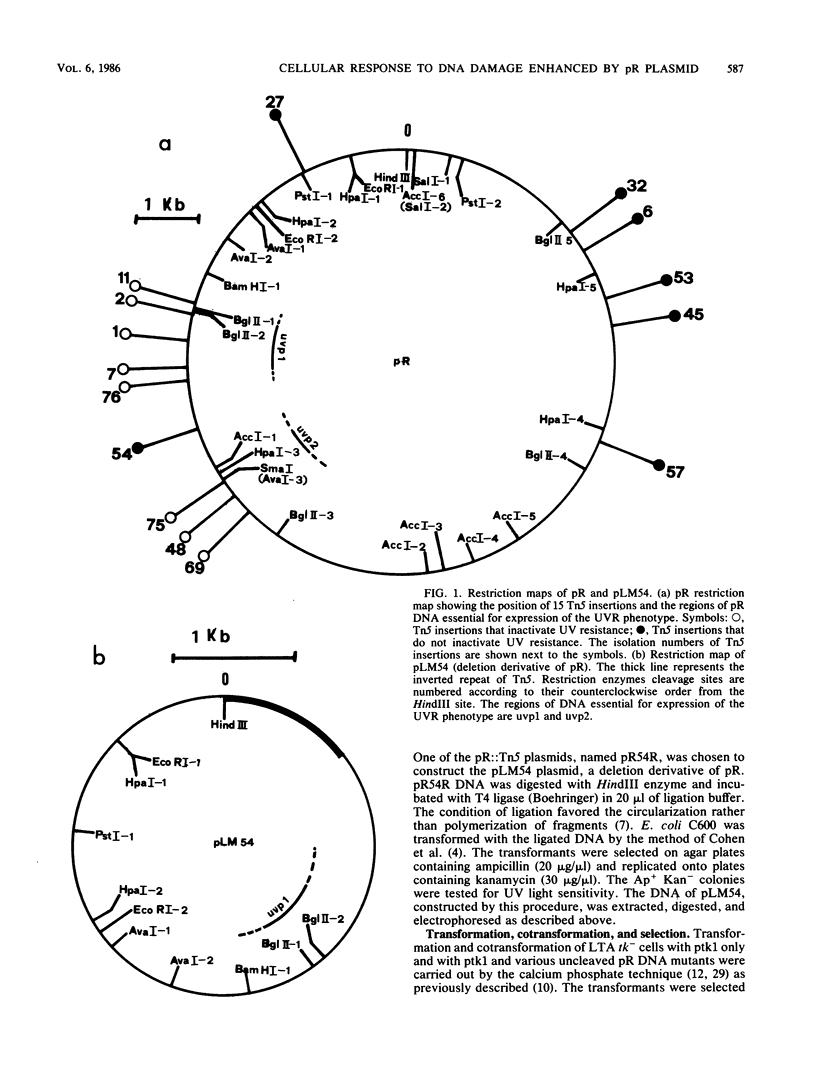
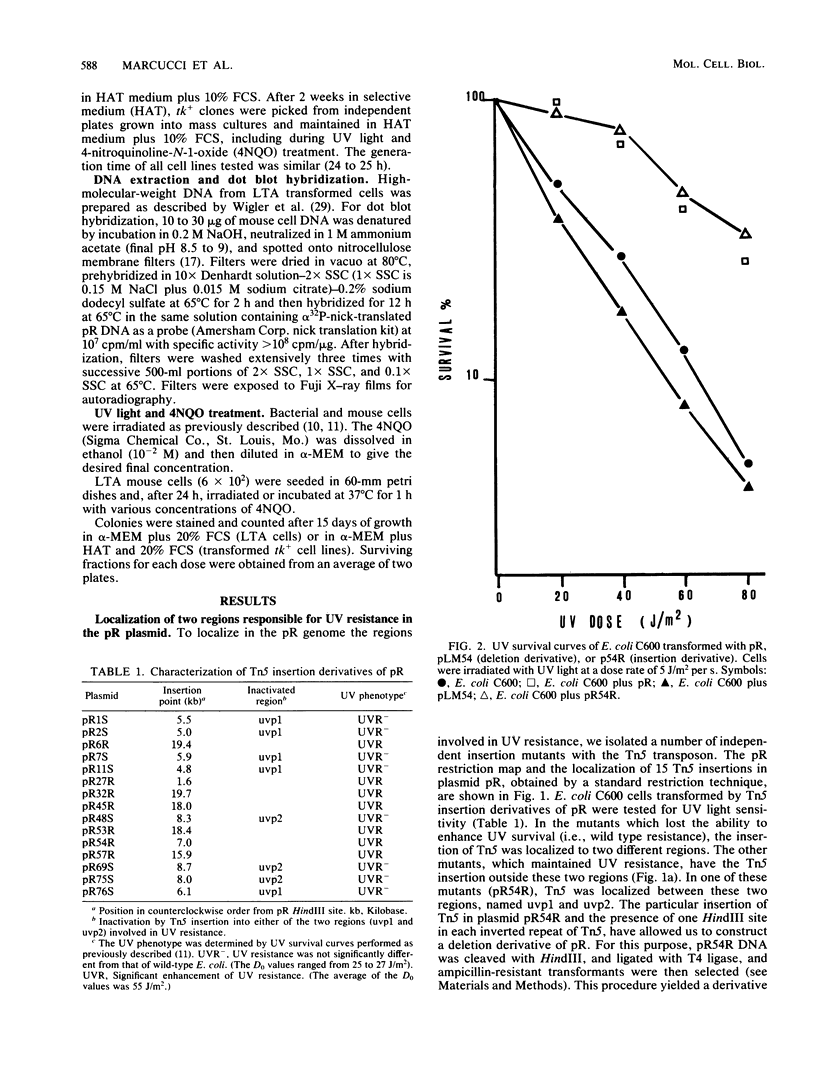
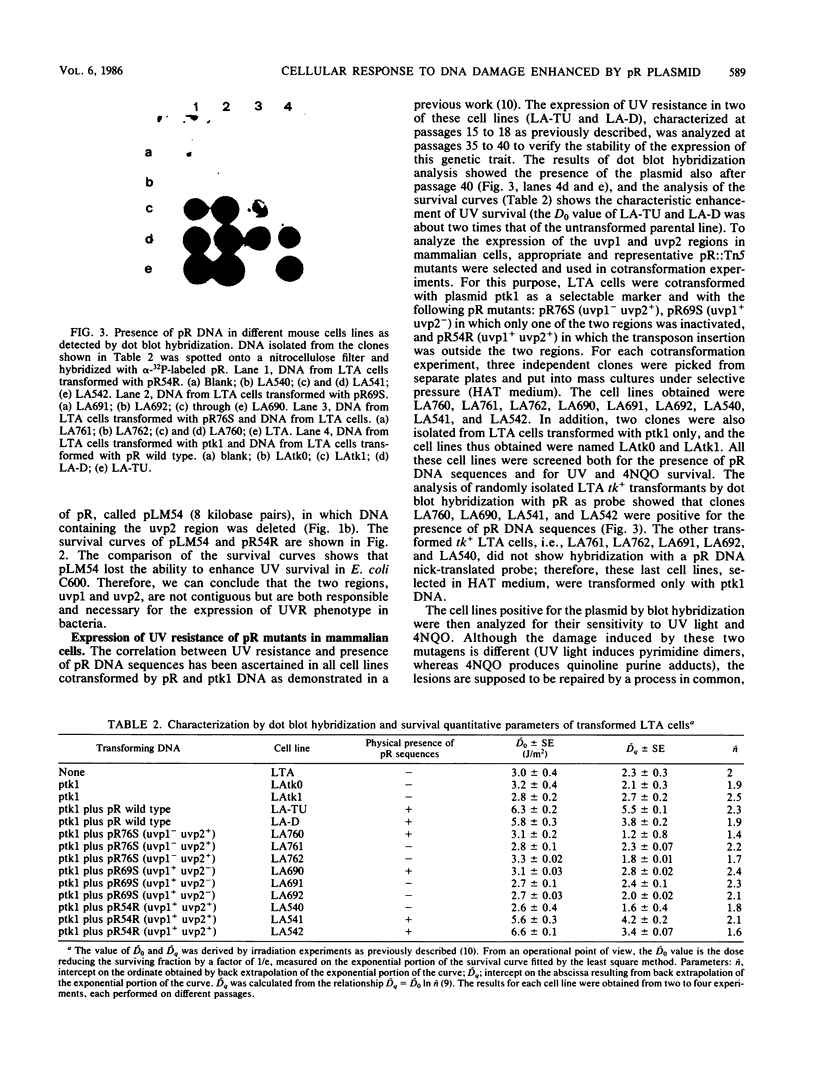


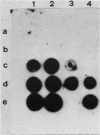
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bockstahler L. E., Lytle C. D. Ultraviolet light enhanced reactivation of a mammalian virus. Biochem Biophys Res Commun. 1970 Oct 9;41(1):184–189. doi: 10.1016/0006-291x(70)90486-9. [DOI] [PubMed] [Google Scholar]
- Cohen J. D., Eccleshall T. R., Needleman R. B., Federoff H., Buchferer B. A., Marmur J. Functional expression in yeast of the Escherichia coli plasmid gene coding for chloramphenicol acetyltransferase. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1078–1082. doi: 10.1073/pnas.77.2.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta U. B., Summers W. C. Ultraviolet reactivation of herpes simplex virus is mutagenic and inducible in mammlian cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2378–2381. doi: 10.1073/pnas.75.5.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugaiczyk A., Boyer H. W., Goodman H. M. Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol. 1975 Jul 25;96(1):171–184. doi: 10.1016/0022-2836(75)90189-8. [DOI] [PubMed] [Google Scholar]
- Elder J. T., Spritz R. A., Weissman S. M. Simian virus 40 as a eukaryotic cloning vehicle. Annu Rev Genet. 1981;15:295–340. doi: 10.1146/annurev.ge.15.120181.001455. [DOI] [PubMed] [Google Scholar]
- Elli R., Marcucci L., Bosi R., Oppi C., Battaglia P. A., Gigliani F. The pR UV+ plasmid, transfected into mammalian cells, enhances their UV survival. Nucleic Acids Res. 1983 Jun 11;11(11):3679–3686. doi: 10.1093/nar/11.11.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliani F., Elli R., Marcucci L., Battaglia P. A. Identification of a protein coded by pR plasmid and involved in SOS repair in E. coli. Nucleic Acids Res. 1981 Feb 11;9(3):623–631. doi: 10.1093/nar/9.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Jeeves W. P., Rainbow A. J. U.V. enhanced reactivation of U.V.-and gamma-irradiated adenovirus in normal human fibroblasts. Int J Radiat Biol Relat Stud Phys Chem Med. 1983 Jun;43(6):599–623. doi: 10.1080/09553008314550721. [DOI] [PubMed] [Google Scholar]
- Jimenez A., Davies J. Expression of a transposable antibiotic resistance element in Saccharomyces. Nature. 1980 Oct 30;287(5785):869–871. doi: 10.1038/287869a0. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. J., Shanabruch W. G., Walker G. C. Functional organization of plasmid pKM101. J Bacteriol. 1981 Mar;145(3):1310–1316. doi: 10.1128/jb.145.3.1310-1316.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Lytle C. D., Goddard J. G., Lin C. H. Repair and mutagenesis of herpes simplex virus in UV-irradiated monkey cells. Mutat Res. 1980 Apr;70(2):139–149. doi: 10.1016/0027-5107(80)90153-0. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Potter A. A., Nestmann E. R., Iyer V. N. Introduction of the plasmid pKM101-associated muc genes into Saccharomyces cerevisiae. Mutat Res. 1984 May-Jun;131(5-6):197–204. doi: 10.1016/0167-8817(84)90025-7. [DOI] [PubMed] [Google Scholar]
- Radman M. Is there SOS induction in mammalian cells? Photochem Photobiol. 1980 Dec;32(6):823–830. doi: 10.1111/j.1751-1097.1980.tb04062.x. [DOI] [PubMed] [Google Scholar]
- Sarasin A., Benoit A. Induction of an error-prone mode of DNA repair in UV-irradiated monkey kidney cells. Mutat Res. 1980 Mar;70(1):71–81. doi: 10.1016/0027-5107(80)90059-7. [DOI] [PubMed] [Google Scholar]
- Sarasin A., Bourre F., Benoit A. Error-prone replication of ultraviolet-irradiated simian virus 40 in carcinogen-treated monkey kidney cells. Biochimie. 1982 Aug-Sep;64(8-9):815–821. doi: 10.1016/s0300-9084(82)80135-1. [DOI] [PubMed] [Google Scholar]
- Takebe H., Furuyama J. I., Miki Y., Kondo S. High sensitivity of Xeroderma pigmentosum cells to the carcinogen 4-nitroguinoline-1-oxide. Mutat Res. 1972 May;15(1):98–100. doi: 10.1016/0027-5107(72)90099-1. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster T. D., Dickson R. C. Direct selection of Saccharomyces cerevisiae resistant to the antibiotic G418 following transformation with a DNA vector carrying the kanamycin-resistance gene of Tn903. Gene. 1983 Dec;26(2-3):243–252. doi: 10.1016/0378-1119(83)90194-4. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]