Background: The molecular mechanisms of endotoxin tolerance remain not well elucidated.
Results: IRG1, up-regulated by LPS and during sepsis, can feedback suppress the Toll-like receptor-triggered inflammatory response by increasing A20 expression via reactive oxygen species (ROS) in LPS-tolerized macrophages.
Conclusion: Inducible IRG1 promotes endotoxin tolerance by increasing A20 expression through ROS.
Significance: Providing new molecular mechanisms regulating hypoinflammation of sepsis and endotoxin tolerance.
Keywords: Inflammation, Innate Immunity, Macrophages, Reactive Oxygen Species (ROS), Toll-like Receptors (TLR), A20, Endotoxin Tolerance, Immune Responsive Gene 1, ROS
Abstract
Sepsis-associated immunosuppression (SAIS) is regarded as one of main causes for the death of septic patients at the late stage because of the decreased innate immunity with a more opportunistic infection. LPS-tolerized macrophages, which are re-challenged by LPS after prior exposure to LPS, are regarded as the common model of hypo-responsiveness for SAIS. However, the molecular mechanisms of endotoxin tolerance and SAIS remain to be fully elucidated. In addition, negative regulation of the Toll-like receptor (TLR)-triggered innate inflammatory response needs further investigation. Here we show that expression of immune responsive gene 1 (IRG1) was highly up-regulated in the peripheral blood mononuclear cells of septic patients and in LPS-tolerized mouse macrophages. IRG1 significantly suppressed TLR-triggered production of proinflammatory cytokines TNF-α, IL-6, and IFN-β in LPS-tolerized macrophages, with the elevated expression of reactive oxygen species (ROS) and A20. Moreover, ROS enhanced A20 expression by increasing the H3K4me3 modification of histone on the A20 promoter domain, and supplement of the ROS abrogated the IRG1 knockdown function in breaking endotoxin tolerance by increasing A20 expression. Our results demonstrate that inducible IRG1 promotes endotoxin tolerance by increasing A20 expression through ROS, indicating a new molecular mechanism regulating hypoinflammation of sepsis and endotoxin tolerance.
Introduction
Sepsis, sepsis-induced hyperinflammation, and subsequent sepsis-associated immunosuppression (SAIS)4 are important causes of death worldwide. During sepsis, patients may die early from bacteremia or hyperinflammation, an uncontrolled overactivation of the innate immune system with high amounts of circulating proinflammatory cytokines, or from sepsis-associated multiorgan failure (1–3). Meanwhile, in addition to eliciting a robust inflammatory response, sepsis also paradoxically renders the host an immunocompromised state at the late stage, which is named SAIS. SAIS often leads many septic patients to die from secondary infections, which is characterized by neutrophil paralysis and lymphopenia. However, the molecular mechanisms for the SAIS remain to be fully investigated. Therefore, better understanding of the precise mechanism of SAIS is of important significance to the development of new approaches to prevent and treat this life threatening disease.
Toll-like receptors (TLRs) are one of the most important sensors of the innate immune system by detecting the conserved pathogen-associated molecular pattern in host defense against infection (4, 5). TLRs induce production of proinflammatory cytokines and type I interferon through MyD88 and TRIF by activating MAPK, NF-κB, and IRF3 pathways (6). It is now known that full activation of TLRs is essential for initiating the innate immune response and enhancing adaptive immunity to eliminate invading pathogens. However, TLR signaling must be precisely controlled to avoid inappropriate activation or overactivation of TLR-triggered inflammatory innate responses, which may cause immune disorders such as septic shock and autoimmune diseases. Up to now, some negative regulators of TLR-triggered inflammatory innate response have been identified, which contribute to maintain the immunological balance (7–9). However, new negative regulators of the TLR-triggered inflammatory innate response should be further identified in the innate cells, especially at the late stage of the inflammatory innate response and even in sepsis, as some molecules may be induced to be up-regulated to feedback inhibit the inflammatory response or sepsis.
It has been shown that suppression of proinflammatory cytokine production in endotoxin-tolerized macrophages closely resembles the phenotype of monocytes from immunocompromised septic patients, which is the well established animal model of human SAIS (10–12). LPS-tolerized macrophages, which are re-challenged by LPS after prior exposure to LPS, are regarded as the common model of hypo-responsiveness for SAIS (13–15). The studies showed that endotoxin tolerance reprograms TLR4 signaling with decreased proinflammatory cytokine production but increased anti-proinflammatory cytokine production by decreasing TLR4 expression, blocking tyrosine phosphorylation of TLR4 and TIRAP, TLR4-MyD88, and IRAK1-MyD88 assemblies, attenuating activation of IRAK4 and IRAK1, inhibiting K63-linked ubiquitination of IRAK1 and TRAF6, or increasing expression of negative regulators IRAK-M, SHIP-1, suppressor of cytokine signaling 1 (SOCS1), and A20 (13, 16–18). Recently, ROS-induced ATF3 expression and TNF-activated GSK3 were found to be related with SAIS by mediating chromatin remodeling (19, 20). However, the molecular mechanisms for endotoxin tolerance such as identification of epigenetic regulation needs to be further investigated.
Immune responsive gene 1 (IRG1), cloned as a LPS-inducible gene in 1994, was found to play important roles in embryonic implantation and neurodegeneration (21, 22). Its biological function in innate immune response remains largely unknown except reports about its inducible expression by stimulation with LPS, infections with Mycobacterium avium subspecies paratuberculosis (23, 24), Chlamydia pneumoniae (GEO microarray data, GDS2651, 25), zymosan (GEO microarray data, GSM147169, 26) or active virus compared with inactive virus (GEO microarray data, GDS1271, 27). IRG1 expression is also found to be dysregulated in autoimmune or inflammatory diseases. According to a set of gene profiling data of spinal cords from EAE mice (GEO microarray data, GSM13053, 28), IRG1 was significantly up-regulated in EAE spinal cords (6-fold in EAE spinal cords relative to control; p < 0.01 by Welch's t test). Therefore, IRG1 is predicated to be involved in pathogenesis of the inflammatory autoimmune diseases. In this study, we found that IRG1 expression was highly up-regulated in peripheral blood mononuclear cells (PBMC) of patients with sepsis. Accordingly, mRNA and protein expression of IRG1 was significantly up-regulated in LPS-tolerized mouse macrophages. Furthermore, we found that knockdown of IRG1 by small interfering RNA (siRNA) did not affect TLR-induced production of proinflammatory cytokines (TNF-α and IL-6) and IFN-β in wild-type macrophages, but could significantly increase the production of these cytokines in LPS-tolerized macrophages. Mechanically, we found that knockdown of IRG1 increased activation of NF-κB and IRF3 accompanied with decreased A20 expression and ROS production. Importantly, increased ROS by H2O2 abrogated the role of IRG1 knockdown in LPS-tolerized macrophages, as evidenced with decreased activation of NF-κB and IRF3, and reduced production of proinflammatory cytokines and IFN-β. ROS was found to increase A20 expression by increasing the H3K4me3 modification of histone on the A20 promoter domain. Therefore, our results provide new mechanistic insight to endotoxin tolerance by demonstrating that IRG1, up-regulated significantly by LPS and during sepsis, can feedback suppress the TLR-triggered inflammatory response by increasing A20 expression via ROS in LPS-tolerized macrophages. Also, our study outlines a potential target to be possibly manipulated to prevent SAIS in clinics.
EXPERIMENTAL PROCEDURES
Subjects
We included 9 subjects with sepsis from the surgical ICU, Changhai Hospital (Shanghai, China), after the study was approved by the local ethics committee of Second Military Medical University, Shanghai, China. The preliminary diagnosis of sepsis was made with well accepted guidelines (29). Therapeutic strategy was carried out according to the standard protocol for sepsis (30, 31). Exclusion criteria included pregnancy, age <18 years, a history of chronic heart failure, or chronic renal failure. On ICU admission, the mean SOFA scores were 7.8. Ten ml of whole blood was collected from subjects within 24 h after the diagnosis of sepsis (acute sepsis group), or 1 day after leaving the ICU with body recovery (after sepsis group). Whole blood from five healthy volunteers served as controls.
Mice and Cell Culture
C57BL/6J mice were from Joint Ventures Sipper BK Experimental Animals Co. (Shanghai, China). All mice were bred in specific pathogen-free conditions. All animal experiments were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of Second Military Medical University, Shanghai. Thioglycollate-elicited mouse peritoneal macrophages were prepared and cultured in endotoxin-free RPMI1640 medium with 10% FCS as described previously (32).
Plasmids Construction and Stable Transfection
Recombinant vectors encoding murine Irg1 (NM_008392) was constructed by PCR-based amplification of cDNA from primary peritoneal macrophages of C57BL/6J mice, and then subcloned into the pcDNA3.1 eukaryotic expression vector (Invitrogen). The RAW264.7 cells with IRG1 stable overexpression were selected as previously reported (33).
Reagents
LPS (0111:B4), Pam3Cys4, and N-acetylcysteine (NAC) were from Sigma. Antibodies specific to anti-IRG1 (ab122624), anti-TAK1 phosphorylated at Tyr-187 (ab79583) were from Abcam. Antibodies against Erk phosphorylated at Thr-202/Tyr-204 (E10), Jnk phosphorylated at Thr-183/Tyr-185 (G9), p38 phosphorylated at Thr-180/Tyr-182 (9211), IRF3 phosphorylated at Ser-396 (4D4G), IKKα/β phosphorylated at Ser-176/Ser-180 (16A6), IκBα (4812), A20 (4625), and IRAK-M (4369) were from Cell Signaling Technology. Antibodies specific to SOCS1 (N-18), p65 (sc372), SHIP (sc-8425), and β-actin (sc-130656) were from Santa Cruz.
RNA Interference
Small interfering RNA targeting Irg1 and A20 were from Dharmacon. siRNA duplexes were transfected into primary peritoneal macrophages or RAW264.7 cells using INTERFERin Reagent from Polyplus as described previously (34). Cells were cultured for an additional 36 h before LPS stimulation.
RNA Quantification and Quantitative Real-time PCR
Total RNA was extracted with TRIzol reagent (Invitrogen) following the manufacturer's instructions. RNA concentrations were determined with a NanoDrop instrument (NanoDrop Technologies). Quantitative real-time PCR (Q-PCR) analysis was performed by Light Cycler (Roche Applied Science) and the SYBR RT-PCR kit (Takara) as described previously (33). The primers used for IRG1 were: hIRG1 forward, 5′-CGTGTTATTCAGAGGAGCAAGAG-3′, reverse, 5′-AGCATATGTGGGCGGGAG-3′; mouse Irg1 forward, 5′-GCGAACGCTGCCACTCA-3′, reverse, 5′-ATCCCAGGCTTGGAAGGTC-3′. The relative expression level of mRNAs was normalized by the level of β-actin expression in each sample.
Immunoblot
Cells were lysed with cell lysis buffer supplemented with protease inhibitor mixture (Calbiochem). Protein concentrations of the extracts were measured with BCA assay (Pierce). The isolation of nuclear proteins and immunoblot analysis were performed as described previously (34).
Cytokine Detection
TNF-α, IL-6, and IFN-β in the supernatants and serum were measured with ELISA kits (R&D Systems).
ROS Measurement
Peritoneal macrophages (1 × 106 cell/assay) were incubated with 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA) (Invitrogen, 10 μg/ml) for 30 min, and then stimulated with LPS for the indicated times. The fluorescence intensity, reflecting intracellular ROS levels, was immediately measured using LSR II (BD Biosciences). The level of ROS was expressed as mean fluorescence intensities generated by counting 10,000 cells.
Chromatin Immunoprecipitation Assay (ChIP)
A ChIP assay kit was used according to the manufacturer's instructions (Millipore) for these assays. Macrophages (2 × 107 cells) were fixed with 1% (v/v) formaldehyde and incubated for 5 min at 37 °C. Cells were lysed and chromatin was sheared by liquid phase sonication of samples in Eppendorf tubes immersed in ice-cold water as described previously (35). H3K4me3 antibodies to precipitate the chromatin fragments were prebound to Invitrogen Dynabeads in 0.5% BSA in PBS. Diluted chromatin preparations were incubated overnight with Dynabeads-antibody complexes. Immunoprecipitated DNA and input DNA were analyzed by quantitative real-time PCR and results are presented as percent of input. The following primers were used for amplification of A20 promoters: 5′-GATCTCACTCTGCACTGCATCC-3′ and 5′-GGCTTTGAAGTCTGGGCTGT-3′.
Statistical Analysis
The statistical significance of comparisons between two groups was determined with Student's t test. A p value < 0.05 was considered statistically significant.
RESULTS
High Inducible Expression of IRG1 in LPS-tolerized Mouse Macrophages and PBMCs of Septic Patients
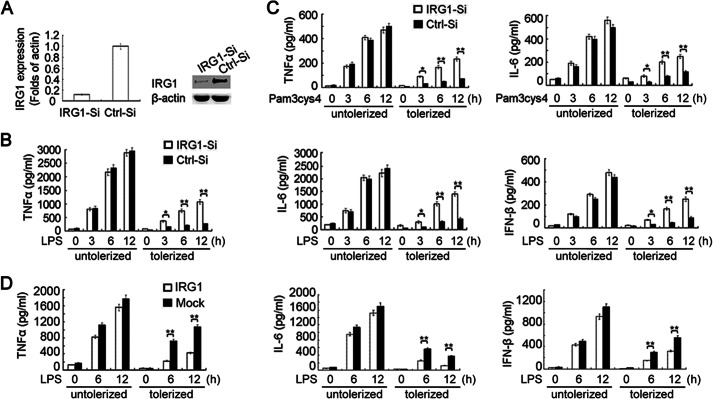
We first explored IRG1 expression in LPS-tolerized mouse macrophages. Consistent with previous reports, LPS stimulation vigorously induced up-regulation of Irg1 (Fig. 1A). The mRNA level of Irg1 reached the peak (866-fold of unstimulated control) at 6 h and decreased gradually at 12 h after LPS stimulation. After stimulating with LPS for 12 h, the culture medium was removed and macrophages were re-stimulated with the second round of LPS. The mRNA level of Irg1 was significantly induced, with even higher expression, in similar kinetics as the first round of stimulation (Fig. 1A). The LPS-induced protein level of IRG1 showed the same pattern in LPS-untolerized and -tolerized mouse macrophages (Fig. 1B). To investigate whether IRG1 is related to sepsis, we collected the PBMCs from patients during and after acute sepsis. The mRNA expression level of IRG1 was much higher in the PBMCs from patients with acute sepsis, and IRG1 expression also remained at a higher level in these septic patients even after recovery (Fig. 1C). The inducible expression pattern of IRG1 in LPS-tolerized mouse macrophages and PBMCs of septic patients indicated that IRG1 may function in a hypoinflammation state of sepsis and in the LPS-tolerized model.
FIGURE 1.
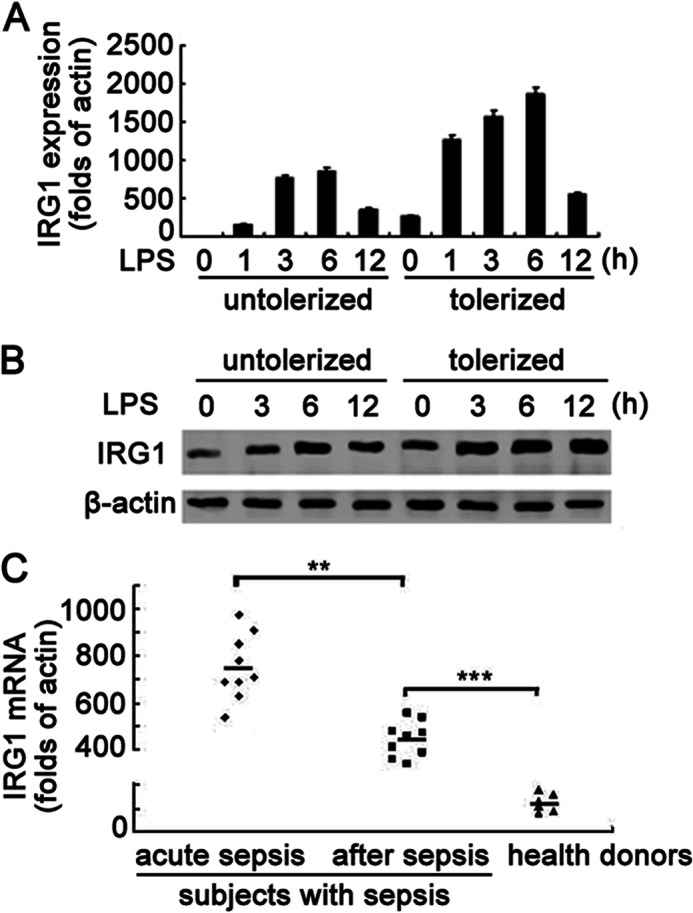
IRG1 expression is significantly up-regulated in LPS-tolerized mouse macrophages and PBMCs from septic patients. A, macrophages were left untreated (untolerized) or treated with LPS (100 ng/ml) for 12 h (tolerized), and then challenged with a second round of LPS stimulation (100 ng/ml) as indicated. The mRNA expression levels of Irg1 in macrophages were analyzed by Q-PCR. Data were normalized to the relative expression of the β-actin. B, Western blot of IRG1 protein expression in macrophages as in A, with β-actin as a loading control. C, Q-PCR analysis of the mRNA expression of IRG1 in PBMCs of subjects within 24 h after the diagnosis of sepsis (acute sepsis group), or 1 day after leaving the ICU with body recovery (after sepsis group), as well as healthy donors. Data were normalized to the relative expression of the β-actin reference gene. Data are shown as mean ± S.E. of three independent experiments (A and C) or are representative of three independent experiments with similar results (B). **, p < 0.01; ***, p < 0.001.
IRG1 Suppresses Proinflammatory Cytokines and IFN-β Production in LPS-tolerized Macrophages
Taking into account the vigorously inducible expression of IRG1, we next determined whether knockdown of IRG1 expression by siRNA could affect the TLR-triggered production of proinflammatory cytokines and IFN-β in both LPS-untolerized and -tolerized macrophages. The knockdown efficiency of IRG1 was determined 6 h after the secondary LPS challenge, at which time point the induction of IRG1 expression was usually most vigorous. The endogenous Irg1 mRNA expression level in macrophages was decreased to 15% of control by siRNA, which was consistent with the substantially decreased protein level confirmed by Western blot analysis (Fig. 2A). Knockdown of IRG1 did not significantly affect the TLR4-induced production of proinflammatory cytokines (TNF-α and IL-6) and IFN-β in LPS-untolerized macrophages with the first round of LPS stimulation (Fig. 2B). However, knockdown of IRG1 increased TLR4-induced production of proinflammatory cytokines and IFN-β in LPS-tolerized macrophages after the second LPS stimulation (Fig. 2B). The mRNA level of proinflammatory cytokines (TNF-α and IL-6) and IFN-β in LPS-tolerized macrophages re-challenged with LPS were also increased by knockdown of IRG1 (data not shown).
FIGURE 2.
Knockdown of IRG1 increases production of proinflammatory cytokines and IFN-β in LPS-tolerized macrophages. A, Q-PCR of Irg1 mRNA expression or immunoblot assay of the IRG1 protein expression level in macrophages transfected with Irg1-specific siRNA (IRG1-Si) or scramble control (Ctrl-Si). B, ELISA of cytokine production in supernatants from LPS-tolerized (100 ng/ml) or -untolerized macrophages with IRG1 silencing or non-silencing, and re-stimulated with a secondary round of LPS (100 ng/ml) as indicated. C, ELISA of cytokine production in supernatants from LPS-tolerized (100 ng/ml) or -untolerized macrophages with IRG1 silencing or non-silencing, and re-stimulated with Pam3Cys4 (1 μg/ml) as indicated. D, ELISA of cytokine production in supernatants from LPS-tolerized (100 ng/ml) or -untolerized RAW264.7 cells stably transfected with Myc-tagged IRG-1, and re-stimulated with secondary LPS (100 ng/ml) as indicated. Data are representative of three independent experiments with similar results or are shown as mean ± S.D. of three independent experiments. *, p < 0.05; **, p < 0.01.
We further observed whether knockdown of IRG1 affected TLR2-triggered production of proinflammatory cytokines and IFN-β in LPS-tolerized macrophages, which were tolerized with LPS for 12 h and re-challenged with the TLR2 ligand Pam3Cys4. Similarly, knockdown of IRG1 did not affect the TLR2-triggered production of proinflammatory cytokines in LPS-untolerized macrophages stimulated directly with Pam3Cys4, but significantly increased the production of proinflammatory cytokines in LPS-tolerized macrophages (Fig. 2C).
We next investigated whether overexpression of IRG1 could inhibit TLR4-induced proinflammatory cytokine production. The Myc-tagged IRG-1 was stably overexpressed in macrophage cell line RAW264.7, and overexpression of IRG-1 was confirmed by Western blot analysis (data not shown). Overexpression of IRG1 inhibited TLR4-induced production of proinflammatory cytokines and IFN-β in LPS-tolerized macrophages, whereas not in untolerized macrophages (Fig. 2D). These results indicated that IRG1 might suppress proinflammatory cytokine and IFN-β production in LPS-tolerized macrophages, thus possibly promoting immune suppressiveness or hypo-responsiveness in septic patients.
IRG1 Suppresses NF-κB and IRF3 Activation in LPS-tolerized Macrophages
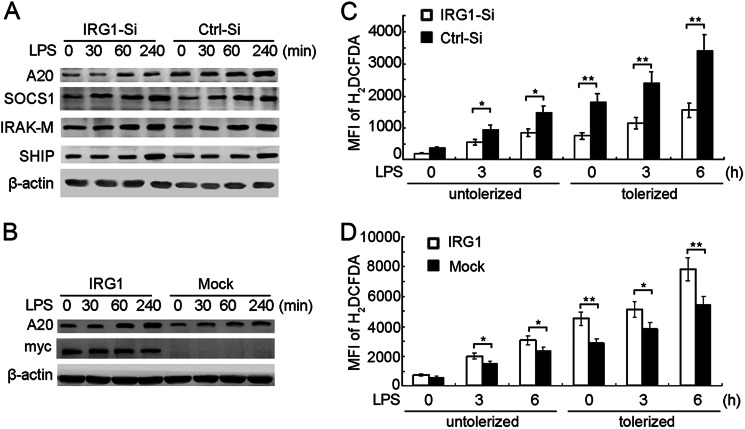
To further confirm the function of inducible IRG1 in a LPS-tolerized macrophage model, we observed the effects of IRG1 silencing on TLR4-triggered activation of MAPK, NF-κB, and IRF3 pathways in LPS-tolerized macrophages. Knockdown of IRG1 did not affect the activation of TAK1, JNK, and p38, but increased activation of IKKα/β, p65, ERK, and IRF3 (Fig. 3A). The degradation of IκBα was also more vigorous in IRG1-silenced macrophages, which led to more activation of the transcription factor NF-κB. Knockdown of IRG1 increased nuclear translocation of transcriptors p65 and IRF3 (Fig. 3B). Accordingly, overexpression of IRG1 inhibited nuclear translocation of p65, AP-1, and IRF3 in LPS-tolerized macrophages (Fig. 3C). These results indicated that IRG1 might suppress p65 and IRF3 activation in LPS-tolerized macrophages, thus contributing to suppression of TLR4-triggered production of proinflammatory cytokines and IFN-β.
FIGURE 3.
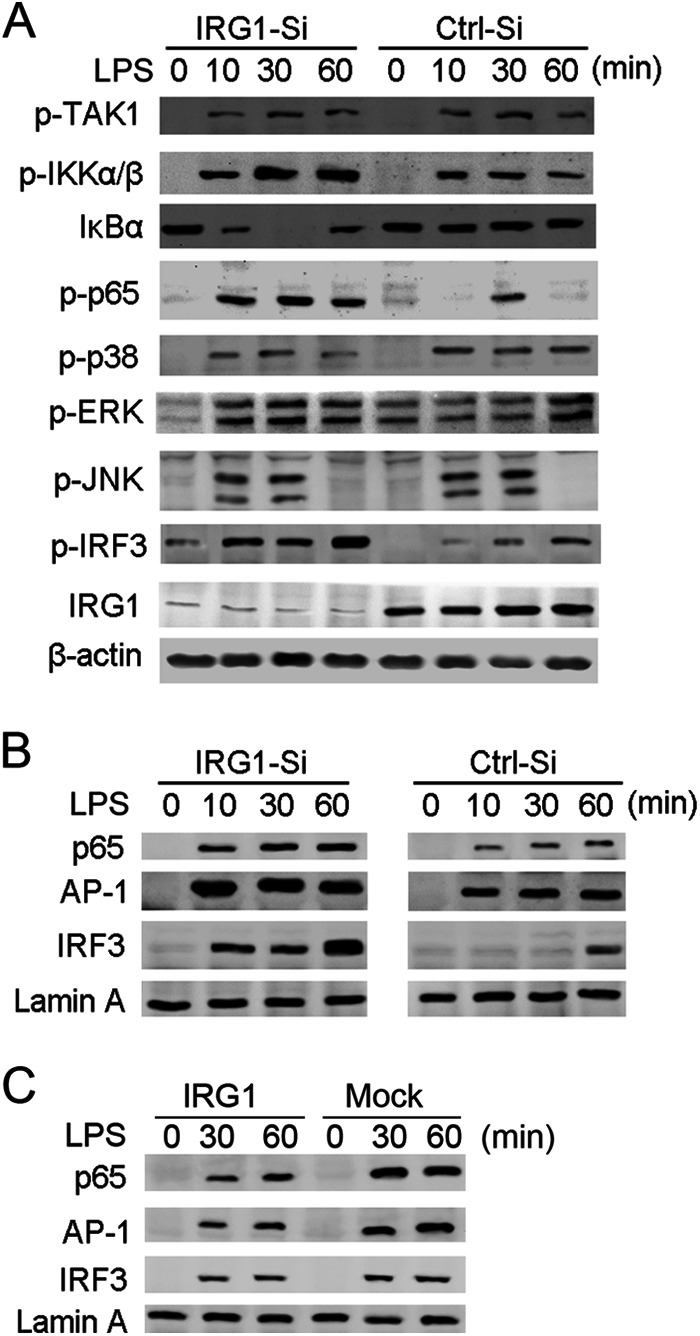
Knockdown of IRG1 increases NF-κB and IRF3 activation in LPS-tolerized macrophages. A, immunoblot analysis with the indicated antibodies of cell lysis from macrophages transfected with Irg1-specific siRNA (IRG1-Si) or scramble control (Ctrl-Si), tolerized with LPS (100 ng/ml), and re-stimulated with a second round of LPS (100 ng/ml) as indicated. B, immunoblot analysis with the indicated antibodies of nuclear proteins from macrophages as in A. C, immunoblot analysis with the indicated antibodies of cell lysis from RAW264.7 cells stably transfected with Myc-tagged IRG1, tolerized with LPS, and re-stimulated with a second round of LPS as indicated. Data are representative of three independent experiments with similar results.
IRG1 Promotes A20 Expression and ROS Production in LPS-tolerized Macrophages
The above data suggested that knockdown of IRG1 did not affect TAK1 activation, but enhanced downstream IKKα/β activation, indicating that IRG1 might function downstream of TAK1 and upstream of IKKα/β. A20, SOCS1, IRAK-M, and SHIP were identified as major inhibitors in endotoxin tolerance (13). We then observed whether knockdown of IRG1 affected the expression of A20, SOCS1, IRAK-M, and SHIP in LPS-tolerized macrophages. Interestingly, we found that expression of A20 was potently decreased in LPS-tolerized macrophages by knockdown of IRG1, whereas the expression of SOCS1, IRAK-M, and SHIP were not affected, suggesting a nonredundant role of A20 in IRG1-mediated endotoxin tolerance (Fig. 4A). Overexpression of IRG1 increased TLR-4-induced expression of A20 in LPS-tolerized macrophages (Fig. 4B). Considering that A20 negatively regulates NF-κB activation by deubiquitinating the K63-linked chain of NEMO, which is the indispensible molecule for IKKα/β activation (36, 37), we proposed that regulation of A20 expression by IRG-1 might be important in the LPS-tolerized macrophage model.
FIGURE 4.
IRG1 increases A20 expression and ROS production in LPS-tolerized macrophages. A, immunoblot analysis with the indicated antibodies of cell lysis from macrophages transfected with Irg1-specific siRNA (IRG1-Si) or scramble control (Ctrl-Si), tolerized with LPS and re-stimulated with a second round of LPS as indicated. B, immunoblot analysis with the indicated antibodies of cell lysis from RAW264.7 cells stably transfected with Myc-tagged IRG1, tolerized with LPS, and re-stimulated with a second round of LPS as indicated. C, FACS analysis of macrophages as in A stained with H2DCFDA. D, FACS analysis of macrophages as in B stained with H2DCFDA. Data are representative of three independent experiments with similar results (A and B) or were present as mean fluorescence index (MFI) of H2DCFDA and mean ± S.E. (C and D). *, p < 0.05; **, p < 0.01.
Novel insights have led to the suggestion that ROS and ROS-induced genes are important regulators of inflammation and are also involved in endotoxin tolerance (18). Whether IRG1 knockdown could affect ROS production was determined by FACS analysis. IRG1 knockdown decreased LPS-induced ROS production in LPS-untolerized macrophages, and the decrease of ROS production by IRG1 knockdown was much more remarkable in LPS-tolerized macrophages (Fig. 4C). Accordingly, IRG1 overexpression increased LPS-induced ROS production in LPS-untolerized macrophages, and the increase of ROS production by IRG1 overexpression was much more remarkable in LPS-tolerized macrophages (Fig. 4D). These results indicated that IRG1 might function in LPS-tolerized macrophages through up-regulation of A20 expression and ROS production.
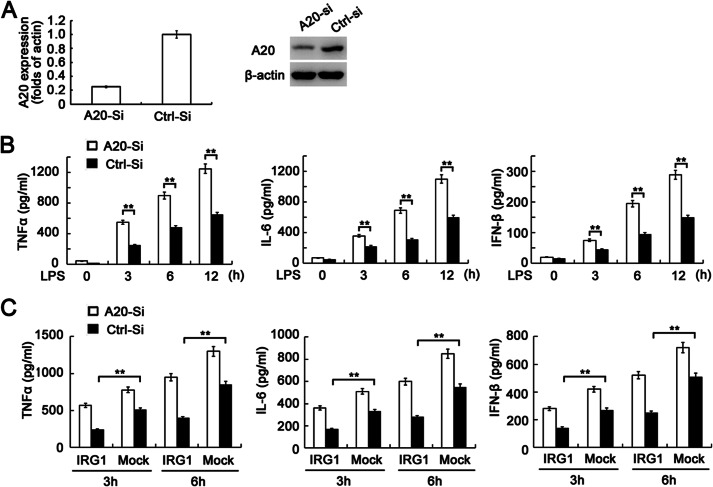
A20 and ROS Production Is Critical for IRG1 Function in LPS-tolerized Macrophages
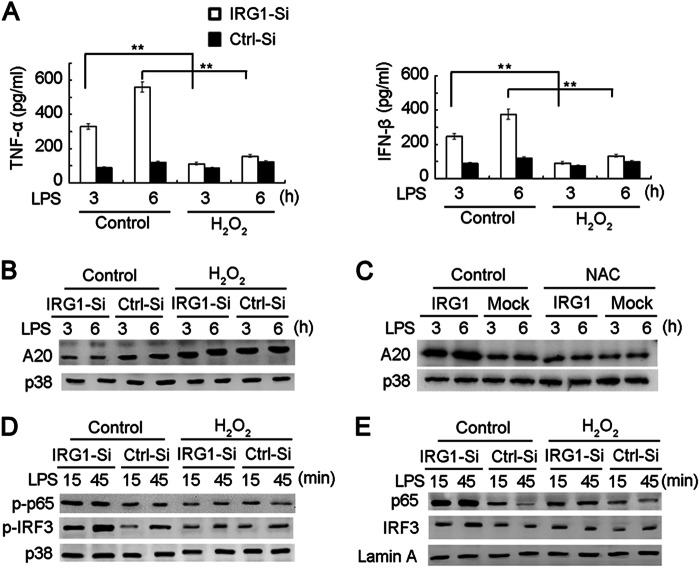
We next investigated the relationship between A20 and ROS in the function of IRG1 in an endotoxin-tolerized model. A20 expression was silenced in RAW264.7 cells by specific siRNA (Fig. 5A). Consistent with previous reports (13), knockdown of A20 expression increased LPS-induced production of proinflammatory cytokines and IFN-β in LPS-tolerized macrophages (Fig. 5B). To further explore the role of A20 in IRG1-induced endotoxin tolerance, we silenced A20 expression in IRG1-overexpressing RAW264.7 cells, and found that overexpression of IRG1 significantly inhibited LPS-induced production of proinflammatory cytokines and IFN-β in LPS-tolerized macrophages. However, the inhibitory effect was abolished when A20 was silenced. The data indicate the increased A20 expression is crucial for IRG1 function in LPS-tolerized macrophages (Fig. 5C). Taking into account that IRG1 knockdown led to decreased ROS production in LPS-tolerized macrophages, we supplemented hydrogen peroxide (H2O2, 20 μm) into macrophages before the second round of LPS challenge. The H2O2 supplement significantly decreased the production of TNF-α and IFN-β in IRG1-silenced macrophages, but did not significantly affect TNF-α production in control macrophages, in which IRG1 was highly expressed (Fig. 6A), suggesting the role of ROS in IRG1-promoted endotoxin tolerance. The supplement of H2O2 also abrogated the decrease of A20 expression in IRG1-silenced macrophages (Fig. 6B). Accordingly, increased A20 expression in IRG1-overexpressing RAW264.7 cells was abrogated by the ROS scavenger NAC (Fig. 6C), indicating that ROS is indispensable for IRG1-induced up-regulation of A20 expression. Moreover, the increased activation and nuclear translocation of p65 and IRF3 in IRG1-silenced macrophages were also abrogated with the supplement of H2O2 (Fig. 6, D and E). These results indicate that IRG1 might up-regulate A20 expression by LPS-tolerized macrophages increasing ROS production.
FIGURE 5.
Knockdown of A20 expression abrogates the decrease of cytokine production in LPS-tolerized macrophages by IRG1 overexpression. A, Q-PCR analysis of A20 mRNA expression or immunoblot assay of the A20 protein expression level in RAW264.7 cells transfected with A20-specific siRNA (A20-Si) or scramble control (Ctrl-Si). B, ELISA of cytokine production in supernatants from LPS-tolerized (100 ng/ml) RAW264.7 cells with A20 silencing or non-silencing, and re-stimulated with a secondary round of LPS (100 ng/ml) as indicated. C, IRG1-overexpressing RAW264.7 cells were transfected with A20-specific siRNA, and tolerized with LPS (100 ng/ml) for 12 h, then re-stimulated with secondary LPS (100 ng/ml) as indicated. Cytokine production in supernatants was detected by ELISA. Data are representative of three independent experiments with similar results or are shown as mean ± S.D. of three independent experiments. **, p < 0.01.
FIGURE 6.
Supplement of ROS abrogates the increase of cytokine production and signal pathways by IRG1 knockdown in LPS-tolerized macrophages. A, ELISA of cytokine production in supernatants from macrophages that were transfected with Irg1-specific siRNA (IRG1-Si) or scramble control (Ctrl-Si), tolerized with LPS and supplemented with or without H2O2 (20 μm) before re-stimulation with a second round of LPS as indicated. B–E, immunoblot analysis with the indicated antibodies in cell lysis (B and D) or nuclear proteins (E) from macrophages as in A. Immunoblot analysis with the indicated antibodies in cell lysis from RAW264.7 cells stably expressing IRG1, tolerized with LPS, and supplemented with or without NAC (20 mm) before re-stimulation with a second round of LPS are as indicated (C). Data are shown as mean ± S.D. of three independent experiments (A) or are representative of three independent experiments with similar results (B–E). **, p < 0.01.
ROS Production Increases A20 Transcription in LPS-tolerized Macrophages by Mediating Histone Modification
We next addressed how ROS could up-regulate A20 expression. We first observed whether the mRNA of A20 is affected by ROS. Consistent with the above results, the supplement of H2O2 up-regulated mRNA expression of A20 in IRG1-silenced macrophages (Fig. 7A), whereas the addition of the ROS scavenger NAC decreased A20 mRNA expression in IRG1-overexpressing macrophages (Fig. 7B), indicating that ROS increases A20 transcription. Taking into account that NF-κB is an important transcriptor of A20 (37, 38), and IRG1 knockdown leads to increased NF-κB activation (Fig. 3) but decreased A20 expression (Fig. 4), we thus excluded the possibility that NF-κB is responsible for A20 transcription. We next determined whether alternated chromatin histone modification is responsible for increased A20 transcription by the ChIP assay. The methylated H3K4me3 of chromatin histone was pulled down following quantization of the A20 promoter domain by Q-PCR. IRG1 knockdown decreased methylation of H3K4, whereas supplement of H2O2 abrogated the phenomena in LPS-tolerized macrophages (Fig. 7C). Accordingly, the increased methylation of H3K4 in IRG1-overexpressing RAW264.7 cells was abolished in the presence of NAC (Fig. 7D). These results indicate that IRG1 increased A20 expression through ROS-medicated histone modification of the A20 promoter domain in an epigenetic level.
FIGURE 7.
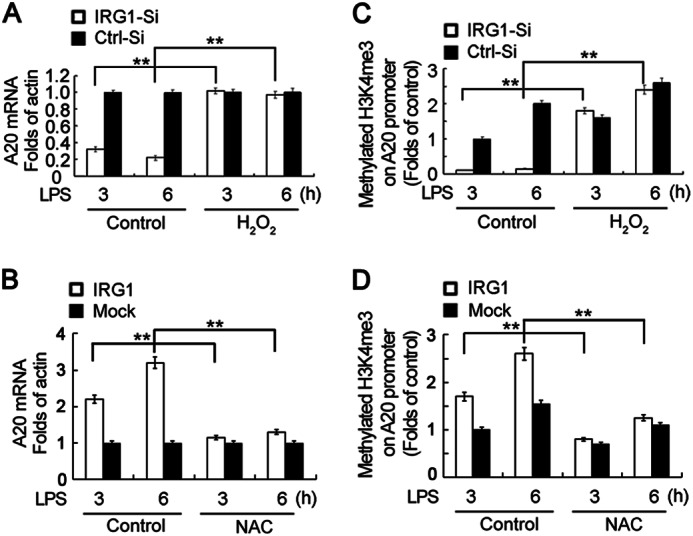
IRG1 enhances A20 transcription by increasing H3K4me3 of the A20 promoter through ROS in macrophages. A, Q-PCR analysis of the A20 mRNA expression level in macrophages that were transfected with Irg1-specific siRNA (IRG1-Si) or scramble control (Ctrl-Si), tolerized with LPS, and supplemented with or without H2O2 before re-stimulation with a second round of LPS as indicated. B, Q-PCR analysis of the A20 mRNA expression level in RAW264.7 cells stably expressing IRG1, tolerized with LPS, and supplemented with or without NAC before re-stimulation with a second round of LPS as indicated. C, Q-PCR of the A20 promoter domain in CHIP production precipitated with H3K4me3 antibody from macrophages as in A. D, Q-PCR of the A20 promoter domain in CHIP production precipitated with H3K4me3 antibody from macrophages as in B. Data are shown as mean ± S.E. of three independent experiments. **, p < 0.01.
DISCUSSION
Sepsis is a life threatening problem worldwide. In the early period, patients may die from hyperinflammation with cytokine storm induced by bacteria, which emphasize the importance of negative regulation of the innate system (9, 16, 17, 32, 34). Numerous agents aimed to suppress hyperinflammation with the cytokine storm in this initial phase have improved clinical outcome and increased survival of the majority of patients during this early period (39). However, in the late period of sepsis, the foregoing hypoinflammation status of the innate immune system often leads to SAIS and secondary infection with organisms not typically pathogenic in the immunocompetent host (1, 2), indicating the importance of an endotoxin-tolerant mechanism. Up to now, there are very few effective approaches verified to address this problem at late stage of sepsis with hypoinflammation or endotoxin tolerance. In this study, we investigated whether Irg1, one of the LPS-inducible genes, was involved in sepsis, sepsis-associated immunosuppression, and endotoxin tolerance. We have demonstrated that IRG1 is important in the maintenance of the reduced inflammatory cytokine production in LPS-tolerized macrophages by promoting A20 expression through a ROS-dependent manner.
IRG1 was highly up-regulated in PBMCs from patients during sepsis and gradually decreased after recovery from sepsis. The significant up-regulation of IRG1 was also observed in LPS-stimulated mouse macrophages, which indicated that IRG1 might be involved in sepsis, SAIS, and endotoxin tolerance. However, how TLR signals triggered Irg1 transcription or up-regulation remains controversial. Several previous studies about mouse dendritic cells and macrophages have shown that LPS-induced Irg1 transcription was dependent via TLR4 in MyD88-dependent and IFN-β-independent manners (40–42). More recently, using LPS-stimulated macrophages as a cell model, the Irg1 gene was found to be induced in MyD88−/− and TRIF−/− macrophages but not in MyD88−/−TRIF−/− macrophages, indicating that both MyD88 and TRIF signal pathways that can lead to IRG1 expression (43). However, most recently, Shi et al. (44) found that M. tuberculosis-induced IRG1 expression was TLR2 and TLR4 independent, but required IFNαβR and STAT1. Furthermore, with its AU-rich element in the 3′-UTR, Irg1 expression was also shown to be modulated by a post-transcriptional mechanism (23). None of these studies, however, investigated the downstream molecular mechanisms and transcription factors that lead directly to the induced Irg1 mRNA expression, which needs further investigation.
The extremely high expression of IRG1 draws us to choose knockdown of IRG1 to observe its function in endotoxin tolerance. Until now, to our knowledge, no exact function of IRG1 in the regulation of innate immune responses has been reported, although many previous reports declared increased IRG1 expression during various infections and TLR ligand stimulation (23, 24). Our data revealed that IRG1 knockdown increased both TLR4- and TLR2-triggered production of proinflammatory cytokines (TNF-α and IL-6) and IFN-β in LPS-tolerized macrophages, other than in LPS-untolerized macrophages, suggesting a selective role of IRG1 in endotoxin tolerance but not the regulation of TLR signaling. Accordingly, the increased cytokines was further confirmed by the observation of the increased p65 and IRF3 activation in LPS-tolerized macrophages once silenced of IRG1. Thus, we hypothesized that the induction of IRG1 expression during sepsis may not contribute to the host from excessive inflammatory responses (“cytokines storm”) in the early phase hyper-responsive period by suppressing cytokines production, but importantly, inducible IRG1 may function in the late hypo-responsive period, causing decreased antibacterial immunity and leading to opportunistic infection of the survivors from sepsis.
Although the molecular mechanism of negative regulation of TLR signaling has been intensively investigated, the molecular mechanism of endotoxin tolerance remains to be further illuminated. SOCS1, IRAK-M, SHIP, and A20 have been linked to LPS-induced tolerance (13, 16, 17). A20 is an early NF-κB-responsive gene that encodes a ubiquitin-editing protein that deubiquitinating K63-lined and ubiquitinating K48-linked chains onto substrate proteins (36, 45). A20 is involved in the negative feedback regulation of NF-κB and IRF3 activation in TLR signaling. Moreover, A20 is not only indispensable for restricting inflammation in response to bacterial infection but also seems to control the immune response to viral infection (46). In our data, silence of IRG1 increases cytokine production in LPS-tolerized macrophages and thus disrupts endotoxin tolerance, with increased A20 production, but did not interfere with the expression of SOCS1, IRAK-M, or SHIP, suggesting a nonredundant role of A20 in IRG1-mediated LPS tolerance.
As NF-κB triggers de novo transcription of A20 mRNA (37, 38), and NF-κB activation was decreased with up-regulation of IRG1 expression in LPS-tolerized macrophages, thus IRG1 may not up-regulate A20 expression through NF-κB activation. Furthermore, the hypo-responsiveness to the secondary LPS challenge in LPS-tolerized macrophages was mediated by the coordinated action of two inhibitory mechanisms: the suppression of the TLR-triggered signaling pathways and the modulation of chromatin remodeling (13, 47, 48). We further found that decreased ROS production is responsible for decreased H3K4me3 modification and A20 expression in this model. Supplement of ROS production abrogated the IRG1 knockdown function in breaking endotoxin tolerance by increasing A20 transcription through H3K4 methylation of histone on the A20 promoter domain.
In conclusion, our present work demonstrates that the highly inducible expression of IRG1 feedback promotes endotoxin tolerance by increasing A20 expression at the epigenetic level through ROS production. Our study provides IRG1 as a new molecule involved in sepsis and endotoxin tolerance, which throws new lights on the molecular mechanism for hypoinflammation of sepsis and endotoxin tolerance. Also, these results suggest a new mechanism of A20 expression at the epigenetic level, and also suggest ROS as a novel drug-targeting substrate to treat the hypo-responsiveness in sepsis.
Acknowledgments
We thank Panpan Ma, Yan Li, and Mei Jin for technical assistance, and Dr. Jin Hou for helpful discussion.
This work was supported by National Key Basic Research Program of China Grants 2013CB530503 and 2012CB910202, National Natural Science Foundation of China Grants 81230074, 81123006, and 31070789, and the Key Project of the “Twelfth Five-year Plan” for Medical Science Development of PLA (BWS12J027).
- SAIS
- sepsis-associated immunosuppression
- IKK
- IκB kinase
- IRAK
- interleukin 1-associated kinase
- IRF
- IFN regulatory factor
- IRG1
- immune responsive gene 1
- NAC
- N-acetylcysteine
- ROS
- reactive oxygen species
- SOCS1
- suppressor of cytokine signaling 1
- TAK1
- TGF-β activated kinase 1
- TRIF
- TIR-domain-containing adaptor protein inducing IFN-β
- PBMC
- peripheral blood mononuclear cell
- Q-PCR
- quantitative real-time PCR
- TLR
- Toll-like receptor
- DCFDA
- 2′,7′-dichlorodihydrofluorescein diacetate.
REFERENCES
- 1. Hotchkiss R. S., Opal S. (2010) Immunotherapy for sepsis. A new approach against an ancient foe. N. Engl. J. Med. 363, 87–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riedemann N. C., Guo R. F., Ward P. A. (2003) Novel strategies for the treatment of sepsis. Nat. Med. 9, 517–524 [DOI] [PubMed] [Google Scholar]
- 3. Boomer J. S., To K., Chang K. C., Takasu O., Osborne D. F., Walton A. H., Bricker T. L., Jarman S. D., 2nd, Kreisel D., Krupnick A. S., Srivastava A., Swanson P. E., Green J. M., Hotchkiss R. S. (2011) Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306, 2594–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barton G. M., Kagan J. C. (2009) A cell biological view of Toll-like receptor function. Regulation through compartmentalization. Nat. Rev. Immunol. 9, 535–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akira S., Uematsu S., Takeuchi O. (2006) Pathogen recognition and innate immunity. Cell 124, 783–801 [DOI] [PubMed] [Google Scholar]
- 6. Kawai T., Akira S. (2010) The role of pattern-recognition receptors in innate immunity. Update on Toll-like receptors. Nat. Immunol. 11, 373–384 [DOI] [PubMed] [Google Scholar]
- 7. Liu J., Liu S., Cao X. (2012) Highlights of the advances in basic immunology in 2011. Cell. Mol. Immunol. 9, 197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kondo T., Kawai T., Akira S. (2012) Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 33, 449–458 [DOI] [PubMed] [Google Scholar]
- 9. O'Neill L. A. (2008) When signaling pathways collide. Positive and negative regulation of Toll-like receptor signal transduction. Immunity 29, 12–20 [DOI] [PubMed] [Google Scholar]
- 10. Pena O. M., Pistolic J., Raj D., Fjell C. D., Hancock R. E. (2011) Endotoxin tolerance represents a distinctive state of alternative polarization (M2) in human mononuclear cells. J. Immunol. 186, 7243–7254 [DOI] [PubMed] [Google Scholar]
- 11. Cavaillon J. M., Adib-Conquy M. (2006) Bench-to-bedside review. Endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit. Care 10, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freudenberg M. A., Galanos C. (1988) Induction of tolerance to lipopolysaccharide (LPS)-d-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect. Immun. 56, 1352–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Biswas S. K., Lopez-Collazo E. (2009) Endotoxin tolerance. New mechanisms, molecules and clinical significance. Trends Immunol. 30, 475–487 [DOI] [PubMed] [Google Scholar]
- 14. Dangi A., Sumpter T. L., Kimura S., Stolz D. B., Murase N., Raimondi G., Vodovotz Y., Huang C., Thomson A. W., Gandhi C. R. (2012) Selective expansion of allogeneic regulatory T cells by hepatic stellate cells. Role of endotoxin and implications for allograft tolerance. J. Immunol. 188, 3667–3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Broug-Holub E., Toews G. B., van Iwaarden J. F., Strieter R. M., Kunkel S. L., Paine R., 3rd, Standiford T. J. (1997) Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia. Elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect. Immun. 65, 1139–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mansell A., Smith R., Doyle S. L., Gray P., Fenner J. E., Crack P. J., Nicholson S. E., Hilton D. J., O'Neill L. A., Hertzog P. J. (2006) Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat. Immunol. 7, 148–155 [DOI] [PubMed] [Google Scholar]
- 17. Boone D. L., Turer E. E., Lee E. G., Ahmad R. C., Wheeler M. T., Tsui C., Hurley P., Chien M., Chai S., Hitotsumatsu O., McNally E., Pickart C., Ma A. (2004) The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 5, 1052–1060 [DOI] [PubMed] [Google Scholar]
- 18. Ho P. C., Tsui Y. C., Feng X., Greaves D. R., Wei L. N. (2012) NF-κB-mediated degradation of the coactivator RIP140 regulates inflammatory responses and contributes to endotoxin tolerance. Nat. Immunol. 13, 379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoetzenecker W., Echtenacher B., Guenova E., Hoetzenecker K., Woelbing F., Brück J., Teske A., Valtcheva N., Fuchs K., Kneilling M., Park J. H., Kim K. H., Kim K. W., Hoffmann P., Krenn C., Hai T., Ghoreschi K., Biedermann T., Röcken M. (2012) ROS-induced ATF3 causes susceptibility to secondary infections during sepsis-associated immunosuppression. Nat. Med. 18, 128–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park S. H., Park-Min K. H., Chen J., Hu X., Ivashkiv L. B. (2011) Tumor necrosis factor induces GSK3 kinase-mediated cross-tolerance to endotoxin in macrophages. Nat. Immunol. 12, 607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee C. G., Demarquoy J., Jackson M. J., O'Brien W. E. (1994) Molecular cloning and characterization of a murine LPS-inducible cDNA. J. Immunol. 152, 5758–5767 [PubMed] [Google Scholar]
- 22. Cheon Y. P., Xu X., Bagchi M. K., Bagchi I. C. (2003) Immune-responsive gene 1 is a novel target of progesterone receptor and plays a critical role during implantation in the mouse. Endocrinology 144, 5623–5630 [DOI] [PubMed] [Google Scholar]
- 23. Basler T., Jeckstadt S., Valentin-Weigand P., Goethe R. (2006) Mycobacterium paratuberculosis, Mycobacterium smegmatis, and lipopolysaccharide induce different transcriptional and post-transcriptional regulation of the IRG1 gene in murine macrophages. J. Leukoc. Biol. 79, 628–638 [DOI] [PubMed] [Google Scholar]
- 24. Degrandi D., Hoffmann R., Beuter-Gunia C., Pfeffer K. (2009) The proinflammatory cytokine-induced IRG1 protein associates with mitochondria. J. Interferon Cytokine Res. 29, 55–67 [DOI] [PubMed] [Google Scholar]
- 25. Rodríguez N., Mages J., Dietrich H., Wantia N., Wagner H., Lang R., Miethke T. (2007) MyD88-dependent changes in the pulmonary transcriptome after infection with Chlamydia pneumoniae. Physiol. Genomics 30, 134–145 [DOI] [PubMed] [Google Scholar]
- 26. Goodridge H. S., Simmons R. M., Underhill D. M. (2007) Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J. Immunol. 178, 3107–3115 [DOI] [PubMed] [Google Scholar]
- 27. Tyner J. W., Uchida O., Kajiwara N., Kim E. Y., Patel A. C., O'Sullivan M. P., Walter M. J., Schwendener R. A., Cook D. N., Danoff T. M., Holtzman M. J. (2005) CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nat. Med. 11, 1180–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spach K. M., Pedersen L. B., Nashold F. E., Kayo T., Yandell B. S., Prolla T. A., Hayes C. E. (2004) Gene expression analysis suggests that 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by stimulating inflammatory cell apoptosis. Physiol. Genomics 18, 141–151 [DOI] [PubMed] [Google Scholar]
- 29. Levy M. M., Fink M. P., Marshall J. C., Abraham E., Angus D., Cook D., Cohen J., Opal S. M., Vincent J. L., Ramsay G. (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 31, 1250–1256 [DOI] [PubMed] [Google Scholar]
- 30. Pan H., O'Brien T. F., Zhang P., Zhong X. P. (2012) The role of tuberous sclerosis complex 1 in regulating innate immunity. J. Immunol. 188, 3658–3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hicks P., Cooper D. J. (2008) The Surviving Sepsis Campaign. International guidelines for management of severe sepsis and septic shock, 2008. Crit. Care Resusc. 10, 8. [PubMed] [Google Scholar]
- 32. Han C., Jin J., Xu S., Liu H., Li N., Cao X. (2010) Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat. Immunol. 11, 734–742 [DOI] [PubMed] [Google Scholar]
- 33. Liu X., Zhan Z., Li D., Xu L., Ma F., Zhang P., Yao H., Cao X. (2011) Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase Btk. Nat. Immunol. 12, 416–424 [DOI] [PubMed] [Google Scholar]
- 34. Xu S., Liu X., Bao Y., Zhu X., Han C., Zhang P., Zhang X., Li W., Cao X. (2012) Constitutive MHC class I molecules negatively regulate TLR-triggered inflammatory responses via the Fps-SHP-2 pathway. Nat. Immunol. 13, 551–559 [DOI] [PubMed] [Google Scholar]
- 35. Chen W., Han C., Xie B., Hu X., Yu Q., Shi L., Wang Q., Li D., Wang J., Zheng P., Liu Y., Cao X. (2013) Induction of Siglec-G by RNA viruses inhibits the innate immune response by promoting RIG-I degradation. Cell 152, 467–478 [DOI] [PubMed] [Google Scholar]
- 36. Zhang S. Q., Kovalenko A., Cantarella G., Wallach D. (2000) Recruitment of the IKK signalosome to the p55 TNF receptor. RIP and A20 bind to NEMO (IKKγ) upon receptor stimulation. Immunity 12, 301–311 [DOI] [PubMed] [Google Scholar]
- 37. Coornaert B., Carpentier I., Beyaert R. (2009) A20. Central gatekeeper in inflammation and immunity. J. Biol. Chem. 284, 8217–8221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krikos A., Laherty C. D., Dixit V. M. (1992) Transcriptional activation of the tumor necrosis factor α-inducible zinc finger protein, A20, is mediated by κB elements. J. Biol. Chem. 267, 17971–17976 [PubMed] [Google Scholar]
- 39. Levy M. M., Dellinger R. P., Townsend S. R., Linde-Zwirble W. T., Marshall J. C., Bion J., Schorr C., Artigas A., Ramsay G., Beale R., Parker M. M., Gerlach H., Reinhart K., Silva E., Harvey M., Regan S., Angus D. C. (2010) The Surviving Sepsis Campaign. Results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 36, 222–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fairfax B. P., Davenport E. E., Makino S., Hill A. V., Vannberg F. O., Knight J. C. (2011) A common haplotype of the TNF receptor 2 gene modulates endotoxin tolerance. J. Immunol. 186, 3058–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takeuchi O., Kawai T., Mühlradt P. F., Morr M., Radolf J. D., Zychlinsky A., Takeda K., Akira S. (2001) Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13, 933–940 [DOI] [PubMed] [Google Scholar]
- 42. Hoshino K., Kaisho T., Iwabe T., Takeuchi O., Akira S. (2002) Differential involvement of IFN-β in Toll-like receptor-stimulated dendritic cell activation. Int. Immunol. 14, 1225–1231 [DOI] [PubMed] [Google Scholar]
- 43. Hirotani T., Yamamoto M., Kumagai Y., Uematsu S., Kawase I., Takeuchi O., Akira S. (2005) Regulation of lipopolysaccharide-inducible genes by MyD88 and Toll/IL-1 domain containing adaptor inducing IFN-β. Biochem. Biophys. Res. Commun. 328, 383–392 [DOI] [PubMed] [Google Scholar]
- 44. Shi S., Blumenthal A., Hickey C. M., Gandotra S., Levy D., Ehrt S. (2005) Expression of many immunologically important genes in Mycobacterium tuberculosis-infected macrophages is independent of both TLR2 and TLR4 but dependent on IFN-αβ receptor and STAT1. J. Immunol. 175, 3318–3328 [DOI] [PubMed] [Google Scholar]
- 45. Heyninck K., Beyaert R. (2005) A20 inhibits NF-κB activation by dual ubiquitin-editing functions. Trends Biochem. Sci. 30, 1–4 [DOI] [PubMed] [Google Scholar]
- 46. Lin R., Yang L., Nakhaei P., Sun Q., Sharif-Askari E., Julkunen I., Hiscott J. (2006) Negative regulation of the retinoic acid-inducible gene I-induced antiviral state by the ubiquitin-editing protein A20. J. Biol. Chem. 281, 2095–2103 [DOI] [PubMed] [Google Scholar]
- 47. Foster S. L., Hargreaves D. C., Medzhitov R. (2007) Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447, 972–978 [DOI] [PubMed] [Google Scholar]
- 48. Foster S. L., Medzhitov R. (2009) Gene-specific control of the TLR-induced inflammatory response. Clin. Immunol. 130, 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]